Abstract
The sterile sponges may lose a significant amount of lint during their routine use in surgery, which is usually neglected. We designed an experimental model to evaluate the potential of sterile sponges to lose lint and to induce foreign body reaction in surgically created wounds. In 15 Wistar rats, separate subcutaneous pockets were created. Left‐sided pockets were temporarily packed with sterile sponges, while the right‐sided pockets remained empty. All the pockets were then irrigated with sterile saline. The presence of microscopic sponge particles was evaluated in the irrigation materials. After 4 weeks, the presence of inflammation and foreign body reaction were evaluated histologically. Microscopic sponge remnants were present in 14 of 15 samples collected from the left sides. The presence of foreign body giant cells with ingested sponge particles and formation of well‐defined foreign body granulomas were observed only in samples collected from the left sides. A statistically significant difference was observed between the two sides when the intensity of inflammation was graded (P= 0·012). Cotton sponges used in surgery are shown to have a potential of losing invisible microscopic particles that have a potential to induce foreign body reaction.
Keywords: Foreign body giant cell, Foreign body reaction, Granuloma, Sterile sponge
Introduction
Sterile gauze/sponges made of cotton are widely used in the practice of medicine. They are applied to cover open wounds, to stop bleeding by compression, to clear away excess fluids during surgical interventions and to handle slippery tissue parts. As a consequence of their widespread use, they have become the most frequently retained foreign body during surgical procedures (1).
The concept that sterile sponges have a potential to induce inflammation and foreign body reaction when they are retained within the body is not new. Numerous reports have documented the frustrating outcomes of forgotten sterile sponges and towels almost in every possible place within the body. Nonetheless, many of these cases are not reported in the literature due to legal issues, so a true incidence of the situation is not clear 1, 2, 3, 4, 5, 6, 7, 8.
Our aim in designing this study was to evaluate whether the common cotton sponges used in surgery have a potential to lose microscopic lint, which stays within the wound through and after the operation, and whether the retained microscopic particles have a further potential to induce a foreign body reaction and alter the intensity of the inflammatory response already initiated by surgical trauma.
Methods
The study proposal was approved by the Ethical Committee of Mersin University Medical Faculty on 10 December 2004 (number 18/08). All animals used in the study received humane care in compliance with the guidelines established by the Ethical Committee; were caged two by two under standard environmental conditions at room temperature, with a 12‐hour light/dark cycle; and were maintained on commercially available balanced rodent food ad libitum.
Experimental model
Fifteen Wistar rats each weighing 200–250 g were used in the study. Anaesthesia was achieved with the intramuscular injections of ketamine hydrochloride (50 mg/kg) and xylazine (5 mg/kg). Hair removal was performed on both sides of the anterior abdominal wall using a hair clipper with its blade adjusted to a cutting length of 0·1 mm (Wahl® Moser™ Acro Professional Animal Clipper, Wahl (UK) Ltd, Kent, UK), followed by meticulous rinsing of cut hair pieces with tap water and shampoo. Surgical cleansing was performed with 10% povidone–iodine solution.
Two separate pockets were planned, one to the right and the other to the left of the anterior abdominal wall, each with a dimension of 2 × 2 cm. After the pockets to be dissected were topographically marked on the abdominal skin, vertical skin incisions of 2 cm located 2·5 cm lateral to the midline were made on both sides of the anterior abdominal wall. Blunt dissection in the subcutaneous plane was carried out to create the planned pockets.
Sterile sponges made of 100% cotton that were identical in structure to the ones used clinically at our institution were used for the study. These sponges are obtained by our institution according to the guidelines established by the Turkish Ministry of Health. They are made of 100% cotton, are naturally white without being processed with any dye, are hydrophilic, do not have any sutured parts, and are neither acidic nor alkaline. The sponges were obtained in sizes of 2 × 1 cm to be suitable for experimental use in the rats (Figure 1). The sponges were folded in such a manner to secure the free cut edges inside, and further folded to reduce their size to fit inside the prepared subcutaneous pockets. The left‐sided pockets were packed with folded sterile sponges, while the right‐sided pockets remained empty. The sponges were kept in the pockets for 15 minutes and then removed. The pockets were then inspected for the presence of macroscopically visible remnants, which were taken out if found. Both right‐ and left‐sided pockets were then irrigated with a 5 cc of constant amount of sterile saline. All the irrigation material was collected in separate glass tubes to investigate the presence of tiny sponge remnants microscopically. The wounds were closed using 4/0 silk sutures, and the animals were returned to their cages.
Figure 1.

Standard‐sized sterile sponge made of 100% cotton and folded in such a manner to secure the cut edges inside.
The animals were reoperated at the end of the fourth week. Anaesthesia was achieved with the use of the same drugs via the same route as discussed earlier. Tissue samples of 1 × 1 cm from the same topographical locations were obtained en bloc with all tissue layers from the skin to the peritoneum without redissecting the previously created subcutaneous planes.
Evaluation parameters
Before starting the experiment, to avoid confusion between the microscopic appearance of sponge lint and hair debris, both sponge lint and rat hair samples were collected in separate tubes filled with saline. After centrifugation and alcohol fixation, the samples were stained with Papanicolaou dye.
The evaluations under light microscope were performed, and the differences between the appearances of sponge lint and rat hair were observed. The microscopic evaluation of the hair debris revealed linear straight filaments with sharp edges. This pattern was clearly different from the ribbon‐like pattern without sharp borders of the sponge fibres evaluated microscopically. Then, the pathologist evaluated ten samples of microscopic sponge lint and hair in a randomised blinded fashion and was able to differentiate them correctly with a 100% success rate.
The presence or absence of macroscopically visible sponge particles in the wound was recorded right after the removal of the packed sterile sponges from the created pockets.
The collected irrigation samples were centrifuged using Leica Cytospin 3 (Leica Microsystems GmbH, Wetzlar, Germany) at 1000 rpm for 1 minute. The sediment was alcohol fixated and stained with Papanicolaou dye, which is one of the well‐studied and trusted methods used in the cytological examination of alcohol‐fixated swabs, fine‐needle aspirates, irrigation samples from body fluids and so on 9, 10. Furthermore, it has been successfully used to stain inorganic materials such as cotton fibre, hair debris and inorganic crystals 11, 12.
The same pathologist who was unaware of the groups all through the process performed the microscopic evaluations for the experiment under the light microscope. First, the presence of sponge particles in the wound irrigation samples was evaluated and then digital pictures of the particles were obtained.
Tissue samples obtained at 4 weeks after the initial surgery were kept in 10% formalin for 24 hours. Macroscopic samples were obtained from the central parts of the surgical specimens, dehydrated and embedded in paraffin as standard, and three consecutive serial sections were obtained with 20‐μm distance between each section. The sections were stained with haematoxylin and eosin, and all were evaluated.
The presence and the intensity of the encountered inflammation were recorded. The intensity of the inflammation was graded according to the abundance of the accumulating inflammatory cells and the extent of the foreign body reaction as mild, moderate or severe. If no foreign body giant cells were encountered or if only one to three giant cells were scattered within the inspected inflammatory area, accompanied by a small number of accumulating lymphocytes of not more than ten, the reaction was graded as mild.
If small groups of three to six foreign body giant cells were found in the presence of a modest lymphocyte infiltration, 10–20 lymphocytes, the reaction was graded as moderate.
If the typical foreign body granuloma formation was encountered with the accumulation of more than six foreign body giant cells surrounded by more than 20 lymphocytes, the reaction was regarded as severe.
Statistical analysis
The statistical analysis was conducted using SPSS 11·5 software for Windows® (SPSS Inc., Chicago, IL, USA). Wilcoxon signed rank test was performed to analyse the significance of the observed difference in terms of the intensity of the evoked inflammatory reaction between the two groups.
Results
Table 1 shows the number of macroscopically detected sponge remnants and the presence of microscopically detected sponge remnants and the presence and intensity of the evoked inflammatory response.
Table 1.
The number of macroscopic gauze remnants and the presence of microscopic sponge particles, and the intensity of the inflammatory reaction pertaining to the left and right sides are listed. The inflammatory reaction intensity is graded as mild (1), moderate (2) or severe (3)
| Rat number | Number of macroscopic gauze remnants | Presence of microscopic gauze remnants (irrigation samples) | Intensity of inflammatory reaction | |||
|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | |
| 1 | 2 | 0 | + | − | 3 | 1 |
| 2 | 2 | 0 | + | − | 2 | 1 |
| 3 | 2 | 0 | + | − | 2 | 2 |
| 4 | 1 | 0 | ‐ | − | 2 | 1 |
| 5 | 2 | 0 | + | − | 3 | 1 |
| 6 | 1 | 0 | + | − | 1 | 1 |
| 7 | 1 | 0 | + | − | 1 | 1 |
| 8 | 2 | 0 | + | − | 3 | 1 |
| 9 | 1 | 0 | + | − | 2 | 1 |
| 10 | 1 | 0 | + | − | 1 | 2 |
| 11 | 2 | 0 | + | − | 1 | 1 |
| 12 | 1 | 0 | + | − | 2 | 1 |
| 13 | 1 | 0 | + | − | 1 | 1 |
| 14 | 2 | 0 | + | − | 3 | 1 |
| 15 | 1 | 0 | + | − | 2 | 1 |
At least one piece of macroscopically visible sponge particle was detected right after the removal of the sponges from the left‐sided pockets, with an average of 1·47 ± 0·52 particles detected per pocket. No sponge particle was detected in the right‐sided pockets.
Microscopic sponge particles were detected in the 14 irrigation samples collected from the left‐sided pockets (Figure 2), while the microscopic evaluation of the irrigation samples from the right‐sided pockets revealed no sponge remnants.
Figure 2.
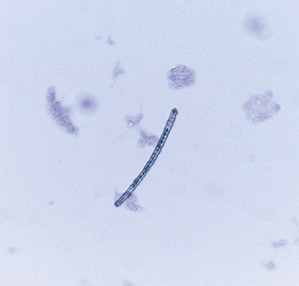
A sponge particle detected by the light microscopy from an irrigation sample collected from the left‐sided pockets (Papanicoulau dye ×100 magnification).
The inflammation was detected by the histological evaluation of all tissue samples, no matter if they were collected from the left or the right sides, but the intensity of the inflammatory response varied.
Foreign body giant cells were observed in every sample obtained from the left‐sided pockets, whereas they were only seen in two samples collected from the right‐sided pockets. A severe inflammatory reaction with the presence of multiple foreign body giant cells forming well‐defined foreign body granulomas was recorded in four samples collected from the left sides (Figures 3a, 3b). In six samples from the left‐sided pockets, the inflammatory reaction was moderate, and in the remaining five samples only a mild reaction was recorded.
Figure 3.
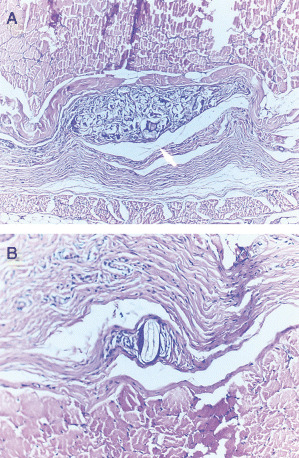
(A) The presence of a well‐defined foreign body granuloma (white arrow), (haematoxylin and eosin ×100 magnification). (B) A foreign body giant cell with an ingested sponge particle (haematoxylin and eosin ×200 magnification).
In the evaluation of the samples collected from the right sides, a moderate inflammatory reaction was recorded in two samples, whereas in the other samples, only a mild reaction was recorded, with no foreign body granulomas observed.
When the intensity of the inflammation was graded, the mean grade of the samples from the left side was 1·93 ± 0·80 and of the right was 1·13 ± 0·35; the statistical evaluation of the difference between the two sides revealed a significant difference (P < 0·05) (Figure 4).
Figure 4.
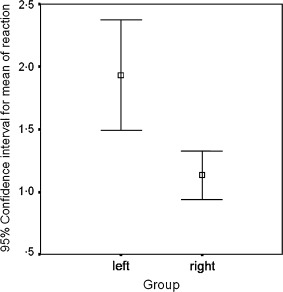
When the intensity of the inflammation was graded, a statistically significant difference was found between the results obtained from the left and the right sides (P < 0·05).
Discussion
Gossypium is a genus including 39–40 species of shrubs from which the commercial fabric cotton is obtained. Surgical sponges are made from cotton fibres and are used for various medical purposes after being sterilised. The cotton fibres are composed of natural polymers of pure cellulose, which is an indigestible material if ingested.
Any foreign substance infiltrating the organism, whether large or invisible to the naked eye, has a potential to induce an inflammatory response within the body; yet, the severity and clinical manifestations of the inflammatory process may vary due to the type and size of the foreign material (13). The antigenic structure and the digestibility of the foreign material are the most important factors guiding the type of the immunological response and the final inflammatory outcomes. The presence of poorly digestible irritants appears to be the pathophysiological basis for the formation of a granulomatous inflammation against a foreign body (14). As surgical sponge is composed of a cellulose structure that cannot be digested by tissue macrophages, it evokes a cascade of inflammatory reactions resulting in the formation of foreign body granulomas 15, 16. The reaction is totally aseptic if the retained sterile gauze is in no means contact with the outside environment, but if it gets somehow contaminated, the inflammatory reaction shifts to a suppurative one and the patient faces the risk of developing a serious infection 7, 8, 17, 18, 19.
The clinical manifestations of retained surgical sponges may vary from the presence of silent masses only causing discomfort to disastrous conditions such as intestinal obstruction and abscess formation 17, 20, 21. Sometimes, the clinical presentation may be so vigorous that the term ‘pseudotumor’ has also been used to denote the possible devastating outcomes of the situation 7, 8. As in these situations, when the sponge left behind is big enough to detect clinically or radiologically, it is not too hard to relate the clinical scenario with the presence of a foreign body.
The problem arises with the presence of tiny, microscopic particles left behind, especially when the dissection field is too large as in a dermolipectomy, breast augmentation, reduction mammaplasty, or an intraperitoneal procedure. In these situations, tens to hundreds of microscopic gauze fibres may be left within the wound just by the routine use of gauze pads for wiping the bleeding and handling tissue parts. Especially in procedures such as breast augmentation and dissection of large flaps, the surgical sponges are kept inside the created pockets for prolonged time to help haemostasis during the dissection of the other sides. During these manipulations, the sponges get fully saturated with blood and their edges may become shredded. Sturdy et al. had documented that the risk of retaining a sponge particle was increased with the use of worn resterilised sponges multiple times and of dry sponges (22). Today, the surgical sponges are disposable and not used for more than one operation, but still loose particles may fall on the operative field particularly when dry sponges with free cut margins are vigorously used.
Subcutaneous tissue was regarded as the second most common site, after the intraperitoneal cavity, where surgical sponges are retained in a series of 58 clinical cases reported by Sturdy et al. (22). The authors noticing the presence of ragged birefringent particles of surgical sponges in the routine microscopic evaluation of granulomas and adhesions, designed an experimental model and investigated the potential of sponge particles to induce a granulomatous reaction and adhesion formation when they were retained within the abdominal cavity of rats. Their results showed that not only a small gauze particle that was retained but also the lint lost from worn gauze by rubbing it within the wound may induce the formation of intra‐abdominal granulomas and adhesions.
Our study showed that there is an established risk of retaining microscopic sponge particles within a surgically created wound, as microscopic remnants were found in 14 of the 15 samples evaluated. This condition is true when the sponges are not only wiped robustly within the wound but also gently placed within the created tissue planes. Even if the macroscopically visible particles were picked up one by one, the surgeon must be aware of the fact that the number of invisible tiny particles may exceed the number of visible ones. Copious irrigation may diminish the number of the particles left; yet, it cannot warrant a totally clear wound, devoid of all foreign material.
In their study, Sturdy et al. opened an abdominal cavity, rubbed the wound with a surgical sponge vigorously, and documented the microscopic granuloma formation in 21 of 29 animals studied (22). The findings of Sturdy et al. in intra‐abdominal wounds were similar to what we have found when the sponge particles remained within subcutaneous planes. These microscopic particles were ingested by foreign body giant cells, which come together and form foreign body granulomas.
In conclusion, this study proved that even if the surgical sponges are gently placed inside a surgically created tissue pocket, they have a potential to lose lint within the wound. Even if the macroscopically visible particles were picked, there will be microscopic particles left behind, which still have a potential to induce a foreign body inflammatory response. Whether the evoked foreign body reaction may interfere with the quality of final wound healing or may have adverse effects on the final success of the surgical procedure performed has to be discussed.
References
- 1. Tacyildiz I, Aldemir M. The mistakes of surgeons: gossypiboma. Acta Chir Belg 2003;103:71–5. [DOI] [PubMed] [Google Scholar]
- 2. Gawande AA, Studdert DM, Orav EJ, Brennan TA, Zinner MJ. Risk factors for retained instruments and sponges after surgery. N Engl J Med 2003;348:229–23. [DOI] [PubMed] [Google Scholar]
- 3. Chaimoff C. Intra‐abdominal retained surgical gauze pad mimicking malignant tumor. Isr J Med Sci 1989;25:656–7. [PubMed] [Google Scholar]
- 4. Ahmad G, Attiq‐ur‐Rehman S, Anjum MZ. Retained sponge after abdominal surgery. J Coll Physicians Surg Pak 2003;13:640–43. [PubMed] [Google Scholar]
- 5. Alotti N, Kecskes G, Simon J, Tomcsanyi J, Papp L. Gauze swabs left intrapericardially following cardiac surgery. J Cardiovasc Surg (Torino) 1999;40:825–7. [PubMed] [Google Scholar]
- 6. Bani‐Hani KE, Gharaibeh KA, Yaghan RJ. Retained surgical sponges (gossypiboma). Asian J Surg 2005;28:109–15. [DOI] [PubMed] [Google Scholar]
- 7. Sahin‐Akyar G, Yagci C, Aytac S. Pseudotumour due to surgical sponge: gossypiboma. Australas Radiol 1997;41:288–91. [DOI] [PubMed] [Google Scholar]
- 8. Serra J, Matias‐Guiu X, Calabuig R, Garcia P, Sancho FJ, La Calle JP. Surgical gauze pseudotumor. Am J Surg 1988;155:235–7. [DOI] [PubMed] [Google Scholar]
- 9. Gammon RR, Kharazi M, Brandt JT, Keyhani‐Rofagha S. Correlation between the papanicolaou stain and the Wright‐Giemsa stain in body fluids: a quality assurance study. Anal Quant Cytol Histol 2006;28:171–4. [PubMed] [Google Scholar]
- 10. Walker TL, Owen M, Donaldson S, McGuire LJ. Bronchoalveolar lavage. Comparison of nucleopore filters and direct smear preparations. Diagn Cytopathol 1997;17:160–4. [DOI] [PubMed] [Google Scholar]
- 11. Van Hoeven KH, Bertolini PK. Prevalence, identification and significance of fiber contaminants in cervical smears. Acta Cytol 1996;40:489–49. [DOI] [PubMed] [Google Scholar]
- 12. Nicol KK, Ward WG, Pike EJ, Geisinger KR, Cappellari JO, Kilpatrick SE. Fine‐needle aspiration biopsy of gouty tophi: lessons in cost‐effective patient management. Diagn Cytopathol 1997;17:30–5. [DOI] [PubMed] [Google Scholar]
- 13. Truscott W. Impact of microscopic foreign debris on post‐surgical complications. Surg Technol Int 2004;12:34–46. [PubMed] [Google Scholar]
- 14. Kumar V, Cotran RS, Robbins SL. Acute and chronic inflammation. In: Kumar V, Cotran RS, Robbins SL, editors: Basic pathology. 5th ed. Philadelphia: WB Saunders, 1992:25–46. [Google Scholar]
- 15. Turgut M, Akyuz O, Ozsunar Y, Kacar F. Sponge‐induced granuloma (“gauzoma”) as a complication of posterior lumbar surgery. Neurol Med Chir (Tokyo) 2005;45:209–11. [DOI] [PubMed] [Google Scholar]
- 16. Al‐Shahwan S, Edward DP. Foreign body granulomas secondary to retained sponge fragment following mitomycin C trabeculectomy. Graefes Arch Clin Exp Ophthalmol 2005;243:178–81. [DOI] [PubMed] [Google Scholar]
- 17. Lone GN, Bhat AH, Tak MY, Garcoo SA. Transdiaphragmatic migration of forgotten gauze sponge: an unreported entity of lung abscess. Eur J Cardiothorac Surg 2005;28:355–7. [DOI] [PubMed] [Google Scholar]
- 18. Chang YS, Ho CL, Tseng SH. Socket infection due to retained gauze after evisceration. Ophthalmic Surg Lasers Imaging 2004;35:503–6. [PubMed] [Google Scholar]
- 19. Grainger J, McClelland D, Neil NC. Retained surgical swab: a case report. Case Rep Clin Prac Rev 2004;5:474–6. [Google Scholar]
- 20. Gencosmanoglu R, Inceoglu R. An unusual cause of small bowel obstruction: gossypiboma – case report. BMC Surg 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cruz RJ Jr, Poli de Figueiredo LF, Guerra L. Intracolonic obstruction induced by a retained surgical sponge after trauma laparotomy. J Trauma 2003;55:989–91. [DOI] [PubMed] [Google Scholar]
- 22. Sturdy JH, Baird RM, Gerein AN. Surgical sponges: a cause of granuloma and adhesion formation. Ann Surg 1967;165:128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]


