Abstract
In mammals, profound changes in the populsation and functions of adult stem cells occur with age and these changes are thought to underlie functional decline and pathophysiology at the tissue and organismal levels associated with aging. SIRT1, a member of the conserved sirtuin family, functions as an anti-aging regulator for adult stem cells. Mediated through its regulatory roles in AMPK and mTORC1 pathways as well as gene expression, SIRT1 modulate the activities of genes maintaining stem cell functions and delays cellular senescence. Further investigation of the cross-talk between SIRT1 and other longevity target genes under different physiological conditions of stem cells may help us better design intervention strategies to antagonize stem cells aging.
Keywords: Sirtuin, SIRT1, Aging, Stem cells, Metabolism, Epigenetics
1. Introduction
One of the reasons why hydra polyp appears to never age is that its adult stem cells have the indefinite self-renewal capacity (Boehm et al., 2012). Transplantation of mesenchymal stem cells (MSCs) isolated from young mice into old mice significantly slow the loss of bone density and prolong the lifespan for old mice (Shen et al., 2011). This is just one of those clear evidence that biologically young adult stem cells can improve tissue homeostasis against aging-related degeneration and hence the state of adult stem cells may be one of the determinants for longevity.
In mammals, aging is associated with profound changes in adult stem cells. Certain populations of stem cells may diminish and even become exhausted in aged animals, such as neural stem cells (NSCs) (Lopez-Otin et al., 2013). For other stem cells, such as hematopoietic stem cells (HSCs), dramatic functional declines and changes are observed: aged HSCs are more likely to differentiate towards the myeloid lineage than the lymphoid lineage (Liu and Rando, 2011; Oh et al., 2014). Despite the specific characteristics of aging associated with different types of stem cell, these heterogeneous aging phenotypes could be driven by some common biological events or processes such as cellular senescence, DNA damage response and epigenetic alterations (Schultz and Sinclair, 2016). These three areas of research (genetic mutation, epigenetics changes and extrinsic factors) in stem cell aging have been highlighted and summarized in a recent review (Goodell and Rando, 2015). In this review, we will focus on a specific class of aging regulator, the sirtuins, in particular, SIRT1 in mammals.
Sirtuins are homologs of the yeast Sir2 (Silent Information Regulator 2), which is the first identified NAD+-dependent histone/ protein deacetylase, or class III histone deacetylase (HDAC) (Seto and Yoshida, 2014). The yeast Sir2 primarily deacetylates Histone H4 at lysine 16 and promotes longevity by suppression the generation of extrachromosomal rDNA circles (ERCs) and maintenance of subtelomeric heterochromatin (Qiu et al., 2010). Seven sirtuins, namely SIRT1–7, have been identified in mammals. SIRT1 mainly localizes in the nucleus; SIRT2 localizes in the cytoplasm, primarily targeting FOXO1, PEPCK and tubulin; SIRT3, SIRT4 and SIRT5 localize in mitochondria, targeting various oxidative phosphorylation enzyme complexes and SODs; SIRT6 and SIRT7 localize in nucleus, with SIRT6 targeting histone H3, PARP-1 and p65 (Morris, 2013).
SIRT1 has long been considered an aging regulator, with profound roles in metabolism, DNA repair, epigenetics changes and circulatory system. SIRT1 deacetylates a number of target substrates, including histones, such as H3 lysine 9, lysine 56 and H4 lysine 16, as well as non-histone proteins, such as Foxos, p53, p65 and PGC-1α. SIRT1 is the closest mammalian homolog of the yeast Sir2, and has been shown to extend lifespan in mice when activated or overexpressed. SIRT1 selective activators SRT1720 and SRT2104 extend the lifespan of both mice fed on a standard diet (Mitchell et al., 2014) and obese mice fed on a high fat diet (Minor et al., 2011). Brain-specific overexpression of SIRT1 in mice can also extend lifespan through increased neural activities of hypothalamus (Satoh et al., 2013). These lines of evidence support the anti-aging function of SIRT1 in mammals.
Besides SIRT1, other members of sirtuins family, such as SIRT3, SIRT6 and SIRT7, have been reported as anti-aging factors in hematopoietic stem cells (HSCs): SIRT3 is highly enriched in HSCs and is not required for young HSCs but is indispensable for aged HSCs maintenance. SIRT3 is repressed with age and its activation in aged HSCs improves the regenerative capacity (Brown et al., 2013). Deletion of SIRT6 leads to impaired quiescence and self-renewal ability of HSCs via enhancing Wnt signaling (Wang et al., 2016). SIRT7 inactivation resulted to reduced quiescence, increased mitochondrial protein folding stress and compromised regenerative capacity of HSCs (Mohrin et al., 2015). Despite these new findings, our knowledge of stem cell aging regulation by these sirtuins remains poor and scarce compared to that of SIRT1. Therefore, in this review, we will focus on SIRT1 and discuss recent findings on the molecular mechanisms of SIRT1 in the regulation of stem cell aging.
2. Functions of SIRT1 in stem cells and during their aging
Unlike terminally differentiated cells, stem cells not only uphold the capacity to differentiate into cells of specific functions in response to appropriate environmental cues, but they also harbor the capacity to reproduce and expand the population of stem cells that maintain the undifferentiated status and avoid self-automatic differentiation or premature differentiation. These properties of stem cells are termed pluripotency/multipotency and self-renewal, respectively. Among different types of stem cells, embryonic stem cells (ESCs) possess the highest level of pluripotency and the strongest self-renewal ability, since they are able to differentiate in to all the cell types of an organism and they are almost immortal (Ohgushi et al., 2015). Studies have shown that SIRT1 is highly expressed in human ESCs, at both the mRNA and the protein levels and that its expression is significantly decreased during embryonic differentiation. In SIRT1 knockout (KO) ESCs, the expression of pluripotency markers is decreased and developmental markers are significantly increased in ESCs upon induction of differentiation, suggesting that SIRT1 is responsible for the regulation of the differentiation program (Calvanese et al., 2010; Tang et al., 2014). Moreover, SIRT1 can prevent both human and mouse ESCs from oxidative stress-induced apoptosis by positively regulating autophagy and mitochondria function (Ou et al., 2014). Thus, SIRT1 maintain the stemness of ESCs by not only regulating the transcription program of differentiation, but also controlling the cellular response to intrinsic and extrinsic stresses.
Hematopoietic stem cells (HSCs) can differentiate into all lineages of blood cells and are required for hematopoietic system maintenance throughout life. In the bone marrow of old mice, many HSCs are actively replicating while HSCs in young mice are predominately quiescent. This results in a larger number of HSCs with much reduced clonal complexity in old mice than in young mice (Rossi et al., 2005). Despite their large numbers, HSCs from old mice were only one-quarter as efficient at engrafting into the bone marrow of irradiated recipients (Morrison et al., 1996). Aged HSCs also have lower capacity to differentiate into lymphoid cells (Sudo et al., 2000). HSCs with deficient SIRT1 exhibit major defects similar to those in aged HSCs. SIRT1 ablation causes adult HSC expansion in a hematopoietic stress-dependent manner; and this expansion eventually leads to long-term HSC exhaustion (Singh et al., 2013). Differentiation of young SIRT1-KO HSCs is skewed toward myeloid lineage associated with a significant decline in the lymphoid compartment (Rimmele et al., 2014).
Mesenchymal stem cells (MSCs), a non-hematopoietic stem cell population first discovered in bone marrow, are highlighted for clinical potentials because MSCs have many applications as cell-based therapies, including immune modulation and woundhealing. MSCs are multipotent cells capable of differentiating into osteoblasts, adipocytes and chondrocytes. The aging phenotypes of bone marrow-derived MSCs have been detailed in a recent review, including reduced basal migration capacity, decreased expression MSCs-specific surface antigens and declined differentiation potentials (Baker et al., 2015). Aging phenotypes of umbilical cord-derived MSCs are also observed: MSCs from older donors show decreased proliferative and colony forming capacity accompanied with a decreased osteogenic differentiation potential but an increased adipogenic potential (Huang et al., 2013). MSCs need to be expanded in vitro in order to meet the required cell counts for therapeutic applications. However, prolonged MSCs culturing results in decreased differentiation capabilities and eventually senescence. Overexpression of SIRT1 in human bone marrow-derived MSCs delays cellular senescence and preserves adipogenic and osteogenic differentiation potentials during prolonged culturing (Yuan et al., 2012). In contrast to overexpression, MSC-specific SIRT1 knockout mice (2.2 years old) have a 64% reduction in the subcutaneous fat with smaller adipocytes; the cortical bone thickness and trabecular volume/bone volume are also reduced by 25% and 23% (Simic et al., 2013). These results suggest that SIRT1 plays important roles in both extending replicated lifespan and preserving differentiation capacity of aging MSCs.
Regeneration of skeletal muscle depends on muscle stem cells or satellite cells. Depletion of satellite cells elevates muscle fibrosis, and exacerbates muscle atrophy and type transitions connected to neuromuscular disruption (Liu et al., 2015). Although satellite cells may not contribute to the maintenance of muscle size or fiber type composition during aging, they are important for preventing age-related muscle fibrosis (Fry et al., 2015). The regenerative functions also decline with age in satellite cells. In old mice, quiescent satellite cells fail to activate and expand upon injury and become senescent (Sousa-Victor et al., 2014). A further study has demonstrated that autophagy plays an essential role in maintaining the quiescent state for satellite cells to avoid senescence during aging (Garcia-Prat et al., 2016). SIRT1 is a critical autophagic flux regulator in satellite cells. A deficiency in SIRT1 leads to a delay in satellite cell activation (Tang and Rando, 2014). derepression of muscle developmental genes in satellite cells (Ryall et al., 2015). The various effects of SIRT1 on these different types of stem cells are summarized in Table 1.
Table 1.
Effects of SIRT1 on stem cells.
| Stem cells | SIRT1 knockout (KO) or over expression (OE) | Phenotypes/pathways | References |
|---|---|---|---|
| ESCs | KO | Accelerated mouse embryonic stem cells differentiation in response to retinoic acid/cellular retinoic acid binding protein II pathway | (Tang et al., 2014) |
| ESCs | KO | More apoptosis induced by ROS, reduced autophagy/class III PI3K, Beclin 1 and mTOR pathway | (Ou et al., 2014) |
| ESCs | KO | More apoptosis induced by ROS/PTEN-JNK-FOXO1 pathway | (Chae and Broxmeyer, 2011) |
| HSCs | KO | Skewed differentiation toward myeloid lineage with decline in the lymphoid lineage/Foxo3 pathway | (Rimmele et al., 2014) |
| HSCs | KO | Aberrant expansion under hematopoietic stress, loss of long-term progenitors/H4K16ac-Hoxa9 pathway | (Singh et al., 2013) |
| MSCs | OE | Reversed senescence phenotype and increased cell proliferation of aged MSCs/TERT and TPP1 pathway | (Chen et al., 2014) |
| MSCs | KO | Reduction in subcutaneous fat, bone thickness and trabecular volume/β-catenin pathway | (Simic et al., 2013) |
| MSCs | OE | Delayed senescence, preserved differentiation potential/p16 pathway | (Yuan et al., 2012) |
3. Molecular mechanisms for SIRT1 functions in stem cells
SIRT1 can directly interact with a number of target proteins that are involved in many key aspects of cellular function including metabolism, stress resistance, inflammation, genome stability, cellular senescence and apoptosis. Through its catalytic activity, SIRT1 can remove acetyl groups from these proteins, which can either direct change their catalytic activities or serve as epigenetic signals to alter their stability or their interactions with other proteins.
3.1. Metabolic regulation by SIRT1
The general regulatory roles of SIRT1 in metabolism has been previously reviewed (Li and Kazgan, 2011), and include regulating gluconeogenesis, increasing fatty acid oxidation, decreasing lipogenesis and increasing insulin secretion. Here we focus on a newly found mechanism of SIRT1 in autophagy regulation and discuss the crosstalk patterns between SIRT1 and two other metabolism master regulators (AMPK and mTOR).
Autophagy (“self-eating”), a cyto-protective, rather than a self-destructive, process, allows cytoplasmic substrates delivered to lysosomes for degradation. It is believed that autophagy plays an essential role in lifespan extension (Madeo et al., 2015). Among the components of the autophagy machinery, Beclin1 is required for phagophore (autophagosome precursor) formation and microtubule-associated protein 1 light chain 3 (LC3) is essential for autophagosome formation. Beclin1 is acetylated by p300 and deacetylated by SIRT1 at lysine residues 430 and 437. These acetylations on Beclin1 inhibit autophagosome maturation (Sun et al., 2015). Deacetylation of LC3 at K49 and K51 by SIRT1 allows LC3 to interact with the nuclear protein DOR and relocate from the nucleus to the cytoplasm. Only those LC3 proteins that have been deacetylated by SIRT1 and re-localized to the cytoplasm are active to form autophagosome (Huang et al., 2015). These findings confirm SIRT1 as a critical positive regulator of autophagy and suggest that impaired SIRT1 function would compromise autophagy. Consistent with these findings, compromised autophagy causes increased apoptosis-induced oxidative stress in the ES cells from SIRT1 knockout mice (SIRT1−/−) (Ou et al., 2014).
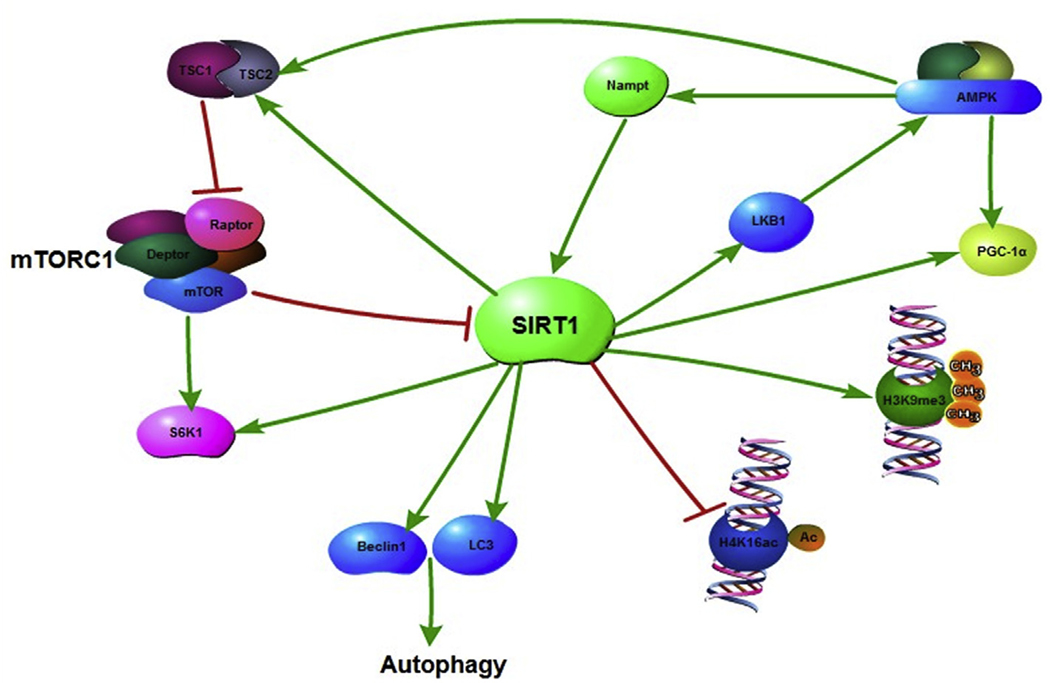
AMP-activated protein kinase (AMPK) is a metabolic fuel gauge sensing changes in the intracellular AMP/ATP ratio to regulate the balance between catabolic and anabolic metabolism. In general, activated AMPK promotes catabolism and inhibits anabolism (Dzamko and Steinberg, 2009). It has been proposed that AMPK is a potent anti-aging target (Ruiz et al., 2016), and research evidence has shown that metformin, an activator of AMPK, has anti-aging and lifespan extension effects in mice (Martin-Montalvo et al., 2013). SIRT1 is another metabolic status sensor and its activity responds to the intracellular NAD+/NADH ratio. It has been suggested that there is a coadjutant relationship between AMPK and SIRT1. On one hand, AMPK activation induced by glucose restriction can up-regulate the rate-limiting NAD+ biosynthesis enzyme NAMPT, which consequently increases SIRT1 activity through elevated NAD+/NADH ratios (Fulco et al., 2008). On the other hand, SIRT1 deacetylates LKB1, a direct activator kinase for AMPK, and promotes LKB1 cytoplasm localization, which in turn activates AMPK (Lan et al., 2008). Therefore, there appears to be a crosstalk and coordination between the activities of SIRT1 and AMPK. This positive coordination between SIRT1 and AMPK is even reflected in their substrates. For example, PGC-1α (peroxisome proliferators-activated receptor α) is a common substrate shared between SIRT1 and AMPK. Both deacetylation by SIRT1 and phosphorylation by AMPK can up-regulate its transcriptional activity and promote mitochondria biogenesis and fatty acid oxidation. Experiments with site-specific mutations in PGC-1α suggest that phosphorylation of PGC-1α by AMPK constitutes a priming signal for subsequent deacetylation by SIRT1 (Canto et al., 2009). For bone marrow derived MSCs, mitochondria homeostasis regulated by SIRT1 and PGC-1α is important to overcome aging-associated defects caused by telomere attrition (Sui et al., 2016).
Unlike the crosstalk between SIRT1 and AMPK, the crosstalk between SIRT1 and mTOR is somewhat complicated. Mammalian Target of Rapamycin (mTOR) is a highly conserved Ser/Thr kinase and is found in two structurally and functionally distinct complexes, mTORC1 and mTORC2. mTORC1 consists of mTOR, Raptor, MLST8, PRAS40, and DEPTOR (Johnson et al., 2013), and is activated by growth factors and amino acids to promote protein synthesis and lipogenesis while inhibiting autophagy and ketogenesis (Cornu et al., 2013). Both genetically knocking down mTOR (Wu et al., 2013) and pharmacologically inhibiting mTOR activity by rapamycin (Harrison et al., 2009) extend the lifespan in mice. A general mTOR signaling pathway can be found in a recent review (Zoncu et al., 2011). SIRT1 has been found to regulate the activities of both mTOR complexes. For mTORC2, SIRT1 positively regulates the transcription of Rictor (a unique mTORC2 component) to increase the mTORC2 activity (Wang et al., 2011). However, the situation is much more complex for mTORC1. The TSC1/TSC2 complex functions as a potent inhibitor for mTORC1 by inactivating a critical mTORC1 activator, a G protein called Rheb. Nutrients and insulin signaling inhibit the TSC1/TSC2 complex through Akt/PKB or MAPK pathways, and hence activate mTORC1. The primary targets of mTORC1 are S6 kinase S6K1 and 4E-BP, both of which, when phosphorylated by mTORC1, activate protein synthesis through either ribosome biogenesis or translation. In this complex signaling network, SIRT1 and mTORC1 regulate each other’s activities. SIRT1 interacts with TSC2, and this interaction negatively regulates mTORC1 activity (Ghosh et al., 2010). Nampt overexpression induces autophagy by inhibiting mTORC1 activity and this effect is also dependent on SIRT1 and TSC2 (Wang et al., 2012). Conversely, SIRT1 is a direct substrate of mTOR, and is phosphorylated at Ser 47. This phosphorylation inhibits the deacetylase activity of SIRT1 (Back et al., 2011). Hence, SIRT1 and mTORC1 appear to be negative regulators for each other. However, SIRT1 also directly deacetylates S6K1, activating its kinase activity (Hong et al., 2014). Since increased S6K1 activity partially mimics activated mTORC1, SIRT1 activation or overexpression also seems to increase mTORC1 signaling in some specific situations. Resveratrol, an activator of SIRT1, improves insulin sensitivity in old mice fed on a standard diet; nonetheless, not only does it fail to improve insulin resistance in old mice receiving a high protein diet, but it causes deleterious inflammation and increased superoxide production in these mice (Baron et al., 2014). Although the reasons behind resveratrol’s contradictory effects remains elusive, it may be caused by simultaneously activating both mTORC1 through the high protein diet and SIRT1 through resveratrol. The intricate crosstalk between SIRT1 and mTOR may also explain why some researchers observed decreased S6K1 activity (Guo et al., 2011), whereas others found the opposite effect (Huang et al., 2008), when the same SIRT1 overexpression was carried out. Caloric restriction (CR) is a robust approach to promote longevity, and it is believed that activating SIRT1 and repressing mTORC1 are key molecular mechanisms responsible for CR benefits. However, a recent study in intestinal stem cells showed that, under CR, activated SIRT1 deacetylates S6K1, and thereby enhances its phosphorylation by mTORC1, resulting in increased protein synthesis (Igarashi and Guarente, 2016a). This study describes a novel molecular mechanism mediating stem cell proliferation under CR conditions (Igarashi and Guarente, 2016b). Fig. 1 summarizes the major pathways discussed here.
Fig. 1.
Metabolic signaling and epigenetic regulation of SIRT1.
Adult stem cells can exist in different states, such as quiescence, active self-renewal and primed differentiation, representing distinct physiological conditions. The crosstalk between SIRT1 and mTOR, as well as other metabolic regulator such as SREBP-1c, can modulate these different physiological conditions and are potentially useful mechanisms to exploit for anti-aging intervention in adult stem cells.
3.2. SIRT1 delays cellular senescence
Cellular senescence is a stress response that permanently stops the cell cycle progression to prevent the proliferation of potential cancerous cells. Besides suppressing tumorigenesis, cellular senescence also regulates tissue repair and induces inflammation, the latter of which accelerates aging and cancer progression (Rodier and Campisi, 2011). Senescent cells accumulate with age, it has been reported that both genetic (Baker et al., 2016) and pharmacological (Chang et al., 2016) clearance of senescence cells in vivo can extend mouse lifespan. Unlike nearly immortal embryonic stem cells, adult stem cells undergo cellular senescence both in vitro and in vivo. Upon prolonged culturing, MSCs become senescent like most primary cells (Piccinato et al., 2015). Oxidative stress also induces MSCs into a premature senescent state (Zhou et al., 2015). In geriatric mice, quiescent satellite cells directly switch to a senescent state without the cell division (Sousa-Victor et al., 2014). The molecular signaling network regulating cellular senescence has been recently reviewed in details (Childs et al., 2015). Briefly, in proliferating cells, progressive telomere erosion, or other DNA-damaging stresses, such as UV, gamma irradiation, oxidative stress and oncogenic Ras overexpression, triggers a DNA damage response (DDR). The DDR leads to the up-regulation of cyclin-dependent kinase inhibitors, such as p21 and p16, which activate RB family proteins and prevent the cell from entering the cell cycle. For the stem cells, becoming senescent means losing both self-renewal and differentiation abilities. It has been show that over-expression or activation of SIRT1 can delay cellular senescence in both somatic cells (Huang et al., 2008) and mesenchymal stem cells (Yuan et al., 2012; Zhou et al., 2015).
Cell senescence is regulated by SIRT1 through a number of pathways. First, SIRT1 deacetylates the longevity gene FOXO3 (Brunet et al., 2004), which is activated upon deacetylation and up-regulates its target antioxidant genes, such as SOD2 (Hori et al., 2013; Ou et al., 2014). Increased oxidative stress response prevents extensive oxidative damages to DNA that induces senescence. Second, SIRT1 promotes the expression of telomerase (TERT), as well as TPP1, a component of the shelterin complex that protects telomeres (Chen et al., 2014). These activities prevent excessive telomere attrition and damage during aging. Third, cyclin-dependent kinase inhibitor p21 is a direct target of the p53 tumor suppressor. SIRT1 deacetylates p53, inhibits its transcriptional activity (Vaziri et al., 2001), and prevents p21-induced cellular senescence (Langley et al., 2002). Another cyclin-dependent kinase inhibitor p16 is also repressed by SIRT1 via both signaling pathways through Akt/S6K1 and epigenetic regulations through histone H3 acetylation at the p16 promoter (Li and Tollefsbol, 2011). Fourth, in addition to these classic cellular senescence regulators, SIRT1 also regulates numerous targets related to cellular stress response and senescence. For example, cyclooxygenase-2 (COX-2), an enzyme producing prostaglandin, a secreted signaling molecule, is expressed under stress, inflammation, and tissue injury. It is an important cellular senescence mediator (Martien et al., 2013) and SIRT1 negatively regulates the expression of COX-2 at the transcription level (Zhang et al., 2010).
3.3. SIRT1 and stemness genes
In human embryonic stem cells, transcription factors OCT4, SOX2 and NANOG constitute the core transcriptional regulatory circuitry that maintains their pluripotency and self-renewal functions (Boyer et al., 2005). Accumulating evidence indicates that these transcription factors also contribute to the stemness in adult stem cells. In the bone marrow, high levels of OCT4 and low levels of p16 expression are hallmarks proliferating MSCs and predictors of greater replicative lifespan and growth potential (Piccinato et al., 2015). Overexpression of OCT4 increases colony formation and enhances both adipogenic and osteogenic differentiation capacity (Hao et al., 2015). Conversely, knocking down SOX2 significantly inhibits their multipotency and proliferation (Yoon et al., 2011). Knocking down OCT4 and NANOG in early passage MSCs also decreases proliferation and differentiation potential while promoting spontaneous differentiation (Tsai et al., 2012).
Research has shown a close relationship between SIRT1 and these stemness genes. On one hand, OCT4 directly binds to the SIRT1 promoter region and up-regulates SIRT1 expression, which in turn inhibits p53 activities and helps maintain pluripotency in stem cells (Zhang et al., 2014). On the other hand, SIRT1 deacetylates OCT4 and the deacetylated OCT4 contributes to naive pluripotency maintenance of stem cells (Williams et al., 2016). SIRT1 also deacetylates SOX2. RNA interference of SIRT1 in MSCs causes hyperacetylation of SOX2, leading to its nuclear export, ubiquitination, and eventual proteasomal degradation (Yoon et al., 2014). This SIRT1-SOX2 axis not only plays important roles in maintaining self-renewal and multipotency for MSCs, it is also required for reprogramming somatic cells into induced pluripotent stem cells (Mu et al., 2015). Furthermore, NANOG expression is directly regulated by SIRT1 in mouse embryonic stem cells (Han et al., 2008). These lines of evidence indicate that SIRT1 plays important roles in maintenance of stem cells through pluripotency factors.
3.4. Histone modification by SIRT1
Histone modifications, such as acetylation and methylation, are critical epigenetic regulation mechanisms that modulate chromatin states and genes expression. They have emerged as markers and regulators of aging at both cellular and organismal levels. In the budding yeast, the levels of histone H4 lysine 16 acetylation (H4K16ac) in the subtelomeric regions determine its replicative lifespan (Dang et al., 2009). Trimethylated lysine 4 of histone H3 (H3K4me3) and trimethylated lysine 27 of histone H3 (H3K27me3) show opposite effects on lifespan in C. elegans (Han and Brunet, 2012). Trimethylated lysine 9 of histone H3 (H3K9me3) is a heterochromatin maker and is dramatically reduced genomewide in MSCs of the premature aging disorder Werner syndrome. The methyltransferase SUV39H1 is responsible for H3K9me3, and targeted knock-in of catalytically inactive SUV39H1 in wild-type MSCs recapitulates accelerated cellular senescence resembling premature aging features found in Werner syndrome MSCs (Zhang et al., 2015).
In vivo, the direct deacetylation targets of SIRT1 are H1K26ac and H4K16ac (Vaquero et al., 2004). These activities result in repression of gene expression and promote the formation of heterochromatin. Deacetylation of H4K16 at target gene promoters by SIRT1 is also important for coordinating metabolic profile adjustments upon differentiation in satellite cells (Ryall et al., 2015). H3K56 acetylation is regulated by SIRT1 indirectly through p300/ CBP (Das et al., 2009; Kong et al., 2011). The processes of H3K56 acetylation and deacetylation are important for genomic stability (Yuan et al., 2009). SIRT1 also modulates histone methylation levels indirectly through various histone methyltransferases and demethylases. Ezh2, the catalytic subunit of the transcription repressor complex PRC2, trimethylates H3K27 and is essential for development and differentiation, including maintenance of stem cell functions (De Haan and Gerrits, 2007). Deacetylation of Ezh2 by SIRT1 inhibits its activity (Wan et al., 2015). SIRT1 deacetylated SUV39H1, which activates its enzymatic activity and facilitates heterochromatin formation (Vaquero et al., 2007). Notably, the interaction between SUV39H1 and SIRT1 in response to oxidative stress is critical to prevent SUV39H1 degradation through the ubiquitin-proteasome system, ensuring genome protection (Bosch Presegue et al., 2011).
3.5. Linking metabolic signaling to epigenetic modification
Cell signaling transduction plays key roles for cells to quickly adjust their physiological status in response to different nutritional conditions, hormones stimulation, temperature changing and other environmental cues. Once these cues disappear, cellular physiological alteration induced by these signals are largely restored. G proteins, kinases and transcription factors are the major cell signaling transduction mediators, such as TSC2-Rheb, AMPK, Akt and Foxo3. Many of their activities are profoundly modulated by SIRT1 (Tonkin et al., 2012). Distinct from the transient cell signaling transduction, the epigenetic modulation renders relatively longer, yet reversible, physiological changes. SIRT1 is a potent epigenetic regulator through both acetylation and methylation of histones (Jing and Lin, 2015). Thus, as both a signaling transduction mediator and an epigenetic regulator, SIRT1 can be considered an important node that integrates the transient cell signaling and the persistent epigenetic modulation into a coordinated regulatory network for complex biological processes.
A study in muscle stem cell is an excellent example demonstrating how SIRT1 coordinates its metabolic signaling and its epigenetic modulation to regulate myogenic differentiation (Ryall et al., 2015). In adults, muscle stem cells are activated to proliferate and differentiate. MyoD, MEF2C and MyoG are master regulators of myogenic differentiation, and SIRT1 inhibits myogenic cells are mainly in a quiescent state. Upon muscle injury, quiescent muscle stem differentiation by deacetylating MyoD (Fulco et al., 2003). The new study demonstrates that muscle stem cells undergo a metabolic shift that increases glycolysis and glutaminolysis during muscle stem cells activation and proliferation. Moreover, this metabolic reprogramming is associated with a decrease in the intracellular NAD+/NADH ratio, resulting in reduced SIRT1 activity towards H4K16ac at the MyoG promoter and hence activation of MyoG transcription and subsequent myogenic differentiation (Ryall et al., 2015). Thus, SIRT1 inhibits myogenic differentiation by linking metabolic signaling to epigenetic regulation of MyoG expression.
Similar integration of signal transduction and epigenetic modulation is also occurring in hematopoietic stem cells (HSCs). Knocking out SIRT1 leads to both increased p53 acetylation level, a cell signaling pathway promoting apoptosis, and increased H4K16ac at the Hoxa9 promoter, an epigenetic modulation activating Hoxa9 expression and consequent aberrant expansion of HSCs (Singh et al., 2013). Furthermore, knocking out SIRT1 and SIRT2 rescues the Ras-PI3K-Akt pathway by reducing the levels of H3K56ac (Liu et al., 2012). All of these lines of evidence suggest that SIRT1 is one of the central players that link cell signaling to epigenetic regulation.
4. Summary
Stem cells display multiple physiological states: quiescent, self-renewal proliferating and differentiating states. During aging, defects and abnormalities are manifested to varying degrees in various pathways regulating these processes. As discussed here, SIRT1 as versatile regulator is intricately involved and integrated in these regulatory networks. Different physiological and pathological conditions, or even pharmacological interventions may alter these networks through changes in protein-protein interactions, subcellular localizations, and post-translational modifications. For example, oxidative stress promotes the interaction between SIRT1 and FOXO3 (Brunet et al., 2004), and in response to resistin treatment, which is an insulin resistance inducing hormone, the interaction between SIRT1 and PGC-1a as well as PPARa decreases (Yu et al., 2013). Therefore, instead of simply overexpressing or activating SIRT1, how to fine tune the regulatory networks around SIRT1, as well as the crosstalk between SIRT1 and other longevity factors, such as IGF-1, FGF21, mTORC1 and p66, in stem cells under different physiological conditions should be carefully considered and thoroughly investigated. Better understanding these mechanisms will help us better optimize our strategies and approaches to manage stem cells aging.
Acknowledgements
We would like to thank members of the Dang lab for critically reading the manuscript and helpful suggestions. This work is supported by a research grant from the Ted Nash Long Life Foundation and the NIH grant R00AG037646. WD is a CPRIT scholar R1306.
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.mce.2017.03.031.
References
- Back JH, Rezvani HR, Zhu Y, Guyonnet-Duperat V, Athar M, Ratner D, Kim AL, 2011. Cancer cell survival following DNA damage-mediated premature senescence is regulated by mammalian target of rapamycin (mTOR)-dependent Inhibition of sirtuin 1. J. Biol. Chem 286, 19100–19108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, et al. , 2016. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530, 184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N, Boyette LB, Tuan RS, 2015. Characterization of bone marrow-derived mesenchymal stem cells in aging. Bone 70, 37–47. [DOI] [PubMed] [Google Scholar]
- Baron S, Bedarida T, Cottart CH, Vibert F, Vessieres E, Ayer A, Henrion D, Hommeril B, Paul JL, Renault G, et al. , 2014. Dual effects of resveratrol on arterial damage induced by insulin resistance in aged mice. J. Gerontol. Ser. A Biol. Sci. Med. Sci 69, 260–269. [DOI] [PubMed] [Google Scholar]
- Boehm AM, Khalturin K, Anton-Erxleben F, Hemmrich G, Klostermeier UC, Lopez-Quintero JA, Oberg HH, Puchert M, Rosenstiel P, Wittlieb J, et al. , 2012. FoxO is a critical regulator of stem cell maintenance in immortal Hydra. Proc. Natl. Acad. Sci. U. S. A 109, 19697–19702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch-Presegue L, Raurell-Vila H, Marazuela-Duque A, Kane-Goldsmith N, Valle A, Oliver J, Serrano L, Vaquero A, 2011. Stabilization of Suv39H1 by SirT1 is part of oxidative stress response and ensures genome protection. Mol. Cell 42, 210–223. [DOI] [PubMed] [Google Scholar]
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, et al. , 2005. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122, 947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K, Xie S, Qiu X, Mohrin M, Shin J, Liu Y, Zhang D, Scadden DT, Chen D, 2013. SIRT3 reverses aging-associated degeneration. Cell Rep. 3, 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, et al. , 2004. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303, 2011–2015. [DOI] [PubMed] [Google Scholar]
- Calvanese V, Lara E, Suarez-Alvarez B, Abu Dawud R, Vazquez-Chantada M, Martinez-Chantar ML, Embade N, Lopez-Nieva P, Horrillo A, Hmadcha A, et al. , 2010. Sirtuin 1 regulation of developmental genes during differentiation of stem cells. Proc. Natl. Acad. Sci. U. S. A 107, 13736–13741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J, 2009. AMPK regulates energy expenditure by modulating NADþ metabolism and SIRT1 activity. Nature 458, 1056–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae HD, Broxmeyer HE, 2011. SIRT1 deficiency downregulates PTEN/JNK/FOXO1 pathway to block reactive oxygen species-induced apoptosis in mouse embryonic stem cells. Stem Cells Dev. 20, 1277–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W, et al. , 2016. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med 22, 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Liu X, Zhu W, Chen H, Hu X, Jiang Z, Xu Y, Wang L, Zhou Y, Chen P, et al. , 2014. SIRT1 ameliorates age-related senescence of mesenchymal stem cells via modulating telomere shelterin. Front. Aging Neurosci 6, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs BG, Durik M, Baker DJ, van Deursen JM, 2015. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat. Med 21, 1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornu M, Albert V, Hall MN, 2013. mTOR in aging, metabolism, and cancer. Curr. Opin. Genet. Dev 23, 53–62. [DOI] [PubMed] [Google Scholar]
- Dang W, Steffen KK, Perry R, Dorsey JA, Johnson FB, Shilatifard A, Kaeberlein M, Kennedy BK, Berger SL, 2009. Histone H4 lysine 16 acetylation regulates cellular lifespan. Nature 459, 802–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das C, Lucia MS, Hansen KC, Tyler JK, 2009. CBP/p300-mediated acetylation of histone H3 on lysine 56. Nature 459, 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Haan G, Gerrits A, 2007. Epigenetic control of hematopoietic stem cell aging the case of Ezh2. Ann. N. Y. Acad. Sci 1106, 233–239. [DOI] [PubMed] [Google Scholar]
- Dzamko NL, Steinberg GR, 2009. AMPK-dependent hormonal regulation of whole-body energy metabolism. Acta Physiol. 196, 115–127. [DOI] [PubMed] [Google Scholar]
- Fry CS, Lee JD, Mula J, Kirby TJ, Jackson JR, Liu F, Yang L, Mendias CL, Dupont-Versteegden EE, McCarthy JJ, et al. , 2015. Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat. Med 21, 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V, 2008. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev. Cell 14, 661–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulco M, Schiltz RL, Iezzi S, King MT, Zhao P, Kashiwaya Y, Hoffman E, Veech RL, Sartorelli V, 2003. Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol. Cell 12, 51e62. [DOI] [PubMed] [Google Scholar]
- Garcia-Prat L, Martinez-Vicente M, Perdiguero E, Ortet L, Rodriguez-Ubreva J, Rebollo E, Ruiz-Bonilla V, Gutarra S, Ballestar E, Serrano AL, et al. , 2016. Autophagy maintains stemness by preventing senescence. Nature 529, 37e42. [DOI] [PubMed] [Google Scholar]
- Ghosh HS, McBurney M, Robbins PD, 2010. SIRT1 negatively regulates the mammalian target of rapamycin. PloS One 5, e9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell MA, Rando TA, 2015. Stem cells and healthy aging. Science 350, 1199e1204. [DOI] [PubMed] [Google Scholar]
- Guo W, Qian L, Zhang J, Zhang W, Morrison A, Hayes P, Wilson S, Chen T, Zhao J, 2011. Sirt1 overexpression in neurons promotes neurite outgrowth and cell survival through inhibition of the mTOR signaling. J. Neurosci. Res 89, 1723e1736. [DOI] [PubMed] [Google Scholar]
- Han MK, Song EK, Guo Y, Ou X, Mantel C, Broxmeyer HE, 2008. SIRT1 regulates apoptosis and Nanog expression in mouse embryonic stem cells by controlling p53 subcellular localization. Cell Stem Cell 2, 241e251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Brunet A, 2012. Histone methylation makes its mark on longevity. Trends Cell Biol. 22, 42e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Q, An JQ, Hao F, Yang C, Lu T, Qu TY, Zhao LR, Duan WM, 2015. Inducible lentivirus-mediated expression of the Oct4 gene affects multilineage differentiation of adult human bone marrow-derived mesenchymal stem cells. Cell. Reprogr 17, 347e359. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, et al. , 2009. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392e395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Zhao B, Lombard DB, Fingar DC, Inoki K, 2014. Cross-talk between sirtuin and mammalian target of rapamycin complex 1 (mTORC1) signaling in the regulation of S6 kinase 1 (S6K1) phosphorylation. J. Biol. Chem 289, 13132e13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori YS, Kuno A, Hosoda R, Horio Y, 2013. Regulation of FOXOs and p53 by SIRT1 modulators under oxidative stress. PloS One 8, e73875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Gan Q, Han L, Li J, Zhang H, Sun Y, Zhang Z, Tong T, 2008. SIRT1 overexpression antagonizes cellular senescence with activated ERK/S6k1 signaling in human diploid fibroblasts. PloS One 3, e1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R, Xu Y, Wan W, Shou X, Qian J, You Z, Liu B, Chang C, Zhou T, Lippincott-Schwartz J, et al. , 2015. Deacetylation of nuclear LC3 drives autophagy initiation under starvation. Mol. Cell 57, 456e466. [DOI] [PubMed] [Google Scholar]
- Huang S, Feng C, Wu Y, Yang S, Ma K, Wu X, Fu X, 2013. Dissimilar characteristics of umbilical cord mesenchymal stem cells from donors of different ages. Cell Tissue Bank. 14, 707e713. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Guarente L, 2016a. mTORC1 and SIRT1 cooperate to foster expansion of gut adult stem cells during calorie restriction. Cell 166, 436e450. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Guarente L, 2016b. The unexpected role of mTORC1 in intestinal stem cells during calorie restriction. Cell Cycle 1e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing H, Lin H, 2015. Sirtuins in epigenetic regulation. Chem. Rev 115, 2350e2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Rabinovitch PS, Kaeberlein M, 2013. mTOR is a key modulator of ageing and age-related disease. Nature 493, 338e345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong S, Kim SJ, Sandal B, Lee SM, Gao B, Zhang DD, Fang D, 2011. The type III histone deacetylase Sirt1 protein suppresses p300-mediated histone H3 lysine 56 acetylation at Bclaf1 promoter to inhibit T cell activation. J. Biol. chem 286, 16967e16975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan F, Cacicedo JM, Ruderman N, Ido Y, 2008. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMPactivated protein kinase activation. J. Biol. Chem 283, 27628e27635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley E, Pearson M, Faretta M, Bauer UM, Frye RA, Minucci S, Pelicci PG, Kouzarides T, 2002. Human SIR2 deacetylates p53 and antagonizes PML/p53induced cellular senescence. EMBO J. 21, 2383e2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Kazgan N, 2011. Mammalian sirtuins and energy metabolism. Int. J. Biol. Sci 7, 575e587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Tollefsbol TO, 2011. p16(INK4a) suppression by glucose restriction contributes to human cellular lifespan extension through SIRT1-mediated epigenetic and genetic mechanisms. PloS One 6, e17421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Rando TA, 2011. Manifestations and mechanisms of stem cell aging. J. Cell Biol 193, 257e266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Wei-LaPierre L, Klose A, Dirksen RT, Chakkalakal JV, 2015. Inducible depletion of adult skeletal muscle stem cells impairs the regeneration of neuromuscular junctions. eLife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang DL, Chen S, Zhao L, Sun FL, 2012. Oncogene Ras/phosphatidylinositol 3-kinase signaling targets histone H3 acetylation at lysine 56. J. Biol. Chem 287, 41469e41480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G, 2013. The hallmarks of aging. Cell 153, 1194e1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo F, Zimmermann A, Maiuri MC, Kroemer G, 2015. Essential role for autophagy in life span extension. J. Clin. Investig 125, 85e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martien S, Pluquet O, Vercamer C, Malaquin N, Martin N, Gosselin K, Pourtier A, Abbadie C, 2013. Cellular senescence involves an intracrine prostaglandin E2 pathway in human fibroblasts. Biochim. Biophys. Acta 1831, 1217e1227. [DOI] [PubMed] [Google Scholar]
- Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, et al. , 2013. Metformin improves healthspan and lifespan in mice. Nat. Commun 4, 2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor RK, Baur JA, Gomes AP, Ward TM, Csiszar A, Mercken EM, Abdelmohsen K, Shin YK, Canto C, Scheibye-Knudsen M, et al. , 2011. SRT1720 improves survival and healthspan of obese mice. Sci. Rep 1, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Martin-Montalvo A, Mercken EM, Palacios HH, Ward TM, Abulwerdi G, Minor RK, Vlasuk GP, Ellis JL, Sinclair DA, et al. , 2014. The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet. Cell Rep. 6, 836e843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohrin M, Shin J, Liu Y, Brown K, Luo H, Xi Y, Haynes CM, Chen D, 2015. Stem cell aging. A mitochondrial UPR-mediated metabolic checkpoint regulates hematopoietic stem cell aging. Science 347, 1374e1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris BJ, 2013. Seven sirtuins for seven deadly diseases of aging. Free Radic. Biol. Med 56, 133e171. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL, 1996. The aging of hematopoietic stem cells. Nat. Med 2, 1011e1016. [DOI] [PubMed] [Google Scholar]
- Mu WL, Wang YJ, Xu P, Hao DL, Liu XZ, Wang TT, Chen F, Chen HZ, Lv X, Liu DP, 2015. Sox2 deacetylation by Sirt1 is involved in mouse somatic reprogramming. Stem Cells 33, 2135e2147. [DOI] [PubMed] [Google Scholar]
- Oh J, Lee YD, Wagers AJ, 2014. Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat. Med 20, 870e880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgushi M, Minaguchi M, Sasai Y, 2015. Rho-signaling-directed YAP/TAZ activity underlies the long-term survival and expansion of human embryonic stem cells. Cell Stem Cell 17, 448e461. [DOI] [PubMed] [Google Scholar]
- Ou X, Lee MR, Huang X, Messina-Graham S, Broxmeyer HE, 2014. SIRT1 positively regulates autophagy and mitochondria function in embryonic stem cells under oxidative stress. Stem cells 32, 1183e1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccinato CA, Sertie AL, Torres N, Ferretti M, Antonioli E, 2015. High OCT4 and low p16(INK4A) expressions determine in vitro lifespan of mesenchymal stem cells. Stem Cells Int. 2015, 369828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Brown KV, Moran Y, Chen D, 2010. Sirtuin regulation in calorie restriction. Biochim. Biophys. Acta 1804, 1576e1583. [DOI] [PubMed] [Google Scholar]
- Rimmele P, Bigarella CL, Liang R, Izac B, Dieguez-Gonzalez R, Barbet G, Donovan M, Brugnara C, Blander JM, Sinclair DA, et al. , 2014. Aging-like phenotype and defective lineage specification in SIRT1-deleted hematopoietic stem and progenitor cells. Stem Cell Rep. 3, 44e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F, Campisi J, 2011. Four faces of cellular senescence. J. Cell Biol 192, 547e556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, Weissman IL, 2005. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc. Natl. Acad. Sci. U. S. A 102, 9194e9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz R, Perez-Villegas EM, Carrion AM, 2016. AMPK function in aging process. Curr. Drug Targets 17 (8), 932e941. [DOI] [PubMed] [Google Scholar]
- Ryall JG, Dell’Orso S, Derfoul A, Juan A, Zare H, Feng X, Clermont D, Koulnis M, Gutierrez-Cruz G, Fulco M, et al. , 2015. The NAD(þ)-dependent SIRT1 deacetylase translates a metabolic switch into regulatory epigenetics in skeletal muscle stem cells. Cell Stem Cell 16, 171e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh A, Brace CS, Rensing N, Cliften P, Wozniak DF, Herzog ED, Yamada KA, Imai S, 2013. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 18, 416e430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz MB, Sinclair DA, 2016. When stem cells grow old: phenotypes and mechanisms of stem cell aging. Development 143, 3e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto E, Yoshida M, 2014. Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harb. Perspect. Biol 6, a018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Tsai YT, Dimarco NM, Long MA, Sun X, Tang L, 2011. Transplantation of mesenchymal stem cells from young donors delays aging in mice. Sci. Rep 1, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simic P, Zainabadi K, Bell E, Sykes DB, Saez B, Lotinun S, Baron R, Scadden D, Schipani E, Guarente L, 2013. SIRT1 regulates differentiation of mesenchymal stem cells by deacetylating beta-catenin. EMBO Mol. Med 5, 430e440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Williams CA, Klarmann K, Burkett SS, Keller JR, Oberdoerffer P, 2013. Sirt1 ablation promotes stress-induced loss of epigenetic and genomic hematopoietic stem and progenitor cell maintenance. J. Exp. Med 210, 987e1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa-Victor P, Gutarra S, Garcia-Prat L, Rodriguez-Ubreva J, Ortet L, RuizBonilla V, Jardi M, Ballestar E, Gonzalez S, Serrano AL, et al. , 2014. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature 506, 316e321. [DOI] [PubMed] [Google Scholar]
- Sudo K, Ema H, Morita Y, Nakauchi H, 2000. Age-associated characteristics of murine hematopoietic stem cells. J. Exp. Med 192, 1273e1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui B, Hu C, Jin Y, 2016. Mitochondrial metabolic failure in telomere attritionprovoked aging of bone marrow mesenchymal stem cells. Biogerontology 17 (2), 267e279. [DOI] [PubMed] [Google Scholar]
- Sun T, Li X, Zhang P, Chen WD, Zhang HL, Li DD, Deng R, Qian XJ, Jiao L, Ji J, et al. , 2015. Acetylation of Beclin 1 inhibits autophagosome maturation and promotes tumour growth. Nat. Commun 6, 7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AH, Rando TA, 2014. Induction of autophagy supports the bioenergetic demands of quiescent muscle stem cell activation. EMBO J. 33, 2782e2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S, Huang G, Fan W, Chen Y, Ward JM, Xu X, Xu Q, Kang A, McBurney MW, Fargo DC, et al. , 2014. SIRT1-mediated deacetylation of CRABPII regulates cellular retinoic acid signaling and modulates embryonic stem cell differentiation. Mol. Cell 55, 843e855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkin J, Villarroya F, Puri PL, Vinciguerra M, 2012. SIRT1 signaling as potential modulator of skeletal muscle diseases. Curr. Opin. Pharmacol 12, 372e376. [DOI] [PubMed] [Google Scholar]
- Tsai CC, Su PF, Huang YF, Yew TL, Hung SC, 2012. Oct4 and Nanog directly regulate Dnmt1 to maintain self-renewal and undifferentiated state in mesenchymal stem cells. Mol. Cell 47, 169e182. [DOI] [PubMed] [Google Scholar]
- Vaquero A, Scher M, Erdjument-Bromage H, Tempst P, Serrano L, Reinberg D, 2007. SIRT1 regulates the histone methyl-transferase SUV39H1 during heterochromatin formation. Nature 450, 440e444. [DOI] [PubMed] [Google Scholar]
- Vaquero A, Scher M, Lee D, Erdjument-Bromage H, Tempst P, Reinberg D, 2004. Human SirT1 interacts with histone H1 and promotes formation of facultative heterochromatin. Mol. Cell 16, 93e105. [DOI] [PubMed] [Google Scholar]
- Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA, 2001. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 107, 149e159. [DOI] [PubMed] [Google Scholar]
- Wan J, Zhan J, Li S, Ma J, Xu W, Liu C, Xue X, Xie Y, Fang W, Chin YE, et al. , 2015. PCAF-primed EZH2 acetylation regulates its stability and promotes lung adenocarcinoma progression. Nucleic Acids Res. 43, 3591e3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Diao D, Shi Z, Zhu X, Gao Y, Gao S, Liu X, Wu Y, Rudolph KL, Liu G, et al. , 2016. SIRT6 controls hematopoietic stem cell homeostasis through epigenetic regulation of Wnt signaling. Cell Stem Cell 18, 495e507. [DOI] [PubMed] [Google Scholar]
- Wang P, Guan YF, Du H, Zhai QW, Su DF, Miao CY, 2012. Induction of autophagy contributes to the neuroprotection of nicotinamide phosphoribosyltransferase in cerebral ischemia. Autophagy 8, 77e87. [DOI] [PubMed] [Google Scholar]
- Wang RH, Kim HS, Xiao C, Xu X, Gavrilova O, Deng CX, 2011. Hepatic Sirt1 deficiency in mice impairs mTorc2/Akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. J. Clin. Investig 121, 4477e4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EO, Taylor AK, Bell EL, Lim R, Kim DM, Guarente L, 2016. Sirtuin 1 promotes deacetylation of Oct4 and maintenance of naive pluripotency. Cell Rep. 17, 809e820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JJ, Liu J, Chen EB, Wang JJ, Cao L, Narayan N, Fergusson MM, Rovira II, Allen M, Springer DA, et al. , 2013. Increased mammalian lifespan and a segmental and tissue-specific slowing of aging after genetic reduction of mTOR expression. Cell Rep. 4, 913e920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon DS, Choi Y, Jang Y, Lee M, Choi WJ, Kim SH, Lee JW, 2014. SIRT1 directly regulates SOX2 to maintain self-renewal and multipotency in bone marrow-derived mesenchymal stem cells. Stem Cells 32, 3219e3231. [DOI] [PubMed] [Google Scholar]
- Yoon DS, Kim YH, Jung HS, Paik S, Lee JW, 2011. Importance of Sox2 in maintenance of cell proliferation and multipotency of mesenchymal stem cells in low-density culture. Cell Prolif. 44, 428e440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu A, Zheng Y, Zhang R, Huang J, Zhu Z, Zhou R, Jin D, Yang Z, 2013. Resistin impairs SIRT1 function and induces senescence-associated phenotype in hepatocytes. Mol. Cell. Endocrinol 377, 23e32. [DOI] [PubMed] [Google Scholar]
- Yuan HF, Zhai C, Yan XL, Zhao DD, Wang JX, Zeng Q, Chen L, Nan X, He LJ, Li ST, et al. , 2012. SIRT1 is required for long-term growth of human mesenchymal stem cells. J. Mol. Med 90, 389e400. [DOI] [PubMed] [Google Scholar]
- Yuan J, Pu M, Zhang Z, Lou Z, 2009. Histone H3-K56 acetylation is important for genomic stability in mammals. Cell Cycle 8, 1747e1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Chen HZ, Liu JJ, Jia YY, Zhang ZQ, Yang RF, Zhang Y, Xu J, Wei YS, Liu DP, et al. , 2010. SIRT1 suppresses activator protein-1 transcriptional activity and cyclooxygenase-2 expression in macrophages. J. Biol. Chem 285, 7097e7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Li J, Suzuki K, Qu J, Wang P, Zhou J, Liu X, Ren R, Xu X, Ocampo A, et al. , 2015. Aging stem cells. A Werner syndrome stem cell model unveils heterochromatin alterations as a driver of human aging. Science 348, 1160e1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZN, Chung SK, Xu Z, Xu Y, 2014. Oct4 maintains the pluripotency of human embryonic stem cells by inactivating p53 through Sirt1-mediated deacetylation. Stem Cells 32, 157e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Chen X, Liu T, Gong Y, Chen S, Pan G, Cui W, Luo ZP, Pei M, Yang H, et al. , 2015. Melatonin reverses H2 O2 -induced premature senescence in mesenchymal stem cells via the SIRT1-dependent pathway. J. Pineal Res 59, 190e205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM, 2011. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol 12, 21e35. [DOI] [PMC free article] [PubMed] [Google Scholar]