Abstract
To undertake a systematic review of all reliable evaluations of the clinical performance and cost‐effectiveness of a film‐forming liquid acrylate in the protection of the chronic ulcer peri‐wound skin. Results from searches were scrutinised by two reviewers to identify possible randomised controlled trials and full reports of these were obtained. Details of eligible studies were extracted and summarised independently by two reviewers using a data extraction sheet. Meta‐analysis was used to combine the results of trials where the interventions and outcome measures were sufficiently similar. A total of nine eligible studies were identified. The review reveals that a liquid‐film forming acrylate (Cavilon no‐sting barrier film, 3M, Minneapolis, MN, USA) is a safe and effective skin barrier to protect the peri‐wound area of chronic ulcers. There is no difference between the protective properties of different barrier methods that are used to protect the peri‐wound skin around chronic ulcers. Compared with no treatment or a placebo, this liquid film‐forming acrylate has a significant impact on the integrity of the peri‐wound skin. In addition, it has significant benefits in terms of pain control and patient comfort, and its use may reduce nursing time.
Keywords: Cavilon, Chronic wound, Meta‐analysis, No‐sting barrier film, Peri‐wound skin, Systematic review
Introduction
A chronic wound represents a break in the integrity of the skin with a dermal or deeper base and a healing process that is delayed. The centre of the wound contains open dermal tissue or deeper tissue with cells and a connective tissue backbone that requires fluid for nourishment. Since the time of Winter (1), moist interactive healing has been shown to facilitate healing. Increased exudate often indicates increased bacterial burden or persistent inflammation resulting in a local fluid imbalance. Excess wound fluid can also result from uncontrolled oedema of the surrounding skin (venous disease, congestive heart failure and low albumen). This increased fluid will compromise the wound margin and peri‐wound skin integrity. The peri‐wound breakdown of the surface keratin results in local maceration and hyper‐hydration of the underlying epidermal cells and dermal components. Moisture balance within a chronic wound requires enough moisture to support cellular and interstitial tissue function but no access to damage the surrounding skin. The prepara‐ tion of the wound bed paradigm (2) is built on the foundation of treating the'whole patient' before the ‘hole in a patient’. We need to correct the cause and address patient‐centred concerns before turning our attention to local wound management. A number of the newer moist interactive dressings will help to control fluid and may contain a partial‐to‐complete fluid lock, but there is always some leakage of fluid below the dressing that can lead to maceration of the surrounding skin. One of the ways to protect the surrounding skin is to apply a barrier before wound dressings are placed over the defect to maintain moisture balance.
Traditionally, barriers have consisted of three traditional modalities, and the more recent addition of a fourth class of film‐forming liquid acrylates (3):
-
•
Petrolatum: Petrolatum provides an external barrier that is occlusive to water. Petrolatum tends to melt, especially with local warmth, and it can also migrate into the wounds. This type of material is incompatible with heavy metals such as silver.
-
•
Zinc oxide paste (ointment): Petrolatum can be made stiffer by a combination of zinc oxide powder forming a zinc oxide paste (a paste is a powder in an ointment). This paste will provide peri‐wound protection and is less likely to soften with excessive heat or moisture. Unfortunately, petrolatum is translucent, whereas zinc oxide ointment is opaque and the condition of the wound margin is often difficult to ascertain.
-
•
Windowed adhesive dressings (thin hydrocolloids, films): If a more permanent barrier is required, a hydrocolloid or film dressing with an adhesive backing can be applied around the wound, and a window over the wound removed to apply a different primary dressing or the occlusive dressing can be used to protect the wound and surrounding skin. When hydrocolloids are used over the wound and surrounding skin, maceration may result from the interaction of the hydrocolloid with the wound surface causing an amorphous hydrophilic combination of wound fluid and dressing residue.
-
•
Film‐forming liquid acrylates: These products have been introduced into wound care over the past 10–15 years in a spray bottle or applicator pad and are applied around the wound. The material solidifies within a few seconds on contact with normal skin and forms an impermeable solid film that prevents maceration from the adjacent wound fluid. The properties of the four types of wound margin barriers are listed in Table 1.
Table 1.
Comparison of skin barrier products (3)
| Barrier | Pros | Cons | Comments |
|---|---|---|---|
| Film‐forming liquid acrylates | Ease of application | Some contain alcohol and/or acetone | Some products can have adhesive |
| Uniform application | (burn/sting on application) | dressing applied over top | |
| Flexible and comfortable | Require provider education regarding frequency | ||
| Visualise underlying skin | and thickness of application | ||
| Resist wash off | |||
| Do not trap contaminants | |||
| Low sensitisation rate | |||
| Adhesive dressings | Form a solid interface between two surfaces | Edges may lift | May need to be used with central window |
| (transparent films, thick hydrocolloids) | May have a long wear time Visualisation of underlying skin may be possible | Trapping of moisture allows bacterial proliferation | requiring training and education |
| Adhesive allergies | |||
| Smell/odour may occurv | |||
| Zinc oxide ointment/paste | Robust (stiff) | Potential allergens and variable consistency | Unable to visualise underlying skin |
| Commonly used and accepted | Messy and difficult to remove | If not contaminated, the old zinc oxide does not need | |
| Inexpensive and readily available | May become contaminated, requiring removal | to be removed, but fill in the spaces with extra | |
| May inhibit absorbency and adhesion | material applied with a tongue depressor | ||
| of overlying dressings/devices | |||
| Petrolatum and related | Inexpensive (petrolatum) and accessible | Potential allergens | Quantity applied variable with users |
| ointments/creams | Visualisation of underlying skin | Product variability | |
| Tend to melt and wash off | |||
| Often inhibit absorbency and adhesion | |||
| of other devices | |||
| Require frequent re‐application |
Chronic wound exudate contains proteases, which break down protein and will actively damage what may be otherwise healthy tissue. These pro‐inflammatory molecules make chronic wound fluid a wounding agent in its own right and therefore potentially more deleterious than moisture alone (4).
Maceration is defined as the softening and breaking down of the skin stratum corneum and epidermis resulting from prolonged exposure to moisture. Peri‐wound maceration can occur in any exudating chronic wound. This maceration can delay healing, lead to enlargement of the wound and may be accompanied by pain. Additional consequences for the patient are a longer treatment time and added discomfort. For the health care system, increased costs result from longer treatment time and additional material resources, as well as increased staff costs.
No‐sting barrier film (NSBF) is a liquid intended for use as a film‐forming product, which upon application to intact or damaged skin forms a long‐lasting waterproof barrier. It acts as a protective interface between skin and wound fluids, body wastes, perspiration, adhesive products and friction. Several research studies have been performed and published on its use for a variety of indications since its introduction in 1992. This systematic review with meta‐analysis provides an overview of the clinical performance of NSBF for peri‐wound protection.
Objective
The aim was to undertake a systematic review of all reliable evaluations of the clinical and cost‐effectiveness of NSBF in the protection of the peri‐wound skin of chronic ulcers.
Criteria for considering studies for this review
Types of studies
Prospective, randomised controlled trials (RCTs) and controlled clinical trials (CCTs) that employed quasi‐random methods of allocation (such as day of the week or surname), which evaluate NSBF in the protection of the peri‐wound skin of chronic ulcers, were eligible for inclusion. Trials were included if they reported an objective measure of outcome, such as reduction of erythema or maceration. Trials, which only report surrogate outcome measures or subjective assessments of improvement/deterioration, were excluded. There was no restriction on articles on the basis of language or publication status.
Types of participants
People of any age with chronic wounds in any care setting.
Types of intervention
Trials, which evaluate any kind of outcome of the use of NSBF in the protection of the peri‐wound area of chronic ulcers, are included.
Search strategy
Electronic searches of Medline, Cinahl, EMBASE, Cochrane Controlled Trials Register, as well as hand searches of conference proceedings and wound care journals were used to identify RCTs and CCTs. Experts in wound care were contacted to enquire about unpublished, ongoing and recently published trials. References within identified papers were scrutinised to identify additional studies.
Methods of the review
Data collection
Details of eligible studies were extracted and summarised using a data extraction sheet. Two reviewers (JS, AB) verified data extraction independently. Any disagreement was resolved by discussion. For the randomised trials, a quality assessment instrument developed by the Cochrane Musculoskeletal Injuries Group (5) was modified and used. Each of the ten questions in this assessment can give a score of 0, 1, 2 or 3. Quality of the included studies was assessed by two reviewers (JS, AB) independently. Any disagreement was resolved by discussion.
Presentation of results
The plots used in this systematic review combine the results of individual studies. Outcomes can be either dichotomous or continuous. For dichotomous data, relative risk has been used rather than odds ratios, as event rates were high in the identified trials and odds ratios would give an inflated impression of the magnitude of effect. Continuous outcomes (e.g. rate of healing) are measured on a continuous scale and are compared statistically by standardised mean differences. For all results in this review, the fixed effects model was used. For each trial, relative risks or standardised mean differences with 95% confidence intervals were calculated for all outcomes. The results of both the relative risk ratios and mean differences are presented in forest plots to allow a quick comparison to be made with the results from other studies. Forest plots were created in Review Manager 4·2.3 (© 2003 The Cochrane Collaboration).
Description of studies
Excluded studies
A total of 49 papers were identified, with NSBF as a treatment. There were six papers that were qualified as expert opinions. Ten studies reported on laboratory testing. Twelve studies reported on incontinence care, three on other indications. Of the remaining 18 papers that reported on the use of NSBF for chronic wounds, seven were case studies on a total of 43 patients. Therefore, possible data from 11 controlled trials were considered for the review. One study was excluded, because the outcome of interest was ulcer healing rather than peri‐wound protection (6). One study was excluded, because the data were also presented in an earlier paper (7).
Included studies
Characteristics of the nine included studies are summarised in Table 2.
Table 2.
Included studies
| Study | Methods | Participants | Interventions | Outcomes | Allocation |
|---|---|---|---|---|---|
| Rolstad et al. 1994 (8) | Randomised controlled trial | 19 patients/30 wounds | Treatment of peri‐wound area of wounds, tubes or stomas with alcohol‐based and siloxane‐based skin protectant | Clinical effectiveness, pain | Not used |
| Hampton 1998 (16) | Randomised controlled trial | 62 patients | NSBF versus conventional treatment: patient isused as its own control by using half of theaffected area for each treatment | Wound healing | Unclear |
| García et al. 2000 (17) | Case‐control study | 26 patients | NSBF | Level of maceration, improvement ofperi‐wound, area, patient comfort, ease of application and adverse events | Not used |
| Coutts et al. 2001 (10) | Case‐control study | 15 patients* | NSBF versus either zinc oxide or petrolatum | Change in wound size, change in wound exudate, change in peri‐wound condition, time to clean and time to apply | Not used |
| Bär and Vanscheidt 2001 (11) | Randomised controlled trial | 20 patients | NSBF versus zinc paste | Ease of use, efficacy and tolerability | Adequate |
| García et al. 2002 (18) | Randomised controlled trial | 60 patients | NSBF and hydrocolloid dressing versus hydrocolloid dressing alone | Maceration | Unclear |
| Cameron et al. 2003 (9) | Randomised controlled trial | 35 patients | NSBF versus zinc paste | Ease of use, efficacy and tolerability | Adequate |
| Neander and Hesse 2003 (12) | Randomised double‐blind controlled trial | 227 patients | NSBF versus placebo | Erythema assessment with a chromameter | Adequate |
| Vin and Bonnetblanc 2003 (19) | Randomised controlled trial | 40 patients | NSBF versus no film. | Erythema control, clinical efficacy, skin condition and adverse events | Adequate |
NBSF, No‐sting barrier film. *Interim analysis; final report, 30 patients had similar results (10).
Methodological quality
The quality of research in this area is generally poor as trials are often too small, follow up is short, recurrence is rarely considered and sometimes multiple ulcers are incorrectly regarded as independent. The mean quality score for the included trials is 19·89 (SD 4·59, range 14–30). Although this score is low, it is much higher than in a large number of reviews in the Cochrane Database, where scores below 10 are no exception. In these reviews, there is no withdrawal of studies because of low quality scores.
Results
Product performance: erythema/maceration control
Erythema or maceration control was reported in eight papers. Some of the data were continuous, some dichotomous. Therefore, two different comparisons were made. Four of the papers compared NSBF with traditional treatment (zinc oxide, petrolatum and alternative barriers) and four with either no treatment or placebo controlled. For both comparisons with traditional treatment, there was no significant difference between the two groups. The results confirm that existing methods for protecting the peri‐wound area of chronic ulcers are satisfactory. When NSBF was compared with a placebo or with no treatment, the difference was significant in the favour of NSBF. For the dichotomous, as well as for the continuous data, the P value was < 0·0001 (1, 2).
Figure 1.

Product performance: erythema/maceration control (continuous data).
Figure 2.

Product performance: erythema/maceration control (dichotomous data).
Wound healing
There was only one study reporting wound healing, comparing NSBF to zinc oxide. There was no significant difference between the two treatment methods (Figure 3).
Figure 3.
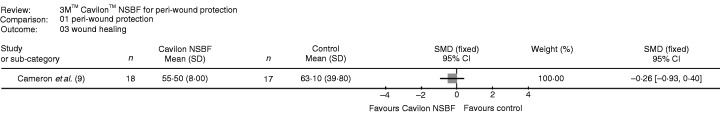
Wound healing.
Cleansing and application time
Two studies reported cleansing time, one also measured application time. There was a significant difference for both measurements, favouring the use of NSBF. The P value for cleaning was < 0·00001, and the time for applying the product had a value of 0·001 (Figure 4).
Figure 4.

Application time.
Pain and patient comfort
Two studies reported on pain. In one study, pain or no pain was reported (8); in the other study, a scale was used on which pain was described as none, mild, uncomfortable, distressing or horrible (9). For the comparison, none and mild were grouped, as well as the three other degrees. There was a significant difference favouring NSBF (P = 0·007). One study reported on patient comfort, and also here, there was a significant difference (P = 0·04), favouring the use of NSBF (9) (Figure 5).
Figure 5.

Pain and patient comfort.
Ease of application
Although the ease of application was mentioned in several included studies as an observational remark, there was no difference calculated (P = 0·07) in one reported study (8) (Figure 6)
Figure 6.

Ease of application.
Discussion
This review revealed that there is no statistically significant difference between the protective properties of different peri – wound skin barriers. This is not really a surprise as zinc paste and petrolatum in various preparations have been used for many years to protect the peri‐wound skin in a satisfactory manner. In several other studies, it is mentioned that zinc oxide as well as petrolatum are difficult to remove. Coutts et al. (10) state that zinc oxide and petrolatum are effective but problematic as these products can interfere with dressing function, including both absorption and adhesion. Some zinc oxide preparations contain common sensitisers (lanolin, perfumes), and we commonly sensitise persons to these allergens, especially when they are applied around leg ulcers. An additional advantage of a barrier film is transparency. It is possible to assess the damaged skin under the film without having to remove it (11). The main conclusion of this review is NSBF has a significant protective effect on the peri‐wound skin when compared with placebo or no treatment.
Other useful study endpoints include peri‐wound skin erythema. Neander and Hesse (12) used a measurable and objective method to measure erythema. They documented peri‐wound skin erythema which completely disappeared after 3 days in 88·1% of the patients and after 4 days in all patients when treated with NSBF. In matched placebo‐treated controls, 99% of the erythema was still present.
Unfortunately, there is no true clinical cost‐effectiveness data available for the NSBF peri‐wound protection. Data are available for the use of NSBF in the treatment of incontinence. Baatenburg de Jong et al. (13), Bale et al. (14) and Zehrer et al. (15), all showed that NSBF has a significant reduction in the overall treatment cost of these patients.
Reviewers' conclusions
Implications for practice
NSBF is a safe and effective barrier to protect the peri‐wound skin of chronic ulcers. Its benefits include: visibility of wound margins, reduction of erythema, pain control, patient comfort and reduced staff time.
Implications for research
Much of the research concerning chronic wound care, and the importance of the peri‐wound area in particular, is of poor quality. The quality scores of the identified trials were generally low, as in most trials truly objective outcome measures were under‐utilised. Randomised trials can be performed, but it is difficult to blind investigators and patients to the treatments because of their obviously different clinical appearances. There is a need for validated peri‐wound skin assessment tools. Research in this area is often hindered by ethical considerations. In the study of Hampton (16), it was agreed that it would be unacceptable to continue if the control tissues (without NSBF) deteriorated during the study and the research tissues (with NSBF) improved. In this case, the film would be applied to both sides of the wound. This consideration limited this study considerably, as it allowed only a small margin for the film to show an improvement before being applied to both sides of the wound. The author concludes that using the film on the entire area was a major but unavoidable fault of the study. The final conclusion of the study was therefore dramatically weakened. Also, available equipment allows an objective and more precise quantification of the peri‐wound area. These methods were used in the study of Neander and Hesse (12) and in that way an example of how research in this area can be performed at a high quality level.
In this study, a large number of patients were studied (227). NSBF was compared with water in a randomised protocol dividing wounds into a 12–6 o'clock and 6–12 o'clock quadrant. Subjective measurement of erythema was replaced with chromameter determinations of colour changes. Fat content was measured using a sebumeter, as increased product‐related fat or oil residue could affect covering wound care product adhesion. The use of the intra‐individual double blind test in this study also guaranteed that the baseline characteristics are fully comparable. Finally, economic evaluations for cost‐effectiveness should be included in future trials.
Potential conflict of interest
Both reviewers are employed by 3M, Germany. Some of the research included in this review was supported by educational grants from 3M Company. Dr Sibbald is a professor of medicine and public health sciences at the University of Toronto. He has been an investigator, consultant and speaker for the 3M Company.
Qualification for authorship: Jan Schuren and Anja Becker reviewed the included papers and completed a data extraction sheet independently. Quality assessment of the included papers was performed by the two authors independently. R Gary Sibbald reviewed the results, edited the manuscript and commented on the clinical significance of the data.
References
- 1. Winter GD. Formation of the scab and the rate of epithelialization of superficial wounds in the skin of the young domestic pig. Nature 1962;193: 293–4. [DOI] [PubMed] [Google Scholar]
- 2. Sibbald RG, Orsted H, Schultz GS, Coutts P, Keast D. Preparing the wound bed 2003: focus on infection and inflammation. Ostomy Wound Manage 2003;49(11):24–51. [PubMed] [Google Scholar]
- 3. Sibbald RG, Campbell K, Coutts P, Queen D. Intact skin – an integrity not to be lost. Ostomy Wound Manage 2003; 49(6):27–41. [PubMed] [Google Scholar]
- 4. Cutting KF, White RJ. Maceration of the skin and wound bed 1: its nature and causes. J Wound Care 2002;11(7):275–8. [DOI] [PubMed] [Google Scholar]
- 5. Gibson JNA, Handoll HHG, Madhok R. Interventions for treating proximal humeral fractures in adults (Cochrane review). The Cochrane Library: Issue 2. Oxford: Update Software, 2002. [DOI] [PubMed] [Google Scholar]
- 6. Wilson Coon J, Hunter M, Czarnata D, Martino S, Anton P, Maksym J. Treatment of early pressure ulcers in critically ill patients. Presentation at the WOCN Conference, Toronto, Canada, 2000.
- 7. Hampton S. Macerated and excoriated tissues: a randomised controlled trial in wound care with case studies. Poster Presentation at EWMA Conference, Harrogate, UK, 1997.
- 8. Rolstad BS, Borchert K, Magnan S, Scheel N. A comparison of an alcohol‐based and a siloxane‐based peri‐wound skin protectant. J Wound Care 1994;3(8):367–8. [DOI] [PubMed] [Google Scholar]
- 9. Cameron J, Hoffman D, Wilson J, Cherry GW. Comparison of two peri‐wound skin protectants in venous leg ulcers: a randomised controlled trial. J Wound Care 2005;14(5):233–6. [DOI] [PubMed] [Google Scholar]
- 10. Coutts P, Sibbald GR, Queen D. Peri‐wound skin protection: a comparison of a new skin barrier vs traditional therapy in wound management. Poster Presentation at the CAWC Conference, London ON, Canada, 2001.
- 11. Bär M, Vanscheidt W. Ulcer edge protection with a polymer protective film. Zeitschrift für Wundheilung 2001;22(1):16–20. [Google Scholar]
- 12. Neander KD, Hesse F. Wound edge protection in chronic wounds. J Wound Care 2003;12(10):369–71. [DOI] [PubMed] [Google Scholar]
- 13. Baatenburg de Jong H, Admiraal H. Comparing cost per use of 3M Cavilon No Sting Barrier Film with zinc oxide oil in incontinence patients. J Wound Care 2004;13(9):398–400. [DOI] [PubMed] [Google Scholar]
- 14. Bale S, Tebble N, Jones V, Price P. The benefits of implementing a new skin care protocol in nursing homes. J Tissue Viability 2004;14(2):44–50. [DOI] [PubMed] [Google Scholar]
- 15. Bliss DZ, Zehrer C, Savik K, Ding L, Hedblom E. An economic evaluation of skin damage prevention regimens among nursing home residents with incontinence: Product and Labor Costs. 2005, 3M Healthcare, submitted for publication. [DOI] [PubMed]
- 16. Hampton S. Film subjects win the day. Nurs Times 1998;94(24):80–2. [PubMed] [Google Scholar]
- 17. García RF, Gago M, Adame S, Romero J, Jiménez A, Vega J. 3M™ Cavilon™ No Sting Barrier Film: an evaluation of peri‐wounds prone to maceration. Poster Presentation at the EPUAP Conference, Pisa, Italy, 2000.
- 18. García González RF, Verdú Soriano J, Gago Fornelis M, Rueda López J, Segovia Gómez T, Peters Gutírrez FS. The use of a No Sting Barrier Film to prevent maceration in pressure ulcers treated with an adhesive hydrocolloid dressing. Poster Presentation at the EWMA Conference, Granada, Spain, 2002.
- 19. Vin F, Bonnetblanc JM. Peri‐wound protection with Cavilon NSBF for patients with chronic venous leg ulcers: a randomised multi‐centre trial 2003, data on file, 3M Healthcare.


