Abstract
The aim of this article was to investigate the effectiveness of testing cold immersion recovery responses in the diabetic foot with neuropathy using a contact thermography system based on thermochromic liquid crystals. A total of 81 subjects with no history of diabetic foot ulceration were assigned to neuropathy, non neuropathy and healthy groups. Each group received prior verbal and written description of the test objectives and subsequently underwent a comprehensive foot care examination. The room temperature and humidity were consistently maintained at 24°C and less than 50%, respectively, with air conditioning. The right foot for each subject was located on the measurement platform after cold immersion in water at 18–20°C. Whole‐field thermal images of the plantar foot were recorded for 10 minutes. Patients with diabetes with neuropathy show the highest ‘delta temperature’, that is difference between the temperature after 10‐minute recovery period and baseline temperature measured independently at all the three sites tested, that is first metatarsal head (MTH), second MTH and heel. This clinical study showed for the first time the evidence of poor recovery times for the diabetic foot with neuropathy when assessing the foot under load. A temperature deficit (because of poor recovery to baseline temperature) suggests degeneration of thermoreceptors, leading to diminished hypothalamus‐mediated activity in the diabetic neuropathic group.
Keywords: Cold immersion, Diabetic foot, Liquid crystal thermography, Recovery rate, Thermometry, Ulcer
Introduction
Diabetic foot is the most commonly occurring complication of diabetes mellitus. The term ‘diabetic foot’ refers to complications of the foot specific to the diabetic, distinctive underlying factors, which include peripheral neuropathy and peripheral vascular disease (PVD). Details related to the important socioeconomic consequences of the diabetic foot disease are widely published (1). Despite technological advances in the prevention and treatment of diabetic foot complications, the incidence remains unacceptably high. Prevention of foot ulcers by identifying individuals at high risk represents the most effective way of reducing the incidence of lower limb amputation in diabetic patients 2, 3, 4.
Current methods for determination of the risk of foot ulceration are the assessment of circulation, neuropathy and foot pressure. These methods are widely used clinically as well as in the research domain. Patients with long‐standing diabetes mellitus are susceptible to nerve damage throughout the body (peripheral, somatic or autonomic) because of poor glycaemic metabolism. However, clinical evidence suggests that peripheral nerves are most commonly affected 5, 6.
Progressive degeneration of sensory nerve pathways is thought to affect thermoreceptors and mechanoreceptors 6, 7. The neuropathic foot is characterised by heightened colouration and increased foot temperature 8, 9. The reactive hyperaemia following a period of loading is impaired in the neuropathic foot 10, 11. There is little evidence of thermally stimulated reactive hyperaemia in the neuropathic foot from other studies 12, 13. In comparison, there is better understanding of hyperaemia in Raynaud’s phenomenon, which commonly affects the hands 14, 15, 16, 17. Assessment of thermal patterns and hyperaemic response in patients with Raynaud’s disease has provided a suitable outcome measure for clinical evaluation 18, 19, 20. On the contrary, foot temperature and response of thermoreceptors are not routinely assessed in the diabetic foot clinic.
A thermometer is obviously a simple assessment tool, and relating temperature to the clinical condition is the problem. Furthermore, assessment of plantar foot temperature in the diabetic foot is complicated by the problem of obtaining data during normal conditions of loading, that is standing and walking. This is important as ulceration is strongly related to tissue loading and does not occur in patients with pressure relief. Thermometry has been shown to be a useful home monitoring tool for preventing diabetic foot ulcers and amputations in high‐risk patients 21, 22, 23. Various techniques relevant to the neuropathic assessment of the diabetic foot have been reviewed (3). Dynamic measurements such as rate of warming, maximum recovery temperature, degree of temperature variation at the anatomical site and lag time (time interval between onset of thermal stress and onset of cooling/warming) are useful when assessing response to thermal stimuli (24).
A collaborative research initiative within the authors’ research group has led to the development of a contact thermography system using thermochromic liquid crystals (TLC) (25). The aim of this article was to investigate the effectiveness of testing cold immersion recovery responses in the diabetic foot with neuropathy using the liquid crystal thermography (LCT) system.
Research design and methods
This study involving 81 subjects in three independent study groups was completed at the MV Hospital for Diabetes and Diabetes Research Centre at Chennai (India). Ethical approval was obtained from the local ethics committee at the centre. A diverse type 2 diabetic patient group (minimum duration 12 months) was assessed, excluding patients with active foot ulceration, PVD, Charcot’s foot deformity or any physical disability.
The study groups included (a) diabetic patients with neuropathy (n = 28), (b) diabetic patients without neuropathy (n = 23) and (c) healthy controls (n = 30). For the neuropathic diabetic group, mean age was 58 years (range 41–71 years), whereas for the non neuropathic diabetic group, mean age was 50 years (range 33–63 years). For healthy controls, mean age was 32 years (range 20–51 years). This group was not well matched in terms of age to the other two groups because of difficulty in recruiting age‐matched subjects. A summary of the composition of study group and parameters such as age, sex, duration of diabetes, % glycosylated haemoglobin (HbA1c) and body mass index is given in Table 1.
Table 1.
Summary of the composition of study group for the clinical study
| Patient group/parameters | Diabetic with neuropathy | Diabetic without neuropathy | Healthy |
|---|---|---|---|
| Number of subjects | 28* | 23* | 30 |
| Male/female | 24/4 | 15/8 | 8/22 |
| Age (years), mean ± SD | 57·92 ± 7·08 | 50·35 ± 9·79 | 32·43 ± 7·3 |
| Duration of diabetes (years), mean ± SD | 14·75 ± 6·8 | 9·45 ± 5·8 | n/a |
| Glycosylated haemoglobin (%), mean ± SD | 9·01 ± 1·81 | 8·79 ± 1·82 | n/a |
| Body mass index (kg/m2), mean ± SD | 25·24 ± 3·77 | 25·31 ± 3·48 | 25·07 ± 4·16 |
A total of 30 subjects per group were included in the clinical study. Two (male) neuropathic diabetic subjects and seven (three male/four female) non neuropathic diabetic subjects were excluded from the final analysis, as they were either recently diagnosed with diabetes or had duration of diabetes less than 12 months.
n/a = Not applicable.
Subjects in the two diabetic groups were selected from the outpatient department of MV Hospital, and appointments were scheduled for inpatients, considering their other routine diagnostic tests. Subjects in the healthy control group were selected from among the hospital staff as well as accompanying family members of the patients. All subjects were given a prior verbal and written description of the test objectives and test procedure. Informed written consent was obtained from all patients before the thermographic examination. All the measurements were performed under controlled conditions in the foot laboratory at MV Hospital for Diabetes and in accordance with the test protocol approved by the ethical committee.
Assessment protocol
A qualified research nurse from the foot care laboratory at MV Hospital assisted during the data collection throughout the study. A comprehensive evaluation of the patient’s foot was performed, typical of the routine foot care programme at the hospital. Visual inspection of the foot followed by sensory neuropathy tests using 10‐g Semmes–Weinstein monofilament and a vibration perception threshold (VPT) test using a biothesiometer were performed by trained nurses. Both tests have been shown clinically useful in previous studies 6, 26. Both tests were made at five sites on the foot, and a VPT threshold for neuropathy was taken as 30 V. Insensitivity to a graded 10‐g nylon monofilament at three or more sites was considered as clinical neuropathy. Furthermore, PVD was assessed by determination of ankle brachial perfusion index, with values at or above 0·9 considered as normal 27, 28. Data for mean HbA1c that indicate glycaemic control over previous 3 months were also recorded and are shown in Table 1.
In order to standardise the patient recruitment process, a clear classification and staging system was adopted using the exclusion criteria as discussed. Therefore, the diabetic subjects were classified into one of the two groups depending on the results of tests for sensory neuropathy and PVD.
The thermal testing procedure commenced with a 20‐minute rest period in order for the plantar temperature to equilibrate with the room temperature. The room temperature and humidity were consistently maintained at 24°C and less than 50%, respectively, with air conditioning. During the waiting period, the patient was seated on a chair with the feet flat on ground. With the patient seated, plantar foot temperature was measured using a digital thermometer (thermocouple probe) at the first metatarsal head (MTH) and the heel before each test. These were used as a reference measurement for each site to compare with the start temperature on the LCT platform. Patients then stood on the measurement platform and their feet positioned using consistent alignment with reference markers. All subjects were advised to avoid movement during the duration of the test and use support from the handrail along the adjacent wall.
For the baseline measurement, 60 static images of the right foot were recorded over a continuous 5‐minute period. The sampling rate was one image every 5 seconds. For the cold immersion test, the patient’s foot was placed in a water bath at 18–20°C for 3 minutes. This variation in the water bath temperature did not affect the measurements as the main purpose was to cool the foot below the ambient temperature at 24°C. Our previous studies of perfusion have shown that there may not be a measurable change below 40 seconds of immersion (10). Furthermore, going beyond 3 minutes (or 180 seconds) could alter the elastic properties of the skin, that is on cooling, the elasticity is reduced by contraction, and on warming, the skin causes relaxation. This change will presumably lead to a redistribution of fluid in the region, thus changing the thermal properties of the skin. Therefore, we optimised the immersion time to 3 minutes in order to achieve a measurable response while minimising the risk of changes in the thermal conductivity of the local tissue because of contraction/relaxation of the bulk tissue. When skin is wet, its temperature can be altered by evaporation. To prevent this problem, the foot was dried thoroughly using a pre‐sterilised towel prior to placing on the measurement platform for the thermal measurements.
Results
The results for evaluation of plantar temperatures following cold immersion in all study groups are presented. 1, 2, 3 show the mean temperatures (°C) at three regions of interest, that is first MTH, second MTH and the heel, respectively. All figures show temporal distribution of the temperature for the 10‐minute duration of recovery after cold immersion.
Figure 1.
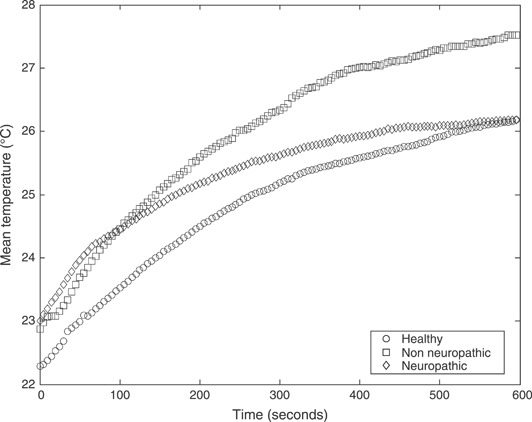
Mean temperatures (°C) following cold immersion under the first metatarsal head for all three study groups: healthy non diabetic patients, diabetic patient with neuropathy and diabetic patients without neuropathy.
Figure 2.
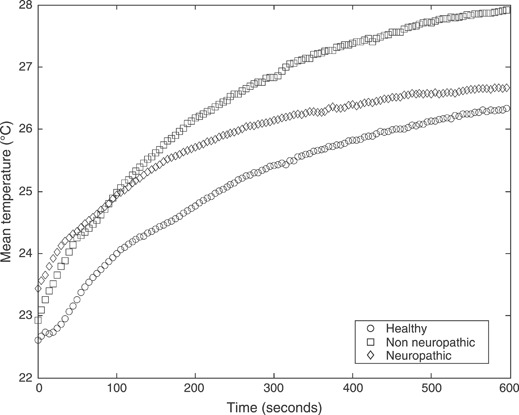
Mean temperatures (°C) following cold immersion under the second metatarsal head for all three study groups: healthy non diabetic patients, diabetic patients with neuropathy and diabetic patients without neuropathy.
Figure 3.
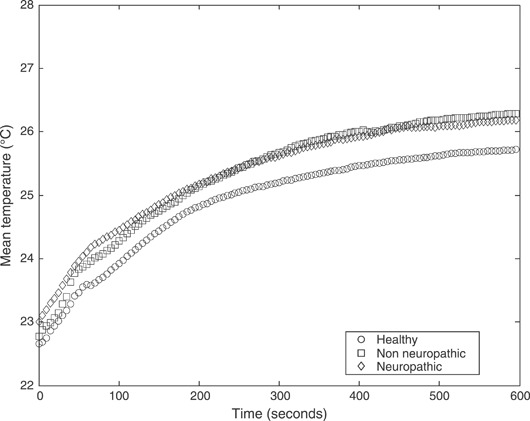
Mean temperatures (°C) following cold immersion under the heel for all three study groups: healthy non diabetic patients, diabetic patients with neuropathy and diabetic patients without neuropathy.
Table 2 lists the preclinical temperatures measured at the first MTH for all study groups using digital thermometer prior to the clinical tests using the LCT system. These measurements were also made at the second MTH and heel. However, no statistical inference can be drawn for the following reasons: (a) limited number of samples in each group and (b) poor accuracy of the digital thermometer (±1·5°C). These results are indicative and considered as reference measurements against the temperatures measured from the LCT system.
Table 2.
Preclinical temperatures measured at the first MTH using digital thermometer prior to the clinical tests using LCT system*
| Study group | Baseline | Cold immersion recovery |
|---|---|---|
| Neuropathic | 28·93 (1·8) | 22·20 (1·06) |
| Non neuropathic | 29·17 (1·18) | 22·00 (1·17) |
| Healthy | 28·45 (1·52) | 21·94 (0·77) |
LCT, liquid crystal thermography; MTH, metatarsal head.
The mean temperatures (°C) and standard deviation are shown for all study groups.
Table 3 provides a detailed summary of the mean temperatures measured by the LCT system at the start and end of the baseline and cold immersion tests. Mean temperatures (°C) and standard deviation among the study groups are listed in Table 3.
Table 3.
Summary of the mean temperature measurement at all regions of interest for three study groups*
| Study group | Baseline | Cold immersion recovery | |
|---|---|---|---|
| Neuropathic | |||
| Start | 26·67 (1·41) | 23·01 (1·01) | First MTH |
| End | 29·65 (1·84) | 26·19 (1·40) | |
| Start | 26·75 (1·54) | 23·43 (1·13) | Second MTH |
| End | 29·68 (1·82) | 26·67 (1·4) | |
| Start | 26·71 (1·23) | 23·01 (1·01) | Heel |
| End | 29·01 (1·43) | 26·19 (1·4) | |
| Non neuropathic | |||
| Start | 26·54 (1·6) | 22·87 (1·1) | First MTH |
| End | 30·26 (2·36) | 27·17 (3·38) | |
| Start | 26·63 (1·44) | 22·924 (0·94) | Second MTH |
| End | 30·2 (2·21) | 27·91 (3·06) | |
| Start | 26·21 (1·48) | 22·78 (0·87) | Heel |
| End | 28·66 (1·73) | 26·11 (1·48) | |
| Non diabetic healthy | |||
| Start | 26·11 (1·02) | 22·29 (0·47) | First MTH |
| End | 28·64 (1·77) | 26·18 (2·2) | |
| Start | 26·12 (1·05) | 22·61 (0·83) | Second MTH |
| End | 28·78 (1·8) | 26·34 (2·00) | |
| Start | 25·90 (1·27) | 22·66 (0·84) | Heel |
| End | 28·01 (1·39) | 25·72 (1·40) | |
MTH, metatarsal head.
Mean temperatures (°C) and standard deviation at the start and end of baseline and cold immersion recovery tests are listed.
Table 4 lists the ‘delta temperatures’, that is difference in mean skin temperature between final temperatures after 10‐minute recovery and final temperatures after 5 minutes of baseline (before immersion control period) test at the three regions of interest for all study groups.
Table 4.
Delta temperatures at the first MTH, second MTH and heel for all study groups
| Healthy (°C) | Non neuropathic (°C) | Neuropathic (°C) | |
|---|---|---|---|
| First MTH | 1·46 | 3·09 | 3·46 |
| Second MTH | 2·44 | 2·29 | 3·01 |
| Heel | 2·29 | 2·55 | 2·82 |
MTH, metatarsal head.
Interestingly, diabetic patients with neuropathy show the highest differences (delta temperature) at all the three sites, as shown in Table 4. This is a key clinical finding, suggesting the impaired response of the thermoreceptors. This finding is further strengthened by qualitative analysis of the recovery curves in 1, 2, which illustrate the saturation of the recovery at the MTH region for the neuropathic group.
Discussion
The results of this study allowed an evaluation of cold immersion recovery responses for plantar tissue under loading using a low‐cost LCT system. It was possible to obtain detailed analysis of the dataset from three measurement sites in each group with the intent to assess the neuropathic diabetic foot.
Evidence from other studies suggests that reperfusion of tissue following removal of load is of clinical interest, especially in the diabetic group 10, 12, 13. Mean blood flow into the tissue, plantar pressure, duration of loading and dynamic response of perfusion have all been evaluated. However, there has been no study related to temperatures under the normally loaded foot. Thermometry has an important role in promoting current understanding of the pathogenesis of diabetic foot ulceration (3).
During standing, the foot sole is exposed to high static pressure, resulting in changes in the microcirculation. It is suggested that the temperature response during the loading period is likely to be dominated by local metabolic factors, perfusion status and physical characteristics of the plantar tissue in contact with the TLC sensor sheet. The above temporal response for measured temperature is linked to a complex interplay between all these factors and their association can only be established by independent assessment of these factors. Vertical loading has a higher impact on superficial blood flow in contrast to shear, which affects perfusion deeper in dermis (29). The measured response in this study only considers vertical loading of the foot.
Although it is clear that the hyperaemic response compensates for the ischaemic state of the tissue upon loading 10, 11, 30, there is no evidence suggesting whether any such response is induced when the duration of loading is longer. Current analysis was restricted to 5 minutes for baseline and 10 minutes for recovery test. This was because of limited capacity for data recording and saturation of the recovery response. However, the recording time is consistent with the commonly used cold stress test for Raynaud’s syndrome where 10‐minute recovery period is observed 14, 31, 32. It would stand to reason that contrary to evidence from other studies, our system is able to detect changes more rapidly. Perhaps this is because there is better blood flow to the foot in weight‐bearing mode. In addition, there could be physiological reasons to do with the patients such as vascular conditions; however, patients with PVD were excluded so that patients would have good lower limb blood flow.
Intuitively, thermal stimulus to plantar tissue will result in recruitment of thermoregulatory shunt flow mediated by the hypothalamus to maintain homeostasis. In the diabetic groups, this ability is compromised because of degeneration of thermoreceptors and autonomic neuropathy. Both sensory neuropathy and autonomic neuropathy can affect perfusion to lower extremities (33) and hence temperature (3). Both neuropathies coexist, and therefore, it is not possible to establish an independent correlation between measured thermal response and each form of neuropathy using the current protocol. It is important to state that the patients were allocated to one of the three study groups based on the results of monofilament testing and VPT testing. Detailed information on the microcirculatory status of the feet was unavailable and therefore the comparison between the three study groups was based on the responses to thermal stimulus. It is shown that by assessing the thermal parameters at the same sites as those of sensory testing, the authors are able to distinguish between clinical and sub‐clinical forms of neuropathy.
Measurements from a digital thermometer (Table 2) were however used as reference and were consistent with the LCT system measurements (Table 3). However, the ability of the LCT system to produce whole‐field images of the foot must be emphasised when considering its relevance as a thermal modality for plantar foot assessment.
A rapid rate of recovery response for the healthy and non neuropathic groups suggests that the response of thermoreceptors is intact. Both groups show good recovery after 10 minutes to baseline temperatures (consistent with the commonly used cold stress test studies), except the non neuropathic group assessment at the first MTH. This may be because of selective degeneration of thermoreceptors in the foot. Thermal cycling tests provide a useful justification of the diminished or absent response of the thermoreceptors for the neuropathic group. The group shows poor recovery to baseline temperatures at all measurement sites, indicating the failure of the hypothalamus‐mediated recovery under the foot following an event of thermal stimulus. These subjects have no clinical evidence of PVD but have clinical evidence of sensory neuropathy; therefore, the response is dominated by the function of thermoreceptors or related signalling pathways.
The response is more pronounced at the MTH region for cold immersion recovery because of punctuate distribution of the sensory receptors. The recovery response at the heel is comparatively slower, which is likely to be because of the presence of adipose tissue that provides thermal insulation. Besides, the geometrical condition of the skin surface may itself modify the processes of emission and absorption of heat (31).
Conclusions
A new multi‐centre collaboration was established for promoting the role of thermography in assessment of the diabetic neuropathic foot. The LCT system extends application of contact thermography in assessment of plantar temperatures under the influence of load that is essential for assessment of the diabetic foot. The important advantages of this system are safety of examination, non invasive technique, simplicity, speed and low cost. Such a system promotes a coupling between prevalent sensory testing modalities and itself aimed to characterise the diabetic neuropathic foot. The consistency in the measured temperatures and temporal variation at three measurement sites during cold immersion recovery responses are useful indications for the patterns associated with diabetes and subsequent neuropathy, which may be clinically asymptomatic but visible when testing the response of thermoreceptors.
Armstrong et al. have suggested cooling of the foot to be a safe and an effective method of reducing inflammation and an interventional tool to reduce skin breakdown risk (34). Current clinical study showed for the first time the evidence of poor recovery times for the diabetic foot with neuropathy when assessing the foot under load. Furthermore, a temperature deficit (because of poor recovery to baseline temperature) suggests degeneration of thermoreceptors, leading to diminished hypothalamus‐mediated activity in the diabetic neuropathic group.
Acknowledgements
The authors are grateful to the Engineering & Physical Sciences Research Council (GR/S0732201) and to the European Foundation for Study on Diabetes for the Albert Renold Travel fellowship (European Foundation for study of Diabetes) for providing funding to carry out this work. The authors also acknowledge Prof. Ann Anderson at Union College (Schenectady, NY, USA) for providing access to the LCT laboratory and technical support.
References
- 1. Gordois A, Scuffham P, Shearer A, Oglesby A. Cost‐of‐illness study: the healthcare costs of diabetic neuropathy in the UK. Diabetic Foot 2003;6:62–73. [Google Scholar]
- 2. Boulton A, Connor H, Cavanagh PR. The foot in diabetes. New York: John Wiley & Sons, 1998. [Google Scholar]
- 3. Bharara M, Cobb J, Claremont D. Thermography and thermometry in the assessment of diabetic neuropathic foot: a case for furthering the role of thermal techniques. Int J Low Extrem Wounds 2006;5:250–60. [DOI] [PubMed] [Google Scholar]
- 4. Reiber G. Diabetic foot care: financial implications and practical guidelines. Diabetes Care 1992;15 Suppl 1:29–31. [DOI] [PubMed] [Google Scholar]
- 5. McNeely JM, Boyko JE, Ahroni HJ, Stensel LV, Reiber EG, Smith GD, Pecoraro ER. The independent contributions of diabetic neuropathy and vasculopathy in foot ulceration. Diabetes Care 1995;18:216–19. [DOI] [PubMed] [Google Scholar]
- 6. Viswanathan V, Snehalatha C, Seena R, Ramachandran A. Early recognition of diabetic neuropathy: evaluation of a simple outpatient procedure using thermal perception. Postgrad Med J 2002;78:541–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ziegler D, Mayer P, Wiefels K. Assessment of small and large fibre function in long term type 1 (insulin dependent) diabetic patients with and without painful neuropathy. Pain 1988;34:1–10. [DOI] [PubMed] [Google Scholar]
- 8. Stess RM, Sisney PC, Moss KM, Graf PM, Louie KS, Gooding GA, Grunfeld C. Use of liquid crystal thermography in the evaluation of the diabetic foot. Diabetes Care 1986;9:267–72. [DOI] [PubMed] [Google Scholar]
- 9. Benbow S, Chan A, Bowsher D, Williams G, Macfarlane I. The prediction of diabetic neuropathic plantar foot ulceration by liquid‐crystal contact thermography. Diabetes Care 1994;17:835–9. [DOI] [PubMed] [Google Scholar]
- 10. Cobb JE. An in‐shoe laser Doppler sensor for assessing plantar blood flow in the diabetic foot. PhD Thesis. Bournemouth University, Bouremouth, 2000. [DOI] [PubMed] [Google Scholar]
- 11. Flynn M, Edmonds M, Tooke J, Watkins P. Direct measurement of capillary blood flow in the diabetic neuropathic foot. Diabetologia 1988;31:652–6. [DOI] [PubMed] [Google Scholar]
- 12. Rayman G, Hassan A, Tooke J. Blood flow in the skin of the foot related to the posture in diabetes mellitus. BMJ 1986;292:87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rayman G, Williams S, Spencer P, Smaje L, Wise P, Tooke J. Impaired microvascular hyperaemic response to minor skin trauma in type 1 diabetes. BMJ 1986;292:1295–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ring E. Raynaud’s phenomenon: assessment by thermography (consensus report, European Association of Thermology). Thermology 1988;3:69–73. [Google Scholar]
- 15. Clark S, Dunn G, Moore T, Jayson M, King T, Herrick A. Comparison of thermography and laser Doppler imaging in the assessment of Raynaud’s phenomenon. Microvasc Res 2003;66:73–6. [DOI] [PubMed] [Google Scholar]
- 16. Ring E, Aarts N, Black C. Raynaud’s phenomenon: assessment by thermography. Thermology 1998;3:69–73. [Google Scholar]
- 17. O’Reilly D, Taylor L, El‐Hadidy K, Jayson M. Measurement of cold challenge responses in primary Raynaud’s phenomenon and Raynaud’s phenomenon associated with systemic sclerosis. Ann Rheum Dis 1992;51:1193–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boignard A, Salvat‐Melis M, Carpentier P, Minson C, Grange L, Duc C, Sarrot‐Reynauld F, Cracowski J. Local hyperemia to heating is impaired in secondary Raynaud’s phenomenon. Arthritis Res Ther 2005;7:1103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Foerster J, Wittstock S, Fleischanderl S, Storch A, Riemekasten G, Hochmuth O, Meffert B, Meffert H, Worm M. Infrared monitored cold response in the assessment of Raynaud’s phenomenon. Clin Exp Dermatol 2006;31:6–12. [DOI] [PubMed] [Google Scholar]
- 20. Foerster J, Kuerth A, Niederstrasser E, Krautwald E, Pauli R, Paulat R, Eweleit M, Riemekasten G, Worm M. A cold response index for the assessment of Raynaud’s phenomenon. J Dermatol Sci 2007;45:113–20. [DOI] [PubMed] [Google Scholar]
- 21. Lavery LA, Higgins KR, Lanctot DR, Constantinides GP, Zamorano RG, Armstrong DG, Athanasiou KA, Agrawal CM. Home monitoring of foot skin temperatures to prevent ulceration. Diabetes Care 2004;27:2642–7. [DOI] [PubMed] [Google Scholar]
- 22. Armstrong DG, Lipsky B, Polis A, Abramson M. Does dermal thermometry predict clinical outcome in diabetic foot infection? Analysis of data from the SIDESTEP* trial. Int Wound J 2006;3:302–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lavery LA, Higgins KR, Lanctot DR, Constantinides GP, Zamorano RG, Armstrong DG, Athanasiou KA, Agrawal CM. Preventing diabetic foot ulcer recurrence in high‐risk patients: use of temperature monitoring as a self‐assessment tool. Diabetes Care 2007;30:14–20. [DOI] [PubMed] [Google Scholar]
- 24. Merla A, Di Donato L, Di Luzio S, Farina G, Pisarri S, Proietti M, Salsano F, Romani G. Infrared functional imaging applied to Raynaud’s phenomenon. IEEE Eng Med Biol Mag 2002;21:73–9. [DOI] [PubMed] [Google Scholar]
- 25. Bharara M. Liquid crystal thermography for neuropathic assessment of the diabetic foot. PhD Thesis. Bournemouth University, Bouremouth, 2007. [Google Scholar]
- 26. Miranda‐Palma B, Sosenko J, Bowker J, Mizel M, Boulton A. A comparison of the monofilament with other testing modalities for foot ulcer susceptibility. Diabetes Res Clin Pract 2005;70:8–12. [DOI] [PubMed] [Google Scholar]
- 27. Pendsey S. Diabetic foot: a clinical atlas, 1st edn. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd, 2003. [Google Scholar]
- 28. NHS . Type 2 diabetes – prevention and management of foot problems. Vol. 2006. National Institutes of Health and Clinical Excellence, UK, 2004. [Google Scholar]
- 29. Tsay D. Pressure distribution in tissue. In: Webster J, editor. Prevention of pressure sores – engineering and clinical aspects. Bristol: Adam Hilger, 1991:19–33. [Google Scholar]
- 30. Meinders M, Lange Ad, Netten P, Wollesheim H. Microcirculation in the foot sole as a function of mechanical pressure. Clin Biomech 1996;11:410–7. [DOI] [PubMed] [Google Scholar]
- 31. Jung A, Zuber J. Thermographic methods in medical diagnostics. Warsaw: MedPress, 1998. [Google Scholar]
- 32. Ring E. Cold stress testing of the hand. In: Ammer K, Ring E, editors. The thermal image in medicine and biology. Vienna: Uhlen Verlag, 1995:237–40. [Google Scholar]
- 33. Flynn M, Tooke J. Diabetic neuropathy and the microcirculation. Diabet Med 1995;12:298–301. [DOI] [PubMed] [Google Scholar]
- 34. Armstrong DG, Sangaland MB, Jolley D, Maben F, Kimbriel HR, Nixon BP, Cohen KI. Cooling the foot to prevent foot wounds – a proof of concept trial. J Am Podiatr Med Assoc 2005;95:103–7. [DOI] [PubMed] [Google Scholar]


