ABSTRACT
Background: An increasing number of chronic wounds in our society require strategies to improve wound healing and wound closure. One of several options is skin transplantation. In this article, we focus on the transplantation of tissue engineered autologous epidermal sheets derived from outer root sheath (ORS) cells of the patients' hair.
Patients and Methods: Out of the stem cells of the ORS of anagen hair, autologous keratinocytes are cultured ex vivo in organotypic cultures to form a multilayered epidermal equivalent (EpiDex™, EuroDerm Biotech & Aesthetics, Stuttgart, Germany). These sheets are placed on the wound bed. Patients were observed twice a week in the first 2 weeks, then once weekly for 4 weeks, then every 4 weeks for up to 12 weeks after transplantation. A total of 23 patients with (n = 18) and without (n = 5) therapeutic improvement were analyzed retrospectively. We evaluated only the effect of a single transplantation in a selected ulcer per patient. Furthermore, a subgroup‐analysis for responder patients with an ulcer area < 25 cm2 (n = 12) was performed.
Results: In the responder patients (n = 18), a total wound reduction of 23% was observed. Patients (n = 12) with ulcer area < 25 cm2 had an improvement of 64%. Complete wound closure in this subgroup after a single transplantation was achieved in 33 % (n = 4) cases.
Conclusions: Autologous keratinocyte transplantation with EpiDex™ can be performed easily and safely in patients with chronic wounds with satisfying results. Our data suggest that patients with small ulcer area < 25 cm2 might profit the most from this method.
Keywords: Tissue engineering, Autologous keratinocyte transplantation, EpiDex™
BACKGROUND
Tissue engineering of skin was developed mostly by the need of skin substitution in patients with severe and large burns, and most of the data and studies have been carried out in this group of patients. In our society, an increasing number of chronic wounds such as venous leg ulcers result in a wide spectrum of socio‐economic problems and create the need to find medical solutions to improve wound healing and wound closure (1). Current strategies include an optimized topical wound treatment, improvement of circumstances for delayed wound healing and combination with autologous skin transplantation. In the last decades, also tissue engineered skin has been used more and more for transplantation in chronic wounds 2, 3, 4, 5. Recently, it has been shown that autologous epidermal equivalents can be as effective as split‐thickness skin autografts in chronic vascular leg ulcers (6). Here, we show our results of a single transplantation of autologous keratinocytes (EpiDex™) for treatment of chronic recalcitrant venous leg ulcers.
PATIENTS
For this evaluation, we retrospectively analysed patients treated with autologous keratinocyte sheets (EpiDex™). All patients suffered from chronic venous insufficiency but most of them suffered from additional diseases and/or contraindications for surgical treatments (Figure 1). Patients with crurobrachial pressure index < 0.9 were not included in this analysis. We selected patients whose ulcers had not shown any improvement despite more than 6 months of intensive wound care and sufficient compression therapy. All wounds had granulation tissue (but to different levels) and did not show any clinical signs of infections at the time of transplantation.
Figure 1.
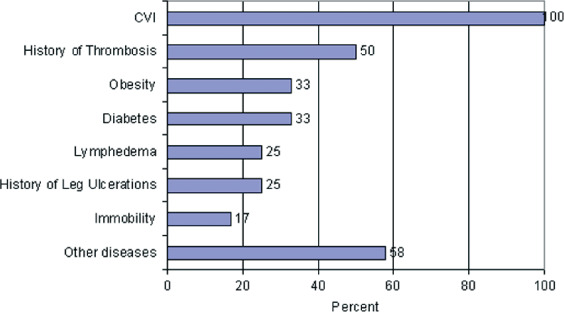
Additional comorbidity of the responding patients.
Only a single transplantation of one indicator ulcer was analysed. Clinical response was defined as reduction in wound area > 5% during the first 12 weeks. Minimal reduction (< 5%), stagnation or progress of wound area was declared as treatment failure.
The group with therapeutic improvement (n = 18) was composed of a female:male ratio of about 2.6:1. The mean age of these responder patients was 73 years with a minimum of 52 years and a maximum of 86 years. Maximum wound area was 314 cm2, minimum 1 cm2, and mean wound area was 45.3 cm2. For this group, prior therapy of the wound was performed with modern wound dressings but non invasive (58%), with split‐skin transplantation (33%) or by invasive surgical treatments such as vein stripping (8%). Each patient in this group suffered from on average three comorbidities with relevance to ulcer pathogenesis or ulcer healing, for example diabetes mellitus, immobility or history of relevant thrombosis (for details, see Figure 1). Treatment of these additional diseases was standard care, for example medicamentously (diabetes mellitus) or by lymphatic drainage. Chronic venous insufficiency was treated in all patients through sufficient compression therapy. ‘Other diseases' were not documented separately because most of them such as high blood pressure, hyperuricaemia, hyperlipoproteinaemia are not thought to influence significantly the chronic venous insufficiency, the leg ulcer or wound healing.
METHOD
Depending on the wound area to be transplanted, we plucked between 45 and 290 anagen hair follicles of the lateral scalp. Epidermal equivalents were generated in vitro from cells of the outer root sheath (ORS) in organotypic culture under good manufacturing practices as described previously 3, 7.
After 4 weeks of cell culture, autologous keratinocyte sheets are fully differentiated. The sheets are about 0.8 cm2 with a multilayered full thickness epidermis similar to normal skin fixed on the reverse side of the silicon sheets. In wounds with fibrinous or necrotic wound covering, debridement was performed before transplantation, it was not performed routinely. About > 50% of the wound area was covered with keratinocyte sheets. Sheets were fixed with wound dressings such as polyurethane (PU) foam (e.g. Allevyn Non adhesive® Smith & Nephew GmbH, Schenefeld, Germany) or gaze (Adaptic® Systagenix Wound Management, Gargrave, UK) combined with a four‐layer‐bandage to assure contact of the keratinocytes to the wound bed. In larger wound areas with strong wound fluid secretion, PU foam wound dressings turned out to be more suitable because they prevented maceration of the keratinocyte sheets.
Patients were observed twice a week in the first 2 weeks, then once weekly for 4 weeks, then every 4 weeks regularly. Additional visits were scheduled depending on the current wound condition. All treatments were carried out on an outpatient basis. We calculated the wound area as a form of an ellipse. Observation time ranged between 28 and 149 days because of different wound conditions. All secondary treatments with EpiDex™ were not included in this evaluation.
RESULTS
Out of the total of 23 patients, n = 18 responded while n = 5 showed no response to autologous keratinocyte transplantation. In the responder group (see Figure 2), a mean reduction in wound area of 23% (= 10.5 cm2; begin of treatment = 45.3 cm2; end of treatment = 34.8 cm2; Table 1) was observed. Subgroup analysis of responder patients (n = 12) with smaller wound areas up to a maximum of 25 cm2 (mean value 8.9 cm2) showed an improvement of 64.4 % (mean relative improvement of 5.7 cm2; Table 1). Total wound closure in this subgroup after a single treatment was achieved in 33% (n = 4) cases.
Figure 2.
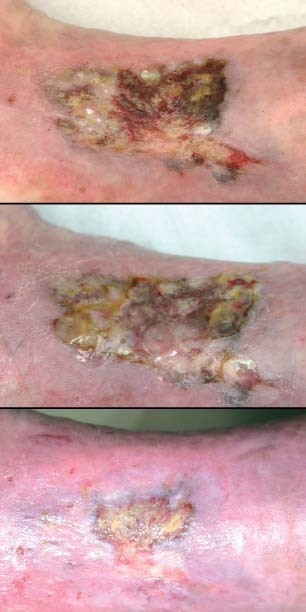
Example of a patient who responded very well to treatment with autologous keratinocytes; photographs are taken on the day of transplantation, after 6 days and after 11 weeks.
Table 1.
Comparison of some basic characteristics of analysed patients responding to treatment and the subgroup of these patients with a wound area < 25 cm2
| Responder group (n = 18) | Subgroup < 25 cm2 (n = 12) | |
|---|---|---|
| Age of patients in years | 73 | 72 |
| Duration of ulceration in years | 7.6 | 5.4 |
| Wound area before treatment in cm2 | 45.3 | 8.9 |
| Wound area after treatment in cm2 | 34.8 | 3.2 |
| Improvement of wound area in total (%) | 23 | 64 |
| Complete wound closure in percentage of patients | 22 | 33 |
According to our photographic documentation, we estimate that in about at least 60–90% epidermal sheets were still visible at the time of the first dressing change. We did not consider this as wound closure. Wound closure was defined as further stable epithelisation of these epidermal isles or wound reduction from the wound edge. At the first dressing change, about 50% of the patients did not show any measurable reduction in wound area (but maybe still visible keratinocyte sheets), the other 50% showed about 20% wound reduction referring to the initial wound area.
Five of our patients showed reduction of wound area < 5%, stable wound area or increasing wound area under treatment (see Figure 3). In these patients, we observed additional complications such as strong exudation (n = 3), necrosis (n = 1) and pain during treatment (n = 1). Severe adverse effects were not observed in any of the patients treated.
Figure 3.
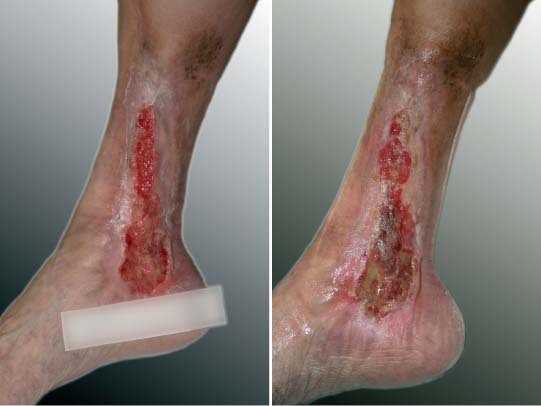
Example of a patient who did not respond to the treatment with autologous keratinocytes; photographs are taken on the day of transplantation and after 23 days. Wound area even increased slightly during this observational period.
To compare our results directly with the published data 8, 9, we calculated for two different values of w (= the width of epithelisation) with the pre‐defined wound area A
before the wound area Aafter with 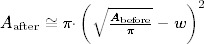 . This mathematical relationship is described by the two lines in Figure 4. We choose the values w = 0.4 cm and w = 0.8 cm according to already described values of the width of epithelisation from the wound edge 8, 9. Our observed different widths of epithelisation are represented by the marks. Thus, the width of epithelisation (edge effect) observed in our study lies within the same range as reported previously for other methods of autologous skin equivalent transplantation 8, 9.
. This mathematical relationship is described by the two lines in Figure 4. We choose the values w = 0.4 cm and w = 0.8 cm according to already described values of the width of epithelisation from the wound edge 8, 9. Our observed different widths of epithelisation are represented by the marks. Thus, the width of epithelisation (edge effect) observed in our study lies within the same range as reported previously for other methods of autologous skin equivalent transplantation 8, 9.
Figure 4.
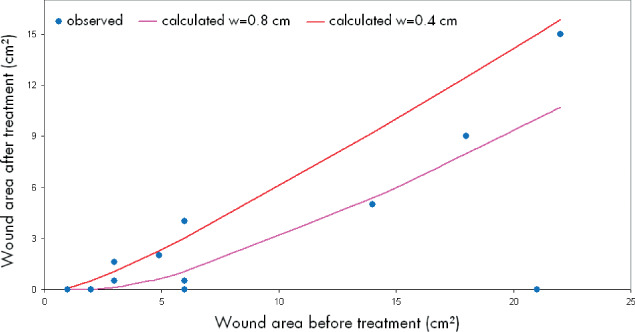
Mathematical calculation of the ‘edge effect'.
DISCUSSION
The current standard for treatment of recalcitrant leg ulcers is the transplantation of split‐thickness skin autografts. This usually requires hospitalisation and has the disadvantage of creating a second wound at the donor sites. Autologous keratinocyte transplantation with EpiDex™ is a painless treatment option for wounds, without the need for surgical procedures or anaesthesia (10).
The proliferation potential of ORS‐derived keratinocytes is not dependent on the age of the patient (3), and the transplanted living keratinocytes can produce growth factors and cytokines to stimulate and influence the wound milieu, which can cause the so called ‘edge‐effect' (11).
In our study, wound area in the responding group (n = 18) could be reduced by 10.5 cm2 from the begin of treatment = 45.3 cm2 to the end of treatment = 34.8 cm2 (= 23% reduction). Total wound closure was observed in 22% of our patients. Using the same methodology, Limat et al. published that 34% of all treated ulcers healed completely (11). One explanation for these discrepant results compared with our observation is that our cohort was mainly composed of patients with hard‐to‐heal wounds and severe comorbidities (see Table 1). Moreover, in Germany only patients who had failed all other interventional therapeutic options (ulcer shaving, vein stripping, skin transplantation) were given an approval from their health insurance company for autologous keratinocyte transplantation.
In our study, some wounds showed continuous but slow wound healing after transplantation. Furthermore in some patients, we observed an acute stimulation of centrifugal re‐epithelialisation from the wound edge (edge effect) within the first 3–4 weeks. This edge effect seems to be because of the secreted growth factors and cytokines from the transplant that stimulates keratinocytes at the wound margin. Moll et al. (8) showed centripetal epithelisation from the wound margin of about 4–7 mm/week, other authors (9) even describe an edge effect up to 10 mm/week. Thus, the ‘edge effect' observed in our study lies within the same range as reported previously for other methods of autologous skin equivalent transplantation 8, 9.
Unfortunately, we have no additional data about the split‐skin‐transplantation because it was not performed in our institution. It was documented as part of the medical history. In particular, we have no additional data about how long‐lasting or how successful split‐skin transplantation was. There might be several reasons why autologous keratinocyte transplantation was successful even in patients in whom split‐skin transplantation had failed. It can be discussed that first we only report short‐term results of the method (maximum 3 years). Second, it is possible that medical care and support for our patients were optimised compared with patients with split‐skin transplantation (i.e. our patients performed nearly every dressing change in our specialised wound care unit in the first 2–4 weeks).
We also compared our results obtained using EpiDex™ with results published for other tissue engineering products. For example, an analysis of data with Apligraf® (Organogenesis Inc., Canton, MA, USA), which is a bi‐layered skin substitute of bovine type 1 collagen, human fibroblasts and human keratinocytes, showed equivalent ulcer healing in ulcers treated with Apligraf® or standard care of less than 1‐year duration (65.5 versus 72.6%) but a subgroup‐analysis showed a distinct benefit for ulcers of greater than 1‐year duration (47 versus 19%) 12, 13. This implies that the effect of autologous transplantation with Apligraf® is most prominent in patients with an ulcer duration of > 1 year. Our data and former clinical evaluations of EpiDex™(14) suggest an increased benefit of EpiDex™ for patients with ulcer duration between 0.5–4 years, but yet the upper limit is not clear. We therefore hypothesise that the duration of ulceration might serve as a predictive marker for treatment success but this hypothesis has to be proven in future prospective studies.
For OrCel® (Forticell Bioscience, New York, NY, USA), a dermis substitute with allogenic fibroblasts in a biodegradable matrix, published data show that 35% of all treated ulcers healed as compared with 20% treated with standard care. It could be shown that in treated ulcers with a wound area < 6 cm2, the OrCel‐treated group of patients had more benefit with 47% versus 23% in standard care (13). This is similar to our subgroup analysis of responder patients (n = 12) with smaller wound areas up to a maximum of 25 cm2 (mean value 8.9 cm2) who achieved an improvement of 64.4% (mean relative improvement of 5.7 cm2) after a single transplantation with EpiDex™. In aggregate, putative predictive markers for a response seem to be duration of ulceration (< 4 years) and/or small wound area (< 25 cm2).
We also tried to characterise the small group of the non responder patients in comparison to the responder group, but both groups were very similar with regard to concomitant diseases, age, mean wound area or the use of post‐treatment wound dressing. Up to now, we were unable to identify robust prognostic markers that might predict a failure of the therapy; however we observed more complications such as excessive wound secretion, pain or necrosis in the non responder group.
It was also not possible for us to identify any differences in the occurrence of complications in using silver or silver‐free PU foam or gauze, and we did not observe any clinical difference between using additional oral antibiotics or not during the first 7 days after transplantation (data not shown).
CONCLUSION
Our results indicate that transplantation of ORS‐derived epidermal sheets (EpiDex™) has a great potential to improve previously inert chronic wounds. Practical advantage of the method is its handling in an outpatient treatment. The transplanted ORS‐derived keratinocytes can stimulate the wound milieu, which can re‐initiate wound healing. Further analysis is needed to identify those patients who will profit the most.
ACKNOWLEDGEMENTS
This work is not supported by any funding source and has not been previously presented. The authors have no conflict of interest to disclose. Each author certifies that all affiliations with or financial involvement (e.g. employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, royalties) with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the article are completely disclosed.
REFERENCES
- 1. Gerngroß H, Minholz R. Wundheilung: kritischer Bericht zur Situation in Deutschland. Trauma Berufskrank 2001;3(Suppl 1):S32–S36. [Google Scholar]
- 2. Leigh IM, Prukis PE, Navsaria HA, Phillips TJ. Treatment of chronic venous ulcers with sheets of cultured allogenic keratincytes. Br J Dermatol 1987;117:591–597. [DOI] [PubMed] [Google Scholar]
- 3. Limat A, Mauri D, Hunziker T. Successful treatment of chronic leg ulcers with epidermal equivalents generated from cultured autologous outer root sheath cells. J Invest Dermatol 1996;107:128–135. [DOI] [PubMed] [Google Scholar]
- 4. Hunziker T, Limat A. Cultured keratinocyte grafts. Curr Probl Dermatol 1999;27:57–64. [DOI] [PubMed] [Google Scholar]
- 5. Gibbs S, Van Den Hoogenband HM, Kirtschig G, Richters CD, Spiekstra SW, Breetveld M, Scheper RJ, De Boer EM. Autologous full‐thickness skin substitute for healing chronic wounds. Br J Dermatol 2006;155:267–74. [DOI] [PubMed] [Google Scholar]
- 6. Tausche AK, Skaria M, Böhlen L, Liebold K, Hafner J, Friedlein H, Meurer M, Goedkoop RJ, Wollina U, Salomon D, Hunziker T. An autologous epidermal equivalent tissue‐engineered from follicular outer root sheath keratinocytes is as effective as split‐thickness skin autograft in recalcitrant vascular leg ulcers. Wound Repair Regen 2003;11:248–252. [DOI] [PubMed] [Google Scholar]
- 7. Limat A, French LE, Blal L, Saurat JH, Hunziker T, Salomon D. Organotypic cultures of autologous hair follicle keratinocytes for the treatment of recurrent leg ulcers. J Am Acad Dermatol 2003;48:207–214. [DOI] [PubMed] [Google Scholar]
- 8. Moll I, Schönfeld M, Jung EG. Applikation von Keratinozyten in der Therapie von Ulcera crurum. Hautarzt 1995;46:548–552. [DOI] [PubMed] [Google Scholar]
- 9. Phillips TJ, Kehinde O, Green H, Gilchrest BA. Treatment of skin ulcers with cultured epidermal allografts. J Am Acad Dermatol 1989;21:191–199. [DOI] [PubMed] [Google Scholar]
- 10. Limat A, Hunziker T. Use of epidermal equivalents generated from follicular outer root sheath cells in vitro and for autologous grafting of chronic wounds. Cells Tissues Organs 2002;172:79–85. [DOI] [PubMed] [Google Scholar]
- 11. Limat A, French L, Salomon D, Daurat JH, Hunziker T. Behandlung therapierefraktärer chronischer Ulcera cruris mit Epidermisäquivalenten aus autologen Keratinzyten der äußeren epithelialen Haarwurzelscheide. Vasomed 1999;11:127–129. [Google Scholar]
- 12. Falanga V, Sabolinski M. A bilayered living skin construct (Apligraf) accerelates complete closure of hard‐to‐heal venous ulcers. Wound Rep Regen 1999;7:201–207. [DOI] [PubMed] [Google Scholar]
- 13. Ehrenreich M, Ruszczak Z. Update on tissue‐engineered biological dressings. Tissue Eng 2006;12:2407–2424. [DOI] [PubMed] [Google Scholar]
- 14. Renner R, Teuwen I, Gebhardt C, Simon J. Int Wound J 2008;5:445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]


