Abstract
Diabetic foot infections are the major cause of morbidity. Infection is the common sequel of diabetic foot ulceration that leads to delayed wound healing. These infections are difficult to control. If not addressed well in time, they may lead to amputation of foot. An attempt has been made to develop simple and effective treatment modality by using citric acid as a sole antimicrobial agent to control diabetic foot infections not responding to conventional treatment. Hundred and fifteen cases of diabetic foot ulcers of different Wagner grades infected with a variety of bacteria were investigated for culture and susceptibility, and susceptibility to citric acid. Citric acid gel was applied to ulcer to determine its efficacy in the management of diabetic foot ulcers with different Wagner grades. Citric acid gel was found effective in the control of foot infections; especially in Wagner grades I and II, the success rate was found to be more than 94%. In Wagner grade III also, it was found effective in complete healing of ulcers without deep osteomyelitis. Citric acid treatment is effective in the control of diabetic foot infections and in successful management of diabetic foot ulcers with Wagner grades I and II, and even with Wagner grade III, without deep osteomyelitis.
Keywords: Citric acid treatment, Diabetic foot ulcers, Wagner grades
INTRODUCTION
Foot infections are the major cause of morbidity in diabetic patients. Nearly 25% of diabetic patients run the risk of developing ulcers (1). Approximately 50 000–60 000 amputations are performed in diabetic patients each year in the USA (2). The vascular insufficiency and associated neuropathy are the important predisposing factors. Once the ulceration occurs, the chances of healing are poor. Infection is the common sequel of diabetic foot ulceration and once established becomes progressively severe and more difficult to treat. Bacteria enter the skin, causing development of small wounds that fail to repair under the body's natural healing process and more infections occur. Eradication of the infectious agent is paramount to the success of healing, if not attended well in time; it may lead to amputation of foot. Hence, identification of foot infections and their control is the critical part in the successful management of diabetic foot, but infections in diabetics are difficult to control. Number of earlier studies show that many antiseptic agents are toxic to the cells involved in wound healing process. These agents by interfering with the normal healing process retard healing and can do more harm than good. Even they may permit more virulent organisms to dominate, and hence should be avoided in diabetic patients 3, 4, 5, 6. The systemic antibiotics have also been showed not to reach adequate tissue levels in chronic granulation tissue and have no effect on the bacterial level in granulating wound (7). Thus, it is very difficult to eliminate the infecting organism from infection site and to control infection, which is paramount to the success of healing. In recent years, the use of citric acid has been reported in the effective management of a variety of chronic wounds including burns infections, pseudomonal wound infections, leprosy ulcers and other chronic wounds caused by various bacteria including multiple antibiotic resistant strains of Escherichia coli, Pseudomonas aeruginosa and other bacteria 8, 9, 10, 11. In a histopathological study also, citric acid has been found to actuate the wound healing process by boosting fibroblastic growth and neovascularisation that in turn increases microcirculation of wound enabling the formation of healthy granulation tissue thereby leading to faster healing of the wound (12).
In view of this, an attempt was made to develop an effective and reliable therapeutic approach to control diabetic foot infections by using citric acid as a sole antimicrobial agent.
PATIENTS AND METHODS
The present retrospective review study was carried out in the Departments of Microbiology, Surgery, Orthopaedics and Medicine, MIMSR Medical College and Y C Rural Hospital, Latur during the period of January 1998 to June 2009. The study was approved by institutional ethical committee. One hundred and fifteen known consecutive cases of type 2 diabetes mellitus with foot ulcers were enrolled for this study after obtaining informed consent. The cases comprised of both newly diagnosed and known old diabetic patients with foot ulcers of different Wagner grades (grade I: 34 cases, grade II: 52 cases and grade III: 29 cases) not responding to conventional therapy for more than 2 weeks to 2 years.
Inclusion criteria
Patients with the following were included:
-
1
Age – age group more than 20 years.
-
2
Sex – both male (79) and female (36) patients.
-
3
Social class – patients belonging to all social classes, but 60% cases were from low socioeconomic status.
-
4
Education – educated as well as non educated patients.
-
5
Life style – smokers/tobacco chewers and non smokers/non tobacco chewers.
-
6
Walking practices – government and private employees – walking less and Agricultural/field workers – walking more were included in the present study.
Exclusion criteria
Patients with the following were excluded:
-
1
Wagner grades IV and V.
-
2
Associated comorbid conditions like severe anaemia, malnutrition, hypoproteinaemia and infections like tuberculosis and human immunodeficiency virus.
-
3
Peripheral vascular disease.
After thorough general and systemic clinical examination, localised infection was ascertained by signs of local inflammation (redness, swelling and warm) and indirectly by raised erythrocyte sedimentation rate (ESR), and leucocytosis on peripheral blood. A specimen from ulcer was collected and processed for culture by conventional techniques (13) and antimicrobial susceptibility testing by Kirby–Bauer disc diffusion method (14). Susceptibility of clinical isolates to citric acid was determined by broth dilution method (15).
The topical application of 3% citric acid was started daily once. Three percent white soft paraffin (100% pure petroleum jelly), a hydrocarbon base not absorbed by the skin, was used as an inert vehicle for citric acid. Citric acid gel was prepared by mechanical mixing in a mortar by taking all sterile precautions. For wound dressing, the wound was first irrigated and cleaned with normal saline. After cleaning, citric acid gel was applied to the wound and dressed with a sterile pad. This treatment modality was used daily until the wound healed completely or showed formation of healthy granulation tissue in wounds with large raw area. No antibiotics were given during this treatment modality except in five cases with systemic symptoms such as fever, toxicity, etc. Formation of healthy granulation tissue, significant reduction in the slough and discharge, and shrinkage of wound margin were taken as criteria for controlled infection.
RESULTS
Culture study yielded Staphylococcus aureus (37·6%) as the most common isolate followed by P. aeruginosa (20·00%), E. coli (16·00%) and other bacteria in decreasing order of frequency. Ciprofloxacin (52·8%) followed by amikacin (33·6%) were found most effective antimicrobial agents in in vitro study. Ampicillin (4·00%) was found to be the least effective antimicrobial agent. All bacterial isolates were found to be inhibited by citric acid. The minimum inhibitory concentration (MIC) of citric acid in vitro was found in the range of 500–2500 µg/ml against different clinical isolates. P. aeruginosa was found to be most susceptible (MIC: 500–1000 µg/ml) and Klebsiella spp. was found to be least susceptible (MIC: 2000–2500 µg/ml) (Table 1).
Table 1.
Clinical isolates, their antibiogram and MIC to citric acid *
| Sr. no. | Name of organism | No. of isolates | Antibiotic susceptibility | MIC to citric acid (µg/ml) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | G | Ak | Cf | Pf | Ca | Ce | ||||
| 1. | Staphylococcus aureus | 47 (37·6) | 03 (6·38) | 12 (25·53) | 12 (25·53) | 33 (70·21) | 13 (27·65) | 08 (17·02) | 17 (36·17) | 900–1000 |
| 2. | Pseudomonas aeruginosa | 25 (20·00) | 00 | 05 (20·00) | 10 (40·00) | 05 (20·00) | 00 | 09 (36·00) | 00 | 500–1000 |
| 3. | Escherichia coli | 20 (16·00) | 00 | 05 (25·00) | 05 (20·00) | 06 (30·00) | 02 (10·00) | 07 (35·00) | 02 (10·00) | 1500–2000 |
| 4. | Klebsiella spp. | 10 (8·00) | 00 | 05 (50·00) | 07 (70·00) | 07 (70·00) | 00 | 00 | 00 | 2000–2500 |
| 5. | Staphylococcus albus | 07 (5·6) | 02 (28·57) | 02 (28·57) | 03 (42·85) | 05 (71·42) | 02 (28·57) | 02 (28·57) | 02 (28·57) | 1200–1500 |
| 6. | Citrobactor spp. | 07 (5·6) | 00 | 03 (42·85) | 03 (42·85) | 05 (71·42) | 03 (42·85) | 00 | 00 | 1500–1600 |
| 7. | Streptococci | 04 (3·2) | 00 | 00 | 00 | 03 (75·00) | 02 (50·00) | 00 | 02 (50·00) | 1000–1500 |
| 8. | Proteus vulgaris | 02 (1·6) | 00 | 02 (100) | 02 (100) | 02 (100) | 02 (100) | 02 (100) | 00 | 1500–1600 |
| 9. | Candida albicans | 03 (2·4) | – | – | – | – | – | – | – | |
| Total | 125 | 05 (4·00) | 34 (27·2) | 42 (33·6) | 66 (52·8) | 24 (19·2) | 28 (22·4) | 23 (18·4) | ||
A, ampicillin; AK, amikacin; Ca, ceftazidime; Ce, ceftriaxone; Cf, ciprofloxacin; G, gentamicin; Pf, pefloxacin.
*Figures in parenthesis indicate percentage.
In patients with ulcers of Wagner grade I, application of 3% citric acid gel resulted in complete healing in 32 cases (94·12%) in 5–30 applications. In two cases, healthy granulation tissue was formed and 95% healing occurred, but on about 5 mm area (in diameter) no skin formation occurred.
In patients with ulcers of Wagner grade II, complete healing was seen in 49 cases (94·23%) in 16–34 applications and healthy granulation tissue in three cases, which required skin grafting for complete closure of ulcer.
In patients with ulcers of Wagner grade III, complete healing was seen in 25 cases (86·21%) in 16–34 applications; however, in one case of uncontrolled diabetes a total of 60 applications were required (1, 2, 3, 4, 5). In four cases, pus and slough was minimised, and healthy granulation tissue was seen. Out of four cases, one case showed complete healing after angioplasty; however in three cases, because of deep osteomyelitis, amputation was carried out (Table 2).
Figure 1.
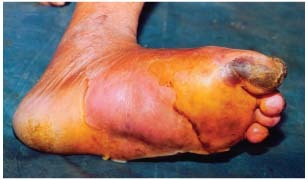
Diabetic foot: before application.
Figure 2.
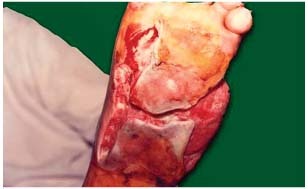
Diabetic foot: after 06 applications.
Figure 3.
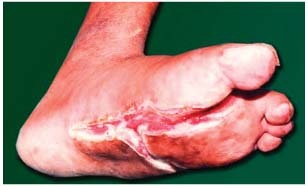
Diabetic foot: after 16 applications.
Figure 4.
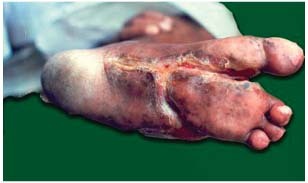
Diabetic foot: after 43 applications.
Figure 5.
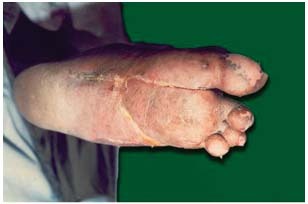
Diabetic foot: after 60 applications.
Table 2.
Distribution of cases according to Wagner grade, no. of applications and final outcome *
| Sr. no. | Wagner grade | No. of cases | No. of applications | Final outcome | ||
|---|---|---|---|---|---|---|
| Healed | Partial healing: healthy granulation, but no epithelisation | Amputation | ||||
| 1. | Grade I | 34 | 5–30 | 32 (94·12) | 02 (5·88) | 00 |
| 2. | Grade II | 52 | 6–34 | 49 (94·23) | 03 (5·76) | 00 |
| 3. | Grade III | 29 | 16–34 (60 in one case) | 25 (86·21) | 04 (13·79) | 03 (10·34) |
| Total | 115 | 106 (92·17) | 09 (7·83) | 03 (02·60) | ||
*Figures in parenthesis indicate percentage.
DISCUSSION
Peripheral neuropathy is a common complication of diabetes, affecting more than 30% of the diabetic population (16). It increases the risk of ulceration, which is the most common cause of foot and leg amputations 17, 18. The presence of macrovascular disease and infection increases the probability of a foot ulcer leading to a lower limb amputation (19). The infections are often difficult to control because of ischaemia, compromised immunity, disturbed glycaemic control and neuropathy.
The healing of ulcers can be enhanced by controlling infections, but infections in diabetics are difficult to control because the commonly used antiseptic agents are not effective in controlling the infection 3, 4, 5, 6 and because of inadequate tissue levels of antibiotics in chronic granulation tissue (7). In addition, the most international guidelines including international diabetic foot working group discourage the use of presently available topical agents in foot wounds. In view of the toxic nature of commonly used antiseptic agents and little lasting effect of antibiotics, an attempt was made to use citric acid as a topical antiseptic agent to eliminate the infecting organism from infection site, which has been reported highly effective in the management of pseudomonal infections of burns and wounds 8, 9, 10, 11, 12. In the present study, its use for the treatment of diabetic foot ulcers caused by a variety of bacteria including multiple drug resistant bacteria was found extremely effective. After the application of citric acid, control of infection was seen immediately after 3–4 applications as evidenced by rapidly minimised pus and sloughing and by rapid granulation and renewal of epithelia. Healthy granulation was observed in all 115 cases even in severely compromised ulcers and ulcers with osteomyelitis, but amputation could not be avoided in cases with deep osteomyelitis in three cases.
The citric acid has antiseptic property as indicated by microbiological studies and by rapid clearing up of infected surfaces (12). This antiseptic property may be because of lowering of pH that makes an environment unsuitable for growth and multiplication of bacteria causing wound infections. It also enhances epithelisation, which is a major factor in wound healing. Hydration, oxygenation and removal of dead tissue ensure good epithelisation. Citric acid keeps wound surface moist and prevents wound desiccation, which retards wound healing. Thus, it reduces dehydration necrosis. Histological studies showed that it increases vascularity of the wound, which helps to remove the dead tissue and makes the wound healthy (12). All these actions increase migration of epithelial cells from surrounding skin and epithelisation acts as stimulus for laying of ground substance. Another possibility is its synergistic antioxidant property (20) that prevents free radical damage and may stabilise lysosomal enzymes needed for collagen synthesis.
These results indicate that citric acid treatment is highly effective in the control of infecting organism, which is paramount to the success of healing. Thus, it helps in the effective control of diabetic foot infections and in the successful management of diabetic foot ulcers with Wagner grades I and II, and even with Wagner grade III without deep osteomyelitis. Citric acid, being a natural product, has no adverse effects.
This being an observational study, we feel further controlled studies are necessary to achieve more concrete conclusions.
ACKNOWLEDGEMENTS
Authors wish to thank Prof. Dr V.D. Karad, Executive President & Director, MAEER's MIT, Pune, Mr. R.K. Karad, Coordinator, Dr N.S. Arvikar, Ex Principal and Dr Sarita Mantri, Principal, MIMSR Medical College, Latur, for their support and encouragement. We are also thankful to Dr P.B. Kulkarni, Prof. of Surgery for clinical support and cooperation. Our thanks are also due to Mr S.S. Gutte, Miss Nameeta Surwase, Mr S.P. Mane, Mr D.L. Ghante and Mr Nagnath Doke for technical assistance.
REFERENCES
- 1. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217–28. [DOI] [PubMed] [Google Scholar]
- 2. Barbul A. Wound healing. In: Brunicardi FC, Andersen DK, Billiar TR, Dunn DL, Hunter JG, Pollock RE, editors. Schwartz's Principles of Surgery, 8th edn. New York: McGrew‐Hill, 2005:223–48. [Google Scholar]
- 3. Branemark P, Albrektsson B, Lindstrom J, Lundberg G. Local tissue effects of disinfectants. Acta Chir Scand 1966;357(Suppl):166–76. [PubMed] [Google Scholar]
- 4. Kramer SA. Effect of povidone – iodine on wound healing: a review. J Vasc Nurs 1999;17:17–23. [DOI] [PubMed] [Google Scholar]
- 5. Pathare NA. Infections and diabetes mellitus. J Diabetic Asso Ind 1998;38:4–6. [Google Scholar]
- 6. Robson MC, Edstrom LE, Krizek TJ, Groskin MG. The efficacy of systemic antibiotics in the treatment of granulating wound. J Surg Res 1974;16:299. [DOI] [PubMed] [Google Scholar]
- 7. Kinghton DR, Halliday B, Hunt TK. Oxygen as an antibiotic: a comparison of the effects of inspired oxygen concentration and antibiotic administration in vivo bacterial clearance. Arch Surg 1986;121:191–95. [DOI] [PubMed] [Google Scholar]
- 8. Nagoba BS, Gandhi RC, Wadher BJ, Deshmukh SR, Gandhi SP. Citric acid treatment of severe electric burns complicated by multiple antibiotic resistant Pseudomonas aeruginosa . Burns 1998;24:481–3. [DOI] [PubMed] [Google Scholar]
- 9. Nagoba BS, Deshmukh SR, Wadher BJ, Mahabaleshwar L, Gandhi RC, Kulkarni PB, Mane VA, Deshmukh JS. Treatment of superficial pseudomonal infections with citric acid – an effective and economical approach. J Hosp Infect 1998;40:155–57. [DOI] [PubMed] [Google Scholar]
- 10. Nagoba BS, Wadher BJ, Rao AK, Kore GD, Gomashe AV, Ingle AB. Simple and effective approach for the treatment of chronic wound infections caused by multiple antibiotic resistant Escherichia coli . J Hosp Infect 2008;69:177–80. [DOI] [PubMed] [Google Scholar]
- 11. Nagoba BS, Wadher BJ, Chandorkar AG. Citric acid treatment of non‐healing ulcers in leprosy patients. Br J Dermatol 2002;146:1101. [DOI] [PubMed] [Google Scholar]
- 12. Nagoba BS, Gandhi RC, Wadher BJ, Potekar RM, Kolhe SM. Microbiological, histopathological and clinical changes in chronic wounds after citric acid treatment. J Med Microbiol 2008;57:681–2. [DOI] [PubMed] [Google Scholar]
- 13. Collee JG, Duguid JP, Fraser AG, Marmion BP, editors. Mackie & McCartney Practical Medical Microbiology, volume 2, 13th edn. London: Churchill‐Livingston, 1989. [Google Scholar]
- 14. Bauer AW, Kirby WMM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 1966;45:493–6. [PubMed] [Google Scholar]
- 15. Baron EJ, Peterson LR, Finegold SM. Methods for testing antimicrobial effectiveness. In: Diagnostic Microbiology, 9th edn. London: Mosby, 1990:168–88. [Google Scholar]
- 16. Young MJ, Boulton AJM, Williams DRR, McLeod AF, Sonksen PH. A multi‐center study of the prevalence of diabetic neuropathy in patients attending UK diabetic clinics. Diabetologia 1993;36:150–4. [DOI] [PubMed] [Google Scholar]
- 17. Boulton AJM. The pathogenesis of diabetic foot problems: an overview. Diabet Med 1996;13:S12–6. [PubMed] [Google Scholar]
- 18. Larsson J, Apelqvist J. Towards less amputation in diabetic patients: incidence, cause, cost, treatment and prevention: a review. Acta Orthop Scand 1995;66:181–92. [DOI] [PubMed] [Google Scholar]
- 19. Pecoraro RE, Riber GE, Burgess EM. Pathways to diabetic limb amputation: basis for prevention. Diabetes Care 1990;13:513–21. [DOI] [PubMed] [Google Scholar]
- 20. Budhavari S. The Merck Index, 11th edn. Rahway: Merck & Co, 1989;2330. [Google Scholar]


