ABSTRACT
Over the last decade Vacuum Assisted Closure® (KCI Licensing, Inc., San Antonio, TX) has been established as an effective wound care modality for managing complex acute and chronic wounds. The therapy has been widely adopted by many institutions to treat a variety of wound types. Increasingly, the therapy is being used to manage infected and critically colonized, difficult‐to‐treat wounds. This growing interest coupled with practitioner uncertainty in using the therapy in the presence of infection prompted the convening of an interprofessional expert advisory panel to determine appropriate use of the different modalities of negative pressure wound therapy (NPWT) as delivered by V.A.C.® Therapy and V.A.C. Instill® with either GranuFoam™ or GranuFoam Silver™ Dressings. The panel reviewed infected wound treatment methods within the context of evidence‐based medicine coupled with experiential insight using V.A.C.® Therapy Systems to manage a variety of infected wounds. The primary objectives of the panel were 1) to exchange state‐of‐practice evidence, 2) to review and evaluate the strength of existing data, and 3) to develop practice recommendations based on published evidence and clinical experience regarding use of the V.A.C.® Therapy Systems in infected wounds. These recommendations are meant to identify which infected wounds will benefit from the most appropriate V.A.C.® Therapy System modality and provide an infected wound treatment algorithm that may lead to a better understanding of optimal treatment strategies.
Keywords: negative pressure wound therapy, NPWT, V.A.C.® Therapy System, Vacuum Assisted Closure®, infected wound care
INTRODUCTION
Infection
Infections complicate the treatment of wounds and impede the healing process by damaging tissue, reducing wound tensile strength and inducing an undesirable inflammatory response 1, 2. It is generally believed that wound infection advances in stages from contaminated, colonized, critically colonized to infected 3, 4. When wound surface bacteria begin replication and increase their metabolic activity, the resulting by‐products, such as endotoxins and metalloproteinases (MMPs), negatively impact all phases of wound healing (5).
The control and prevention of infections is critical in order for the normal wound healing cascade to occur. Heavy wound bioburden increases metabolic requirements, stimulates a proinflammatory environment, and encourages the in‐migration of monocytes, macrophages and leukocytes that can negatively impact healing. Bacteria also secrete harmful cytokines which can lead to vasoconstriction and resultant decreased blood flow to the wound (5). From a healthcare economic perspective, decreased infection rates yield significant overall cost savings, as infections result in significant morbidity, mortality, excess medical expenses and lengthened hospital stays annually (6).
The ancient Egyptians were the first civilization to provide detailed information about management of disease, including wound management with the application of various potions and grease to assist healing. Hippocrates, known as the father of medicine, used vinegar to irrigate open wounds and wrapped dressings around wounds to prevent further injury (7). In 1867, Joseph Lister introduced the antiseptic treatment of wounds which fostered a growing understanding of the importance of technical surgical skill coupled with a reduction in bacterial introduction into surgical wounds (8). During the first half of the 20th century, sulfa drugs and antibiotics (eg, penicillin, streptomycin, etc) were developed for the systemic treatment of infection.
The scale of wound infections has been most evident in times of war. During the American Civil War, arms and legs were subsequently amputated despite a 25–90% risk of amputation residual limb infection (9). World War I produced new wound etiologies such as high‐velocity bullet and shrapnel injuries, as well as those contaminated by mud from the trenches. During World War II, modern surgical reconstructive and tissue‐preserving techniques began to replace amputation. Debridement, irrigation, and closure by delayed primary intention became fundamental management techniques to facilitate wound closure 10, 11. Wars following World War II have used a combination of debridement, aseptic techniques, wound treatment systems, and topical and systemic drugs for the treatment of infection.
In 2001, the United States Centers for Disease Control and Prevention (CDC) estimated that about 290,000 surgical site infections (SSIs) occurred annually, with approximately 8,000 patient deaths resulting from SSIs (12). An SSI occurs when an infection develops at the surgical site within 30 days or within 1 year of an operation if a foreign body (eg, heart pacemaker or artificial joint) is implanted as part of the surgery. About 70% of SSIs are superficial infections involving skin only. However, the remaining infections are more serious and can involve tissues under the skin, organs, or implanted materials (12).
Unfortunately, the quest to eradicate infection is nowhere near being resolved, due to an insurgence of antibiotic‐resistant bacterial strains and the increasingly complex surgical interventions performed in immunocompromised patients and implant surgeries. Therefore, one of the panel's purposes was to offer recommendations for the use of V.A.C.® Therepy modalities on infected wounds.
V.A.C.® Therapy and infected wounds
Negative pressure wound therapy (NPWT), as delivered by Vacuum Assisted Closure® (V.A.C.® Therapy, KCI Licensing, Inc., San Antonio, TX), was cleared for marketing in 1995. This system applies localized negative pressure to the wound bed through a polyurethane reticulated open‐cell foam (ROCF) dressing (V.A.C.® GranuFoam™ Dressing) or a polyvinyl alcohol foam (V.A.C.® WhiteFoam) dressing. When tissue is stretched and drawn into the open pores of the foam dressing under negative pressure, cell mitosis is stimulated, leading to proliferation of reparative granulation tissue (13). When used in conjunction with appropriate antibiotic therapy, NPWT/ROCF has been reported to facilitate wound closure through its ability to reduce localized edema, increase wound edge vascularity, remove inhibitory agents, stimulate granulation tissue formation, provide a moist wound healing environment promote perfusion and promote wound contraction 14, 15, 16, 17.
Current indications of NPWT/ROCF include chronic, acute, subacute and traumatic wounds, pressure and diabetic ulcers, partial‐thickness burns, dehisced wounds, flaps and grafts (18). A large body of literature has been published regarding clinical experience with NPWT/ROCF. A number of these publications have reported the use of adjunctive NPWT/ROCF treatment of contaminated sternal, abdominal, and extremity wounds 19, 20, 21, 22. Reported benefits of NPWT/ROCF in the management of colonized or infected wounds are listed in Table 1.
Table 1.
Reported benefits of NPWT/ROCF on infected wounds
| • Positive patient outcomes |
| • Limb preservation |
| • Positive patient comfort and pain relief |
| • Low number of dressing changes |
| • Less complexity required in surgical procedures |
| • Relatively short time to closure |
| • Short hospital stay |
| • Low failure rate |
| • Low overall costs |
Panel members agreed that treatment of infected wounds or those at high risk for infection requires cautious and judicious use of NPWT/ROCF. This therapy should be provided against a background of best practice with regard to early recognition of infection, administration of appropriate antibiotic therapy, thorough wound irrigation and surgical debridement (23). Despite expanded usage of NPWT/ROCF in contaminated wounds 18, 24, 25, some clinicians avoid use of the therapy in the presence of infection and continue to treat wound infection with standard therapies, including moistened gauze dressings, antiseptics, antibiotics, and enzymatic ointments.
Most studies documenting use of NPWT/ROCF for adjunctive management of complex colonized or infected wounds were performed with V.A.C.® GranuFoam™ Dressing. V.A.C. GranuFoam Silver® (NPWT/ROCF‐silver) Dressing and V.A.C. Instill® Wound Therapy (NPWT with instillation) were developed to specifically provide the antimicrobial benefits of silver and wound irrigation to NPWT/ROCF, respectively. The panel members reviewed three NPWT/ROCF systems during their discussion.
-
1
V.A.C.® Therapy with GranuFoam™ Dressing
-
2
V.A.C.® Therapy with GranuFoam Silver™ Dressing
-
3
V.A.C. Instill® Wound Therapy with GranuFoam™ or GranuFoam Silver™ Dressings
The primary purpose of this article is to: 1) review and evaluate existing evidence regarding use of NPWT/ROCF to manage infected wounds; 2) develop practice recommendations based on published evidence and clinical experience regarding use of the V.A.C.® Therapy Systems on infected wounds; and 3) demonstrate how each of three different V.A.C.® Therapy modalities may be incorporated into an infection treatment algorithm.
BACKGROUND
Pathophysiology of wound healing
Wound healing progresses through a number of highly interdependent phases in an attempt to not only repair but also to compensate for reduced function that has occurred as a result of the wound's loss of tissue integrity. This process can be divided into four general phases that often overlap in time: 1) hemostasis (control of bleeding); 2) inflammation (removal of debris, control of infection, clearance of inflammation); 3) proliferation (angiogenesis, deposition of granulation tissue, contraction); and 4) remodeling (remodeling of the connective tissue matrix, and maturation) 26, 27, 28. Because growth factors, cytokines, proteases, and cellular and extracellular elements all play important roles in different stages of the healing process, imbalances of one or more of these components may account for the impaired healing observed in chronic wounds.
Levels of various MMPs (MMP‐1 [collagenase], MMP‐2 [gelatinase A] and MMP‐9 [gelatinase B]) and serine proteases are markedly increased in fluids from chronic wounds (29), whereas MMP levels are lower in acute wound healing (30). Other proteases, such as neutrophil elastase, have also been observed to be significantly higher in chronic wounds (31). Elevated levels of serine proteases degrade fibronectin, an essential protein involved in the remodeling of the extracellular matrix (ECM) 29, 32.
Although inflammation is part of normal wound healing, healing may be prolonged when inflammation is excessive (33). In chronic wounds, necrotic tissue and slough tend to continually accumulate due to underlying pathogenic abnormalities that alter the biochemical and cellular environment (34). The accumulation of necrotic tissue or slough in a wound promotes bacterial colonization and prevents complete repair of the wound. The wound “stalls” in the inflammatory stage indefinitely, since increasing bacteria in a wound produce pro‐inflammatory mediators such as IL‐1, TNF‐α, prostaglandin E2, and thromboxane (35).
Wound infection continuum and healing
All wounds contain bacteria at levels ranging from contamination to infection. The increased bacterial bioburden may be confined to the superficial wound bed or may be present in deep compartments and the surrounding tissue of the wound margin. Once wounding occurs, whether through surgery, trauma, or endogenous mechanisms, the probability for contamination to occur is 100% 36, 37. Contaminating microorganisms arise from the external environment, surrounding skin, and endogenous sources, such as the gastrointestinal tract 36, 37.
While previously accepted terms of contaminated versus infected seemed clear cut, a more complex “continuum of wound infection” has emerged in recent years. The continuum consists of the surgical wound, contaminated wound (presence of non‐replicating organisms), the colonized wound (replicating organisms without tissue necrosis), the critically colonized wound, and the infected wound. The four terms of interest used in reference to the bacterial bioburden of a wound in wound healing within this context are contaminated, colonized, critically colonized, and infected 3, 4. These terms are defined in detail later in this paper.
The development of an infected wound is an ‘exponential progression’ which may start with sterility, but more often with contamination, colonization, critical colonization, or infection. An untreated superficial infection can potentially become systemic and result in sepsis. All stages are linked and if attempts are not made to stop the progression at any level, bacteria may continue to replicate and produce a polymeric matrix (glycocalyx), or biofilm, that adheres to any inert or living surface and allows bacteria to live protected in an otherwise hostile environment (33). These protected colonies can undergo genetic mutation to alter their sensitivity to antimicrobials and are generally resistant to topical and systemic antibacterials. Thus, debridement is usually necessary to remove a biofilm 33, 38, 39, 40, although complete biofilm eradification cannot be assured.
It has been postulated that the microorganisms in an infected wound consume the nutrients and oxygen that would otherwise be directed toward tissue repair (41). The bacteria release enzymes that break down proteins, a critical component of wound regeneration. Schmidtchen et al reported that elastase‐producing bacterial isolates were shown to significantly degrade plasma proteins and extracellular products of human skin and fibroblasts, and inhibit fibroblast growth (42). These effects, in conjunction with the finding that proteinase production was detected in wound fluid ex vivo, suggest that bacterial proteinases play a pathogenic role in chronic wounds. Reducing the bacterial load of a wound may enable the body to redirect resources from fighting infection to healing.
Extensive debridement (ie, removal of necrotic tissue, foreign material, and bacteria from an acute or chronic wound) and open wound management have been widely advocated to prevent progression to infection in traumatic wounds, contaminated soft tissue defects, postoperative complications and chronic, non‐healing wounds. The goal is removal of necrotic tissue, exudate, and infectious material from the wound bed to enable progression through the normal wound healing phases, assuming that systemic and local factors are functioning normally (43). Aggressively debriding a chronic wound helps convert it to an acute wound. Debridement is accomplished through non‐surgical (eg, biologic, enzymatic, autolytic, or mechanical) and surgical (eg, sharp, hydro‐surgical, or laser) methods.
The combination of debridement to a viable tissue base and the application of topical antimicrobials has been shown to aid in the successful management of complex infected or critically colonized wounds 25, 44, 45. Once the wound has been debrided, adequate vascularization has been confirmed, and appropriate antimicrobial therapy has been initiated, NPWT/ROCF can be applied according to the algorithm outlined in Figure 1 to prepare the wound for primary, secondary, or delayed primary closure.
Figure 1.
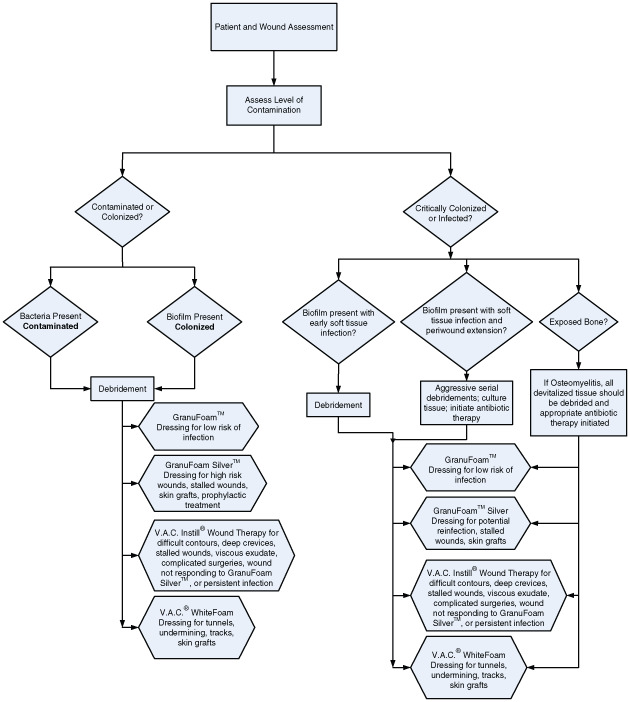
Algorithm for assessment and treatment of wounds with V.A.C.® Therapy System modalities.
Patient assessment
A thorough patient assessment identifying all underlying medical problems that can potentially inhibit the immune system and impact wound healing must first be performed and addressed. Specific systemic risk factors associated with poor wound healing include uncontrolled diabetes mellitus (DM), peripheral vascular disease (PVD), venous insufficiency, malnutrition, alcoholism, neuropathy, generalized edema, previous radiation therapy in the area of the wound and long‐term corticosteroid use (41). A combination of these factors may also cause a remodeled wound to break down.
Peripheral vascular assessment, especially in patients with lower extremity wounds, is imperative. Regardless of the etiology, insufficient blood flow may prevent systemic antibiotics from reaching the wound and may not allow the body to systemically clear microorganisms. If perfusion is inadequate, surgical, endovascular, hyperbaric oxygen and/or pharmacological intervention may be necessary.
Patient age should also be considered a major factor in the wound healing process, based on the biochemical and cytokine milieu that is present with aging 46, 47. There is an up‐regulation of MMP‐2 in normal aged skin, and MMP‐2 and MMP‐9 in acute wounds in aged skin in comparison to that found in skin of young adults 46, 47. This alteration in the cytokine profile is similar to that seen in chronic wounds in younger patients and may affect the wound healing process.
Wound assessment
A complete wound assessment, including size (eg, area, volume, depth, etc), base color, amount and type of exudate, odor, and presence of edema, is necessary to determine the status of the wound. Specifically, wound color can be an initial determinant of infection. Bioburden classification may be made both clinically and by more quantifiable methods. Tissue biopsy and culture has become the “gold standard” for assessing wound bacterial levels 48, 49. However, quantitative culturing is not routinely performed in many institutions as it is less convenient and/or accessible than swab cultures, requires additional practitioner skill, is invasive, takes longer to collect and process and is more costly. Deep bacterial swabs can be used to provide a semi‐quantitative estimate of bacterial growth and may be performed after debridement in the operating room 48, 50. Despite ongoing controversy in measuring bacterial bioburden, current diagnoses of infection have remained largely based on clinical symptoms and signs (Table 2). Currently, the Infectious Diseases Society of America (IDSA) guidelines recommend that two signs of inflammation (eg, redness, warmth, or, swelling) or other clinical signs (eg, purulence, cellulitis, or, pain) must be present to indicate infection (51). Although it is beneficial to have quantitative results, broad‐spectrum antibiotic treatment can be administered, provided cultures have already been taken at the onset of secondary clinical findings, with clinical improvement noted by the time culture results are available.
Table 2.
Clinical and systemic signs of infection
| Signs/symptoms of systemic infection | Signs/symptoms of local infection |
|---|---|
| • Fever | • Pain |
| • Aches | • Redness |
| • Chills | • Pus |
| • Nausea | • Swelling |
| • Vomiting | • Drainage with a foul odor |
| • Weakness | • Heat to the site |
| • Fever |
Deep bacterial swabs, tissue biopsies, and wound cultures may be used to guide antimicrobial therapy. Antibiotics and/or topical antiseptics should be administered with a clear understanding of the treatment goals determined by the clinician. Antibiotics are commonly administered systemically and have a single pharmacologic target, which makes them vulnerable to resistance. On the other hand, antibacterial antiseptics are suitable for topical administration, have broad‐spectrum coverage, and multiple mechanisms of action, which renders them less likely to trigger resistance (52).
WOUND TYPES AND ASSESSMENT ALGORITHM
For the purposes of establishing an NPWT/ROCF algorithm for treating infected wounds, wounds were classified according to level of contamination. The following are detailed descriptions of each of the classifications in the algorithm. (Figure 1)
Contaminated wounds
Wound contamination is defined as the presence of non‐replicating microorganisms that do not impair wound healing 3, 4. Primary therapy goals for contaminated wounds are to prevent further wound deterioration and facilitate closure. Often these wounds are inflamed subsequent to an acute trauma, which can be detrimental to wound healing. Debridement can be performed surgically or non‐surgically to remove non‐replicating microorganisms.
Colonized wounds
Wounds that are making progress toward healing (ie, demonstrating granulation tissue, wound contraction and decreasing wound depth) are described as colonized. That is, the bioburden is low enough that it does not impair the wound's ability to heal. The first step in the management of such wounds is surgical or excisional debridement to restart the inflammatory phase of the wound healing cycle. Most chronic wounds contain more than three species of microorganisms, which increases the risk of infection because they may develop synergies with each other 33, 53, 54, 55. In wounds that are infected with multiple species, distinguishing which is the causative organism may be difficult. In the absence of true quantitative cultures, immediate broad‐spectrum antibiotic coverage and treatment are warranted.
Critically colonized wounds
The term critically colonized was introduced to describe a wound with a level of bacterial burden between the categories of colonized and infected. Chronic wounds, stalled due to high bacterial bioburden, could be considered critically colonized. This relatively new classification, first introduced in 1996 by Davis (56), has been the source of critical comment but has gained increasing acceptance. The term has become synonymous with locally infected. Both describe wounds that do not heal but may not display classic local signs of infection (eg, erythema, warmth, pain, etc) 3, 4. Although these wounds do not demonstrate the normal host responses to infection, they still contain a microbiological impairment to healing.
Critically colonized wounds are at very high risk to become infected. In addition, a confounding factor in the stalling process may be the presence of biofilms, which can give the wound a healthy pink appearance even though the wound may be harboring large colonies of bacteria 38, 39, 40, 57, 58.
Infected wounds
Infection occurs when there is a histological demonstration of tissue invasion by organisms and a subsequent host response 59, 60. Infected wounds may demonstrate any of the classic clinical signs of infection (Table 2). The type and pathogenicity of the organisms may sometimes be as important as the quantity of organisms in increasing the risk of infection 55, 61.
The wound assessment algorithm (Figure 1) defines critically colonized wounds as having biofilm present with early local infection of soft tissue. Infected wounds, by contrast, have biofilm present with soft tissue infection and periwound extension. When infection penetrates to deeper levels, it is important to rule out the spread of infection and/or necrosis along the anatomic planes. With extensive or persisting infection, a return to the operating room for repeated thorough debridements is warranted until the infection is controlled in the wound. If osteomyelitis is suspected, devitalized tissue must be debrided and appropriate antibiotic therapy initiated.
PREREQUISITES FOR USING NPWT/ROCF IN INFECTED WOUNDS
The panel established three important prerequisites before initiating NPWT/ROCF on critically colonized or infected wounds: 1) the patient is free of most systemic signs of gross infection; 2) all necrotic tissue is debrided and abscesses are drained in the wound; and 3) the wound has adequate perfusion (necrosis can occur when negative pressure is applied on an ischemic wound). If there is any doubt, re‐examine the wound in 12‐24 hours before starting NPWT/ROCF. Sound clinical judgment is necessary before placement of NPWT/ROCF and usage should always be in combination with good medical and wound care practices.
PRE‐TREATING AND MONITORING INFECTED WOUNDS
In deep wound infection, NPWT/ROCF should always be used in combination with antibiotics after having obtained a bacterial swab pre‐ or intraoperatively to avoid the consequences of bacteremia (62). Immediate initiation of empiric, broad‐spectrum antibiotic treatment at presentation of infection is recommended, followed by antibiotics to which the cultured microorganisms are sensitive once culture results are received (63). A six‐week antibiotic treatment course when treating spine wound infections is commonly reported in the literature 63, 64.
All infected wounds should be debrided until only normal, soft, and well‐vascularized tissue remains. NPWT/ROCF should be implemented as early as possible after surgical debridement (62). The wound should be assessed at each dressing change in a closely monitored health care setting and under the supervision of an experienced wound care clinician. Although manufacturer guidelines recommend dressing changes 48–72 hours (65), reports in the literature cite periods of 1 to 4 days between dressing changes, depending on type and location of infected wound. Per manufacturer's guidelines, infected wounds should be monitored closely and may require more frequent dressing changes than non‐infected wounds (65).
CONTRAINDICATIONS, PRECAUTIONS AND REPORTED COMPLICATIONS
Caution should be exercised in all cases of wound infection. Based on literature and panel consensus, NPWT/ROCF should not be used in the presence of gross infection, sepsis or recurrent infection. Contraindications, warnings, and precautions for use of NPWT/ROCF as provided by the panel are summarized in Table 3.
Table 3.
NPWT/ROCF systems: contraindications, warnings and precautions recommended by the panel *
| CONTRAINDICATIONS | WARNINGS | PRECAUTIONS |
|---|---|---|
| Gross infection | If bleeding develops, discontinue therapy | Exposed blood vessels, nerves or organs must be protected using overlying fascia, tissue, or other protective barrier |
| Sepsis | Protect vessels and organs (cover with thick layers of natural tissue or meshed non‐adherent material) | Extreme care must be taken in the presence of existing or potential bleeding problems |
| Recurrent deep wound infection | Use therapy cautiously in presence of infected blood vessels | Bone fragments or sharp edges that could puncture protective barriers, vessels, or organs should be managed by debriding sharp edges |
| Presence of a pseudoaneurysm | Smooth and cover any residual sharp edges to decrease risk of serious or fatal injury | Extreme care must be taken to protect vascular anastomoses, including coronary artery bypass grafts, or weakened, irradiated, or sutured blood vessels or organs |
| Malignancy | Monitor hemostasis, anticoagulants, and platelet aggregation inhibitors | |
| Lack of local wound hemostasis | Monitor infected wounds closely; may require more frequent dressing changes than non‐infected wounds | |
| Untreated osteomyelitis | NPWT/ROCF‐silver is not intended to replace use of systemic therapy | |
| Unprotected vascular anastomoses | ||
| Foam placement directly over weakened or irradiated vessels |
This table does not necessarily reflect the contraindications, warnings, and precautions provided in the manufacturer's labeling.
The majority of reported complications (Table 4) reflect inadequate protection of exposed vital structures. One report documents the use of NPWT/ROCF in an infected wound that led to toxic shock syndrome (66). This patient was managed with NPWT/ROCF that had been in place for three days directly over dehisced abdominal fascia. The authors stated that the dressing may have provided an environment that enhanced proliferation or elaboration of the toxigenic Staphylococcus. Another contributing factor may have been that there was no protective layer between the exposed abdominal structures and the foam. The importance of protecting all exposed abdominal or neurovascular structures underneath the foam is detailed in recommended guidelines for NPWT/ROCF use 44, 67, 68.
Table 4.
Complications reported with NPWT/ROCF in infected wounds
| Major complications | Reference | Results |
|---|---|---|
| Toxic Shock Syndrome | Gwan‐Nulla and Casal (66) | GranuFoam™ Dressing placed for 3 days directly over dehisced abdominal fascia. Patient underwent surgical reexploration; NPWT was stopped. Wound was completely healed one year later. |
| Bleeding complications (2/16 patients) | Jones et al (125) | Two patients experienced bleeding complications related to NPWT/ROCF over deep infection of the spine. One patient died of delayed complications related to intraoperative blood loss, blood loss via NPWT/ROCF, and refusal of blood transfusion on religious beliefs. The other patient's wound healed successfully at 6‐month follow‐up. |
| Arterial erosion & hemorrhage of left anterior tibial artery | White (126) | Reexploration of the wound showed lateral wall of the anterior tibial artery eroded and was the source of bleeding; artery ligated; no further hemorrhage. STSG later applied. Wound healed uneventfully. |
| Empyema (2/2 patients) | Patel (127) | Patients were maintained on NPWT/ROCF after diagnosis of empyema, and their wound infections and empyemas subsequently resolved while using NPWT/ROCF. Wounds healed. |
| Uncontrolled sepsis (2/73 patients) | Ploumis et al (63) | NPWT/ROCF placed on 79 wounds from 73 patients undergoing spinal surgery. All but 2 patients achieved clean, closed wounds within 1 year of follow‐up. |
| Sepsis | Chester (128) | Sepsis developed in patient treated with NPWT/ROCF for groin wound dehiscence following inguinal block dissection. Sepsis resolved with antibiotic therapy and NPWT/ROCF cessation. Close surveillance of bacterial flora while using NPWT/ROCF recommended. |
| Cardiac rupture (5/5 patients) | Sartipy (129) | Major bleeding complications due to rupture of the right ventricle while on NPWT/ROCF. This potentially lethal complication may be avoided by covering the heart with protective layers of paraffin gauze dressings. |
SELECTING AN NPWT/ROCF MODALITY
The following are brief descriptions, literature reviews and panel recommendations to assist clinicians in using each type of NPWT/ROCF modality for the management of critically colonized or infected wounds (Figure 1).
NPWT with GranuFoam™ Dressing
The GranuFoam™ Dressing is a polyurethane foam dressing with open reticulated pores measuring 400–600 microns. Its hydrophobic, porous structure helps facilitate exudate removal. The open pores with spatial connections between the pores allow equal distribution of applied negative pressure throughout the foam and wound site (13).
The physiological and biological mechanisms by which negative pressure affects bacterial load in wounds have not yet been elucidated. In their original work in developing NPWT/ROCF, Morykwas and Argenta conducted a study using a swine model in which they deliberately infected the wounds (Staphylococcus aureus and Staphylococcus epidermidis) and measured the number of colony forming units per gram of tissue over time for the saline gauze control group versus NPWT/ROCF group. No antibiotics were used to treat the infection. NPWT/ROCF‐treated wounds showed a significant decrease in the number of bacterial colony forming units from 108 organisms per gram of tissue to 104 by the fifth day of treatment (15).
In contrast to Morykwas and Argenta's findings, two studies have shown no change in quantitative bacterial count with the application of NPWT/ROCF. In a controlled randomized trial, Vuerstaek et al found a decrease in the number of non‐fermentative negative rods and an increase in the number of Staphylococcus aureus in NPWT/ROCF‐treated wounds (69). However, an additional outcome of this trial showed that NPWT/ROCF significantly reduced wound preparation time and time to complete healing compared with traditional treatment modalities.
A second study, a retrospective review of 25 patient charts, found that NPWT/ROCF failed to reduce bacterial colonization of acute wounds. Because this study differs from the original Morykwas study in the frequency of dressing changes (every 3 to 5 days versus daily in the Morykwas study), this outcome may be more a reflection of frequency of dressing changes than of bioburden reduction (70). Further research is needed in this area. Interestingly, all wounds in both studies did progress to complete closure in a significantly accelerated time frame, despite the bacteria levels. These findings are further supported by a prospective randomized clinical trial in which Moues et al found a significant decrease in the number of non‐fermentative negative rods, yet a significant increase in the number of S. aureus in NPWT/ROCF‐treated wounds versus moist gauze therapy. The total quantitative bacterial load was generally stable in both groups. However, the study showed a positive effect of NPWT/ROCF on wound healing, based on significant reduction of wound surface area (71).
A Medline search using the terms “infected” and “vacuum assisted closure” in combination with “sternal wound”, “abdominal wound”, “extremity wound”, “groin wound”, “spinal wound” or “necrotizing fasciitis” yielded over 100 publications focused on the use of NPWT/ROCF in infected wounds. The vast majority of these publications describe the effective use of NPWT/ROCF within a small to medium‐sized, uncontrolled cohort. Studies that describe complications in NPWT/ROCF‐treated infected wounds are described in Table 4. Although more prospective randomized studies are needed to validate hypotheses, the volume of positive anecdotal evidence is compelling and demonstrates wide use of this therapy in diverse infected wound types.
Successful management of critically colonized and infected lower extremity wounds with NPWT/ROCF has also been documented in the literature 24, 67, 72, 73, 74. In a series of 77 Iraqi patients with 88 NPWT/ROCF‐treated high‐energy soft tissue combat wounds, the overall wound complication and infection rates were 0%, compared to an anecdotal infection rate of 80% prior to introduction of NPWT/ROCF treatment. The authors attributed the results to the closed environment of the NPWT/ROCF, which protects the wound from the ward environment, isolates the tissue injury, and keeps the wound clean and free of exudates (75).
GranuFoam™ Dressing is used by itself in the majority of the studies, although occasionally it is used in tandem with other antibacterial products. Song et al has reported on the use of GranuFoam™ Dressing over a silver coated antimicrobial barrier dressing in temporizing complicated mediastinitis wounds (76). Other case reports have documented successful use of silver contact layer dressings between the wound or skin graft and the GranuFoam™ Dressing 77, 78, 79, 80. However, in these cases, microdeformational changes within the wound that are expected with NPWT/ROCF could be limited by the lack of direct GranuFoam™ Dressing contact with the wound surface.
Panel recommendations
The panel recommended use of the GranuFoam™ Dressing in contaminated or colonized shallow wounds with no exposed bone or foreign body. It can also be used either in wounds with low risk of infection or in infected wounds, which can be seen in the algorithm in Figure 1.
The majority of studies report that NPWT/ROCF is effective in the management of colonized or infected wounds. Our review revealed relatively few major complications (Table 4), given the extensive use of NPWT/ROCF. This is consistent with the experience of all panel members who noted a reduction in clinical signs of infection with use of NPWT/ROCF in most colonized and infected wounds. Although the anecdotal evidence is positive and encouraging, there is a growing need for further controlled research in this area of bioburden reduction using NPWT/ROCF. Because of the complexities of wound healing, measuring bacteria and developing well‐designed prospective wound studies, there remains a paucity of controlled evidence from which to draw conclusions regarding NPWT/ROCF and bacterial clearance. As applications for the therapy expand into increasingly complex wounds at high risk for deep infection, there is considerably more to learn and understand about the effect of NPWT/ROCF on wound bioburden.
NPWT with GranuFoam Silver™ Dressing
The GranuFoam Silver™ Dressing has all of the properties of the original GranuFoam™ Dressing, but with the added feature that the foam has been micro‐bonded with metallic silver via a proprietary metallization process (65). With the initiation of NPWT/ROCF‐silver, exposure of the silver dressing to wound fluid causes oxidation of metallic silver to ionic silver, which results in a 99.9% microorganism kill within the first thirty minutes in vitro. An in vitro silver elution study showed that the GranuFoam Silver™ Dressing provides sustained release of ionic silver up to 72 hours (81). The reticulated open‐cell structure of this dressing allows for compression and conformity to the entire wound surface. Exposure of the dressing to wound fluid results in oxidation of metallic silver to ionic silver, allowing the continuous, sustained release of silver ions that act as an effective barrier to bacterial penetration (65).
Silver has been widely used in wound care due to its demonstrated broad‐spectrum antibacterial, antifungal and antiviral properties. Its usage has increased during the past five years, perhaps because of the increased level of bacterial resistance to traditional antibiotics. For example, rates of methicillin‐resistant Staphylococcus aureus (MRSA) increased from about 30% in 1989 to 50–60% in 1999 in most institutions across the US (82). Although coagulase‐negative Staphylococci (CoNS) have been methicillin resistant for many years, the incidence of resistance has also increased from 70% in 1989 to 90–95% in 2001–2002 (83).
In addition to MRSA and other gram positive cocci, and gram negative bacteria, especially Pseudomonas aeruginosa, Serratia marcescens, Acinetobacter, Enterobacter, and Citrobacter, are also problematic for clinicians because of their frequent occurrence and their resistance patterns 84, 85. For years, imipenem has been used to treat infections caused by multidrug‐resistance organisms because of its efficacy and the lack of resistance to it. In 1988, the incidence of Pseudomonas resistance to imipenem was approximately 5%. During the last 5 years, resistance has risen to approximately 11%. In intensive care unit (ICU) settings where it is used frequently, resistance has jumped to approximately 20% (86).
Unlike traditional antibiotics, ionic silver has multiple mechanisms of action, such as inhibiting cellular respiration, denaturing nucleic acids, and altering cellular membrane permeability 87, 88. An adequate concentration of silver, in addition to its various mechanisms of action, makes it difficult for microorganisms to develop resistance because the numerous mutations they would have to undergo in order to develop defense mechanisms against silver's varied attack (5).
However, potential resistance to silver remains an important factor, and the adequate level of silver required for bioburden efficacy remains a controversial and complex issue. Some investigators believe that low levels are enough and others support the view that a higher level of silver is necessary for sufficient bacterial kill. There is no global standard for elution studies, and the literature varies drastically, from 1.0 parts per million (ppm) to over 36 ppm, in the suggested requirements for the proper kill levels (89). A key confounding factor is that all pathogens have multiple strains and different strains require different levels of silver for kill. There are also significant differences in measurement methodology, which complicate data interpretation of silver levels among various silver dressings.
The literature supports that silver release must be measured and sustained over time 2, 89, 90. Research also indicates that the ability of a silver‐containing dressing to conform to the contours of a wound is important to reduce areas of non‐contact where bacteria may proliferate (91).
Some authors have suggested that higher levels of silver delivered by agents such as nanocrystalline silver can be harmful to viable cells. This toxicity of silver is supported in several in vitro studies 92, 93, 94, 95. However, the toxicity is generally associated with frequent and high levels of silver used, and increasing evidence suggests that toxicity results observed in animal and in vitro studies do not seem to play out in actual wounds.
In a study of silver‐containing dressings and their variable effects on cells, Cochrane et al used a fibroblast‐seeded collagen gel model to evaluate the effect of seven different silver‐containing antimicrobial dressings on fibroblast contraction and viability. Three of the seven dressings demonstrated less than 20% cell viability after 96 hours, compared to approximately 70% viability for the remaining four dressings. The authors concluded that toxicity effects of silver are based on numerous characteristics of a dressing, including its fluid handling, antimicrobial, physical and chemical properties (95).
Gabriel et al reported on a prospective case series of 5 consecutive patients with infected wounds managed with NPWT/ROCF‐silver (44). The dressing was in direct and complete contact with the wound bed. The results showed that no complications were experienced during therapy and all wounds progressed to primary or secondary closure. Specifically, the times to infection clearance and wound closure were 7.00 ± 1.58 and 19.20 ± 8.7 days, respectively.
In a case of a 4% total body surface area lower extremity burn, the patient developed a wound infection underneath a partial‐thickness sheet graft with an eventual 40% graft loss. Antibiotics were initiated and on postoperative day 22, NPWT/ROCF‐silver was applied over a soft silicone wound contact layer. The wound was completely re‐epithelialized by day 9 of NPWT use and re‐grafting was avoided (96).
NPWT/ROCF‐silver appears to offer sufficient antimicrobial effect and promote wound healing. The positive outcomes are likely due to a combination of factors including lower nontoxic levels of silver release, optimal wound conformability, exudate removal in a non‐traumatic fashion and the established benefits of NPWT/ROCF. It is important to note that use of V.A.C GranuFoam Silver® is not intended to replace use of systemic therapy but to complement antibiotic treatment protocols.
Panel recommendations
Based on their experiences, panel members suggested that benefits of NPWT/ROCF‐silver include antiseptic, antimicrobial properties without the requirement of an additional adjunctive silver layer. The properties of the silver dressing may minimize tissue adherence to the foam, causing less pain during dressing removal, compared to the original NPWT/ROCF dressing. Some panel members suggested the use of NPWT/ROCF‐silver for a broad range of wounds including cases of complex, colonized, or infected wounds post‐debridement, as well as in acute traumatic wounds for reduction of bacterial bioburden. Some panel members preferred using NPWT/ROCF‐silver dressing in patients who have severe comorbidities and a history of chronic wound colonization to help reduce the risk of recurrent infection. Some panel members recommend that the silver dressing may be considered for use with the following wound types or characteristics: partial‐thickness burns, chronic wounds, exposed mesh, stalled wounds, exposed hardware, dehisced, diabetic ulcers, pressure ulcers (stage III and IV), diabetic amputations, and split‐thickness skin grafts (STSGs).
Caution should be exercised when using silver in wounds that show signs of cellulitis or systemic infection, wounds that are colonized with fungus, patients with leucopoenia or when signs of possible side effects are present, such as erythema multiforme (97). Silver dressings should also not be used with saline or papain‐urea (which has since been removed from the market) debriding ointment. Saline may decrease the amount of silver released (97).
Interface layers are generally not recommended to be used between the wound and the V.A.C. GranuFoam Silver® Dressing since it is the direct contact of the foam with the wound bed that induces micromechanical effects at the dressing‐to‐tissue interface resulting in tissue undulations and cellular microdeformation (13). A non‐adherent layer is needed only to protect exposed blood vessels, anastomatic sites, organs and nerves.
Judicious use of silver dressings is recommended. Duration of NPWT/ROCF‐silver has yet to be clearly defined and varies among clinicians and with clinical presentation. The panel recommends evaluation of the treatment goals and reassessment of progress and changes in the wound after initiation of treatment. Short‐term use of silver‐containing dressings until wound bioburden is controlled and wound healing progress has been reported (41). Notable secondary signs and symptoms of healing would include: change in odor, pain, color and quality of granulation tissue; reversal of stalling; and coverage of structures. Guidelines for duration are multifaceted and should reflect response to treatment. As with any modality, if the wound is not progressing within 1–2 weeks, reevaluation of treatment is warranted. After completing treatment with NPWT/ROCF‐silver, it may be appropriate to switch back to NPWT with GranuFoam™ or V.A.C.® WhiteFoam Dressings for the duration of treatment.
NPWT with timed, automated instillation of fluids
Wound irrigation is an integral step in the management of soft tissue injuries and open fractures, typically following debridement of the injured soft tissues. Several factors, including fluid type, fluid volume, and delivery method, must be considered prior to wound irrigation. While pulsed lavage is perhaps the most common type of irrigation, high pressure pulsed lavage has been shown to be deleterious to bone healing (98). Although the effect of high pressure irrigation systems on some wounds remains controversial, it is well accepted that intermittent irrigation is a requirement to remove debris and adhered bacteria when dealing with heavily contaminated wounds.
V.A.C. Instill® Wound Therapy (NPWT with instillation) was introduced to the US acute care market in 2003 as an evolution of the standard NPWT/ROCF. The instillation device combines the mechanisms of action of the original NPWT/ROCF with timed, intermittent, gravity‐fed delivery of an instilled topical solution through a second ingress tube applied to the foam. Varying lengths of solution infusion and dwell times are set via the microprocessor. NPWT with instillation is a low‐pressure irrigation delivery system indicated for patients who would benefit from Vacuum Assisted Closure® and controlled delivery of topical wound treatment solutions and suspensions over the wound bed 99, 100. NPWT with instillation allows contaminated wounds to be intermittently irrigated in an automated fashion to help remove particulate and bacterial matter and facilitate closure (101).
Surgeons are familiar with the use of topical antibiotics for wound irrigation, and most are aware of the controversy surrounding their efficacy. No single ideal topical solution exists to treat all variations in bacterial colonization, wound types and host factors. An ideal agent would have a broad spectrum of activity with no systemic absorption; it would be painless, inexpensive, nontoxic with minimal wound healing interference, and would be approved for topical use by the US Food and Drug Administration (FDA) (101).
Based on the manufacturer's clinical guidelines, NPWT with instillation is intended for use with topical wound treatment solutions. Various topical agents (such as hydrogen peroxide, which is contraindicated for use with NPWT with instillation) are not intended for extended tissue contact and must not be used with NPWT with instillation. If in doubt about the appropriateness of using a particular solution for NPWT with instillation, contact the solution's manufacturer about its suitability for saturated topical wound irrigation in conjunction with exposure to polyurethane foam. Solutions should not be delivered in conflict with solution manufacturer's Instructions for Use or Prescribing Information (65).
Choices among the short list of antiseptics that are FDA approved (Table 5) for topical use in solution form can have potential drawbacks. Mafenide acetate is FDA approved for use in a 5% solution form. This agent is broadly bacteriostatic against many gram positive and gram negative bacteria as well as some anaerobes. However, the solution has been shown to be systemically absorbed, and complications, such as metabolic acidosis, rare bone marrow depression, and fatal hemolytic anemia with disseminated intravascular coagulation, have been reported 102, 103, 104, 105.
Table 5.
Topical wound irrigation & solutions *
| Drug Name | Mafenide Acetate Example: Sulfamylon | Sodium Oxychlorosene Example: Clorpactin | Sodium Hypochlorite Example: Dakin's Solution | Sodium Hypochlorite Example: Microcyn | Silver Nitrate AgNo3 | Benzalkonium Cl Example: Zephiran |
|---|---|---|---|---|---|---|
| Drug Class | Sulfonamide Antimicrobial Agent | Antiseptic Agent | Antiseptic Agent | Antiseptic Agent | Silver Releasing Agent Antiseptic | Antiseptic Agent |
| Clinical Indications per Manufacturer | Adjunctive topical antimicrobial agent to control bacterial infection when used under moist dressings over meshed autografts on excised burn wounds | Treating localized infections; irrigant for cleansing and disinfecting wounds | Highly diluted, neutral antiseptic solution for cleansing wounds. Consists of sodium hypochlorite (household bleach), sodium bicarbonate and sterile water. | Indicated for management via debridement of wounds such as stage I‐IV pressure ulcers, partial and full‐thickness wounds, diabetic foot ulcers, post surgical wounds, first‐ and second‐degree burns, grafted and donor sites | Antiseptic cleansing | Antisepsis of skin, mucous membranes and wounds |
| Common Irrigation Concentrations (%) and Preparations | 5% topical solution | 0.1–0.4% solution | Concentration commonly used 0.25%, or 0.125%. V.A.C. Instill® recommended concentration is max 0.125% (quarter strength). | Electrolyzed water (99.97%), Sodium Chloride (NaCl) 0.023%, Sodium Hypochlorite (NaOCl) 0.004%, and Hypochlorous Acid (HOCl) 0.003% | 0.5% solution | Available commercially as 1:750 aqueous solution. For irrigation of deep infected wounds; solutions of 1:3,000‐1:20,000 recommended. |
| Effective Against | Pseudomonas aeruginosa and other gram negative and gram positive bacteria and certain anaerobes. | Treatment of localized infections, particularly when resistant organisms are present. Complete spectrum includes bacteria, fungi, viruses, mold, yeast, spores; effective in cases of antibiotic resistance. | Effective against MRSA and Pseudomonas. | Gram positive and gram negative bacteria, some yeast | Fungi, gram positive bacteria, Pseudomonas. | Gram positive bacteria, some viruses, fungi, protozoa. Activity increases with temperature and pH. |
Listing of solution or pharmaceutical product is not an endorsement or recommendation by KCI® for use with the V.A.C. Instill® Wound Therapy. This is a listing of some commonly used topical solutions that fall within the V.A.C. Instill® Wound Therapy's indications for use.
Silver nitrate 0.5% solution has been proposed as one solution for use with NPWT with instillation 45, 101. Silver nitrate 0.5% solution is approved by the FDA and is bactericidal against panresistant bacteria. Silver nitrate has a proven track record at many burn centers throughout the US for the decolonization of burn wounds prior to skin grafting 106, 107, 108. The solution is painless when applied to the wound and is not absorbed into the blood stream, which has been demonstrated through serum level tests (101). This solution does not damage the components of the negative pressure dressing. The silver nitrate solution is prepared from lyophilized powder, which is reconstituted with sterile water. Pharmaceutical grade ready‐to‐use 0.5% silver nitrate can also be purchased (45).
Microcyn® Skin and Wound Cleanser (Oculus Innovative Sciences, Petaluma, CA) with preservatives is an FDA cleared topical wound irrigation solution that has been used successfully with NPWT with instillation 109, 110. It has demonstrated effectiveness by reducing multiple microorganisms including MRSA, VRE, Pseudomonas, and Candida. It is non‐irritating and non‐cytotoxic with a shelf life of 24 months.
Instillation‐vacuum techniques in treating infection have been described in Europe throughout the past two decades 111, 112, 113, 114. Many of these studies have employed the instillation vacuum sealing technique, which involves temporary implantation of the white polyvinyl alcohol foam and connection to redon bottles or an NPWT device and hand‐administered antiseptic or antibiotic instillation at regular, timed intervals. Unfortunately, review of the earlier European literature regarding vacuum sealing was limited largely to abstracts as many of the manuscripts were written in German. In a retrospective review of 27 patients with acute infection of bone and soft tissues, chronic osteomyelitis or chronic wounds treated with this technique for 7 days, only one recurrence of infection was reported during an average 4.2 month follow‐up time (115).
In the US, an initial review of the NPWT with instillation device with case studies was published by Wolvos (2004) (99). In a pilot study of 5 patients, a variety of complex wounds were treated with NPWT and culture‐directed antibiotics. Dilute lidocaine was combined with some of the irrigation solutions. All wounds that cultured positive for infection prior to receiving NPWT with instillation had follow‐up cultures that showed no growth or only normal flora. Culture‐directed antibiotic wound irrigation appeared to decrease the bacterial burden of infected wounds and change the appearance to that of clean wounds. In the two cases that were being treated with traditional NPWT, switching to NPWT with instillation facilitated a clear improvement in wound clinical appearance and culture results.
Gabriel et al reported results of a prospective case series of 15 patients treated with NPWT‐instillation compared to a retrospective historical control of 15 patients treated with moist gauze wound care. Compared with the standard moist wound care therapy control group, patients in the NPWT with instillation group required fewer days of treatment, cleared of clinical infection earlier, had wounds close earlier and had fewer in‐hospital stay days. They noted that the addition of an instilled, irrigating fluid to NPWT/ROCF provided a unique autolytic and mechanical debridement effect that appeared to enhance wound healing over traditional NPWT/ROCF for complex, infected wounds. The authors recommended NPWT with instillation for wounds that could benefit from automated intermittent hydro‐debridement, such as acute traumatic wounds or acutely debrided wounds because of infected soft tissue (45).
In a series of 5 post‐surgical diabetic foot wounds treated with NPWT with instillation, Bernstein et al noted a decrease in hospital stay and amputation rate with the instillation technique (101). The authors noted the addition of instilled solutions lowered viscosity of the wound fluid and allowed more efficient removal into the canister. The system was found to be efficacious in wounds with high levels of exudate and slough content. Benefits of the therapy included aiding in withdrawing remaining infectious material from the wound, enhancing granulation tissue formation, maintaining a moist wound environment, and periodically bathing the wound with antibiotic solution may help to prevent contiguous osteomyelitis or desiccation of exposed bone.
A recent retrospective, case‐control cohort study compared outcomes of patients with osteomyelitis of the pelvis or lower extremity treated with NPWT with instillation (polyhexanide antiseptic solution) and white polyvinyl alcohol foam versus gentamicin polymethylmethacrylate beads and long‐term intravenous antibiotics (116). The rate of recurrence of infection was 3/30 in the Instillation group versus 55/93 in the Bead group (p< 0.0001). The duration of hospital stay and number of surgical procedures were also less in the Instillation group. The authors concluded that in posttraumatic osteomyelitis, NPWT with instillation reduced the need for repeated surgical interventions compared to implanted gentamicin polymethylmethacrylate beads (116).
Brem et al reported use of NPWT with instillation on a polytraumatized patient who suffered a third‐degree open femur defect fracture with substantial loss of the lateral femoral muscles and significant disruption of the soft tissue (117). Critical infection was confirmed and NPWT with instillation was initiated as a last resort to salvage the limb. Lavasept (available outside of the US) was instilled for 6 days and the number of organisms was significantly reduced. The patient continued with NPWT without instillation and was ultimately discharged with an intact leg with good function.
Panel recommendations
The panel‐recommended indications for NPWT with instillation are detailed in Table 6. Topical solutions that can be used with NPWT with instillation include cleansers, antibiotics, antifungals, antiseptics and anesthetics. The system is intended for use with aqueous solutions in a physiologic pH range defined as 6.0–7.4 (101).
Table 6.
Recommendations for use of NPWT with instillation
| • * Diffuse or extensive osteomyelitis is encountered or if large areas of post‐debridement bone or joint are present |
| • * Postoperative infection following total joint arthroplasty |
| • * A wound is suspected to be critically colonized and subsequently has become stalled, yet needs continued NPWT |
| • * An alternative to antibiotic‐impregnated beads is needed |
| • † Wounds at a high risk for a major amputation due to infection |
| • † Open fracture wounds to prevent wound infections or osteomyelitis |
| • † To decrease the viscosity of wound exudate |
| • † Exposed hardware or foreign bodies including mesh |
| • † Infected wounds that remain infected after a trial of NPWT/ROCF |
Adapted from Bernstein et al (101)
†Tom Wolvos, MD, FACS, e‐mail communication, July 21, 2009
A deeper wound with exposed bone or a foreign body (ie, hardware), or appearing grossly contaminated, may require surgical irrigation. Some of the panel members recommend that the wound may benefit from NPWT with instillation with antiseptic solutions, such as 0.5% silver nitrate, Microcyn ® or from NPWT/ROCF‐silver with regular sterile water irrigation. Aggressive brushing of exposed hardware and continuous irrigation with an antiseptic may be effective in decreasing biofilm formation. However, randomized, controlled studies are warranted. Once there is appropriate wound progression, NPWT with instillation can be converted to NPWT with GranuFoam Silver™ or regular GranuFoam™ Dressing.
In critically colonized wounds with early local infection, NPWT with instillation of an antiseptic solution is recommended (Figure 1). Some panel members recommended that once wound exudate and slough levels are minimized and hydro‐debridement is no longer necessary, NPWT with instillation can be converted to NPWT/ROCF with GranuFoam Silver™ or regular GranuFoam™ Dressing. Following short‐term treatment, critically colonized wounds have a strong probability of reverting to a chronic state. Therefore, some panel members suggested that these wounds should be treated with a broad‐spectrum antiseptic, GranuFoam Silver™ Dressings, or regular GranuFoam™ Dressings until full closure is obtained.
Infected wounds may require aggressive serial debridements and may require multiple returns to the operating room. Once all necrotic tissue and abscesses are removed and clinical signs are improving, NPWT with instillation of an antiseptic may be initiated. Some panel members suggest the use of NPWT with instillation using a GranuFoam Silver™ Dressing for both superficial and deep infected wounds to aggressively address infection. As with critically colonized wounds, infected wounds should also be managed with a broad‐spectrum antiseptic modality until full closure is obtained.
When using a silver nitrate solution for instillation, it is important that the pharmacist prepare the solution carefully, as solutions greater than 0.5% can denature proteins 93, 118. Silver nitrate at a concentration of 5% to 10% produces chemical cautery. Minor complications of the use of silver nitrate include staining of bed linens and instability to light. Silver nitrate is hypotonic and not an ideal environment for healing tissue; it should be used for short‐term use only.
Specifically, NPWT with instillation could have a potential role in treating postoperative infection following total joint arthroplasty, a serious and devastating complication. Antibiotic beads have been a mainstay of practice for dead‐space management in infected wounds involving lower and upper extremities 119, 120, 121, 122. Newer bio‐absorbable carriers of antibiotic‐containing hyaluronic acid gel are showing promise for prophylactic treatment or treatment of an actual deep infection following total joint arthroplasty in rabbit and mouse models (119). To develop an effective treatment for this complication, various attempts at the instillation of antibiotic solution into a wound have been made with either passive egress through the suture line or active suction via an exterior pump 123, 124.
CONCLUSION
V.A.C.® Therapy, with its newer adjuncts, may be an optimal initial dressing after debridement of infected wounds because of its combined effects of V.A.C.® Therapy mechanisms, antibacterial treatment and irrigation. The combination therapy has enabled the panel members to temporize wounds, which allow the reconstructive surgeon and the patient time before committing to a definite reconstruction. In addition, utilizing the concept of the reconstructive ladder, this therapy has enabled surgeons to perform less complex reconstructions of major soft tissue defects. This ultimately reduces wound morbidity and decreases anesthesia time—all of which are better tolerated by the patient.
The important point in the management of the described wounds is that bacterial contamination is seen in any open wound, even though low bacterial colony units are reported. All bacteria, whether in an acute or chronic wound, or in a contaminated versus a colonized wound, will produce virulence factors (eg, exotoxins, endotoxins, and MMPs), all of which have deleterious effects to wound healing. They decrease the ability of the wound to progress to the next stage and halt it in a chronic inflammatory phase. Therefore the removal of the cytotoxic cytokines via V.A.C.® Therapy and the decrease of the bacterial bioburden with the use of antiseptic modalities (eg, V.A.C. Instill®Wound Therapy or GranuFoam Silver™), synergistically help in managing these difficult to treat wounds.
Case Studies
Case #1 is an acute wound with exposed muscle at risk for poor progression if adequate wound care is not performed.
Assessment and Level of Contamination
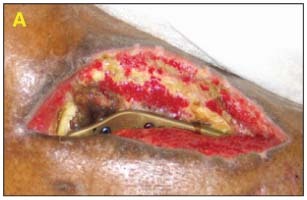
11‐year old male with acute lower extremity soft tissue avulsion injury and tibia/fibula fracture. Exposed bone, muscle, and tendon (A). The patient had a large soft tissue deficit with heavy contamination. The debridement was done in the operating room and the application of external fixation was performed (B).
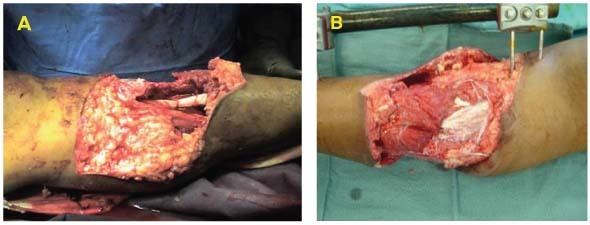
Dressing Selection and Outcome
The V.A.C. GranuFoam Silver® Dressing was applied over the wound and therapy was initiated at ‐125 mmHg continuously. On day 7, the patient returned to the operating room and an STSG was applied. The graft was bolstered with the GranuFoam SilverTM Dressing and therapy was initiated at ‐75mmHg continuously (C). The dressing was removed on day three and there was 100% graft take (D).
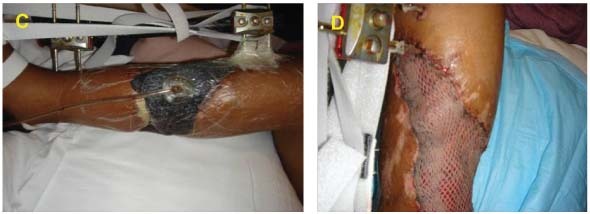
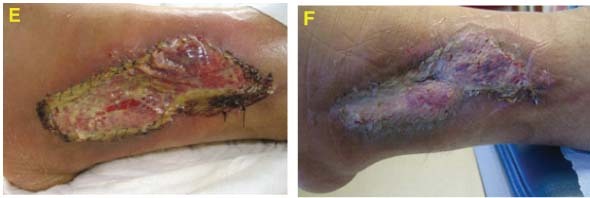
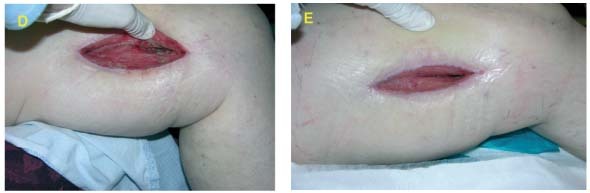
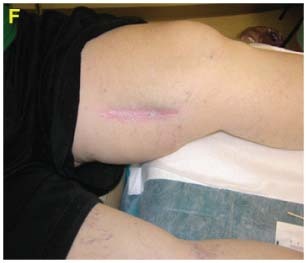
Case #2 is an example of initial treatment with V.A.C. Instill® Wound Therapy and then converting to V.A.C.® Therapy with GranuFoam Silver™ Dressing.
Assessment and Level of Contamination
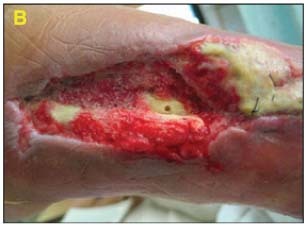
49‐year‐old male with history of distal tibial fracture with deep infection requiring hardware removal. Exposed bone and soft tissue infection noted (A).
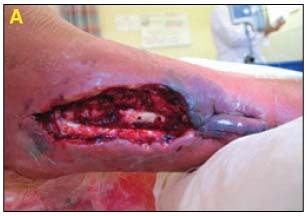
Dressing Selection and Outcome:
Wound was debrided and V.A.C. Instill® Wound Therapy was initiated. Wound progression continued as evidenced by granulation tissue formation and obvious cleansing of the wound (B).
The wound treatment was converted to GranuFoam Silver™ Dressing with ‐125 mmHg pressure (C). Wound continued to progress with healthy granulation tissue formation (D).
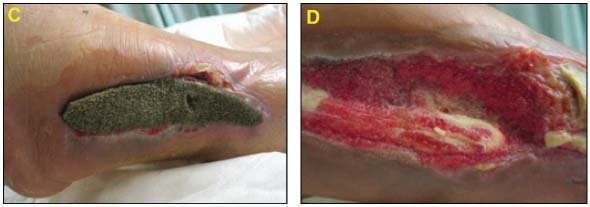
A bilaminate skin substitute was applied, (E) and final coverage with a STSG (F) was performed.
Case #3 is an example of treatment with V.A.C. Instill® Wound Therapy
Assessment and Level of Contamination
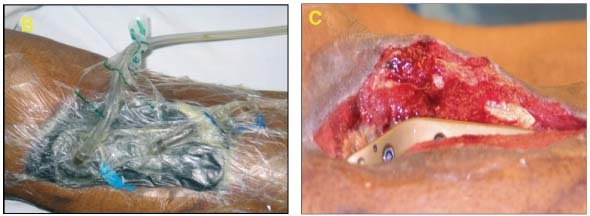
65‐year‐old male with infected open right knee joint with exposed hardware for three months. Deep crevices and slough noted. Wound critically colonized with topical contamination and biofilm formation (A).
Dressing Selection and Outcome
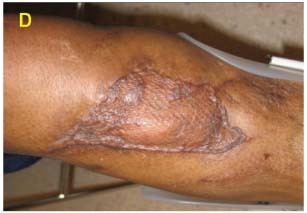
After debridement, it was determined that this wound would benefit from continuous irrigation to break down or prevent biofilm formation and ensure solution reached all crevices and difficult contours. V.A.C. Instill® Wound Therapy was initiated with silver nitrate irrigation (B). Improved healing was evident and the knee was closed via local flap on day 10 (C).
The knee remained closed and the flap viable at 6‐month follow‐up (D).
Case #4 is an example of initial treatment with V.A.C. Instill® Wound Therapy and then converting to V.A.C.® Therapy with GranuFoam™ Dressing.
Assessment and Level of Contamination
70‐year‐old male who underwent an above knee femoral and popliteal artery bypass graft for peripheral vascular disease and osteomyelitis of his left foot; presented 3 months later with fever and cellulitis of the medial distal thigh. An elevated WBC indicated an infection which was revealed by CT scan as fluid in his distal medial thigh. An abscess containing MRSA in patient's thigh was irrigated and debrided (A).
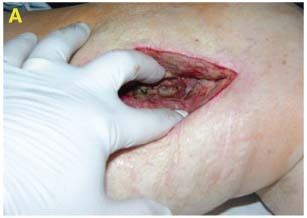
Dressing Selection and Outcome
V.A.C.® WhiteFoam Dressing was placed deep in the wound (B) and covered with GranuFoam™ Dressing over the wound. V.A.C. Instill® (−125 mmHg) was applied (instill 20 seconds, hold 10 minutes, repeat every quarter hour) with Microcyn® as the irrigant (C).
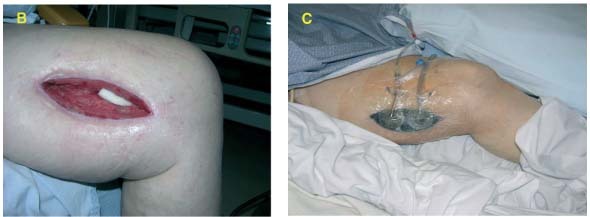
After 9 days, the bypass graft was covered with healthy granulation tissue (D). NPWT/ROCF was applied and patient was discharged from the hospital (E) and allowed to close by secondary intention.
At a 2‐month follow‐up, wound remained healed with no evidence of superficial or deep infection (F).
DISCLAIMER
The information provided is based on an expert advisory panel convened to determine appropriate use of the different modalities of negative pressure wound therapy (NPWT) as delivered by V.A.C.® Therapy and V.A.C. Instill® with either GranuFoam™ or GranuFoam Silver™ Dressings for the treatment of infected wounds. All NPWT systems do not perform in the same manner, and outcomes using other systems may not be the same. Recommendations are not intended as a guarantee of results, outcomes, or performance of the V.A.C.® Therapy Systems. Please note that use of V.A.C.® Therapy does not preclude use of other surgical techniques or modalities to manage wounds. As with any application, consult product labelling and instructions for use prior to initiating V.A.C.® Therapy.
Microcyn® is the trademark of Oculus Innovative Sciences (Petaluma, CA). All other trademarks designated herein are proprietary to KCI Licensing, Inc., its affiliates and/or licensors.
ACKNOWLEDGEMENTS
We would like to thank Andrea Adams, Karen Beach, Alice Goodwin, Ricardo R Martinez and Julissa Ramos for their editorial assistance and preparation of this manuscript.
REFERENCES
- 1. Wright JB, Lam K, Burrell RE. Wound management in an era of increasing bacterial antibiotic resistance: a role for topical silver treatment. Am J Infect Control 1998;26(6):572–7. [DOI] [PubMed] [Google Scholar]
- 2. Yin HQ, Langford R, Burrell RE. Comparative evaluation of the antimicrobial activity of ACTICOAT antimicrobial barrier dressing. J Burn Care Rehabil 1999;20(3):195–200. [DOI] [PubMed] [Google Scholar]
- 3. Chin C, Schultz G, Stacey M. Principles of wound bed preparation and their application to the treatment of chronic wounds. Prim Intention 2003;11(4):171–82. [Google Scholar]
- 4. Schultz GS, Barillo DJ, Mozingo DW, Chin GA. Wound bed preparation and a brief history of TIME. Int Wound J 2004;1(1):19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Warriner R, Burrell R. Infection and the chronic wound: a focus on silver. Adv Skin Wound Care 2005;18(Suppl 1):2–12. [DOI] [PubMed] [Google Scholar]
- 6. Zhan C, Miller MR. Excess Length of Stay, Charges, and Mortality Attributable to Medical Injuries During Hospitalization. JAMA 2003;290(14):1868–74. [DOI] [PubMed] [Google Scholar]
- 7. Breasted JH. The Edwin Smith Surgical Papyrus. Chicago: University of Chicago Press; 1930. [Google Scholar]
- 8. Lister J. On a new method of treating compound fracture, abscess, etc. with observations on the conditions of suppuration. Part I on compound fracture. Lancet 1867;89(2272):323–6. [Google Scholar]
- 9. Singhal H, Kaur K, Zammit C. Wound infection. www emedicine com 2008;August 21.
- 10. Lowry KF, Curtis GM. Delayed suture in the management of wounds; analysis of 721 traumatic wounds illustrating the influence of time interval in wound repair. Am J Surg 1950;80(3):280–7. [DOI] [PubMed] [Google Scholar]
- 11. Haury B, Rodeheaver G, Vensko J, Edgerton MT, Edlich RF. Debridement: an essential component of traumatic wound care. Am J Surg 1978;135(2):238–42. [DOI] [PubMed] [Google Scholar]
- 12. No Author. Surgical Site Infection (SSI). 12‐17‐2008. Atlanta, GA, Centers for Disease Control and Prevention. 7‐23‐2009. Ref Type: Generic. [Google Scholar]
- 13. Saxena V, Hwang CW, Huang S, Eichbaum Q, Ingber D, Orgill DP. Vacuum‐assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg 2004;114(5):1086–96. [DOI] [PubMed] [Google Scholar]
- 14. Lambert KV, Hayes P, McCarthy M. Vacuum assisted closure: a review of development and current applications. Eur J Vasc Endovasc Surg 2005;29(3):219–26. [DOI] [PubMed] [Google Scholar]
- 15. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997;38(6):553–62. [DOI] [PubMed] [Google Scholar]
- 16. Wackenfors A, Sjogren J, Gustafsson R, Algotsson L, Ingemansson R, Malmsjo M. Effects of vacuum‐assisted closure therapy on inguinal wound edge microvascular blood flow. Wound Repair Regen 2004;12(6):600–6. [DOI] [PubMed] [Google Scholar]
- 17. Wackenfors A, Sjogren J, Algotsson L, Gustafsson R, Ingemansson R, Malmsjo M. The effect of vacuum‐assisted closure therapy on the pig femoral artery vasomotor responses. Wound Repair Regen 2004;12(2):244–51. [DOI] [PubMed] [Google Scholar]
- 18. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38(6):563–76. [PubMed] [Google Scholar]
- 19. Sjogren J, Mokhtari A, Gustafsson R, Malmsjo M, Nilsson J, Ingemansson R. Vacuum‐assisted closure therapy for deep sternal wound infections: the impact of learning curve on survival and predictors for late mortality. Int Wound J 2008;5(2):216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fuchs U, Zittermann A, Stuettgen B, Groening A, Minami K, Koerfer R. Clinical outcome of patients with deep sternal wound infection managed by vacuum‐assisted closure compared to conventional therapy with open packing: a retrospective analysis. Ann Thorac Surg 2005;79(2):526–31. [DOI] [PubMed] [Google Scholar]
- 21. DeFranzo AJ, Pitzer K, Molnar JA et al. Vacuum‐assisted closure for defects of the abdominal wall. Plast Reconstr Surg 2008;121(3):832–9. [DOI] [PubMed] [Google Scholar]
- 22. Stannard JP, Robinson JT, Anderson ER, McGwin G, Jr. , Volgas DA, Alonso JE. Negative pressure wound therapy to treat hematomas and surgical incisions following high‐energy trauma. J Trauma 2006;60(6):1301–6. [DOI] [PubMed] [Google Scholar]
- 23. Fleck T, Gustafsson R, Harding K et al. The management of deep sternal wound infections using vacuum assisted closure (V.A.C.) therapy. Int Wound J 2006;3(4):273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Armstrong DG, Attinger CE, Boulton AJ et al. Guidelines regarding negative pressure wound therapy (NPWT) in the diabetic foot: results of the Tucson expert consensus Conference (TECC) on V.A.C. Therapy. Ostomy Wound Manage 2004;50(4 Suppl):3S–27S. [PubMed] [Google Scholar]
- 25. Attinger CE, Janis JE, Steinberg J, Schwartz J, Al‐Attar A, Couch K. Clinical approach to wounds: debridement and wound bed preparation including the use of dressings and wound‐healing adjuvants. Plast Reconstr Surg 2006;117(7 Suppl):72S–109S. [DOI] [PubMed] [Google Scholar]
- 26. Banwell P, Withey S, Holten I. The use of negative pressure to promote healing. Br J Plast Surg 1998;51(1):79. [DOI] [PubMed] [Google Scholar]
- 27. Banwell PE. Topical negative pressure therapy in wound care. J Wound Care 1999;8(2):79–84. [DOI] [PubMed] [Google Scholar]
- 28. Banwell PE, Teot L. Topical negative pressure (TNP): the evolution of a novel wound therapy. J Wound Care 2003;12(1):22–8. [DOI] [PubMed] [Google Scholar]
- 29. Yager DR, Zhang LY, Liang HX, Diegelmann RF, Cohen IK. Wound fluids from human pressure ulcers contain elevated matrix metalloproteinase levels and activity compared to surgical wound fluids. J Invest Dermatol 1996;107(5):743–8. [DOI] [PubMed] [Google Scholar]
- 30. Cook H, Davies KJ, Harding KG, Thomas DW. Defective extracellular matrix reorganization by chronic wound fibroblasts is associated with alterations in TIMP‐1, TIMP‐2, and MMP‐2 activity. J Invest Dermatol 2000;115(2):225–33. [DOI] [PubMed] [Google Scholar]
- 31. Grinnell F, Zhu M, Parks WC. Collagenase‐1 complexes with alpha2‐macroglobulin in the acute and chronic wound environments. J Invest Dermatol 1998;110(5):771–6. [DOI] [PubMed] [Google Scholar]
- 32. Wlaschek M, Peus D, Achterberg V, Meyer‐Ingold W, Scharffetter‐Kochanek K. Protease inhibitors protect growth factor activity in chronic wounds. Br J Dermatol 1997;137(4):646. [DOI] [PubMed] [Google Scholar]
- 33. Davey ME, O’toole GA. Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev 2000;64(4):847–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Falabella AF, Carson P, Eaglstein WH, Falanga V. The safety and efficacy of a proteolytic ointment in the treatment of chronic ulcers of the lower extremity. J Am Acad Dermatol 1998;39(5 Pt 1):737–40. [DOI] [PubMed] [Google Scholar]
- 35. Ladwig GP, Robson MC, Liu R, Kuhn MA, Muir DF, Schultz GS. Ratios of activated matrix metalloproteinase‐9 to tissue inhibitor of matrix metalloproteinase‐1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair Regen 2002;10(1):26–37. [DOI] [PubMed] [Google Scholar]
- 36. Sibbald RG, Orsted H, Schultz GS, Coutts P, Keast D. Preparing the wound bed 2003: focus on infection and inflammation. Ostomy Wound Manage 2003;49(11):24–51. [PubMed] [Google Scholar]
- 37. Sibbald RG. Topical antimicrobials. Ostomy Wound Manage 2003;49(5A Suppl):14–8. [PubMed] [Google Scholar]
- 38. Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet 2001;358(9276):135–8. [DOI] [PubMed] [Google Scholar]
- 39. Costerton JW, Stewart PS. Battling biofilms. Sci Am 2001;285(1):74–81. [DOI] [PubMed] [Google Scholar]
- 40. Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science 1999;284(5418):1318–22. [DOI] [PubMed] [Google Scholar]
- 41. Sussman C, Bates‐Jensen B. Management of the Wound Environment with Dressings and Topical Agents. In: Sussman C, Bates‐Jensen B, editors. Wound Care: A Collaborative Practice Manual for Health Professionals. 3 ed. Lippincott Williams and Wilkins; 2006;p. 250–67. [Google Scholar]
- 42. Schmidtchen A, Holst E, Tapper H, Bjorck L. Elastase‐producing Pseudomonas aeruginosa degrade plasma proteins and extracellular products of human skin and fibroblasts, and inhibit fibroblast growth. Microb Pathog 2003;34(1):47–55. [DOI] [PubMed] [Google Scholar]
- 43. Kirshen C, Woo K, Ayello EA, Sibbald RG. Debridement: a vital component of wound bed preparation. Adv Skin Wound Care 2006;19(9):506–17. [DOI] [PubMed] [Google Scholar]
- 44. Gabriel A, Heinrich C, Shores JT, Baqai WK, Rogers FR, Gupta S. Reducing bacterial bioburden in infected wounds with vacuum assisted closure and a new silver dressing—a pilot study. Wounds 2006;18(9):245–55. [Google Scholar]
- 45. Gabriel A, Shores J., Heinrich C et al. Negative pressure wound therapy with instillation: a pilot study describing a new method for treating infected wounds. Int Wound J 2008;5(3):399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ashcroft GS, Herrick SE, Tarnuzzer RW, Horan MA, Schultz GS, Ferguson MW. Human ageing impairs injury‐induced in vivo expression of tissue inhibitor of matrix metalloproteinases (TIMP)‐1 and ‐2 proteins and mRNA. J Pathol 1997;183(2):169–76. [DOI] [PubMed] [Google Scholar]
- 47. Ashcroft GS, Horan MA, Herrick SE, Tarnuzzer RW, Schultz GS, Ferguson MW. Age‐related differences in the temporal and spatial regulation of matrix metalloproteinases (MMPs) in normal skin and acute cutaneous wounds of healthy humans. Cell Tissue Res 1997;290(3):581–91. [DOI] [PubMed] [Google Scholar]
- 48. Sibbald RG, Woo K, Ayello EA. Increased bacterial burden and infection: the story of NERDS and STONES. Adv Skin Wound Care 2006;19(8):447–61. [DOI] [PubMed] [Google Scholar]
- 49. Tenenhaus M, Bhavsar D, Rennekampff HO. Diagnosis and Surgical Management in Wound Bacterial Burden. In: Granick MS, Gamelli RL, editors. Surgical Wound Healing and Management. 1 ed. New York: Informa Healthcare USA, Inc.; 2007;p. 29–38. [Google Scholar]
- 50. Bill TJ, Ratliff CR, Donovan AM, Knox LK, Morgan RF, Rodeheaver GT. Quantitative swab culture versus tissue biopsy: a comparison in chronic wounds. Ostomy Wound Manage 2001;47(1):34–7. [PubMed] [Google Scholar]
- 51. Lipsky BA, Berendt AR, Deery HG et al. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2004;39(7):885–910. [DOI] [PubMed] [Google Scholar]
- 52. Gilbert P. Avoiding the resistance pitfall in infection control. Does the use of antiseptic products contribute to the spread of antibiotic resistance? Ostomy Wound Manage 2006;52(10A Suppl):1S–3S. [PubMed] [Google Scholar]
- 53. Facklam R, Washington J. Streptococcus and elated catalase‐negative gram‐positive cocci. In: Balows A, Hausler WJ, Jr , Herrmann KL, Isenberg HD, Shadomy HJ, editors. Manual of Clinical Microbiology. 5 ed. Washington, D.C.: American Society for Microbiology; 1991;p. 238. [Google Scholar]
- 54. Bucknall TE. The effect of local infection upon wound healing: an experimental study. Br J Surg 1980;67(12):851–5. [DOI] [PubMed] [Google Scholar]
- 55. Dow G, Browne A, Sibbald RG. Infection in chronic wounds: controversies in diagnosis and treatment. Ostomy Wound Manage 1999;45(8):23–40. [PubMed] [Google Scholar]
- 56. Davis E. Don't deny the chance to heal! Presented at the 2nd Joint Meeting of the Wound Healing Society and the European Tissue Repair Society, May 15‐19, 1996;Boston, MA. 5‐15‐1996. Ref Type: Abstract.
- 57. Potera C. Biofilms invade microbiology. Science 1996;273(5283):1795–7. [DOI] [PubMed] [Google Scholar]
- 58. Potera C. Forging a link between biofilms and disease. Science 1999;283(5409):1837–9. [DOI] [PubMed] [Google Scholar]
- 59. Schultz GS, Sibbald RG, Falanga V et al. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003;11(2 Suppl):S1–S28. [DOI] [PubMed] [Google Scholar]
- 60. Orsted H, Sibbald RG. A coordinated approach to chronic wound care. Ostomy Wound Manage 2001;47(10):6–8. [PubMed] [Google Scholar]
- 61. Robson MC, Duke WF, Krizek TJ. Rapid bacterial screening in the treatment of civilian wounds. J Surg Res 1973;14(5):426–30. [DOI] [PubMed] [Google Scholar]
- 62. Sumpio BE, Allie DE, Horvath KA et al. Role of negative pressure wound therapy in treating peripheral vascular graft infections. Vascular 2008;16(4):194–200. [DOI] [PubMed] [Google Scholar]
- 63. Ploumis A, Mehbod AA, Dressel TD, Dykes DC, Transfeldt EE, Lonstein JE. Therapy of Spinal Wound Infections Using Vacuum‐assisted Wound Closure: Risk Factors Leading to Resistance to Treatment. J Spinal Disord Tech 2008;21(5):320–3. [DOI] [PubMed] [Google Scholar]
- 64. Mehbod AA, Ogilvie JW, Pinto MR et al. Postoperative Deep Wound Infections in Adults After Spinal Fusion: Management with Vacuum‐Assisted Wound Closure. J Spinal Disord Tech 2005;18(1):14–7. [DOI] [PubMed] [Google Scholar]
- 65. Therapy Clinical V.A.C. Guidelines: A reference source for clinicians. 7‐1‐2007. Ref Type: Pamphlet.
- 66. Gwan‐Nulla DN, Casal RS. Toxic shock syndrome associated with the use of the vacuum‐assisted closure device. Ann Plast Surg 2001;47(5):552–4. [DOI] [PubMed] [Google Scholar]
- 67. DeFranzo AJ, Argenta LC, Marks MW et al. The use of vacuum‐assisted closure therapy for the treatment of lower‐extremity wounds with exposed bone. Plast Reconstr Surg 2001;108(5):1184–91. [DOI] [PubMed] [Google Scholar]
- 68. Gupta S, Gabriel A, Shores J. The perioperative use of negative pressure wound therapy in skin grafting. Ostomy Wound Manage 2004;50(4A Suppl):32–4. [PubMed] [Google Scholar]
- 69. Vuerstaek JD, Vainas T, Wuite J, Nelemans P, Neumann MH, Veraart JC. State‐of‐the‐art treatment of chronic leg ulcers: A randomized controlled trial comparing vacuum‐assisted closure (V.A.C.) with modern wound dressings. J Vasc Surg 2006;44(5):1029–38. [DOI] [PubMed] [Google Scholar]
- 70. Weed T, Ratliff C, Drake DB. Quantifying bacterial bioburden during negative pressure wound therapy. Does the wound VAC enhance bacterial clearance? Ann Plast Surg 2004;52(3):276–9. [DOI] [PubMed] [Google Scholar]
- 71. Moues CM, Vos MC, Van Den Bemd GJ, Stijnen T, Hovius SE. Bacterial load in relation to vacuum‐assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen 2004;12(1):11–7. [DOI] [PubMed] [Google Scholar]
- 72. Armstrong DG, Boulton AJM, Banwell P. Negative pressure wound therapy in treatment of diabetic foot wounds: a marriage of modalities. Ostomy Wound Manage 2004;50(4A):9–12. [PubMed] [Google Scholar]
- 73. Armstrong DG, Lavery LA, Abu‐Rumman P et al. Outcomes of subatmospheric pressure dressing therapy on wounds of the diabetic foot. Ostomy Wound Manage 2002;48(4):64–8. [PubMed] [Google Scholar]
- 74. Attinger CE, Bulan E, Blume PA. Surgical debridement. The key to successful wound healing and reconstruction. Clin Podiatr Med Surg 2000;17(4):599–630. [PubMed] [Google Scholar]
- 75. Leininger BE, Rasmussen TE, Smith DL, Jenkins DH, Coppola C. Experience With Wound VAC and Delayed Primary Closure of Contaminated Soft Tissue Injuries in Iraq. J Trauma 2006;61(5):1207–11. [DOI] [PubMed] [Google Scholar]
- 76. Song DH, Wu LC, Lohman RF, Gottlieb LJ, Franczyk M. Vacuum assisted closure for the treatment of sternal wounds: the bridge between debridement and definitive closure. Plast Reconstr Surg 2003;111(1):92–7. [DOI] [PubMed] [Google Scholar]
- 77. Reeves I, Plourde M, Authier S, Doddridge C. [Combined effect of Acticoat and VAC on wound healing]. Perspect Infirm 2005;2(5):10–5. [PubMed] [Google Scholar]
- 78. Melano E, Rodriguez HL, Carillo R, Dillon L. The effects of Panafil when using topical negative pressure to heal an infected sternal wound. J Wound Care 2004;13(10):425–6. [DOI] [PubMed] [Google Scholar]
- 79. Totaro P, Rambaldini M. Efficacy of antimicrobial activity of slow release silver nanoparticles dressing in post‐cardiac surgery mediastinitis. Interact Cardiovasc Thorac Surg 2008. [DOI] [PubMed] [Google Scholar]
- 80. Lumenta DB, Kamolz LP, Frey M. Adult Burn Patients With More Than 60% TBSA Involved‐Meek and Other Techniques to Overcome Restricted Skin Harvest Availability‐The Viennese Concept. J Burn Care Res 2009;30(2):231–42. [DOI] [PubMed] [Google Scholar]
- 81. Ambrosio A, Barton K, Ginther D. VAC GranuFoam Silver dressing ‐ A new antimicrobial silver foam dressing specifically engineered for use with the VAC therapy. White Paper , 1‐15. 1‐1‐2006. San Antonio, TX, Kinetic Concepts, Inc. Ref Type: Generic.
- 82. Fridkin SK, Gaynes RP. Antimicrobial resistance in intensive care units. Clin Chest Med 1999;20(2):303–16, viii. [DOI] [PubMed] [Google Scholar]
- 83. Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004;39(3):309–17. [DOI] [PubMed] [Google Scholar]
- 84. Diekema DJ, BootsMiller BJ, Vaughn TE et al. Antimicrobial resistance trends and outbreak frequency in United States hospitals. Clin Infect Dis 2004;38(1):78–85. [DOI] [PubMed] [Google Scholar]
- 85. McDonald LC. Trends in antimicrobial resistance in health care‐associated pathogens and effect on treatment. Clin Infect Dis 2006;42(Suppl 2):S65–S71. [DOI] [PubMed] [Google Scholar]
- 86. Gaynes R, Edwards JR, National Nosocomial Infections Surveillance System. Overview of nosocomial infections caused by gram‐negative bacilli. Clin Infect Dis 2005;41(6):848–54. [DOI] [PubMed] [Google Scholar]
- 87. Ovington LG. The truth about silver. Ostomy Wound Manage 2004;50(9A Suppl):1S–10S. [PubMed] [Google Scholar]
- 88. Driver VR. Silver dressings in clinical practice. Ostomy Wound Manage 2004;50(9A Suppl):11S–5S. [PubMed] [Google Scholar]
- 89. Brett DW. A discussion of silver as an antimicrobial agent: alleviating the confusion. Ostomy Wound Manage 2006;52(1):34–41. [PubMed] [Google Scholar]
- 90. Hall RE, Bender G, Marquis RE. Inhibitory and cidal antimicrobial actions of electrically generated silver ions. J Oral Maxillofac Surg 1987;45(9):779–84. [DOI] [PubMed] [Google Scholar]
- 91. Jones SM, Banwell PE, Shakespeare PG. Interface dressings influence the delivery of topical negative‐pressure therapy. Plast Reconstr Surg 2005;116(4):1023–8. [DOI] [PubMed] [Google Scholar]
- 92. Ip M, Lui SL, Poon VK, Lung I, Burd A. Antimicrobial activities of silver dressings: an in vitro comparison. J Med Microbiol 2006;55(Pt 1):59–63. [DOI] [PubMed] [Google Scholar]
- 93. Poon VK, Burd A. In vitro cytotoxity of silver: implication for clinical wound care. Burns 2004;30(2):140–7. [DOI] [PubMed] [Google Scholar]
- 94. Drosou A, Falabella A, Kirsner RS. Antiseptics on wounds: an area of controversy. Wounds 2003;15(5):149–66. [Google Scholar]
- 95. Cochrane CA, Walker M, Bowler P, Parsons D, Knottenbelt DC. The effect of several silver‐containing wound dressings on fibroblast function in vitro using the collagen lattice contraction model. Wounds 2006;18(2):29–34. [Google Scholar]
- 96. Poulakidas S, Kowal‐Vern A. Facilitating residual wound closure after partial graft loss with vacuum assisted closure therapy. J Burn Care Res 2008;29(4):663–5. [DOI] [PubMed] [Google Scholar]
- 97. Swezey L. Antimicrobial wound dressings ‐ silver. Ezine Articles. 7‐14‐2009. Ref Type: Generic.
- 98. Bhandari M, Schemitsch EH. High‐pressure irrigation increases adipocyte‐like cells at the expense of osteoblasts in vitro. J Bone Joint Surg Br 2002;84(7):1054–61. [DOI] [PubMed] [Google Scholar]
- 99. Wolvos T. Wound instillation ‐ The next step in negative pressure wound therapy. Lessons learned from initial experiences. Ostomy Wound Manage 2004;50(11):56–66. [PubMed] [Google Scholar]
- 100. Wolvos T. Wound instillation with negative pressure wound therapy. Ostomy Wound Manage 2005;51(2A Suppl):21S–6S. [PubMed] [Google Scholar]
- 101. Bernstein BH, Tam H. Combination of subatmospheric pressure dressing and gravity feed antibiotic instillation in the treatment of post‐surgical diabetic foot wounds: A case series. Wounds 2005;17(2):37–48. [Google Scholar]
- 102. Skulan TW, Hoppe JO. Preliminary observations on the toxicity of the hydrochloride and acetate salts of mafenide (sulfamylon r). Life Sci 1966;5(24):2279–82. [DOI] [PubMed] [Google Scholar]
- 103. McCauley RL, Linares HA, Pelligrini V, Herndon DN, Robson MC, Heggers JP. In vitro toxicity of topical antimicrobial agents to human fibroblasts. J Surg Res 1989;46(3):267–74. [DOI] [PubMed] [Google Scholar]
- 104. Cooper ML, Boyce ST, Hansbrough JF, Foreman TJ, Frank DH. Cytotoxicity to cultured human keratinocytes of topical antimicrobial agents. J Surg Res 1990;48(3):190–5. [DOI] [PubMed] [Google Scholar]
- 105. McCauley RL, Li YY, Poole B et al. Differential inhibition of human basal keratinocyte growth to silver sulfadiazine and mafenide acetate. J Surg Res 1992;52(3):276–85. [DOI] [PubMed] [Google Scholar]
- 106. Richards RM, Mahlangu GN. Therapy for burn wound infection. J Clin Hosp Pharm 1981;6(4):233–43. [DOI] [PubMed] [Google Scholar]
- 107. Klasen HJ. Historical review of the use of silver in the treatment of burns. I. Early uses. Burns 2000;26(2):117–30. [DOI] [PubMed] [Google Scholar]
- 108. Klasen HJ. A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver. Burns 2000;26(2):131–8. [DOI] [PubMed] [Google Scholar]
- 109. Wolvos TA. Advanced Wound Care with Stable, Super‐Oxidized Water. A look at how combination therapy can optimize wound healing. Wounds 2006;18(Suppl 1):11–3. [Google Scholar]
- 110. Wolvos T. Treatment of Diabetic Wounds with Negative Pressure Wound Therapy. In: Nassan S., editor. Pre‐ and Post‐Operative Services for the Amputee with Diabetes. American Diabetes Association; 2007;p. 87–93. [Google Scholar]
- 111. Labler L, Trentz O. V.A.C. instillation: in vitro model. Part 1. Biomed Tech (Berl) 2005;50(12):413–8. [DOI] [PubMed] [Google Scholar]
- 112. Labler L, Trentz O. V.A.C. instillation: in vitro model. Part 2. Biomed Tech (Berl) 2006;51(1):30–7. [DOI] [PubMed] [Google Scholar]
- 113. Moch D, Fleischmann W, Westhauser A. [Instillation vacuum sealing–report of initial experiences]. Langenbecks Arch Chir Suppl Kongressbd 1998;115:1197–9. [PubMed] [Google Scholar]
- 114. Kirr R, Wiberg J, Hertlein H. [Clinical experience and results of using the V.A.C. instill therapy in infected hip‐ and knee prosthetics]. Zentralbl Chir 2006;131(Suppl 1):S79–S82. [DOI] [PubMed] [Google Scholar]
- 115. Fleischmann W, Russ M, Westhauser A, Stampehl M. [Vacuum sealing as carrier system for controlled local drug administration in wound infection]. Unfallchirurg 1998;101(8):649–54. [DOI] [PubMed] [Google Scholar]
- 116. Timmers MS, Graafland N, Bernards AT, Nelissen RG, Van Dissel JT, Jukema GN. Negative pressure wound treatment with polyvinyl alcohol foam and polyhexanide antiseptic solution instillation in posttraumatic osteomyelitis. Wound Repair Regen 2009;17(2):278–86. [DOI] [PubMed] [Google Scholar]
- 117. Brem MH, Blanke M, Olk A et al. [The vacuum‐assisted closure (V.A.C.) and instillation dressing : Limb salvage after 3 degrees open fracture with massive bone and soft tissue defect and superinfection.]. Unfallchirurg 2008;111(2):122–5. [DOI] [PubMed] [Google Scholar]
- 118. Lansdown AB. Silver in health care: antimicrobial effects and safety in use. Curr Probl Dermatol 2006;33:17–34. [DOI] [PubMed] [Google Scholar]
- 119. Matsuno H, Yudoh K, Hashimoto M et al. A new antibacterial carrier of hyaluronic acid gel. J Orthop Sci 2006;11(5):497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Joseph TN, Chen AL, Di Cesare PE. Use of antibiotic‐impregnated cement in total joint arthroplasty. J Am Acad Orthop Surg 2003;11(1):38–47. [DOI] [PubMed] [Google Scholar]
- 121. Neut D, Van De Belt H, Van Horn JR, Van Der Mei HC, Busscher HJ. Residual gentamicin‐release from antibiotic‐loaded polymethylmethacrylate beads after 5 years of implantation. Biomaterials 2003;24(10):1829–31. [DOI] [PubMed] [Google Scholar]
- 122. Taggart T, Kerry RM, Norman P, Stockley I. The use of vancomycin‐impregnated cement beads in the management of infection of prosthetic joints. J Bone Joint Surg Br 2002;84(1):70–2. [DOI] [PubMed] [Google Scholar]
- 123. Rand RP, Cochran RP, Aziz S et al. Prospective trial of catheter irrigation and muscle flaps for sternal wound infection. Ann Thorac Surg 1998;65(4):1046–9. [DOI] [PubMed] [Google Scholar]
- 124. Willenegger H. A. die spul‐saugdrainage. Indikation, wirkungsweise und technik der spul‐saugdrainage. Aktuelle Probl Chir Orthop 1979;12:68–70. [PubMed] [Google Scholar]
- 125. Jones GA, Butler J, Lieberman I, Schlenk R. Negative‐pressure wound therapy in the treatment of complex postoperative spinal wound infections: complications and lessons learned using vacuum‐assisted closure. J Neurosurg Spine 2007;6(5):407–11. [DOI] [PubMed] [Google Scholar]
- 126. White RA, Miki RA, Kazmier P, Anglen JO. Vacuum‐assisted closure complicated by erosion and hemorrhage of the anterior tibial artery. J Orthop Trauma 2005;19(1):56–9. [DOI] [PubMed] [Google Scholar]
- 127. Patel NV, Shah RD, Welsh RJ, Chmielewski GW. Empyema–a complication of vacuum‐assisted closure of infected thoracotomy wounds in two consecutive cases. Am Surg 2009;75(4):349–50. [PubMed] [Google Scholar]
- 128. Chester DL, Waters R. Adverse alteration of wound flora with topical negative‐pressure therapy: a case report. Br J Plast Surg 2002;55(6):510–1. [DOI] [PubMed] [Google Scholar]
- 129. Sartipy U, Lockowandt U, Gabel J, Jideus L, Dellgren G. Cardiac rupture during vacuum‐assisted closure therapy. Ann Thorac Surg 2006;82(3):1110–1. [DOI] [PubMed] [Google Scholar]


