Abstract
A prospective, single‐centre, randomized controlled study was performed to evaluate the effectiveness of Graftjacket, a human acellular regenerative tissue matrix as a treatment option for chronic non healing lower extremity wounds. Twenty‐eight diabetic patients with full‐thickness wounds that had been present for at least 6 weeks were treated with sharp debridement and randomized to a single application of Graftjacket tissue matrix plus mineral oil‐soaked fluff compression dressing or to a control treatment of wound gel with gauze dressings. All patients were seen weekly. By week 16, 12 of 14 patients treated with Graftjacket tissue matrix demonstrated complete wound closure compared with 4 of 14 patients in the control group. Patients treated with Graftjacket tissue matrix showed a statistically significant higher percentage of wound healing with respect to wound area, and clinically significant differences in wound depth and wound volume. This comparison is not performed to demonstrate that the application of the Grafjacket is more effective than sharp debridement. This study is done to help assign a role to the use of Graftjacket matrix in lower extremity wound care.
Keywords: Chronic wounds, Diabetic foot ulcer, Human tissue matrix
Introduction
Management of ulcers in the diabetic foot can often present challenging and complex problems. It is estimated that 20·8 million people suffer from diabetes in the Unites States alone (1), and this number grows every year. Diabetic foot ulcers are a common problem for these patients. Approximately, 15% of the diabetic population will be afflicted with these slow‐to‐heal, frequently recurring ulcers at some point in their lifetime, and these ulcers remain the primary cause of hospitalizations for all diabetes patients.
Difficult to heal diabetic foot ulcers leave the patient susceptible to infection, which oftentimes leads to amputation. More than 60% of non traumatic lower‐limb amputations occur in people with diabetes (1), and 85% of amputations are preceded by ‘non healing’ foot ulcers (2). In 2002, about 82 000 non traumatic lower‐limb amputations were performed in people with diabetes (1), making successful and proper treatment of foot ulcers extremely important.
Open wounds occur when an injury to the foot such as a small cut or blister goes unnoticed, often secondary to peripheral neuropathy. This neuropathy affects the nerves of the feet and can lead to loss of sensation, making the feet more susceptible to damage. It is this loss of sensation that allows the patient to ignore the wound.
Treatment options for diabetic foot ulcers vary but most often include frequent debridement and dressing changes as well as proper off‐loading to remove pressure. Quick wound closure is desired to lower the incidence of amputation as well as recurrent ulceration. Advancements in tissue engineering have resulted in several graft options for the treatment of wounds that may achieve quicker results compared with conventional treatment.
Several human skin matrixes are available for the treatment of diabetic foot wounds. In a 12‐week study, 50% of patients treated with Dermagraft (Smith and Nephew, Largo, FL, USA) healed compared with 8% of patients treated with no administration of the cultured dermis tissue (3). Another randomized study showed 30% of patients healed with Dermagraft treatment compared with 18% of the control patients 4, 5. A 12‐week study using another tissue matrix, Graftskin (Apligraf; Organogenesis, Canton, MA, USA and Novartis Pharmaceuticals, East Hanover, NJ, USA), demonstrated that 56% of Graftskin‐treated patients healed compared with 38% for the control group (6). In all these studies, the foot ulcers were limited to Wagner grade‐1 foot ulcers (7). Over 50% of the patients treated with Graftskin required five applications of the tissue matrix 10, 11, 12. In the Dermagraft studies, up to eight applications of the tissue matrix were required (4). Because of the need for multiple applications and the limitation of the tissue matrix to superficial full thickness foot ulcers, an alternative tissue matrix was investigated.
Graftjacket (Wright Medical Technology, Inc, Arlington, TN, USA) is an acellular dermal graft that is derived from human tissue. It has been processed to remove the living cells and preserve an intact matrix that can be re‐vascularized and re‐populated by the recipient following transplantation. Extensive safety testing is performed to ensure graft safety and compliance to the requirement of the Food and Drug Administration (FDA) and the American Association of Tissue Banks (AATB).
A prospective, single‐centre, randomized, controlled study of 4‐week duration was performed to determine the effectiveness of this novel tissue matrix on various lower extremity wounds in diabetic patients (8). Full‐thickness wounds on various locations of the foot and leg were treated with either sharp debridement and Graftjacket tissue matrix or sharp debridement only to determine the safety and efficacy of this new matrix product. Four‐week data showed significant rates of wound reduction and closure compared with the control group. The percent change in wound area over a 4‐week period has been shown to be a good indicator of complete healing in a 12‐week study (9). It was therefore hypothesized that after 12 weeks, 18 of the 20 Graftjacket‐treated patients would be healed compared with two of the 20 debridement‐only‐treated patients.
This study reports the results of a 16‐week prospective, randomized, control trial to assess the safety and effectiveness of Graftjacket acellular tissue matrix on the treatment of full‐thickness (Wagner grade‐2) lower extremity wounds compared with sharp debridement‐only treatment.
Materials and Methods
Patients were enrolled after the study was discussed, and an informed consent was signed. Each patient underwent a complete medical history, a lower extremity physical examination and a full assessment of the wound. They were eligible for the study if they had a full‐thickness chronic wound for at least 6 weeks without epidermal coverage. In addition, the wound did not have evidence of active infection, and the patient had a palpable/audible pulse to the affected lower extremity. Patients were then randomized to one of two treatment groups, sharp debridement (weekly for duration of study) or sharp debridement followed by Graftjacket tissue matrix onlay.
Wound measurements were determined using wound tracing on wound film. Digital calipers were used for exact measurement of the tracing. Wound depth was evaluated using disposable sterile rulers. All measurements were taken at the point of greatest length, width and depth. Complete healing was defined as full epithelialization of the wound with the absence of drainage.
Patients in the control group were treated with sharp debridement using typical full‐thickness wound debridement technique. All necrotic tissue was removed from the wound, and a bleeding wound base was created. Curasol (Healthpoint Ltd, Forth Worth, TX, USA) wound gel was applied, and the wound covered with gauze dressings. Debridement occurred weekly through the duration of the study or until wound closure was achieved. Off‐loading of the wound was performed using an elastic‐wrapped walking boot with an insole cut out (13). Patients were seen weekly by the surgeon. Moist dressings were applied until the wound was considered healed. Off‐loading of the wound was continued until the wound was considered healed for 2 weeks. The patients were then fitted for custom‐molded diabetic shoes.
Surgical technique
Patients in the Graftjacket treatment group underwent similar sharp debridement. All necrotic tissue was removed from the wound, and a bleeding wound base was created. Once the wound was evaluated for length, width and depth, the GraftJacket was measured and cut to fit the defect. When cutting the graft, it was important to accommodate for the depth of the wound. The GraftJacket was then prepared for implantation. A 10‐minute bath in sterile saline was used to rehydrate the scaffold. The graft was noted to be ready for application, when the paper covering the basement membrane surface of the scaffold detached from the graft. The scaffold was then applied with the reticular surface (shiny side) against the wound bed. The basement membrane surface (dull surface) was superficial and exposed to the compression dressing. Using skin staples or sutures, the scaffold was circumferentially affixed to the wound margins, making sure to contour the entire wound bed with the scaffold. To maintain a moist environment for healing, a mineral oil‐soaked fluff compressive dressing was applied as the postoperative dressing. This dressing was changed and reapplied at days 5, 10 and 15. After day 15, the scaffold was assessed for the amount of granular incorporation. If the matrix was firmly incorporated into the wound, the area was covered with a dry sterile dressing and non adherent cover. In special circumstances, when it was determined that the scaffold was not firmly incorporated into the wound bed, a mineral oil‐soaked fluff compressive dressing was reapplied. The wound was reassessed 1 week later using the same criteria. The patient was also off‐loaded using the instant total contact cast technique described above (13).
Results
A total of 28 patients were enrolled in the study, with 14 patients in each treatment arm. All 28 patients completed the 16‐week study. Table 1 summarizes the demographics, ulcer location, size and age upon enrolment. All wounds were classified as Wagner grade‐2. There were no statistical differences between the two treatment arms with respect to age of the patient or the size (area, depth, and volume) of the starting ulcer using the Mann – Whitney rank sum test.
Table 1.
Patient demographics and wound location
| Statistic | Graftjacket (n = 14) | Debridement (n = 14) | P |
|---|---|---|---|
| Age | |||
| n | 14 | 14 | |
| Mean (SD) | 61·43 (7·18) | 66·21 (4·37) | 0·066 |
| Median | 63 | 66 | |
| Minimum, maximum | (42, 71) | (59, 73) | |
| HBA1C | |||
| n | 14 | 14 | 0·837 |
| Mean (SD) | 8·09 (0·98) | 7·89 (0·60) | |
| Median | 7·8 | 7·85 | |
| Minimum, maximum | (6·9, 10·1) | (7·1, 9·1) | |
| Foot | |||
| Right | 8 (57·14%) | 7 (50%) | 15 (53·57%) |
| Left | 6 (42·86%) | 7 (50%) | 13 (46·43%) |
| Location | |||
| Dorsal foot | 1 (7·14%) | 1 (3·57%) | |
| Dorsum 1st metatarsophalangeal joint | 1 (7·14%) | 1 (3·57%) | |
| Dorsum foot | 1 (7·14%) | 3 (21·43%) | 4 (14·29%) |
| Lateral ankle | 1 (7·14%) | 1 (3·57%) | |
| Lateral foot | 1 (7·14%) | 1 (3·57%) | |
| Lateral hindfoot | 1 (7·14%) | 1 (3·57%) | |
| Medial amputation site | 1 (7·14%) | 1 (3·57%) | |
| Medial ankle | 1 (7·14%) | 1 (7·14%) | 2 (7·14%) |
| Medial first ray | 2 (14·29%) | 2 (7·14%) | |
| Medial instep | 1 (7·14%) | 1 (3·57%) | |
| Medial leg | 1 (7·14%) | 1 (3·57%) | |
| Plantar foot | 3 (21·43%) | 2 (14·29%) | 5 (17·86%) |
| Plantar heel | 1 (7·14%) | 1 (3·57%) | |
| Plantar lateral foot | 1 (7·14%) | 1 (7·14%) | 2 (7·14%) |
| Plantar medial foot | 1 (7·14%) | 1 (3·57%) | |
| Submet | 1 (7·14%) | 1 (7·14%) | 2 (7·14%) |
| Transmetatarsal amputation stump | 1 (7·14%) | 1 (3·57%) | |
Figure 1a shows the average percentage of the wound area that healed each week between the debridement‐only group and Graftjacket‐treated group. Although the differences were significant, the area does not take into consideration the depth of the wound. Because full‐thickness ulcers can have significant differences in depth, the average decrease in the depth of the wound was also analysed (Figure 1b) as well as the reduction in wound volume (Figure 1c). The largest differences occurred when wound depths were taken into consideration. Clinically significant differences between the control‐treated group and the Graftjacket‐treated group were observed when considering wound reduction for length, width, area, depth and volume.
Figure 1.
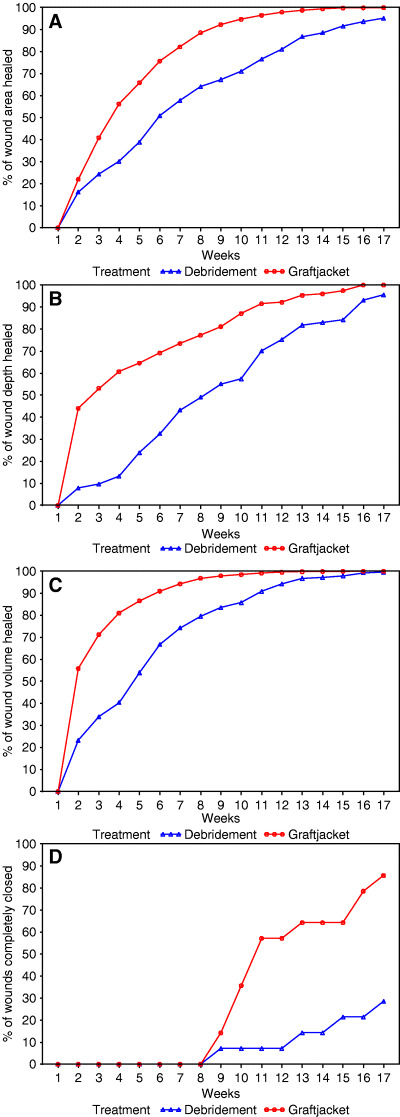
(A) Analysis of wound healing by study visit. The percentage of healing that was achieved by each study visit for the debridement‐only treatment group and the Graftjacket treatment group is shown. (A) Percentage of wound area healed, (B) percentage of wound depth healed, (C) percentage of wound volume healed, (D) percentage of patients with wounds completely closed as determined by compete epithelialization.
Complete wound closure was defined as complete epithelialization without drainage. Figure 1d shows the percentage of wounds completely healed through the treatment course. Twelve of 14 patients treated with Graftjacket were healed by 16 weeks. Only four patients in the control group were healed by 16 weeks. The average time for Graftjacket patients to heal was 11·92 weeks and 13·50 weeks for the control. There was not a relationship between the volume or depth and the ulcers that healed. Statistical analyses on the healing parameters were performed using the Mann – Whitney rank sum test. The results are presented in Table 2. Statistical significance with a P ≤ 0·001 was obtained for the final ulcer area, depth, volume and the number of ulcers healed in the favour of the Graftjacket treatment arm.
Table 2.
Comparison of wound dimensions at the end of the trial
| Parameter | Graftjacket (n = 14) | Debridement (n = 14) | P |
|---|---|---|---|
| Final ulcer area | |||
| Mean (SD) | 1·00 (2·57) | 31·14 (43·74) | 0·005 |
| Median | 0·0 | 7·0 | |
| Minimum, Maximum | (0, 8) | (0, 132) | |
| Final ulcer depth | |||
| Mean (SD) | 0·00 (0·00) | 0·21 (0·43) | 0·090 |
| Median | 0 | 0 | |
| Minimum, Maximum | (0, 0) | (0, 1) | |
| Final ulcer volume | |||
| Mean (SD) | 0·00 (0·00) | 13·50 (36·43) | 0·091 |
| Median | 0·0 | 0 | |
| Minimum, Maximum | (0, 0) | (0, 132) | |
| Ulcer healed | |||
| Total healed | 12 (85·71%) | 4 (28·57%) | 0·006 |
| Depth healed | 14 (100·00%) | 11 (78·57%) | 0·222 |
| Time to healing (weeks) | 11·92 (2·87) | 13·50 (3·42) | |
Completely healed: depth = 0 and width = 0 and length = 0
Adverse events from all 28 randomized patients were evaluated. None of the patients in this study experienced a systemic infection that required intravenous antibiotic treatment or hospital stay. Infection at the wound site such as peri‐wound erythema or local cellulitis occurred in five patients from the debridement‐only treatment group and three patients from the Graftjacket treatment group. This is consistent to other studies, where 17–50% of treated patients, regardless of treatment regime, developed some form of infection at the site of the ulcer 3, 4, 5, 6, 9, 10.
A mild seroma formation at the first visit in a patient receiving a Graftjacket application was noted. The seroma was aspirated, and it was resolved by the next week. It is unclear if the seroma was due to a mild allergic reaction to the tissue matrix or a result of the sharp debridement treatment.
There were minimal complications observed with respect to the application and treatment of the tissue matrix. It was noted that the margins of the graft were dry in one patient after week 1. Another patient had distal and proximal grafts slightly dry after week 2, and a third patient's graft was very dry at the week 3 visit. It was also noted that one graft was over moisturized at week 2. The healing of the wound was not compromised in these patients. Additional graft applications were not required for any of the patients in the study.
Discussion
The treatment of diabetic extremity wounds is a challenging medical problem. As the United States' population ages and continues a trend towards higher incidences of obesity, the treatment of complications associated with diabetes will consume more and more of health care cost. While educating diabetics on the potential medical issues of this disease is a worthwhile goal, it is also necessary to develop treatment modalities to reduce the risks of severe extremity wounds that lead to amputation. Rapid treatment of foot ulcers at their beginning stages can potentially eliminate ulcer progression. However, because of peripheral neuropathy, the presence of a significant wound is not noted in the patient until extensive damage to the surrounding tissue has taken place. For this reason, it is important to identify treatment regimes that can reverse ulcer progression as rapidly as possible.
In this prospective clinical trial, we have investigated the safety and efficacy of Graftjacket acellular tissue matrix for the treatment of various diabetic extremity wounds. Although ulcers on the plantar surface are the most difficult to treat due to off‐loading issues, an attempt was made to mimic the range of extremity wounds that would be seen by a surgeon who have diabetic patients. The wound locations varied from the plantar surface of the foot, the ankles and legs, and to wounds on amputation stumps. The incidence rates of adverse events were comparable between the treatment arms and were similar to levels seen in other studies involving diabetic ulcers. No complications specifically associated with the use of the Graftjacket tissue matrix were noted.
The study demonstrates that the use of Graftjacket tissue matrix in conjunction with sharp debridement results in faster healing of lower extremity ulcers than the use of sharp debridement only. Other skin substitutes have been shown to outperform sharp debridement. Graftskin was 56% effective in healing Wagner grade‐I foot ulcers (6), while Dermagraft was 53–71% effective in healing similar‐sized wounds 3, 4, 9. By contrast, Graftjacket does have the ability to treat non infected wounds with exposed bone, tendon and ligament, as well as wounds only through skin and subcutaneous tissue.
Conclusion
Patients with chronic ulcers of various aetiologies who were treated with Graftjacket, a human acellular regenerative tissue matrix, showed a statistically significant higher percentage of wound closure by week 16 than the patients treated with sharp debridement only. The author recognizes that comparing these treatment arms can lead to questions due to the extreme differences in treatment technique, however, this study was performed to assess whether the Graftjacket scaffold can be used as a valuable tool when treating difficult‐to‐heal lower extremity wounds. It is the authors' belief that this scaffold is a safe and viable tool when treating wounds with extreme size and depth. The way by which this product allows for healing also has raised questions by the author. Because this scaffold causes regeneration of the subcutaneous tissue with complete incorporation of the scaffold into the host tissue, the author hypothesizes that this may lead to a better quality of newly formed tissue, decreasing the chance of re‐ulceration. A long‐term study would be beneficial in this assessment.
Findings from this study strongly support the hypothesis that Graftjacket tissue matrix is a safe and an effective treatment for lower extremity wounds, regardless of location or depth.
Acknowledgement
Stephen A. Brigido, DPM is a consultant for Wright Medical Technology.
References
- 1. www.ada.org.
- 2. Sanders LJ, Reiber GE. Diabetic Foot Ulcers and Amputations. American Diabetes Association, Inc, 2001. [Google Scholar]
- 3. Gentzkow GD, Iwasaki SD, Hershon KS, Mengel M, Prendergast JJ, Ricotta JJ, Steed DP, Lipkin S. Use of dermagraft, a cultured human dermis, to treat diabetic foot ulcers. Diabetes Care 1996;19:350–4. [DOI] [PubMed] [Google Scholar]
- 4. Marston WA, Hanft J, Norwood P, Pollak R. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care 2003;26:1701–5. [DOI] [PubMed] [Google Scholar]
- 5. Grey JE, Lowe G, Bale S, Harding KG. The use of cultured dermis in the treatment of diabetic foot ulcers. J Wound Care 1998;7:324–5. [DOI] [PubMed] [Google Scholar]
- 6. Veves A, Falanga V, Armstrong DG, Sabolinski ML. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care 2001;24(290–295):2001. [DOI] [PubMed] [Google Scholar]
- 7. Brigido SA, Boc SF, Lopez RC. Effective management of major lower extremity wounds using an acellular regenerative tissue matrix: a pilot study. Orthopedics 2004;27:s145–9. [DOI] [PubMed] [Google Scholar]
- 8. Wagner FW. The dysvascular foot: a system for diagnosis and treatment. Foot Ankle 1981;2(2):64–122. [DOI] [PubMed] [Google Scholar]
- 9. Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4‐week period is a robust predictor of complete healing in a 12‐week prospective trial. Diabetes Care 2003;26:1879–82. [DOI] [PubMed] [Google Scholar]
- 10. Hanft JR, Surprenant MS. Healing of chronic foot ulcers in diabetic patients treated with a human fibroblast‐derived dermis. J Foot Ankle Surg 2002;41:291–9. [DOI] [PubMed] [Google Scholar]
- 11. Falanga V, Sabolinski M. A bilayered living skin construct (APLIGRAF) accelerates complete closure of hard‐to‐heal venous ulcers. Wound Repair Regen 1999;7:201–7. [DOI] [PubMed] [Google Scholar]
- 12. Veves A, Falanga V, Armstrong DG, Sabolinski ML. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathis diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care 2001;24: 290–5. [DOI] [PubMed] [Google Scholar]
- 13. Armstrong DG, Short B, Espensen EH, Abu‐Rumman PL, Nixon BP, Boulton AJ. Technique for fabrication of an ‘instant total‐contact cast’ for treatment of neuropathic diabetic foot ulcers. J Am Podiatr Med Assoc 2002;92(7):405–8. [DOI] [PubMed] [Google Scholar]


