Abstract
The aim of this study was to assess the performance of Opsite™ Post‐Op Visible as a post‐surgical dressing in a typical clinical setting. In this multi‐centre clinical evaluation, patients who underwent clean surgery were treated with Opsite Post‐Op Visible dressing. Duration of dressing wear, visibility through the dressing and ability to handle exudate were assessed and the product was rated in comparison with those normally used. A total of 64 patients were recruited. Mean wear time was 4·5 days. Exudate management was rated very good or good at 96% of assessments. Visibility of the incision site was rated as very good or good at 72%, and as acceptable at 24%, of assessments. Patient comfort was rated very comfortable (63%) or comfortable (37%) at all assessments. Dressings were generally rated as satisfactory or exceeding expectations with clinicians stating that the Opsite Post‐Op Visible dressing was better than the dressing they routinely used for 92% of patients. Opsite Post‐Op Visible dressing is an innovative dressing combining good visibility with exudate management and patient comfort. It was found to have adequate wear time, visibility and exudate management properties making it suitable for use on a variety of surgical incision sites.
Keywords: Dressings, Exudate, Opsite, Surgical site infections, Visibility
INTRODUCTION
The National Institute for Health and Clinical Excellence (NICE) estimates that there are 4 million operations performed per year in the UK. At least 5% of these procedures will give rise to a surgical site infection (SSI) (1). However, in the UK, infection rates vary greatly between different types of surgery, ranging from 1% to 2%, such as abdominal hysterectomy, knee and hip prosthesis to 10% and over for surgery involving the bowel, limb amputation and surgery of the liver, pancreas and bile duct (2).
SSIs can have a significant effect on a patient's quality of life, a higher risk of morbidity, prolonged stay in hospital and higher hospital costs. In the USA, patients with SSIs were found to have a higher mortality [relative risk (RR) 2·2], higher rate of readmission (RR 5·5), higher length of stay (mean 12 days per patient) with higher associated costs (mean $5038 per patient at 1991–1995 prices) (3). In the UK, patients with an SSI had an extra length of hospital stay of 11 days (4). NICE estimates that, at an infection rate of 5% and an estimated cost of £3500 per episode, the cost to the National Health Service (NHS) of managing SSIs is around £700 million (1).
Therefore, it is important for clinicians to be able to regularly inspect and monitor post‐operative surgical incision sites to ensure that they remain free from infection and other complications. The NICE clinical guidance on SSIs states that ‘the main purposes of surgical dressings are to allow appropriate assessment of the wound post‐operatively, to absorb exudates, to ease pain and to provide protection for newly formed tissue’. Traditional dressings such as gauze are to be avoided because of the disruption of healing tissue when the dressing is changed. In addition to the disruption caused at scheduled dressing changes, dressings are often removed at least once a day to enable various members of the clinical team to inspect the wound site.
Opsite Post‐Op Visible (Opsite™ is a trademark of Smith & Nephew) is a waterproof, bacteria‐proof dressing with a see‐through absorbent pad which was developed to allow clinicians to inspect the incision site and peri‐wound area without having to lift or remove the dressing. The dressing is made up of a see‐through lattice of highly absorbent Allevyn™ foam which provides good exudate handling properties without obscuring the wound bed below.
The aim of this study was to assess the performance of Opsite Post‐Op Visible in a typical clinical setting with particular reference to duration of wear, visibility of the incision site, absorbency, comfort and conformability.
MATERIALS AND METHODS
This multi‐centre clinical evaluation was performed between March 2008 and January 2009. A collection of case studies were documented using a standard data collection form, allowing the data to be pooled and summarised to give an insight into the uses and performance of the dressings in a clinical setting on multiple surgical wound types.
Patients were recruited prospectively from the adult (≥18 years of age) population routinely seen by clinicians participating in the study. Eligible patients had undergone a clean surgery [as defined by the National Nosocomial Infection Surveillance (NNIS) programme] and a surgical procedure in the operating room (OR) with a surgical incision of less than 18 cm. They were expected to remain in‐patients for at least 48 hours following surgery.
The NNIS defines clean surgery as ‘Uninfected operative wounds in which no inflammation is encountered, and respiratory, alimentary, genital, or uninfected urinary tracts are not entered. In addition, clean wounds are primarily closed and, if necessary, drained with closed drainage’.
Patients with any signs of infection at the incision site were excluded, as well as the patients who were undergoing treatment which is likely to impair wound healing – such as chemotherapy or radiation. Also excluded were patients with disorders which are likely to impair healing.
Ethics approval was not sought as the evaluation involved no change to patient treatment, but informed consent was obtained from all the patients and the study was conducted in accordance with basic ethical principles, with all data collected and stored confidentially.
The primary objective was to measure the duration of dressing wear, and the secondary objectives were to assess visibility of the wound through the dressing and the ability of the dressing to handle exudate. Other secondary objectives were to assess factors relating to patient acceptability and ease of use: condition of surrounding skin, patient comfort, dressing conformability, ease of application and removal, and factors relating to infection: clinical signs and incidence of infection at the surgical site. The number of dressings applied was monitored, and participating clinicians were asked to rate the dressing in comparison with those normally used and to grade their satisfaction with the dressing performance.
Treatment regimen
The incision site was treated according to local protocol, and the Opsite Post‐Op Visible dressing was applied according to the standard centre protocols following manufacturer's instructions. Ancillary products used to treat the incision site were recorded.
Dressings were to be removed only if dictated by clinical need, local hospital protocol or the clinical judgement of the investigator. The end of evaluation was dictated by: no further requirement for dressings, end of evaluation period (21 days after the surgical procedure) and discharge or withdrawal of the patient.
Patients were withdrawn if the test product was not in place for 3 days or more; if it was necessary to change the dressing type on clinical grounds; if the clinician believed it was necessary (e.g. because of adverse events, poor compliance); or if the patient requested withdrawal.
There were no restrictions on concomitant medications or treatments.
Assessments
Baseline demographic characteristics were recorded, along with details of the surgical procedure to be carried out and the closure method.
Assessments were carried out at every dressing assessment, whether the dressing was changed or not, and data relating to each of the objectives specified above were collected. When the dressing was changed clinicians were asked to record the exudate level (none, slight, moderate or heavy), signs of infection and the condition of surrounding skin (healthy, inflamed, macerated, dry and flaky or other) both pre‐ and post‐dressing removal, so that the visibility (very good, good, acceptable, poor or very poor) of the dressing could be assessed. Additionally, patient comfort (very comfortable, comfortable, acceptable, uncomfortable, very uncomfortable), level of pain experienced by the patient at the removal of the dressing (none, mild, moderate or severe), whether or not the dressing conformed to the skin, and whether it was easy to apply or remove were recorded. At the end of the evaluation, satisfaction with the performance of the dressing (exceeded expectations, satisfied or dissatisfied) and comparison with the clinician's usual dressing (better, same or worse) were recorded in accordance with various performance parameters.
Total cost per patient was calculated as total dressing costs for each patient. Total cost per patient per week was calculated using the formula: (total dressing cost × 7)/evaluation duration.
Statistics
All data summaries and statistical analysis were conducted using SAS version 9·1. Cochran‐Mantel‐Haenszel test stratified by patients was used to test for the difference in the level of exudate between baseline and final assessment. Statistical tests were two‐sided and conducted at the 5% significance level.
RESULTS
Patient and wound characteristics
A total of 64 patients from four centres in Belgium and Ireland were recruited into the study. Three patients (5%) were withdrawn during the study at their own request, 20 (31%) did not require any further dressing changes and 41 (64%) were discharged from hospital. There were six protocol violations throughout the study. All were related to the patient having a surgical incision length greater than 18 cm. In five of these patients, this was because of treating more than one surgical incision site. These patients were dressed with more than one evaluation dressing.
The mean age of patients was 61·1 years (range: 21–95) and 55% were male. Mean height was 1·7 m (range: 1·5–2·0) and mean weight was 85·2 kg (range: 44–187).
Most of the patients had undergone abdominal surgery (26; 41%), with the remainder having undergone vascular surgery (11; 17%), orthopaedic surgery (11; 17%) or cholecystectomy (6 laparoscopic, 1 open; 11%). ‘Other’ surgery (9; 14%), where known, included mastopexy, hernia, neurological, removal of lipomas and laparoscopic appendectomy.
The surgical incision site was located on the abdomen (35; 55%), head/neck (2; 3%), chest (3; 5%), back (3; 5%), groin and legs (21; 33%); 18 (49%) were on the left side, 17 (46%) on the right and the remaining 2 (5%) on both left and right. The mean estimated length of surgical incision was 12 cm (range: 2–54). The most common method of closure was sutures (37; 58%) or staples (27; 42%).
Primary objective
Wear time
The mean wear time (per patient) was 4·5 days (range: 0–18) – see Table 1.
Table 1.
Mean wear time
| Mean wear time (days) | Orthopedic | Abdominal | Vascular | Cholecystectomy | Other/Unknown | Total |
|---|---|---|---|---|---|---|
| Mean | 5·5 | 3·2 | 1·9 | 8·3 | 7·3 | 4·5 |
| Median | 4·5 | 3 | 2 | 9 | 8 | 4 |
| Min | 2 | 0 | 1 | 7 | 1 | 0 |
| Max | 18 | 6 | 3·3 | 9 | 10 | 18 |
| N | 11 | 26 | 11 | 7 | 9 | 64 |
Secondary objectives
Reason for dressing change
Where the dressing was changed, the reason is given in Table 2.
Table 2.
Reason for dressing change
| Reason for dressing change | Total (%) |
|---|---|
| Incision site not visible | 2 (2·6%) |
| Dressing saturated | 8 (10·5%) |
| Strike‐through | 2 (2·6%) |
| Leakage | 1 (1·3%) |
| Suture/Staple removal | 17 (22·4%) |
| Patients request | 6 (7·9%) |
| Surgeons request | 7 (9·2%) |
| Surgeons request and incision site not visible | 1 (1·3%) |
| Routine inspection of the wound before discharge | 22 (28·9%) |
| Routine inspection of the wound before discharge and incision site not visible | 1 (1·3%) |
| Dressing non conforming and Strike‐through | 1 (1·3%) |
| Other | 8 (10·5%) |
| N | 76 (100%) |
Visibility
Overall, the visibility of the incision site through the evaluation dressing was rated by the clinicians to be very good or good at 98/136 (72%) assessments, acceptable at 33 (24%) and poor at 5 (4%) assessments. Where the visibility was rated as poor, the clinician commented that this was because of exudate.
Exudate management and level of exudate
Overall, exudate management was rated as very good or good at 127/133 (96%) assessments and acceptable at 3 (2%). The remaining 3 (2%) were rated as poor.
There was a significant reduction in the level of exudate from baseline to final assessment (P < 0·001); 48/50 (96%) had no exudate at the final assessment compared with 32/64 (50%) at baseline.
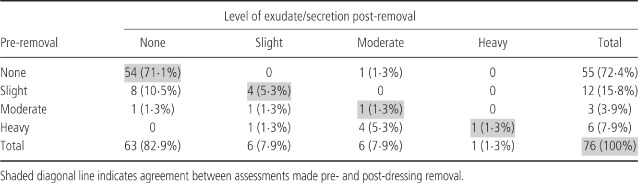
The level of exudate pre‐ and post‐dressing removal is shown in Table 3. The pre‐ and post‐removal assessment of the level of exudate or secretion was in agreement in 60/76 (79%) assessments.
Table 3.
Level of exudate/secretion pre and post‐dressing removal
Signs of infection
With the exception of local erythema and ‘other’ signs (haematoma, redness at the insertion point of staples), there were no clinical signs of infection observed either pre‐ or post‐dressing removal. The clinician's judgement of the presence of signs of infection was in agreement pre‐ and post‐removal at 74/78 (95%) assessments (Table 4).
Table 4.
Clinical signs of infection
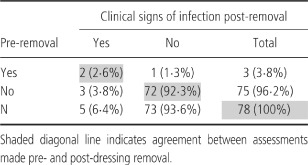
Where there were some clinical signs of infection after removal of the dressing, the clinician had recorded these before removal of the dressing in 2/5 (40%) cases. In the other three cases, local erythema was noted post‐removal.
Condition of surrounding skin
The clinician's judgement of the condition of the surrounding skin was in agreement pre‐ and post‐removal at 72/78 (92%) assessments for healthy skin, 76 (97%) for inflamed skin, 78 (100%) for macerated skin, 78 (100%) for dry and flaky skin, and 75 (96%) for other skin conditions.
Comfort and conformability
Patient comfort during wear was rated to be either very comfortable (86/136; 63%) or comfortable (50; 37%) at all assessments. All of the dressings conformed to the area around the incision site upon dressing application. During wear, the majority of dressings had conformed between assessments (132/135; 98%). Where the dressing had not conformed, the surgical sites were located on the groin, abdomen and a combination of groin, and upper and lower leg.
Pain at dressing removal
Patients reported no pain at 58/76 (76%) of dressing removals. At the remaining dressing removals, patients experienced mild pain (18; 24%).
Ease of use
All of the dressings were rated as easy to apply (58/58; 100%) and remove (76/76, 100%).
Overall product performance
With the exception of one patient, the dressings were rated exceeding expectations (37/64; 58%) or satisfactory (26; 41%). In one case (2%) the clinician was dissatisfied with performance because of inadequate absorption capacity.
Figure 1 shows the satisfaction ratings for each product performance characteristic. For the majority of patients, clinicians rated the evaluation dressings as satisfactory or exceeding expectations in all parameters measured.
Figure 1.
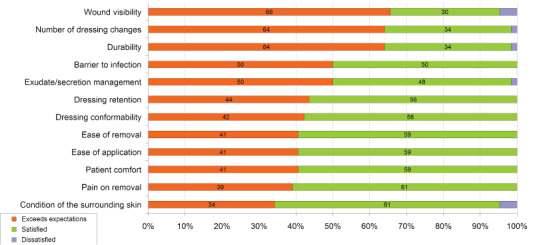
Product performance characteristics.
Comparison with usual dressing
Clinicians rated the dressing as better overall than the dressing routinely applied in 59 out of 64 patients (92%), the same in four patients (6%) and worse than usual in just one (2%) patient.
In most cases, clinicians rated the Opsite Post‐Op Visible dressing as better or similar in all parameters measured (Figure 2).
Figure 2.
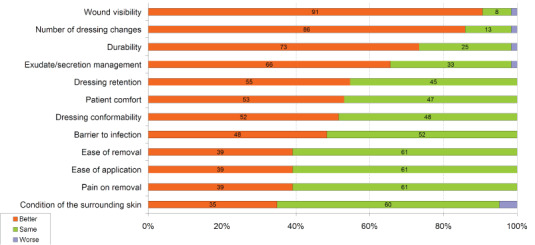
Product performance compared with usual dressing.
For the majority of patients (52/64; 81%), clinicians stated that film and pad dressings would have been routinely used to treat the type of incision site assessed prior to the evaluation. For the remaining patients, non woven dressings (9; 14%), hydrocolloid (1; 2%) and other (2; 3%) would have been routinely used.
Health economic considerations
The mean dressing cost per patient was £1·87 (range: £0·83–£9·20). Cholecystectomy patients had the lowest mean cost per patient of £0·83 (range: £0·83–£0·83) and vascular surgery patients had the highest of £3·72 (range: £1·15–£9·20).
The mean evaluation dressing cost per week was £2·99 (£0·45–£11·62), with cholecystectomy patients having the lowest cost (£0·71, range: £0·65–£0·83) and vascular surgery patients the highest (£5·57, range: £1·74–£10·73). The cost of Opsite Post‐Op Visible was taken from the NHS supply chain catalogue (April 2009).
Safety
Four product complaints from four patients were reported in this study; all were related to an imprint of the matrix foam pad on the skin. This was thought to be because of post‐surgical swelling, and no skin breaks or blistering were reported. There were no safety concerns with the products.
DISCUSSION
NICE clinical guidelines on the management of SSIs recommend that incisions because of heal by primary intention should be ‘covered with a film membrane, with or without a central absorbent pad’(1). The intention clearly is to provide a barrier between the skin and the environment to help reduce contamination by exogenous or endogenous microbes. In this respect, Opsite Post‐Op Visible has an advantage over traditional dressings such as gauze, or non woven products like Mepore™ (Mepore™ is a trademark of Molnlycke Health Care), as the film has been shown to provide a bacterial barrier to most common pathogens as well as meathicillin resistant staphylococcus aureus (MRSA) 5, 6, 7, 8.
The guidelines also emphasise the importance of being able to assess the wound post‐operatively, to absorb exudate, ease pain, and provide protection for developing tissue. Ideally dressings should also maintain a moist wound environment, while avoiding maceration of the surrounding skin (1).
Opsite Post‐Op Visible dressing has been designed with a highly absorbent foam layer, featuring a lattice structure that allows clinicians to see through the dressing to the skin surface below. In this study, the majority of dressings were left in place for a number of days (mean per patient 4·5 days) with the surgeon inspecting the incision site through the dressing, without lifting or removing it. Most dressing removals were routine and performed because the surgeon needed to inspect the wound before discharging the patient, or because staples and sutures required removal. This could suggest that the potential wear time of Opsite Post‐Op Visible dressings may be greater, because the majority of dressings were not changed because of the clinical need or issues with visibility.
The visibility of a dressing has an impact on the costs of care. Traditional dressings such as sterile gauze and Mepore™ have a lower product cost but require replacement each time they are removed to allow inspection of the wound. Using dressing prices taken from the NHS supply chain catalogue, and a conservative estimate for wear time of non barrier dressings of one day (taken from the most recent NICE guidelines on prevention and treatment of SSIs (1)), it has been possible to estimate the resource impact of using Opsite Post‐Op Visible dressing as the first‐line dressing for surgical incision sites, compared with that for a standard film and pad dressing (Tegaderm™+ pad, Tegaderm™ is a trademark of 3M Healthcare), Mepore™ and sterile gauze.
Nursing time per dressing change was also taken from the NICE guidelines, where it was estimated that dressing changes took 10 minutes at a cost per hour of £22. Over a 10‐day treatment period, both Opsite Post‐Op Visible dressing and a standard film dressing would be more cost effective than either of the passive dressings (saline soaked gauze): £10–£11 compared with £38–£39. A typical UK acute hospital performing around 1000 surgical procedures annually could therefore expect to save in the region of 1300 nurse hours, at a cost of around £28,500.
The visibility of the dressing allows the clinician to see through the wound and peri‐wound area without removing it, thus eliminating the possibility that this could cause a disturbance to the healing of wound bed. This also minimises exposure to the environment, reducing opportunities for colonisation by microbes. However, with Opsite Post‐Op Visible dressing transparency has not been gained at the expense of absorbency. Film dressings allow inspection of the wound site, but are unable to handle exuding wounds; while previous absorbent dressings do not offer the facility of transparency. This evaluation confirmed that the Opsite Post‐Op Visible dressing allows inspection of the wound, without the visibility being impaired by the presence of exudate.
Comments from clinicians highlighted the benefits of the dressings for both surgeons and nurses: ‘As Opsite Post‐Op Visible dressing allows visibility of the incision site, there is no need to remove the dressing for inspection, thus prolonging the wear time as well as being a valuable tool in preventing infections'. With occlusive dressings, it is necessary to lift the dressing in order to inspect a surgical incision site for signs of infection. Good clinical practice and infection management protocols dictate that if a dressing is lifted, it should then be replaced. In this evaluation study, clinicians found that it was possible in most cases to inspect the incision site through the dressing.
Overall, these results suggest that Opsite Post‐Op Visible dressing is suitable for the treatment of post‐operative wounds, and it can eliminate the need to lift or remove the dressing for visual inspection of the wound and peri‐wound skin. This will help avoid unnecessary dressing changes that could offer a number of benefits: unnecessary disturbance to the wound will be reduced, as will the potential for exposure to infection; the patient will experience less discomfort and inconvenience, and increased wear time will provide economic benefits for the purchaser.
CONCLUSION
In this study, Opsite Post‐Op Visible was successfully used on a variety of surgical wound types and locations, and clinicians stating that the Opsite Post‐Op dressing was better than the dressing they routinely used in 92% of patients.
ACKNOWLEDGEMENT
This study was funded by Smith & Nephew Wound Management.
REFERENCES
- 1. Clinical Guideline. Surgical site infection: prevention and treatment of surgical site infection. London: National Institute for Health and Clinical Excellence, 2008. [Google Scholar]
- 2. Health Protection Agency. Surveillance of surgical site infection in England October 1997–September 2005. London: Health Protection Agency, 2006. [Google Scholar]
- 3. Kirkland K, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical‐site infections in the 1990s: attributable mortality, excess length of hospitalisation and extra costs. Infect Control Hosp Epidemiol 1999;20:725–30. [DOI] [PubMed] [Google Scholar]
- 4. Coello R, Charlett A, Wilson J, Ward V, Pearson A, Borriello P. Adverse impact of surgical site infections in English hospitals. J Hosp Infect 2005;60:93–103. [DOI] [PubMed] [Google Scholar]
- 5. Smith D. An in‐vitro assessment of the bacterial properties of Allevyn adhesive. Report reference 060722. Data on file. Hull, UK: Smith & Nephew, 2006. [Google Scholar]
- 6. Bacterial barrier testing of Opsite™ Post‐Op Visible dressings against MRSA. Report reference WRP‐TWO42‐361. Data on file. Hull, UK: Smith & Nephew, 2004. [Google Scholar]
- 7. Hammond V. Opsite™ Post‐Op Visible evaluation of product from PQ batches. Report reference DS/07/209. Data on file. Hull, UK: Smith & Nephew, 2007. [Google Scholar]
- 8. Foster D. Bacterial barrier properties of dressing top films. Data on file. Hull, UK: Smith & Nephew, 2007. [Google Scholar]


