Abstract
Disordered cell function within chronic wounds generates many parameters that can be measured to differentiate between healing and non healing status. Theoretically, these may form the basis of a wound assessment system to define disease severity and response to treatment. In a review of tissue, wound exudate and microbiology studies of venous leg ulcers, we identify many such parameters that are associated with healing status. These include cytokines, proteases and their inhibitors, senescence markers, oxidative stress markers and microbiological status defined by culture. Some of these, such as protease level in wound exudate, have been proposed as prognostic indicators of healing status and many more could be considered potential markers to incorporate into a wound assessment system. However, no published data are available that validate known wound components to accurately reflect wound progression on a single patient basis. Rather than further characterisation of the expression of known wound biomarkers, the development of an accurate and objective test for prediction of chronic wound outcome requires identification of an appropriate combination of novel molecules that vary coordinately with healing status.
Keywords: Chronic wound, Diagnostic test, Healing outcome, Prognostic test, Venous leg ulcer
Introduction
Treatment of chronic wounds such as venous leg ulcers (VLUs), diabetic foot ulcers (DFUs) and pressure ulcers presents a significant challenge to patient and health care systems (1). Although many patients with chronic wounds will respond to appropriate standard care (2), a significant number require adjunctive therapies to achieve wound closure. In order to maximise the benefit of such treatment, it would be of value to identify hard‐to‐heal wounds prior to commencing treatment and also to be able to objectively identify non responding wounds early in treatment. Some progress towards these objectives has been made by developing algorithms that relate initial wound area to wound duration (3) and wound area change (4) during treatment. Using these inputs, it has proven possible to identify potential or actual non responsive wounds, with a prediction rate accuracy of 75%. However, methods used to measure wound area require operator skill, and it is still not widely adopted in routine practice (5).
The potential exists to develop alternative objective assessment methodology utilising samples collected from the wound surface to measure biomarkers (see Box) whose concentration varies and can be related to subsequent healing outcome.
Biomarker
In the context of this review, a biomarker may be defined as a substance that can be measured and used to indicate clinical status and response to treatment. Examples from other pathologies are blood glucose for monitoring diabetes or the enzymes alanine transaminase and aspartate transaminase, which are measured as components of a liver function test.
Such a test system would be analogous to other pathologies such as diabetes where an understanding of disease pathology allows measurement of molecules that are linked to disease progression and control of the disease.
Chronic wounds have been the subject of many studies where they are compared with healing wounds. This has generated a large body of literature where the data provided demonstrate many differences between the healing and the non healing wound microenvironment (6). These data may be used to develop models of chronic wound pathogenesis and hypotheses describing which factor(s) may be involved in prolonging the chronic non healing wound. If achieved, the identification of such key factors may be of value in developing novel bioactive therapies and analytical tools for predicting and monitoring healing outcome. However, despite ever more sophisticated analytical techniques being available for wound analysis, ‘we risk the possibility of expending considerable time, effort and money to measure with extreme precision a parameter that is only peripheral to the biologically important processes of tissue repair’(7).
In this article, we review studies of the VLU to identify biomarkers that may characterise wound healing status and consider their potential for incorporation in wound assessment systems. This ultimate goal awaits the development of a panel of appropriate marker molecules linked to healing outcome and its validation in clinical practice.
Characterisation of the chronic wound environment
From the perspective of diagnostic test development, one would hope to identify markers of healing in peripheral blood or urine because of the ease of sampling. However, most efforts have been expended on identifying the components of the wound microenvironment that reflect the healing status, and this review focuses on data generated by analysis of samples taken directly from the wound itself.
Histological studies
Using histological analysis 8, 9, healing is conventionally divided into early and late inflammatory, proliferative and remodelling phases that follow a reproducible temporal sequence in the normal wound. Whereas the inflammatory response diminishes readily in a non infected healing wound, the chronic wound is characterised histologically by a chronic non resolving inflammation in which macrophages, neutrophils and T lymphocytes are the predominant cell types 10, 11, 12. The presence and role of mast cells in inflammation are rarely described in studies of chronic wounds. Experimental studies indicate that they play a role in normal healing (13) and when enumerated in human wounds, chymase‐positive mast cells are found to decrease in number with healing, whereas no change is observed for tryptase‐positive mast cells (14). In VLUs, by contrast, more tryptase‐positive mast cells are found in association with the basement membrane at the wound margin, suggesting that their presence may be associated with hyperproliferation of the epidermis at the margin of VLUs.
The dense infiltration of T lymphocytes and macrophages found at the margin of VLUs is associated with a strong up‐regulation of intra‐cellular adhesion molecule‐1 (15) and vascular cell adhesion molecule (16) by colocated blood vessels, giving a picture of a typical chronic inflammatory response. Expression of leucocyte‐associated adhesion molecules lymphocyte function‐associated antigen‐1 (LFA‐1) and very late antigen‐4 (VLA‐4) is also enhanced on cells within the microvasculature.
Pericapillary fibrin cuffs with an asymmetric capillary distribution are a predominant histological feature of VLUs. In addition to fibrin, the cuffs are composed of actin, collagen IV and extravasated factor XIIIa and α2‐macroglobulin (17). Immunohistochemical localisation of transforming growth factor (TGF)‐β was also observed, possibly as a consequence of α2‐macroglobulin acting as a scavenger for TGF‐β(18). Typically neutrophils and pro‐collagen‐I‐positive fibroblasts are also associated with the cuffs. By contrast, in normally healing skin graft donor sites, vessels are non tortuous, cuffs are absent and factor XIIIa and α2‐macroglobulin are restricted to vessel lumina with TGF‐β and pro‐collagen‐I present in granulation tissue. Capillary walls in VLUs express the terminal complement complex (19)– an indication of activation of the complement system. Codeposition of terminal complement complex was always observed in association with clusterin, vitronectin and the complement component C3d. Protectin was absent from capillary walls expressing the terminal complement complex. Protectin is a complement regulatory protein (also known as CD59) widely expressed by cells in all tissues. It acts to protect cells from the membrane attack complex generated by inappropriate complement activation (20). Its absence in VLU capillaries may contribute to persistent inflammation seen in chronic wounds. Both cyclooxygenase‐1 and ‐2, rate‐limiting enzymes in prostaglandin synthesis, are up‐regulated in VLU (21). Prostaglandin is a key mediator of inflammation, and its increased synthesis is therefore also likely to exacerbate the chronic inflammation seen in VLU.
Many histological observations suggest a disordered regulation of the healing process rather than a diminution in cellular activity leading to defective healing. Aberrant distribution and amounts of growth factors, cytokines and enzymes such as inducible nitric oxide synthase (22) can be identified. Thus, although TGF‐β‐1, ‐2 and ‐3 can be found in the peri‐lesional skin, these ligands are absent in non healing VLU. Additionally, the receptor for TGF‐β‐1 receptor is strongly expressed in VLU, but no TGF‐β‐2 receptor is present at the protein level, although low‐level expression of messenger RNA (mRNA) can be detected (23). By contrast, in healing VLUs, positive immunostaining for all the TGFs and the type I and type II receptors is detected. Similarly, disordered proteolytic enzyme activity and consequential matrix degradation can also be observed. Levels of neutrophil‐derived elastase (24), membrane‐type matrix metalloprotease (MMP), extracellular MMP inducer (25), soluble MMPs (26) and urokinase‐type plasminogen activator (uPA) (27), which is an activator of MMP‐2, detected by immunohistochemistry are all elevated in VLUs. Tissue inhibitors of MMPs in contrast are decreased (26). This net increase in proteolytic activity is associated with defects in the extracellular matrix and one of the most notable is a deficiency of fibronectin that is required as a provisional matrix for keratinocyte migration (28). The importance of this defect is demonstrated by the observation that within 2 weeks of initiating compression bandaging for VLUs, an acute inflammatory response is initiated followed by deposition of fibronectin and epithelial migration (29).
Wound exudate analysis
Histological studies are particularly useful to generate structural information but suffer from the limitation that they require an invasive procedure that offers only a single time point for analysis, with little opportunity for study of temporal relationships by repeated sampling of the same site. Quantitation is especially difficult for soluble antigens present within the wound matrix. These limitations may be addressed by analysis of wound exudate that can be collected at shorter time intervals from the wound surface (30). Wound exudate is assumed to represent the interstitial fluid of the tissue from which it is harvested and thus to contain metabolites derived from the cellular milieu reflecting the functional status of those cells (31).
Proteases
Whereas acute wound fluid contains intact fibronectin, chronic wound fluid contains only fibronectin degradation products of a mass <125 kDa. Their appearance in chronic wound exudate correlates with elevated levels of elastase and cleavage of the proteinase inhibitors α2‐macroglobulin and α1‐proteinase inhibitor (32). Specific inhibition of exudate neutrophil elastase, but not MMPs, prevents fibronectin degradation by chronic wound exudate in vitro.
MMPs are present as part of the normal healing process, but activity rapidly decreases after the acute inflammatory response resolves and healing is initiated (33). In contrast, it is well documented that elevated levels of MMPs persist in the chronic VLU with associated decreased levels of tissue inhibitor of metalloproteases (TIMPs) (34). The 30‐fold elevation of MMP activity over that found in acute wounds decreases concomitant with a positive response to treatment (35). The concentration of MMP‐2, MMP‐9 and neutrophil elastase in VLU exudates has been examined as a possible prognostic indicator (30). Samples were taken from differing sites within single wounds and deterioration or improvement for each site defined. Using these paired data, pro‐MMP‐9 and neutrophil elastase levels were statistically significantly elevated in static or deteriorating areas of the ulcer. Elevation of pro‐MMP‐9, pro‐MMP‐2 and activated MMP‐2 were also identified as markers of overall ulcer severity.
Protease activity is determined by a balance between production of active enzyme and presence of enzyme inhibitors. uPA occupies a potentially significant role in regulation of chronic wound protease activity because it activates plasminogen to form plasmin that in turn activates collagenases. uPA activity is controlled by its own inhibitor, which is found in higher levels at the ulcer edge and base in healing VLUs than in non healers (36). When uPA expression in wound exudate switches from an active to inhibitor‐bound form, there follows a decrease in MMP‐9 expression. This suggests that the presence of a proteolytic cascade initiated by the plasminogen activator/plasmin system during wound healing is directly linked to activation of MMP‐9. Analyses of uPA and MMP‐9 together have been proposed as possible biomarkers to determine wound healing status (37).
Cytokines and growth factors
Wound exudate contains a diverse array of cytokines and growth factors that can be quantitated by immunoassay or bioassay. However, cytokine bioactivity can be regulated by a number of factors additional to the absolute amount present. It is necessary to consider the presence of specific cytokine inhibitors, membrane or soluble expression of receptors and cytokine bioavailability when assessing their role in healing. For example the pro‐inflammatory cytokine tumour necrosis factor (TNF)‐α has the potential to play a prominent role in wound chronicity by virtue of its diverse bioactivities including modulation of endothelial cell function, anticoagulant and prothrombotic activity, chemoattractant for neutrophils and induction of synthesis of other cytokines/chemokines, including interleukin (IL)‐1, colony‐stimulating factor, interferon‐γ and prostaglandin‐E2 (38). TNF‐α bioactivity in solution can be regulated by binding to soluble TNF‐α receptors that pre‐empt binding to membrane‐expressed receptors.
Although total levels of immunoreactive TNF‐α are significantly higher in wound fluid from non healing VLUs than in wound exudate from healing ulcers, levels of bioactive TNF‐α are not elevated (39). Levels of soluble TNF‐α p75 receptors are significantly higher in non healing wound fluid than in healing wound fluid; these levels are theoretically inadequate to substantially neutralise the bioactivity of the accompanying TNF‐α. Further complexity in interpreting the biological significance of wound cytokine data is introduced by consideration of the counter‐regulatory activity of cytokines. For instance IL‐10 expression by keratinocytes has been proposed as a possible mechanism for suppression of cell‐mediated immunity in the VLU (40). However, IL‐10 is just one component in maintaining the balance of immunoregulation, and it is necessary to consider what other cytokines are present that may inhibit its bioactivity, for example the counter‐regulatory pro‐inflammatory IL‐12.
Recognition of the complexity of interacting cytokine networks has led investigators to analyse multiple factors in the context of healing versus non healing wounds. One example is a study (41) where multiple chemokines and cytokines were quantified by ELISA in wound exudates and tissue extracts from VLUs undergoing compression therapy. As the ulcers healed, a steady increase in the IL‐1β:IL‐1 receptor antagonist protein ratio and conversion of an overall non resolving chronic inflammatory cytokine profile to a resolving acute inflammatory one was observed. In contrast, measurement of platelet‐derived growth factor, granulocyte–macrophage colony‐stimulating factor, IL‐1α, IL‐1β, IL‐6 and basic fibroblast growth factor from healing and non healing ulcers demonstrated no statistically significant differences between the two healing states (42).
Other potential biomarkers
The bioactivity of a particular wound exudate is likely to be the aggregate total function of all its components. Therefore, a bioassay may provide a more relevant output reflecting the individual inputs of all the interacting factors present. Even here problems arise, possibly because of differences in assay methodology. For example, chronic wound exudate has been shown to inhibit newborn dermal fibroblast proliferation (43) but stimulate or exert a minimal effect on proliferation of fibroblasts isolated from the edge of VLUs. Fibroblast mitogenic activity is also dependant on the phase of healing. Thus, exudates taken 4 days after experimental wounding are stimulatory, whereas later exudates taken at days 10–14 inhibit fibroblast proliferation (44) and exert differential effects on hyaluronic acid and glycosaminoglycan synthesis.
Bioactivity may not necessarily be a consequence of cytokine function alone. The concentration of amino acids within wound exudate has been demonstrated to influence stimulation of fibroblast proliferation by epidermal growth factor (45). Wound fluid differs from serum in other biochemical parameters such as glucose, bilirubin, total protein, potassium, uric acid, bicarbonate, alkaline phosphatase, α‐globulins, complement components, lactate and lactate dehydrogenase (46). Decreased glucose and increased lactate in wound tissue compared with surrounding normal tissue are also found in DFU (47). These observations are likely to be linked to cellular energy generation in that a decrease in microenvironmental glucose in a reduced PO2 environment (48) will cause a switch to anaerobic respiration with production of lactate and a consequent induction of lactate dehydrogenase production. A number of the measured components, bicarbonate, glucose, total protein, albumin and γ‐globulin were found to be higher in healing than in non healing VLUs, while C‐reactive protein was decreased.
Many of the components characterising chronic wound exudate are present as a consequence of the chronic inflammatory process. An additional manifestation of this process may be the disturbed oxidant/antioxidant profile, leading to a state of oxidative stress that is considered to contribute to the pathophysiology of VLU (49). Another contributory factor may be the generation of free radicals by the Fenton reaction by excess iron present in chronic wound exudate. Mean total iron in VLU exudate measured by atomic absorption spectroscopy is threefold higher than that found in acute wound fluid such as mastectomy drain fluid or blister fluid (50). Ferritin is elevated and transferrin decreased in VLU exudate, with concomitant increases in 8‐isoprostane, a measure of lipid peroxidation (51). Decreased levels of albumin (52), which can act as an antioxidant, and an increase in the oxidative stress marker allantoin:uric acid ratio (53) are found in VLUs compared with acute wounds although levels of the latter marker did not differ between healing and non healing ulcers. It has been suggested that the low level of albumin found in VLU exudate may be a consequence of its oxidation at the ulcer site (54) although a contributory factor may also be patient nutritional status (55).
Fibroblast senescence
As fibroblasts approach the end of their replicative life span, they can assume a senescent phenotype that is characterised by telomere shortening; resistance to apoptotic death; elevated production of collagenase, elastase and stromelysin and decreased levels of the metalloproteinase inhibitors TIMP‐1 and TIMP‐3 (56). Cellular senescence can also result from exposure to stress‐inducing factors known to be present in the chronic wound environment such as oxidative stress (57) or the pro‐inflammatory cytokines IL‐1 and TNF‐α(58).
It is possible that the extracellular matrix (ECM)‐degrading phenotype expressed by accumulated senescent fibroblasts is one of the factors contributing to ulcer non healing. This hypothesis is supported by the observation of a positive relationship between higher levels of fibroblasts with a senescent phenotype identified in VLU and poor healing outcomes 59, 60, 61. The ECM‐degrading phenotype expressed by senescent fibroblasts is the same as the proteolytic enzyme:tissue inhibitor relationship that is observed by analysis of chronic wound exudate (35). It is a reasonable assumption, therefore, that at least a proportion of the components measured in chronic wound exudate will have originated from senescent fibroblasts and their concentration may, in part, be a reflection of the number of senescent cells present.
Wound microbiology
The proliferation of pathogenic bacteria in wound tissue is a significant and frequent factor contributing to healing failure (62). Wound bacteria originate from exogenous sources such as the environment, eg airborne bacteria, the skin surrounding the wound and endogenous sources such as the gastrointestinal, oropharyngeal and genitourinary mucosae. It is unsurprising, therefore, that long‐standing dermal ulcers, although exhibiting no signs of clinical infection, often demonstrate the presence of colonising bacteria (63). Such organisms are likely to play a significant role in modifying the wound microenvironment either indirectly by stimulating an inflammatory reaction or by release of bacterial products. The magnitude of such an effect is likely to be dependant upon an interaction between bacterial bioburden, virulence and intensity of the host response in wound tissue (64).
Wounds that are on a healing trajectory with no clinical evidence of infection but with bacteria present can be described as colonised and this is not considered detrimental to healing. It is however difficult to define when a wound moves from a benign colonisation to an infected state that will impair healing. There is a little consensus to define whether definitive wound microbiology is of use in guiding clinical decisions and one of the major difficulties is to define the most appropriate sample format (62). The value of a surface swab has been seriously questioned on the basis that the data generated only reflect superficial organisms and not those present within the lower regions of granulation tissue (65) where one might predict a greater impact on healing. Swabbing may have some utility in characterising the complexity of the microbial flora that is known to be polymicrobial in nature (66). Although composition of the surface flora changes with healing, the only substantive relationship that has been found is that if more than four different bacterial groups are present, then the chance of healing failure is increased (46). This observation may be related to the concept that synergistic interactions between aerobes and anaerobes contribute to the pathogenesis of VLU infection (67).
Tissue biopsy for quantitative microbiology is considered the most appropriate sampling method to identify wound infection and causative organisms (62). It has the advantage that organisms within the tissue are identified and quantified on a per gram tissue basis. Wound infection has been defined as when >105 cfu/g of tissue are present in biopsies taken in such a way as to prevent contamination of the biopsy with bacteria from the wound surface (68). Because of its virulence, the presence of β‐haemolytic streptococci is considered to have impact on healing at levels as low as103 cfu/g of tissue.
A diverse range of organisms are potentially present within VLU. For instance in a study of 58 VLUs, 69 species were identified (63). Staphylococcus aureus is the most frequently identified potential pathogen from VLU, but within clinically infected ulcers, a significantly greater mean number of anaerobic bacteria per ulcer (particularly Peptostreptococcus spp. and Prevotella spp.) are found compared with non infected ulcers. Anaerobes represent 49% of the total microbial composition in infected ulcers compared with 36% in non infected leg ulcers (66). In addition to the mix of species and their quantitative tissue loading, consideration has to be given to the effect of phenotypic changes as bacteria proliferate within wound tissue. Bacterial biofilms have been associated with chronic infections and demonstrated to be more resistant to the host immune response and antimicrobial agents (69). There is some evidence that biofilms are implicated in wound infection, and it has been suggested that their presence should be treated by use of agents that prevent the formation or promote detachment of biofilms (70).
Discussion
The majority of diagnostic and prognostic tests in current clinical use have been developed from an understanding of pathology or observations associated with disease processes. One of the earliest tests was the detection by taste of glucose in the urine of diabetics noted in 1674 by the British physician Thomas Willis (70). The more recent evolution of blood glucose estimation from a colorimetric single test through to its analysis by rapid throughput multichannel analysers and finally to current user‐friendly point‐of‐care real‐time analysis systems (71) demonstrates the evolutionary process diagnostic tests may follow. It is also an exemplar of hypothesis‐driven development that cannot currently be applied to a multifactorial lesion such as the chronic wound.
Non healing chronic wounds exhibit many clinical, biochemical, histological and microbiological differences when compared with healing surgical wounds or chronic wounds responding to appropriate treatment. The data available (Figure 1) can be used to build a conceptual model of the chronic wound where chronic inflammation, driven at least partially by bacterial colonisation, results in a degradative microenvironment characterised by excess protease activity, oxidative stress and disordered cytokine/growth factor regulation of cellular function. In a research setting, microenvironmental parameters can be quantified within samples of wound biopsy tissue or wound exudate, and it is attractive to hypothesise that they may be incorporated into a wound assessment system. Such a system would be of prognostic value for identification of those wounds that will not respond to standard treatment modalities, to monitor healing response and possibly act as a surrogate endpoint once treatment has been initiated.
Figure 1.
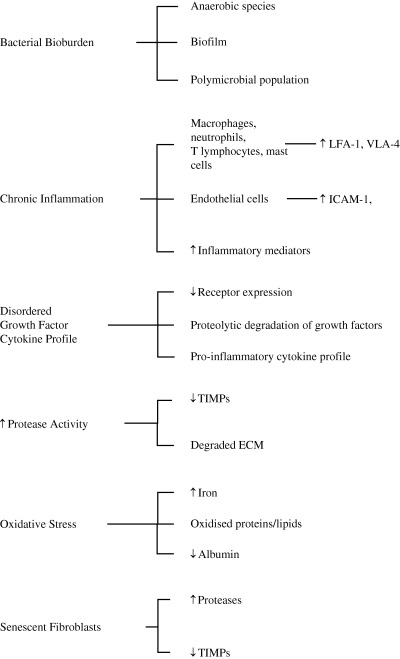
Microenvironmental factors potentially related to chronic wound healing status. ECM, extracellular matrix; ICAM, intercellular adhesion molecule; LFA‐1, lymphocyte function‐associated antigen‐1; TIMPs, tissue inhibitor of metalloproteases; VLA‐4, very late antigen‐4.
Some success has been achieved by incorporating clinically measurable parameters such as wound duration, initial wound area and change in wound area over a 3‐ to 4‐week period to predict healing outcome or monitor treatment response (72). Although this approach can achieve an accuracy of approximately 75%, it may be relatively insensitive as change in wound area is the resultant of many underlying biological processes. For example, a change from a chronic inflammatory cytokine profile to an acute inflammatory profile or a decrease in proteolysis may occur some time in advance of observable reepithelialisation. Thus, enhanced predictive capacity may be generated by measuring those processes that will have impact on subsequent observable changes in healing.
It is clear that given a well‐defined biomarker with clear relationship to clinical course, a wealth of technology exists for development of laboratory‐based rapid throughput or point‐of‐care measurement systems. The challenge when evaluating the wealth of data characterising the chronic wound microenvironment is selection of an appropriate molecule(s) to measure. Many parameters can be measured without understanding the pathogenesis or the complexity of interacting systems within the chronic wound (6). The sophistication of analytical methodology may generally exceed the requirements of clinical wound healing and emphasis has been laid on the need to identify ‘robust measurement techniques which can be applied to such a heterogeneous system’(7).
A large number of parameters have been shown to be differentially expressed between healing and non healing wounds. However, no single biomarker or combination of biomarkers has been demonstrated to accurately reflect wound progression on a single patient basis although claims have been made that changes in the concentration of proteases and fibronectin fragments in wound exudate may be used to indicate clinical infection (73). Because of the ill‐defined nature of the cell biology of chronic wound healing, patient heterogeneity and the lack of good animal models, it is has not been possible to prove any wound prognostic hypothesis at the single molecule level. For example, although angiogenesis can be shown histologically to be defective, no concomitant deficit of vascular endothelial growth factor or its receptor can be demonstrated at the mRNA or protein level in non healing VLU compared with healing ulcers, suggesting that an unidentified inhibitor(s) may be present in the wound environment (74). The development of an accurate and objective test for prediction of chronic wound outcome thus remains dependant not upon further characterisation of the expression of known wound biomarkers but upon identification of an appropriate combination of novel molecules that vary coordinately with healing status.
References
- 1. Ruckley CV. Socioeconomic impact of chronic venous insufficiency and leg ulcers. Angiology 1997;48:67–9. [DOI] [PubMed] [Google Scholar]
- 2. Franks PJ, Moffatt CJ, Connolly M, Bosanquet N, Oldroyd MI, Greenhalgh RM, McCollum CN. Factors associated with healing leg ulceration with high compression. Age Ageing 1995;24:407–10. [DOI] [PubMed] [Google Scholar]
- 3. Margolis DJ, Allen‐Taylor L, Hoffstad O, Berlin JA. The accuracy of venous leg ulcer prognostic models in a wound care system. Wound Repair Regen 2004;12:163–8. [DOI] [PubMed] [Google Scholar]
- 4. Kantor J, Margolis DJ. A multicentre study of percentage change in venous leg ulcer area as a prognostic index of healing at 24 weeks. Br J Dermatol 2000;142:960–4. [DOI] [PubMed] [Google Scholar]
- 5. Flanagan M. Wound measurement: can it help us to monitor progression to healing? J Wound Care 2003;12:189–94. [DOI] [PubMed] [Google Scholar]
- 6. Mani R. Science of measurements in wound healing. Wound Repair Regen 1999;7:330–4. [DOI] [PubMed] [Google Scholar]
- 7. Linblad WJ. How sophisticated do we need to be. Wound Repair Regen 1999;7:329. [DOI] [PubMed] [Google Scholar]
- 8. Dyson M, Young S, Pendle CL, Webster DF, Lang SM. Comparison of the effects of moist and dry conditions on dermal repair. J Invest Dermatol 1998;91:435–9. [DOI] [PubMed] [Google Scholar]
- 9. Berry DP, Harding KG, Stanton MR, Jasani B, Ehrlich P. Human wound contraction: collagen organization, fibroblasts, and myofibroblats. Plast Reconstr Surg 1998;102:124–31. [DOI] [PubMed] [Google Scholar]
- 10. Loots MA, Lamme EN, Zeegelaar J, Mekkes JR, Bos JD, Middelkoop E. Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. J Invest Dermatol 1998;111:850–7. [DOI] [PubMed] [Google Scholar]
- 11. Moore K, Ruge F, Harding KG. T‐lymphocytes and the lack of activated macrophages in wound margin biopsies from chronic leg ulcers. Br J Dermatol 1997;137:188–94. [DOI] [PubMed] [Google Scholar]
- 12. Rosner K, Ross C, Karlsmark T, Petersen AA, Gottrup F, Vejlsgaard GL. Immunohistochemical characterization of the cutaneous cellular infiltrate in different areas of chronic leg ulcers. APMIS 1995;103:293–9. [DOI] [PubMed] [Google Scholar]
- 13. Weller K, Foitzik K, Paus R, Syska W, Maurer M. Mast cells are required for normal healing of skin wounds in mice. FASEB J 2006;20:2366–8. [DOI] [PubMed] [Google Scholar]
- 14. Huttunen M, Aalto ML, Harvima RJ, Horsmanheimo M, Harvima IT. Alterations in mast cells showing tryptase and chymase activity in epithelializating and chronic wounds. Exp Dermatol 2000;9:258–65. [DOI] [PubMed] [Google Scholar]
- 15. Hahn J, Junger M, Friedrich B, Zuder D, Steins A, Hahn M, Klyscz T. Cutaneous inflammation limited to the region of the ulcer in chronic venous insufficiency. VASA 1997;26:277–81. [PubMed] [Google Scholar]
- 16. Weyl A, Vanscheidt W, Weiss JM, Peschen M, Schopf E, Simon J. Expression of the adhesion molecules ICAM‐1, VCAM‐1, and E‐selectin and their ligands VLA‐4 and LFA‐1 in chronic venous leg ulcers. J Am Acad Dermatol 1996;34:418–23. [DOI] [PubMed] [Google Scholar]
- 17. Higley HR, Ksander GA, Gerhardt CO, Falanga V. Extravasation of macromolecules and possible trapping of transforming growth factor‐beta in venous ulceration. Br J Dermatol 1995;132:79–85. [DOI] [PubMed] [Google Scholar]
- 18. O’Connor‐McCourt MD, Wakefield LM. Latent transforming growth factor‐beta in serum. A specific complex with alpha 2‐macroglobulin. J Biol Chem 1987;262:14090–9. [PubMed] [Google Scholar]
- 19. Balslev E, Thomsen HK, Danielsen L, Sheller J, Garred P. The terminal complement complex is generated in chronic leg ulcers in the absence of protectin (CD59). APMIS 1999;107:997–1004. [DOI] [PubMed] [Google Scholar]
- 20. Davies A, Lachmann PJ. Membrane defence against complement lysis: the structure and biological properties of CD59. Immunol Res 1993;12:258–75. [DOI] [PubMed] [Google Scholar]
- 21. Abd‐El‐Aleem SA, Ferguson MW, Appleton I, Bhowmick A, McCollum CN, Ireland GW. Expression of cyclooxygenase isoforms in normal human skin and chronic venous ulcers. J Pathol 2001;195:616–23. [DOI] [PubMed] [Google Scholar]
- 22. Luk PP, Sinha SN, Lord R. Upregulation of inducible nitric oxide synthase (iNOS) expression in faster‐healing chronic leg ulcers. J Wound Care 2005;14:373–5, 378–81. [DOI] [PubMed] [Google Scholar]
- 23. Cowin AJ, Hatzirodos N, Holding CA, Dunaiski V, Harries RH, Rayner TE, Fitridge R, Cooter RD, Schultz GS, Belford DA. Effect of healing on the expression of transforming growth factor beta(s) and their receptors in chronic venous leg ulcers. J Invest Dermatol 2001;117:1282–9. [DOI] [PubMed] [Google Scholar]
- 24. Claudy AL, Mirshahi M, Soria C, Soria J. Detection of undegraded fibrin and tumor necrosis factor‐alpha in venous leg ulcers. J Am Acad Dermatol 1991;25:623–7. [DOI] [PubMed] [Google Scholar]
- 25. Norgauer J, Hildenbrand T, Idzko M, Panther E, Bandemir E, Hartmann M, Vanscheidt W, Herouy Y. Elevated expression of extracellular matrix metalloproteinase inducer (CD147) and membrane‐type matrix metalloproteinases in venous leg ulcers. Br J Dermatol 2002;147:1180–6. [DOI] [PubMed] [Google Scholar]
- 26. Vaalamo M, Weckroth M, Puolakkainen P, Kere J, Saarinen P, Lauharanta J, Saarialho‐Kere UK. Patterns of matrix metalloproteinase and TIMP‐1 expression in chronic and normally healing human cutaneous wounds. Br J Dermatol 1996;135: 52–9. [PubMed] [Google Scholar]
- 27. Herouy Y, Trefzer D, Hellstern MO, Stark GB, Vanscheidt W, Schopf E, Norgauer J. Plasminogen activation in venous leg ulcers. Br J Dermatol 2000;143:930–6. [DOI] [PubMed] [Google Scholar]
- 28. Clark RA, Folkvord JM, Wertz RL. Fibronectin, as well as other extracellular matrix proteins, mediate human keratinocyte adherence. J Invest Dermatol 1985;84:378–83. [DOI] [PubMed] [Google Scholar]
- 29. Herrick SE, Sloan P, McGurk M, Freak L, McCollum CN, Ferguson MW. Sequential changes in histologic pattern and extracellular matrix deposition during the healing of chronic venous ulcers. Am J Pathol 1992;141:1085–95. [PMC free article] [PubMed] [Google Scholar]
- 30. Tarlton JF, Bailey AJ, Crawford E, Jones D, Moore K, Harding KD. Prognostic value of markers of collagen remodeling in venous ulcers. Wound Repair Regen 1999;7:347–55. [DOI] [PubMed] [Google Scholar]
- 31. Staiano‐Coico L, Higgins PJ, Schwartz SB, Zimm AJ, Goncalves J. Wound fluids: a reflection of the state of healing. Ostomy Wound Manage 2000;46(1A Suppl):85–93S [PubMed] [Google Scholar]
- 32. Grinnell F, Ho CH, Wysocki A. Degradation of fibronectin and vitronectin in chronic wound fluid: analysis by cell blotting, immunoblotting, and cell adhesion assays. J Invest Dermatol 1992;98:410–6. [DOI] [PubMed] [Google Scholar]
- 33. Agren MS. Gelatinase activity during wound healing. Br J Dermatol 1994;131:634–40. [DOI] [PubMed] [Google Scholar]
- 34. Bullen EC, Longaker MT, Updike DL, Benton R, Ladin D, Hou Z, Howard EW. Tissue inhibitor of metalloproteases‐1 is decreased and activated gelatinases are increased in chronic wounds. J Invest Dermatol 1995;104:236–40. [DOI] [PubMed] [Google Scholar]
- 35. Trengove NJ, Stacey MC, MacAuley S, Bennett N, Gibson J, Burslem F, Murphy G, Schultz G. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen 1999;7:442–52. [DOI] [PubMed] [Google Scholar]
- 36. Stacey MC, Mata SD. Lower levels of PAI‐2 may contribute to impaired healing in venous ulcers – a preliminary study. Cardiovasc Surg 2000;8:381–5. [DOI] [PubMed] [Google Scholar]
- 37. Wysocki AB, Kusakabe AO, Chang S, Tuan TL. Temporal expression of urokinase plasminogen activator, plasminogen activator inhibitor and gelatinase‐B in chronic wound fluid switches from a chronic to acute wound profile with progression to healing. Wound Repair Regen 1999;7:154–65. [DOI] [PubMed] [Google Scholar]
- 38. Camussi G, Albano E, Tetta C, Bussolino F. The molecular action of tumor necrosis factor‐alpha. Eur J Biochem 1991;202:3–14. [DOI] [PubMed] [Google Scholar]
- 39. Wallace HJ, Stacey MC. Levels of tumor necrosis factor‐alpha (TNF‐alpha) and soluble TNF receptors in chronic venous leg ulcers– correlations to healing status. J Invest Dermatol 1998;110:292–6. [DOI] [PubMed] [Google Scholar]
- 40. Li YQ, Doyle JW, Roth TP, Dunn RM, Lawrence WT. IL‐10 and GM‐CSF expression and the presence of antigen‐presenting cells in chronic venous ulcers. J Surg Res 1998;79:128–35. [DOI] [PubMed] [Google Scholar]
- 41. Fivenson DP, Faria DT, Nickoloff BJ, Poverini PJ, Kunkel S, Burdick M, Strieter RM. Chemokine and inflammatory cytokine changes during chronic wound healing. Wound Repair Regen 1997;5:310–22. [DOI] [PubMed] [Google Scholar]
- 42. Harris IR, Yee KC, Walters CE, Cunliffe WJ, Kearney JN, Wood EJ, Ingham E. Cytokine and protease levels in healing and non‐healing chronic venous leg ulcers. Exp Dermatol 1995;4:342–9. [DOI] [PubMed] [Google Scholar]
- 43. Phillips TJ, Al‐Amoudi HO, Leverkus M, Park HY. Effect of chronic wound fluid on fibroblasts. J Wound Care 1998;7:527–32. [DOI] [PubMed] [Google Scholar]
- 44. Jalkanen M, Haapanen T, Lyytikainen AM, Larjava H. Wound fluids mediate granulation tissue growth phases. Cell Biol Int Rep 1983;7:745–53. [DOI] [PubMed] [Google Scholar]
- 45. Gartner MH, Shearer JD, Bereiter DF, Mills CD, Caldwell MD. Wound fluid amino acid concentrations regulate the effect of epidermal growth factor on fibroblast replication. Surgery 1991;110:448–55. [PubMed] [Google Scholar]
- 46. Trengove NJ, Stacey MC, McGechie DF, Mata S. Qualitative bacteriology and leg ulcer healing. J Wound Care 1996;5:277–80. [DOI] [PubMed] [Google Scholar]
- 47. Stolle LB, Riegels‐Nielsen P. The metabolism of the diabetic foot: in vivo investigation with microdialysis. Acta Orthop Scand 2004;75:106–8. [DOI] [PubMed] [Google Scholar]
- 48. Nemeth AJ, Eaglstein WH, Falanga V. Clinical parameters and transcutaneous oxygen measurements for the prognosis of venous ulcers. J Am Acad Dermatol 1989;20:186–90. [DOI] [PubMed] [Google Scholar]
- 49. Wlaschek M, Scharffetter‐Kochanek K. Oxidative stress in chronic venous leg ulcers. Wound Repair Regen 2005;13:452–61. [DOI] [PubMed] [Google Scholar]
- 50. Wenk J, Foitzik A, Achterberg V, Sabiwalsky A, Dissemond J, Meewes C, Reitz A, Brenneisen P, Wlaschek M, Meyer‐Ingold W, Scharffetter‐Kochanek K. Selective pick‐up of increased iron by deferoxamine‐coupled cellulose abrogates the iron‐driven induction of matrix‐degrading metalloproteinase 1 and lipid peroxidation in human dermal fibroblasts in vitro: a new dressing concept. J Invest Dermatol 2001;116:833–9. [DOI] [PubMed] [Google Scholar]
- 51. Yeoh‐Ellerton S, Stacey MC. Iron and 8‐isoprostane levels in acute and chronic wounds. J Invest Dermatol 2003;121:918–25. [DOI] [PubMed] [Google Scholar]
- 52. James TJ, Hughes MA, Cherry GW, Taylor RP. Simple biochemical markers to assess chronic wounds. Wound Repair Regen 2000;8:264–9. [DOI] [PubMed] [Google Scholar]
- 53. James TJ, Hughes MA, Cherry GW, Taylor RP. Evidence of oxidative stress in chronic venous ulcers. Wound Repair Regen 2003;11:172–6. [DOI] [PubMed] [Google Scholar]
- 54. Moseley R, Hilton JR, Waddington RJ, Harding KG, Stephens P, Thomas DW. Comparison of oxidative stress biomarker profiles between acute and chronic wound environments. Wound Repair Regen 2004;12:419–29. [DOI] [PubMed] [Google Scholar]
- 55. Wipke‐Tevis DD, Stotts NA. Nutritional risk, status, and intake of individuals with venous ulcers: a pilot study. J Vasc Nurs 1996;14: 27–33. [DOI] [PubMed] [Google Scholar]
- 56. Campisi J. The role of cellular senescence in skin aging. J Invest Dermatol Symp Proc 1998;3:1–5. [PubMed] [Google Scholar]
- 57. Chen J‐H, Stoeber K, Kingsbury S, Ozanne SE, Williams GH, Hales CN. Loss of proliferative capacity and induction of senescence in oxidatively stressed human fibroblasts. J Biol Chem 2004;47:49439–46. [DOI] [PubMed] [Google Scholar]
- 58. Dumont P, Balbeur L, Remacle J, Toussaint O. Appearance of biomarkers of in vitro ageing after successive stimulation of WI‐38 fibroblasts with IL‐1alpha and TNF‐alpha: senescence associated beta‐galactosidase activity and morphotype transition. J Anat 2000;197:529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mendez MV, Stanley A, Park HY, Shon K, Phillips T, Menzoian JO. Fibroblasts cultured from venous ulcers display cellular characteristics of senescence. J Vasc Surg 1998;28:876–83. [DOI] [PubMed] [Google Scholar]
- 60. Raffetto JD, Mendez MV, Phillips TJ, Park HY, Menzoian JO. The effect of passage number on fibroblast cellular senescence in patients with chronic venous insufficiency with and without ulcer. Am J Surg 1999;178:107–12. [DOI] [PubMed] [Google Scholar]
- 61. Stanley A, Osler T. Senescence and the healing rates of venous ulcers. J Vasc Surg 2001;33:1206–11. [DOI] [PubMed] [Google Scholar]
- 62. Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev 2001;14:244–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hansson C, Hoborn J, Moller A, Swanbeck G. The microbial flora in venous leg ulcers without clinical signs of infection. Repeated culture using a validated standardised microbiological technique. Acta Derm Venereol 1995;75:24–30. [DOI] [PubMed] [Google Scholar]
- 64. Kingsley A. The wound infection continuum and its application to clinical practice. Ostomy Wound Manage 2003;49(7A Suppl):1–7. [PubMed] [Google Scholar]
- 65. Gradon J, Adamson C. Infections of pressure ulcers: management and controversies. Infect Dis Clin Pract 1995;1:11–6. [Google Scholar]
- 66. Bowler P. The anaerobic and aerobic microbiology of wounds: a review. Wounds 1998;10:170–8. [Google Scholar]
- 67. Bowler PG, Davies BJ. The microbiology of infected and noninfected leg ulcers. Int J Dermatol 1999;38:573–8. [DOI] [PubMed] [Google Scholar]
- 68. Robson MC, Mannari RJ, Smith PD, Payne WG. Maintenance of wound bacterial balance. Am J Surg 1999;178:399–402. [DOI] [PubMed] [Google Scholar]
- 69. Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin‐Scott HM. Microbial biofilms. Annu Rev Microbiol 1995;49:711–45. [DOI] [PubMed] [Google Scholar]
- 70. Foster WD. Pathology as a profession in Great Britain. London: Royal College of Pathologists, 1981. [Google Scholar]
- 71. Tierney MJ, Tamada JA, Potts RO, Jovanovic L, Garg S. Clinical evaluation of the GlucoWatch biographer: a continual, non‐invasive glucose monitor for patients with diabetes. Biosens Bioelectron 2001;16:621–9. [DOI] [PubMed] [Google Scholar]
- 72. Moore K, McCallion R, Searle RJ, Stacey MC, Harding KG. Prediction and monitoring of the therapeutic response of chronic dermal wounds. Int Wound J 2006;3:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cullen B. Wound monitoring. International Patent Publication No WO 03/040406, 2003. [Google Scholar]
- 74. Drinkwater SL, Burnand KG, Ding R, Smith A. Increased but ineffectual angiogenic drive in nonhealing venous leg ulcers. J Vasc Surg 2003;38:1106–12. [DOI] [PubMed] [Google Scholar]


