Abstract
Ulceration is the most common complication of haemangioma and occurs in 5–15% of cases. The present study was carried out to evaluate the clinical features of ulcerated haemangioma and efficacy of the management protocol adopted by us over a period of 20 years. All patients with ulcerated haemangioma were evaluated on the basis of age at presentation, sex, rural or urban distribution and site of haemangioma. Treatment included application of topical antibiotic and systemic antibiotic and analgesic for pain. The total number of patients was 608. The male to female ratio was 1: 2·28. The rural:urban distribution was 2·43:1. The most common site of involvement was head and neck. Mean age of patients was 5·60 ± 2·44 months. Mean size of haemangioma and ulceration was 47·30 ± 20·67 cm2 and 7·49 ± 4·52 cm2, respectively. The mean time for ulcer healing was 40·06 ± 19·41 days. Ulcer size of more than 10 cm2 took more time to heal. Response to treatment was satisfactory. Ulcerated haemangioma usually occurs before completion of 1 year of age; hence, every patient with haemangioma needs careful attention. Adequate treatment and regular follow up brings satisfactory response in the patients.
Keywords: Haemangioma, Management of ulcerated haemangioma, Therapy, Ulcerated haemangioma
Introduction
Haemangiomas are the most common tumours of infancy. The incidence in the general newborn population is between 1·0% and 2·6% and as high as 10% among whites (1). Clinically, haemangioma can be extremely heterogeneous with size, location and rate of proliferation having a significant effect on the risk of complications (2). Ulceration is the most common complication of haemangioma and occurs in 5–15% of cases (3). It generally develops during the proliferative phase of the haemangioma life cycle. It can be a particularly difficult problem because of associated pain, infection, haemorrhage and subsequent scarring (4). Although there are different options for managing the ulceration, none has been found to be universally effective (3).
The present study was carried out to evaluate the clinical features of ulcerated haemangioma and efficacy of the management protocol adopted by us over a period of 20 years.
Material and methods
This retrospective study included all the patients with haemangioma who attended the Hemangioma Clinic with ulcerated lesion during the study period. Ulceration was defined as breach in the continuity of the surface epithelium with or without the presence of infection.
The patients were evaluated on basis of age at presentation, sex, rural or urban distribution and site of haemangioma. Rural population was defined as those living in the countryside. Urban population are those living in the cities with adequate health and education facilities. The size of the haemangioma and the ulceration within the haemangioma were measured with a measuring tape in their two maximum dimension perpendicular to each other. The time taken by the ulcer to heal was recorded along with total number of visits and total duration of follow up.
Our treatment protocol included application of topical antibiotic (mupirocin, fusidic acid, sisomycin or metronidazole ointment) and systemic antibiotic for ulcer size of more than 10 cm2 (co‐amoxyclav 20–40 mg/kg/day in two divided doses orally). For control of pain, suspension of paracetamol was given as per the requirement (40–50 mg/kg/day in four to six divided doses). No other treatment modality to treat the haemangioma was started before the ulcer healed. When the ulcer had healed, steroid either oral or intralesional was given for treating the haemangioma.
Statistical analysis was carried out using SPSS 12.0 version for Windows. Results were analysed by chi‐square test and one‐way analysis of variance. P < 0·05 was considered statistically significant.
Results
The study period ranged from June 1986 to June 2006 (20 years). The total number of patients attending the clinic with adequate record was 2013. The total number of patients presenting with ulceration of haemangioma was 608 (30·2%). Fifty‐nine (9·70%) patients presented with bleeding from the ulcer that was not significant.
The male to female ratio was 1: 2·28. The rural:urban distribution was 2·43:1. The site of involvement was head and neck in 274 (45·06%), trunk in 128 (21·05%), upper extremity in 118 (19·41%) and lower extremity in 88 (14·47%) patients. The rural:urban distribution was not related to sex, site of haemangioma, size of haemangioma or ulcer and time required for response to occur (P > 0·05).
Mean age of patients was 5·60 ± 2·44 months (range 1–11 months). The occurrence of ulceration in various age groups was 113 (18·6%) in 1–3 months, 283 (46·5%) in 3–6 months, 190 (31·3%) in 6–9 months and 22 (3·6%) in 9–12 months. A total of 187 patients (30·75%) were localised as superficial type and 149 (24·51%) as mixed type. There was no deep type of haemangioma that presented with ulceration and 272 (44·74%) had haemangioma with extensive involvement.
The mean size of haemangioma in the superficial, mixed and extensive group was 29·40 ± 10·69 cm2 (range 13–61 cm2), 34·13 ± 11·21 cm2 (range 12–56 cm2) and 66·81 ± 8·09 cm2 (46–101 cm2), respectively. The difference in the size of haemangioma was statistically insignificant between superficial and mixed type (P > 0·05) but statistically significant from the extensive involvement (P < 0.05). The mean size of haemangioma was 47·30 ± 20·67 cm2..
The mean size of ulcer in the superficial, mixed and extensive group was 4·41 ± 2·78 cm2 (range 1–19 cm2), 5·81 ± 3·20 cm2 (range 1–12 cm2) and 10·52 ± 4·19 cm2 (2–25 cm2), respectively. The difference in ulcer size was significantly higher in the extensive involvement group (P < 0.05). Overall mean size of ulceration was 7·49 ± 4·52 cm2.·
The time taken by the ulcer to heal in the superficial, mixed and extensive group was 32·63 ± 13·06 days (range 16–78 days), 42·89 ± 19·89 days (16–81 days) and 57·03 ± 16·12 days (range 16–96 days), respectively. This difference among the groups was statistically significant (P < 0.05). Overall mean time was 40·06 ± 19·41 days. The time taken by ulcer to heal was more in ulcer size of more than 10 cm2 than the ulcer size of less than 10 cm2, and the difference was statistically significant (P < 0.05).
All the patients responded to the ulcer management protocol adopted by us, and after the response they were given the steroid according to the haemangioma size. The mean number of visits till the final response of haemangioma was 11·87 ± 1·11 (range 8–14) and the mean duration of follow up was 5·10 ± 0·85 years (range 4–8 years).
Discussion
The exact cause of ulceration in haemangioma is not known. Maceration and frictional stress are probable contributing factors (3). It is also thought to occur when the haemangioma outgrows its blood supply (4). Ulcerated haemangiomas are more likely to bleed either spontaneously or following minor trauma, but the bleeding is rarely profuse and can generally be controlled by applying local pressure (1) that was also noticed by us during the management of these lesions.
The male to female ratio in our study was 1:2·28, which is close to the study of Wananukul and Chatproedprai (5) in which the ratio was 1:2·2; however, Kim et al. (6) had a ratio of 1: 4·5 in their study. Although we have classified the study population into rural and urban groups, it had no effect on the age of patients, size of the haemangioma or ulcer, etc. As more of our population lives in villages, higher rural ratio is justified.
Head and neck was the most common site of ulceration (Figure 1) and has been noticed by others 5, 6. All patients presenting with ulceration were less than 1 year of age and most of the patients were in the 3‐ to 6‐month age group in which haemangioma progression is rapid, suggesting higher propensity of ulceration during the proliferative period of the haemangioma that has been observed by other workers 5, 6, 7.
Figure 1.
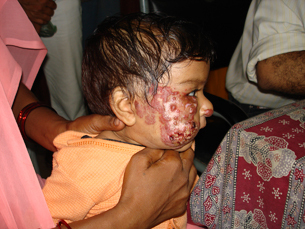
Ulcerated haemangioma of the face.
None of our patients with ulceration had deep type of haemangioma, thus supporting the hypothesis that ulceration results from friction and maceration. This is also supported by the fact that ulceration occurred in haemangioma of larger size (30 cm2 or more) where there are higher chances of friction injury. The introduction of extensive group as a separate entity was to emphasise the importance of size in its relation to ulceration.
The ulcer size was significantly larger in the extensive group suggesting that ulcer size relates to the size of haemangioma that has also been observed by others (7). Healing of ulcer was related to its size and was statistically significant, thereby suggesting the need for careful management of the ulceration especially with the larger size.
An important aspect of the ulcerated haemangioma is local wound care. There are reports of various treatment modalities used for treating it that include topical and/or systemic antibiotic. Other therapies that have been used are topical, intralesional and/or systemic corticosteroids, dressings like hydrocolloids and alginate, pastes and gels and pulsed‐dye laser. For refractory cases, surgical excision has been advocated 5, 6. Radiotherapy had been advocated in the past (8), but it carries the risk of malignancy (9).
We used only topical and systemic antibiotic in the management of wound healing and had satisfactory response in all the patients (2, 3). No attempt was made to expedite the regression of haemangioma during the ulcerative stage with the use of steroids or any other measure, our only concern was healing of the ulcer. We also believe that treatment of ulcerated haemangioma during the active phase by steroid may predispose to increased chances of infection. After healing of the ulcer, therapy to regress the haenangioma was started. No complicated form of dressing and wound management was required as the ulceration in our patients was not deep but only superficial loss of epithelium. Although literature is flooded with successful reports of use of laser 3, 5, 10, 11, we had no experience of it. We did not need the hydrocolloids and alginate dressings, pastes and gels for ulcer healing. Recently, there has been successful use of becaplermin gel in the treatment of ulcerated haemangioma (3), but it is not available to us.
Figure 2.
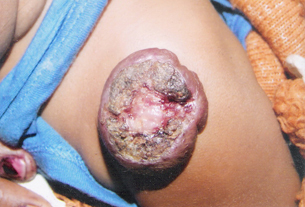
Ulcerated haemangioma before initiation of the treatment.
Figure 3.
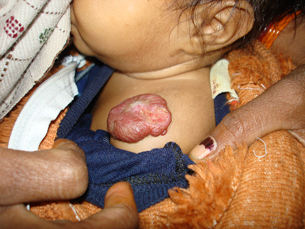
Patient after satisfactory response to the conservative treatment.
The management of pain is an important aspect of ulceration and it has been given due recognition by other authors (6). Various agents used for pain control are oral acetaminophen, topical lignocaine ointment and even polyurethane film 6, 12; however, pain control in our patients was satisfactory with the paracetamol suspension alone.
We did not do the routine culture from the ulcerated haemangioma, yet prescribed the prophylactic antibiotics in all the patients. It was because of the presumed infection in the ulceration as majority of our patients belong to lower socioeconomic strata where they do not care for the wound if not specifically asked for and chances of infection are high. The satisfactory responses of ulcerated lesions do confirm our affirmation.
There have been reports of associated syndromes with haemangioma, namely PHACES (posterior fossa brain malformations, hemangiomas, arterial anomalies, coarctation of the aorta and cardiac defects, and eye abnormalities) (13), PELVIS (perineal hemangioma, external genitalia malformations, lipomyelomeningocele, vesicorenal abnormalities, imperforate anus, and skin) (14), SACRAL (Spinal dysraphism, Anogenital anomalies, Cutaneous anomalies, Renal and urologic anomalies, associated with Angioma of Lumbosacral localization) (15). The PHACES syndrome was first described in 1996 and the other two have been described very recently. It may be a possibility that we may have inadvertently missed case of the PHACES syndrome but to best of our knowledge we had not encountered any such syndrome.
To conclude, ulcerated haemangioma usually occurs before completion of 1 year of age, mostly the first 6 months and hence every patient of haemangioma needs careful attention. Adequate treatment of the ulceration by antibiotics and regular follow up brings satisfactory response in the patients.
References
- 1. Metry DW, Hebert AA. Benign cutaneous vascular tumors of infancy when to worry, what to do. Arch Dermatol 2000;136:905–14. [DOI] [PubMed] [Google Scholar]
- 2. Chan YC, Giam YC. Guidelines of care for cutaneous hemangiomas. Ann Acad Med Singapore 2005;34:117–23. [PubMed] [Google Scholar]
- 3. Metz BJ, Rubenstein MC, Levy ML, Metry DW. Response of ulcerated perineal hemangiomas of infancy to becaplermin gel, a recombinant human platelet‐derived growth factor. Arch Dermatol 2004;140:867–70. [DOI] [PubMed] [Google Scholar]
- 4. David LR, Malek MM, Argenta LC. Efficacy of pulse dye laser therapy for the treatment of ulcerated hemangiomas: a review of 78 patients. Br J Plast Surg 2003;56:317–27. [DOI] [PubMed] [Google Scholar]
- 5. Wananukul S, Chatproedprai S. Ulcerated hemangiomas: clinical features and management. J Med Assoc Thai 2002;85:1220–5. [PubMed] [Google Scholar]
- 6. Kim HJ, Colombo M, Frieden IJ. Ulcerated hemangiomas: clinical characteristics and response to therapy. J Am Acad Dermatol 2001;44:962–72. [DOI] [PubMed] [Google Scholar]
- 7. Chamlin SL, Haggostrom AN, Drolet BA, Baselga E, Frieden IJ, Garzon MC, Horii KA, Lucky AW, Metry DW, Newell B, Nopper AJ, Mancini AJ. Multicenter prospective study of ulcerated hemangiomas. J Pediatr 2007;151:684–9, 689.e1 [epub 24 August 2007]. [DOI] [PubMed] [Google Scholar]
- 8. Podliashchuk EL. Radiotherapy of ulcerated hemangioma. Med Radiol (Mosk) 1983;28:26–30. [PubMed] [Google Scholar]
- 9. Lindberg S, Karlsson P, Arvidsson B, Holmberg E, Lunberg LM, Wallgren A. Cancer incidence after radiotherapy for skin hemangioma during infancy. Acta Oncol 1995;34:735–40. [DOI] [PubMed] [Google Scholar]
- 10. Achauer BM, Vander Kam VM. Ulcerated anogenital hemangioma of infancy. Plast Reconstr Surg 1991;87:861–8. [DOI] [PubMed] [Google Scholar]
- 11. Morelli JG, Tan OT, Weston WL. Treatment of ulcerated hemangiomas with the pulsed tunable dye laser. Am J Dis Child 1991;145:1062–4. [DOI] [PubMed] [Google Scholar]
- 12. Oranje AP, De Waard‐van der Spek FB, Devillers AC, De Laat PC, Madern GC. Treatment and pain relief of ulcerative hemangiomas with a polyurethane film. Dermatology 2000;200:31–4. [DOI] [PubMed] [Google Scholar]
- 13. Frieden IJ, Reese V, Cohen D. PHACE syndrome. The association of posterior fossa brain malformations, hemangiomas, arterial anomalies, coarctation of the aorta and cardiac defects, and eye abnormalities. Arch Dermatol 1996;132:307–11. [DOI] [PubMed] [Google Scholar]
- 14. Girard C, Ignore M, Guillot B, Bessis D. PELVIS syndrome. Arch Dermatol 2006;142:884–8. [DOI] [PubMed] [Google Scholar]
- 15. Stockman A, Boralevi F, Taieb A, Leaute‐ Labreze C. SACRAL syndrome: spinal dysraphism, anogenital, cutaneous, renal and urologic anomalies, associated with an angioma of lumbosacral localization. Dermatology 2007;214:40–5. [DOI] [PubMed] [Google Scholar]


