Abstract
Negative pressure wound therapy (NPWT) is an established modality in the treatment of challenging wounds. However, most existing clinical evidence is derived from the use of open‐cell polyurethane foam at −125 mmHg. Alternative negative pressure systems are becoming available, which use gauze at a pressure of −80 mmHg. This study describes clinical results from a retrospective non comparative analysis of 30 patients treated with Chariker‐Jeter gauze‐based negative pressure systems (V1STA™, Versatile‐1™ and EZ‐Care™; Smith & Nephew, Inc.) in a long‐term care setting. The mean age of the patients was 72 years. The wounds consisted of chronic (n = 11), surgical dehiscence (n = 11) and surgical incision (n = 8). Wound volume and area were recorded at commencement and at the cessation of therapy. Discontinuation of therapy was instigated upon closure through secondary intention or when size and exudate were sufficiently reduced that the wounds could be managed by conventional wound dressing (median 41 days). An overall median reduction in wound volume of 88·0% (P < 0·001) and a 68·0% reduction in area (P < 0·001) compared with baseline were observed over the course of NPWT. The overall rate of volume reduction (15·1% per week) compares favourably with published data from foam‐based systems.
Keywords: Gauze, Negative pressure wound therapy, Chariker‐Jeter
Introduction
Negative pressure wound therapy (NPWT) has become an extensively adopted modality in the past 10 years for the treatment of a wide range of soft‐tissue defects 1, 2. NPWT consists of a wound filler material covered with an adherent airtight drape, connected to a source of negative pressure such as a pump. NPWT appears to act through multiple mechanisms including exudate management, removal of oedema, increases in wound bed blood flow and stimulation of granulation tissue formation 3, 4, 5, 6.
NPWT has predominantly been delivered using a specific porous open‐cell polyurethane foam at a recommended set pressure of −125 mmHg (V.A.C.® system; KCI, San Antonio, TX) 1, 2. However, in 1989, Chariker et al. (7) described an alternative NPWT system that used medical gauze at around −80 mmHg negative pressure. Although the system described by Chariker was not widely adopted at the time, recent clinical case studies have been published and commercial systems have become available 8, 9, 10, indicating that clinical efficacy of NPWT may not be confined to the use of specific wound filler materials or use of a specific level of negative pressure 11, 12. In this study, we review retrospectively the clinical results from a mixed group of patients with challenging wounds in a long‐term care setting to see whether the reductions in wound size and volume achieved using gauze‐based NPWT are comparable with published data from polyurethane foam‐based systems.
Materials and methods
Delivery of NPWT
NPWT was delivered using the V1STA™, Versatile‐1™ or EZ‐Care™ systems (Smith & Nephew, Largo, FL). These systems were used at a negative pressure of −80 mmHg. The wound filler used to transmit negative pressure to the wound bed was saline‐moistened antimicrobial gauze (Kerlix‐AMD; Tyco, Gosport, UK) regardless of the specific device used and was provided as part of a tailored dressing kit from the manufacturer and applied to the wound using the Chariker‐Jeter method of application (7). In all cases, NPWT was delivered using continuous pressure for the stated duration of therapy. Wounds were inspected and dressings changed at 2‐ to 3‐day intervals.
Data assessment
Data were captured from a case series of patients undergoing NPWT using regulatory approved devices in appropriate indications at the Bethany Health and Rehab Center and Trevecca Health and Rehab Center, both part of the Avalon Health Care Group located in Nashville, TN, USA. Patients enrolled in this study were undergoing an approved care pathway, and data were gathered as part of an outcomes tracking exercise. Patients provided written consent upon admission to the facilities to allow the use of their photographs in public domain communications, including research articles, and all clinical data were collected in an anonymous manner. A retrospective analysis was carried out to consolidate the data from this case series. Data were collected on patient demographics, wound type, duration of NPWT, measurements of wound dimensions, reasons for discontinuation of NPWT and device‐related complications.
Data were grouped according to their wound type into the following categories: chronic wounds (including pressure ulcers, diabetic foot ulcers, arterial ulcers and venous leg ulcers), dehisced surgical wounds and surgical incision wounds (wounds that were created by a surgical incision that remained open for closure by secondary intention). Wound area (by width × length) and volume (from depth estimations) using sterile probes were calculated at baseline (when NPWT was initially prescribed) and at the end of therapy. The reason for discontinuation of therapy was captured, and the patient cohort was divided into patients whose therapy was discontinued because of adequate progression of the wound (i.e. further NPWT was not warranted and remaining wounds were taken to closure by other means) and patients whose therapy was discontinued for different reasons.
Statistical analysis
A Wilcoxon signed‐rank test was conducted to determine whether the median percentage reduction in wound volume, area and depth was significantly different from zero. The Hodges–Lehmann estimates for the median and the corresponding 95% confidence intervals are quoted. The median values quoted in the subgroup analysis are actual median values. The Kaplan–Meier estimator was used to determine the median days to adequate wound progression over all indications and separately for each indication. An accelerated failure time model including a term for baseline area was used to compare the time to adequate progression between indications.
Results
Wound and patient demographics
There were 30 patients included in the study who were treated with NPWT for a median of 33 days (range 5–86 days). Patient demographics are shown in Table 1. The mean patient age was 72·3 years. Common comorbidities within the patient population included diabetes (73%), venous disease (40%), cancer (20%) and renal disease (23%). Of the 30 patients, 20 (67%) received therapy until wounds had progressed adequately and NPWT was deemed to be no longer clinically necessary. Of these patients, two wounds were then treated with Apligraf® (Organogenesis Inc., Canton, MA). Only a single wound was taken to complete healing using NPWT. The intent to treat for the remaining wounds was healing by secondary intention to a point where the wounds could be adequately managed by other means (e.g. advanced wound dressings). Final wound closure was not tracked. Of the remaining ten patients, six patients were discharged to an alternative facility or home where the therapy was not provided. One patient was discharged to a hospital facility because of development of a fistula not related to the NPWT device, one patient died, one patient was withdrawn because of non compliance and one patient was transferred onto end of life care because of worsening comorbidities. These patients were lost to further follow‐up. However, measurements of their final wound dimensions were made upon discontinuation of therapy and contributed towards the analysis where specified.
Table 1.
Patient demographics*
| Number of wounds | Male:female | Mean age in years (range) | |
|---|---|---|---|
| Chronic | 11 | 4:7 | 76·5 (66–96) |
| Surgical dehiscence | 11 | 2:9 | 71·3 (55–84) |
| Surgical incision | 8 | 3:5 | 67·8 (32–78) |
| Total | 30 | 9:21 | 72·3 (32–96) |
Patients were grouped according to their wound type into the following categories: chronic wounds (including pressure ulcers, diabetic foot ulcers, arterial ulcers and venous leg ulcers), dehisced surgical wounds and surgical incision wounds (wounds that were created by a surgical incision that remained open for closure by secondary intention).
Effect of NPWT on reduction in wound dimensions
All wounds decreased significantly in volume, area and depth over the duration of therapy compared with baseline measurements (Table 2, left‐hand columns). Median baseline volume was 43·9 cm3, area was 20·2 cm2 and depth was 1·9 cm. The median percentage reduction in wound volume from baseline was 88·0% (P < 0·001, n = 17). The median percentage reduction in wound area was 68·0% (P < 0·001, n = 26) and the median percentage reduction in wound depth was 57·4% (P < 0·005, n = 17).
Table 2.
Median percentage reduction in wound size (volume, area and depth)*
| Combined data (95% CI) | P value | Progressed until NPWT no longer required | Including NPWT discontinued prematurely | |
|---|---|---|---|---|
| % reduction in volume | 88·0 (59·8–94·1) (n = 17) | <0·001 | 92·2 (n = 11) | 63·7 (n = 6) |
| % reduction in area | 68·0 (55·6–78·6) (n = 26) | <0·001 | 81·7 (n = 17) | 44·1 (n = 9) |
| % reduction in depth | 57·4 (38·1–72·7) (n = 17) | <0·005 | 71·8 (n = 10) | 46·7 (n = 7) |
95% CI, 95% confidence interval; NPWT, negative pressure wound therapy.
Wound size was measured at the onset of NPWT and immediately following discontinuation of the therapy. Median percentage reduction in wound size from initial measurements and 95% CI were calculated. Statistical significance of the reduction in size was calculated compared with the baseline measurement. A subanalysis was carried out to assess the percentage reduction in wound size according to whether therapy was discontinued because the wound had progressed to a stage where NPWT was no longer required or was discontinued for non wound‐related factors.
A subanalysis was carried out to assess the percentage reduction in wound size in the wounds that were treated with NPWT until it was no longer deemed clinically necessary (in this study termed adequate progression) and is shown in Table 2 (right‐hand columns). The median percentage reduction in wound volume of patients who were observed until NPWT was no longer required was 92·2%; n = 11 (compared with 63·7% in patients where therapy was discontinued early or was lost to follow‐up; n = 6). The median wound volume in patients who had adequately progressed to healing was 2·2 cm3. The percentage reduction of wound area of patients who had progressed adequately was 81·7%; n = 17 (compared with 44·1% in patients where therapy was discontinued early or was lost to follow up; n = 9). The median wound area for patients who had progressed to an adequate stage of healing was 2·3 cm2. The percentage reduction of wound depth in patients who had progressed adequately was 71·8%; n = 10 (compared with 46·7% in patients where therapy was discontinued early or was lost to follow up; n = 7). The median wound depth in patients who had adequately progressed to healing was 0·4 cm.
Effect of indication on reduction in wound size
The data were assessed to see whether the type of wound affected the extent of the reduction in volume, area or depth of the wound (Figure 1). All the box plots are clustered around the same percentage reduction. No obvious trends were observed with this analysis to suggest that certain indications progressed faster than others. No statistical analysis was carried out because of the relatively low numbers present in each group and the lack of any obvious trend (not shown).
Figure 1.
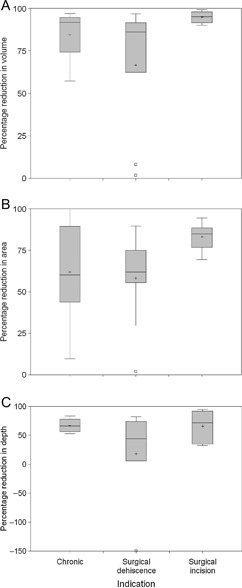
Percentage reduction in wound dimensions divide by indication. The percentage reduction in volume (A), area (B) and depth (C) were calculated and box plots generated. Within each box, the horizontal line is the median and the cross indicates the mean. The box indicates the interquartile range, and the cut‐off lines above and below extend 1·5 times the interquartile range from the 25th or 75th percentile rolled back to where there are data. Those patients who lie outside this distance are represented by a square (outliers).
Progression of wounds over time
The percentage reduction in wound volume and area observed per week of therapy is shown in Table 3. This analysis contained data from patients where therapy was discontinued prematurely as well as those who had progressed to a point where NPWT was no longer required. The overall median percentage reduction per week in wound volume was 15·1% and in wound area was 14·3%.
Table 3.
Timescales of achieving adequate wound progression*
| Indication | Median days to adequate progression | Median % reduction in volume per week | Median % reduction in area per week |
|---|---|---|---|
| Chronic | 41·0 | 15·7 | 13·5 |
| Surgical dehiscence | 44·0 | 12·8 | 19·9 |
| Surgical incision | 40·0 | 15·2 | 14·1 |
| Total | 41·0 | 15·1 | 14·3 |
The median time to achieve adequate progression is shown for each indication (overall n = 30). The median percentage reduction in wound volume (overall n = 16) and area (total n = 25) per week was calculated taking into account the wounds that did not reach adequate progression and are expressed as median values.
The time taken to achieve adequate wound progression (i.e. where the wound reached that stage where it was no longer deemed necessary to treat with NPWT) was assessed according to indication (Table 3). Information regarding patients who reached adequate progression and those whose therapy was terminated prematurely was included in this analysis. Overall, median number of days of therapy to achieve adequate progression was 41·0 days. There was no evidence (P = 0·98) that the time to achieve adequate progression differed between chronic wounds, dehisced surgical wounds and surgical incision wounds. The median time to adequate progression was 41 days for chronic wounds, 44 days for dehisced surgical wounds and 40 days for surgical incision wounds.
Kaplan–Meier plots of the probability of achieving adequate progression by NPWT treatment time are presented for the chronic wounds, dehisced surgical wounds and surgical incision wounds in Figure 2. Similar traces were shown between all three indications showing that wounds treated with NPWT progressed at a similar rate despite different aetiology.
Figure 2.
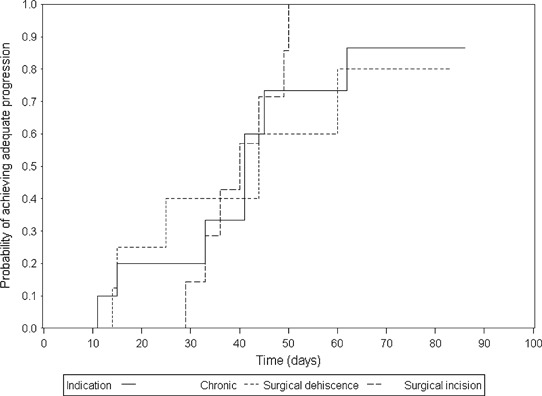
Kaplan–Meier plot showing the probability of achieving adequate progression over time in different indications. The Kaplan–Meier estimator was used to determine the median days to adequate wound progression over all indications and separately for chronic, surgical dehiscence and surgical incision indications.
Example cases
A pressure ulcer in a 66‐year‐old female on the right lower lateral leg caused by a knee immobiliser is shown in Figure 3. The patient had significant comorbidities including history of ovarian carcinoma, diabetes mellitus, hypertension, peripheral venous insufficiency, femur fracture, anaemia, peripheral neuropathy and right deep vein thrombosis. In addition, MRSA was identified at the wound site. NPWT was applied using gauze as a wound filler using the Chariker‐Jeter application technique (7), and pressure was applied at −80 mmHg for the duration of therapy. Dressings were changed twice weekly for a duration of 45 days. During this period, the wound reduced in size by 92% by secondary intention before NPWT was discontinued because it was no longer required. The wound was then adequately managed by alternative advanced wound dressings (not tracked).
Figure 3.
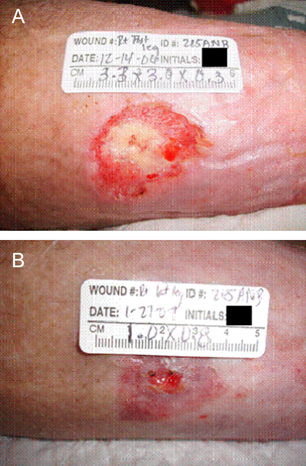
Pressure ulcer in a 66‐year‐old female. This wound was treated with NPWT at −80 mmHg for a duration of 45 days, resulting in a 92% reduction in wound area (3·3 × 3·0–1·0 × 0·8 cm) and corresponding decrease in volume (depth 0·3 to 0 cm). The wound prior to initiation of therapy is shown in (A) and after discontinuation of therapy in (B). Therapy was discontinued because of its adequate progression and ability to manage by other means.
The second case shows progression of a dehisced surgical wound following treatment with NPWT (Figure 4). A 75‐year‐old female had undergone open reduction internal fixation for a humeral fracture. Osteomyelitis developed in the wound site. Removal of the hardware was carried out, and multiple surgical debridement episodes were undertaken prior to application of the NPWT device. Comorbidities included diabetes mellitus, anaemia, depression, status post bilateral nephrectomy caused by renal cell carcinoma, end‐stage renal disease with haemodialysis and coronary artery disease. NPWT was applied using gauze as the wound filler using the Chariker‐Jeter technique (7), and pressure was applied at −80 mmHg for the duration of therapy. Dressings were changed twice weekly for 14 days in which time, an 86% reduction in wound volume was observed. The patient was discharged from the facility prior to wound closure because of the ability to manage the wound at home with alternative wound dressings.
Figure 4.
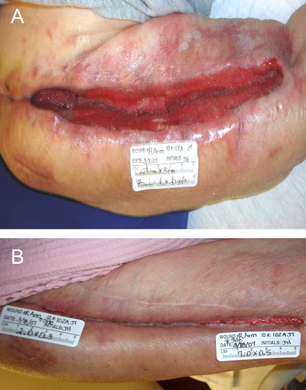
Dehiscence of surgical incision site in a 75‐year‐old female. This wound arose from a previously treated open reduction internal fixation in the arm and subsequent removal of hardware because of infection. The dehisced wound underwent several debridement episodes prior to treatment with NPWT at −80 mmHg for a duration of 15 days, resulting in a 89% reduction in wound area and corresponding decrease in volume.
Generally, the NPWT devices were well tolerated, and no major complications relating directly to its use such as wound infection were noted. No minor complications related to use of the device such as pain, maceration and bleeding were noted. Therapy was discontinued in one patient because of non compliance and patient’s unwillingness to continue treatment. This was because of the patient being unhappy with her level of mobility.
Discussion
NPWT has been widely used in recent years for the treatment of diverse wound types. Until recently, the clinical data have been limited to application of NPWT using predominantly open‐cell polyurethane (black) foam as a wound filler, although closed‐cell polyvinyl alcohol (white) foam has been used where the growth of granulation tissue into open‐cell foam causes pain and tissue damage on removal. The evidence base for the clinical efficacy of foam‐based wound fillers has been accumulating over the past 10 years 2, 6. Typically, pressures are targeted at −125 mmHg although there are no clinical data to identify an optimum pressure, and in fact in a recent report, reduction in wound volume was unrelated to pressures between −50 and −125 mmHg (12). Recently, gauze has been rediscovered as a filler material for NPWT 6, 9, 10. This communication is the first non comparative retrospective analysis of a variety of wounds treated with alternative gauze‐based NPWT systems (V1STA, Versatile‐1 or EZ‐Care systems; Smith & Nephew).
In this study, wound progression was observed in all cases with the use of NPWT. The degree and timescale of wound resolution in this study compare favourably with previous publications where the main objective for application of the NPWT device was to progress wounds as far as possible towards closure by secondary intention 12, 13. The median percentage reduction in wound volume across all indications following NPWT was measured as 88·0%. The median rate of reduction in volume per week across all indications, including patients for whom therapy was discontinued prematurely, was 15·1% per week. McCord et al. (12) observed an 80% reduction in wound volume at the end of NPWT on a paediatric patient cohort treated with open‐cell polyurethane foam (KCI, San Antonio, TX). A study on decubitus ulcers (13) showed a 51·8% reduction in volume over a 6‐week period of foam‐based negative pressure therapy, which equates to 8·6% per week.
Other studies have been conducted based on the common objective of preparing the wound bed for definitive closure by surgical flapping or grafting techniques. Studies with this objective are often conducted in a shorter time frame and resulted in a smaller overall reduction in wound size compared with this study 14, 15. For example, diabetic foot ulcer wounds treated by NPWT were successfully prepared for surgical closure (flapping and grafting) within the relatively short time span of 11 days during which time they reduced by 19% (14). Interestingly, the reduction in wound area per week (19% reduction in 11 days, equivalent to 12·1% per week) is close to the overall median reduction in area of 14·3% per week observed in this study.
In a recent study of NPWT in a paediatric population (12), overall, the treated wounds tended to reduce in volume more rapidly than the wounds observed in this study. This is most likely because of the significant comorbidities present in the present patient cohort, which are known to impact on the efficiency of wound healing. However, wounds with chronic aetiologies treated in both studies (there were a number of pressure ulcers in the paediatric study) adequately progressed to either grafting or healing by secondary intention in approximately the same length of time; 40 days (12) and 41 days for the chronic wounds group in this study, which also consisted largely of pressure ulcers. These data are remarkably consistent.
In conclusion, evidence presented in this study suggests that delivery of NPWT using a gauze‐based dressing as a wound filler material is efficacious at reducing wound volume over an appropriate length of time in medically compromised patients. Further comparative analysis would be required to assess the relative efficacy of gauze‐based NPWT systems with systems that use alternative wound filler materials such as porous foam and the advantages and disadvantages of different wound fillers in different clinical situations.
Acknowledgements
We thank Laura Alvis and Kristi Ukley for data collection with some of the case studies. We also thank Lisa Keyser (Independent Wound Consultant, Nashville, TN, USA, for assistance and Robin Martin (Smith & Nephew) for help with the manuscript. The authors are employees of Smith & Nephew.
This study was performed when PEC was an independent clinical practitioner at Bethany Health and Rehab Center and Trevecca Health and Rehab Center, both part of the Avalon Health Care Group located in Nashville, TN, USA.
References
- 1. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563–77. [PubMed] [Google Scholar]
- 2. Argenta LC, Morykwas MJ, Marks MW, DeFranzo AJ, Molnar JA, David LR. Vacuum‐assisted closure: state of clinic art. Plast Reconstr Surg 2006;117:127S–142S. [DOI] [PubMed] [Google Scholar]
- 3. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997;38:553–62. [DOI] [PubMed] [Google Scholar]
- 4. Plikaitis CM, Molnar JA. Sub‐atmospheric pressure wound therapy and the vacuum‐assisted closure device: basic science and current clinical successes. Expert Rev Med Devices 2006;3:175–84. [DOI] [PubMed] [Google Scholar]
- 5. Morykwas MJ, Simpson J, Punger K, Argenta A, Kremers L, Argenta J. Vacuum‐assisted closure: state of basic research and physiologic foundation. Plast Reconstr Surg 2006;117 (7 Suppl):121S–126S. [DOI] [PubMed] [Google Scholar]
- 6. Hunter JE, Teot L, Horch R, Banwell PE. Evidence‐based medicine: vacuum‐assisted closure in wound care management. Int Wound J 2007;4:256–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chariker ME, Jeter KF, Tintle TE, Ottsford JE. Effective management of incisional and cutaneous fistulae with closed suction wound drainage. Contemp Surg 1989;34:59–63. [Google Scholar]
- 8. Campbell PE. Surgical wound case studies with the versatile‐1 wound vacuum system for negative pressure wound therapy. J Wound Ostomy Continence Nurs. Int Wound J 2006;33:176–80. [DOI] [PubMed] [Google Scholar]
- 9. Miller MS, Ortegon M, McDaniel C. Negative pressure wound therapy: treating a venomous insect bits. Int Wound J 2007;4:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Timmons J. The use of the versatile‐1 wound vacuum system to treat a patient with a challenging grade 3 pressure ulcer in continuing care. Wounds 2006;2:125. [Google Scholar]
- 11. Miller MS, Lowery CA. Negative pressure wound therapy: a rose by any other name. Ostomy Wound Manage 2005;51:44–9. [PubMed] [Google Scholar]
- 12. McCord SS, Naik‐Mathuria BJ, Murphy KM, McLane KM, Gay AN, Bob Basu C, Downey CR, Hollier LH, Olutoye OO. Negative pressure therapy is effective to manage a variety of wounds in infants and children. Wound Repair Regen 2007;15:296–301. [DOI] [PubMed] [Google Scholar]
- 13. Ford CN, Reinhard ER, Yeh D, Syrek D, De Las Morenas A, Bergman SB, Williams S, Hamori CA. Interim analysis of a prospective, randomized trial of vacuum‐assisted closure versus the healthpoint system in the management of pressure ulcers. Ann Plast Surg 2002;49:55–61. [DOI] [PubMed] [Google Scholar]
- 14. Etöz A, Özgenel Y, Özcan M. The use of negative pressure wound therapy on diabetic foot ulcers: a preliminary controlled trial. Wounds 2004;16:264–9. [Google Scholar]
- 15. Vuerstaek JD, Vainas T, Wuite J, Nelemans P, Neumann MH, Veraart JC. State‐of‐the‐art treatment of chronic leg ulcers: a randomized controlled trial comparing vacuum‐assisted closure (V.A.C.) with modern wound dressings. J Vasc Surg 2006;44:1029–37. [DOI] [PubMed] [Google Scholar]


