ABSTRACT
This study investigated the number and type of chronic wounds actually treated by Dutch nursing home physicians (NHPs). It was also the goal to know how many of the treated chronic wounds they considered infected. The NHPs were asked to choose and rank their top five out of several provided criteria for chronic wound infection. After this, the ranking was compared with the choices an international multidisciplinary Delphi group of wound experts made in 2005. A cross‐sectional descriptive survey was conducted using the information from a self‐reported questionnaire in a representative sample of Dutch NHPs. About 361 NHPs (25%) were sent a questionnaire. Of the 361 physicians, 139 (38.5%) filled in and returned the questionnaire of which 121 were valid. Of the NHPs, 73.5% actually treated at least one chronic pressure ulcers (PU), whereas 26.5% did not treat any. All NHPs treated three or less chronic post‐surgical wounds, whereas 68.4% treated none. Chronic venous leg ulcers, arterial ulcers and diabetic ulcers scored infrequently and less than the other two sorts of chronic wounds. Of the Dutch NHPs, 53% considered that none of the PU infected. The other chronic wounds were judged far less frequently to be infected. Dutch NHPs appeared to use more ‘traditional’ criteria such as ‘puss/abscess' and ‘malodour’ to identify infection and did not change their criteria by wound type. According to this study, NHPs do not frequently see many chronic wounds. The most frequent type of wounds treated was PU. For NHPs, the identification of infection of all types of chronic wounds is difficult. The use of criteria that is not in line with consensus documents may lead to ineffective treatment and even seriously damage patients: the clinical identification of infection is still dependent on experts' opinion. Further research on triggers for the suspicion of wound infection and the development of an evidence‐based guideline is necessary.
Keywords: Chronic wounds, Wound infection, Clinical criteria, Nursing home, Dutch nursing home physicians
INTRODUCTION
Wound healing can be defined as the process of tissue repair involving the tissue response to injury (1). The normal wound‐healing process can be divided into a series of artificially defined events. It starts with haemostasis and then involves an inflammatory response, the formation of connective tissue, covering of the wound with epithelium and remodelling of the wound. These different phases normally proceed without difficulty and uncomplicated small wounds are expected to heal and reepithelialise within a period of 3 weeks (1).
However, the process of normal wound healing can be fraught with problems and altered at many points. Both intrinsic and extrinsic factors may contribute to wound recalcitrance. Determining these factors is essential in formulating a successful treatment plan (2). Although an accurate history may help to determine the initial aetiology of the wound, chronicity of dermal wound healing seems more related to secondary factors such as infection and vascular insufficiency (3). Chronic wounds affect more than 1% of the UK population and cost society at least £1 billion per year (4). The costs, both financially and emotionally, are indisputably high.
In Dutch nursing homes, approximately 44 000 new patients are admitted each year. In general, these are very frail and disabled elderly people characterized by considerable care dependency, (co)morbidity and frequent disabilities and at an average age of 80 years 5, 6.
Since 1968, the Dutch nursing home has evolved from a place for discharged hospital patients for chronic nursing care to a facility where nursing is integrated with continuing paramedical and medical care. Patients are supported to reach an optimal level of functioning. Since 1989, ‘nursing home medicine’ is an officially recognized medical discipline in the Netherlands, unique in the world 6, 7.
Chronic wounds tend to occur as result of poor local factors (arterial insufficiency, venous hypertension and trauma) and/or presence of a systemic disease, such as diabetes mellitus or rheumatoid arthritis (8). Wound infection interferes with normal wound healing and occurs when microbes invade viable tissue. This causes a prolonged and excessive inflammatory response, delays collagen synthesis, retards epithelialisation and more tissue damage 8, 9, 10, 11, 12. If wound infection is allowed to progress naturally, local infection may place the patient at risk for systemic complications, sepsis and osteomyelitis and finally may lead to death.
Wound infection and associated delayed healing present a considerable challenge for clinicians, with regard to the clinical identification of infection and the choice of an appropriate treatment. Pain, erythema, edema and warmth are known as ‘classical’ signs of inflammation. In contrast to the often overt signs and symptoms of infection in acute wounds, in chronic wounds these signs and symptoms may be quite subtle (13).
For quantifying bacteria in wounds, quantitative tissue biopsies or swab samples can be used. Research by Bendy et al. and Robson et al. formed the basis of the ‘105 guideline’ in wound care 14, 15, 16, 17. Meanwhile ‘the 105 guideline’ has been disputed as an indicator of chronic wound infection 18, 19. The number of micro organisms seems less significant than the presence of particular species with the potential of quorum sensing to improve their virulence and persistence 10, 18, 19
In order to explore the more subtle signs and symptoms associated with identifying infection in chronic wounds, Cutting et al. (2005) recruited an international multidisciplinary Delphi group of 54 members. Panel members, allocated to one of six panels related to their individual area of expertise, were asked to list the clinical indicators of infection relevant to one wound type group. Criteria were grouped in three bands according to their scores: 4–5 (important), 6–7 (very important), 8–9 (diagnostic). The structure of the bandings was driven by the data (20). This paper applies this work to the nursing home sector in the Netherlands.
This article focuses on wounds that have become chronic as a result of infection, with special reference to their occurrence and assessment in the nursing home setting in the Netherlands. The following questions will be addressed:
-
•
What is the prevalence of chronic pressure ulcers (PU), chronic post‐traumatic wounds, chronic venous ulcers (VLU) and chronic arterial and diabetic ulcers in Dutch nursing homes treated by Dutch nursing home physicians (NHPs)?
-
•
How many of the treated chronic wounds are considered by NHPs to be infected?
-
•
Are the top five of clinical signs and/or symptoms of infection for different types of chronic wounds ranked by Dutch NHPs comparable with the choice an international multidisciplinary Delphi group made in 2005 (20)?
METHODS
A cross‐sectional descriptive survey was conducted using the information from self‐reported questionnaires in a representative sample of all 1433 Dutch NHPs, taken from the list of the Dutch Association of Nursing Home physicians (N.V.V.A at Utrecht, the Netherlands). Every fifth record from the list of Dutch Association members was taken systematically. Between December 2006 and March 2007, 361 (25%) sampled NHPs were sent the questionnaire together with an accompanying supportive explanatory letter.
The questionnaire was designed to collect quantitative and qualitative information. The NHPs were asked to indicate the number of chronic PU, chronic post‐surgical wounds, chronic venous leg ulcers, chronic arterial ulcers and chronic diabetic ulcers they treated. The other questions addressed NHPs' knowledge in diagnosing chronic wound infection. NHPs were asked to consider the clinical symptoms of chronic wound infection and to choose their five most specific signs and symptoms per chronic wound type from a selected list of criteria. The signs and symptoms selected were the same as those used in the European Wound Management Association position document (20).
As patients did not participate, there was no need for ethical approval or permission from a Medical Ethics Committee under the ethical framework used in the Netherlands. However, the anonymity and privacy of participants was respected and kept confidential and secure.
The data from the questionnaire were processed using SPSS13 for Windows, 2004 (SPSS Inc, Chicago, IL, USA).
RESULTS
Of the 361 physicians, 139 (38.5%) filled in and returned the questionnaire. Of the respondents, 15 (4.2%) were excluded because they did not work as a NHP. One envelope (0.25%) was returned unopened and one respondent (0.25%) returned the questionnaire unanswered and 19 (5.3%) of the returned questionnaires were not filled in completely. Finally, 121 (33.5%) of the returned questionnaires were useful (Fig. 1). From the responders, 66% were females and 34% were males. Of the non responders, 61% were females and 39% were males. Of all Dutch NHPs (1177) in 2005, 58% were females and 42% were males (21). The answered questionnaires were returned equally from all the countries of the Netherlands.
Figure 1.
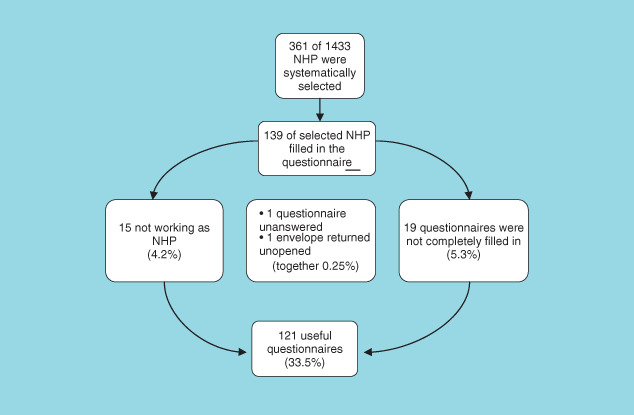
Flow diagram of responding nursing home physicians to the questionnaire.
Quantitative assessment of (infected) chronic wounds
Pressure ulcers
Of the responding Dutch NHPs, 73.5% actually treated at least one chronic PU at the time of the survey, whereas 26.5% did not have any on their caseload (Table 1). Of the NHPs, 53% considered none of the chronic PU to be infected (Table 2).
Table 1.
Type and frequency of chronic wounds treated by Dutch nursing home physicians
| Number of patients with chronic wounds | Pressure ulcers (%) | Post‐surgical wounds (%) | Venous ulcers (%) | Arterial ulcers (%) | diabetic ulcers (%) |
|---|---|---|---|---|---|
| 0 | 26.5 | 68.4 | 76.9 | 82.9 | 80.2 |
| 1 | 20.5 | 21.3 | 20.5 | 16.2 | 14.7 |
| 2 | 19.7 | 10.3 | 2.6 | 0.8 | 4.3 |
| 3 | 16.2 | — | — | — | 0.9 |
| 4 | 10.3 | — | — | — | — |
| 5 | 0.8 | — | — | — | — |
| >5 | 6.0 | — | — | — | — |
Table 2.
Chronic wounds thought to be infected
| Number of patients with chronic wounds | Pressure ulcers (%) | Post‐surgical wounds (%) | Venous ulcers (%) | Arterial ulcers (%) | Diabetic ulcers (%) |
|---|---|---|---|---|---|
| 0 | 53 | 83.8 | 88.1 | 90.5 | 91.4 |
| 1 | 27.4 | 12 | 11.1 | 8.6 | 6.8 |
| 2 | 11.1 | 4.2 | 0.8 | 0.9 | 0.9 |
| 3 | 4.3 | — | — | — | 0.9 |
| 4 | 3.4 | — | — | — | — |
| 5 | 0.8 | — | — | — | — |
| > 5 | — | — | — | — | — |
Post‐surgical wounds
All NHPs treated three or less chronic post‐surgical wounds, whereas 68.4% of the NHPs treated none (Table 1). Of the NHPs, 12% considered one of the chronic post‐surgical wounds infected, whereas 83.8% considered none to be infected (Table 2).
Chronic VLU
Of the NHPs, 97.4% treated one or less chronic VLU, whereas 76.9% treated none (Table 1), and 88.1% of the NHPs considered none of the treated chronic VLU to be infected (Table 2).
Chronic arterial ulcers
Almost all NHPs (99%) treated two or less chronic arterial ulcers (Table 1) and 90.5% considered none of the arterial ulcers to be infected (Table 2).
Chronic diabetic ulcers
Many NHPs (94.9%) treated one or less chronic diabetic ulcer, whereas 80.2% treated none (Table 1). In 91.4% the NHPs considered none of the chronic diabetic ulcers to be to be infected (Table 2).
Qualitative assessment of signs and/or symptoms of chronic wound infection
About 121 Dutch NHPs choose their top five of signs and/or symptoms of infection for different chronic wound types, from a list consisting of 25 symptoms discussed earlier by Cutting et al. (Table 3) (20). In Table 3, the ranking of chosen signs and/or symptoms by NHPs is marked by colours. Different symptoms scoring the same frequency are shown simultaneously.
Table 3.
The five most important signs and symptoms of infection by wound type
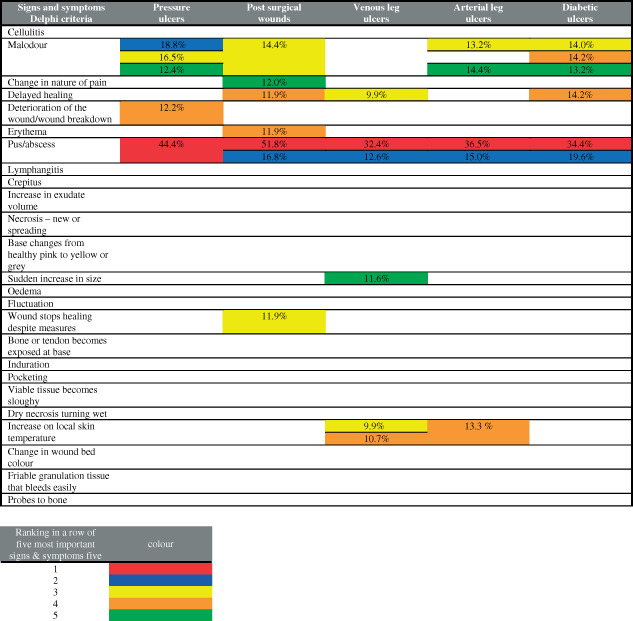
|
Chronic PU
Of the NHPs, 44.4% judged ‘pus/abscess' as the number one symptom they used to suspect wound infection, and 18.8%, 16.5% and 12.4% of the NHPs rated ‘malodour’ in the ranking of either position two, three or five. ‘Deterioration of the wound/wound breakdown’ became number four in the ranking because 12.2% of the NHPs choose this sign (Table 3).
The ranking of the first two signs and/or symptoms was missed by four NHPs, whereas six did not complete the ranking concerning the third and fourth symptom. Eight NHPs did not mention their ‘least important' symptom.
Chronic post‐surgical wounds
The responding NHPs regarded ‘pus/abscess' as most specific sign/symptom of infection: 51.8% chose it as the number one and 16.8% set it at number two; 14.4% of the NHPs ranked ‘malodour’ as third; and ‘Erythema’ and ‘delayed healing’ were both chosen by 11.9%, as the fourth symptom, whereas ‘change in nature of pain’ was set as the fifth symptom by 12.0% of the NHPs (Table 3).
Respectively, 9, 8, 10, 12 and 13 of the responding NHPs did not fill in their ranking of each of the five most important symptoms.
Chronic VLU
Of the NHPs, 32.4% considered ‘pus/abscess' as the most specific symptom for infection of a chronic VLU and 12.6% also ranked ‘pus/abscess' as the second symptom. ‘Increase in local skin temperature’ and ‘delayed/arrested wound healing despite measures' were both chosen in the same measure by 9.9% and became the third most important symptom.
Of the NHPs, 10.7% choose ‘increase in local skin temperature’ as number four, whereas ‘sudden increase in size’ was chosen as number five by 11.6% (Table 3).
Ten NHPs did not fill in their choice of symptoms for the first three ranks. Eleven and twelve of the NHPs failed to choose rank four and five.
Chronic arterial ulcers
‘Pus/abscess' was considered as the most specific symptom by 36.5% of the Dutch NHPs, demonstrating the presence of infection, and was also chosen as the second symptom by 15% of the NHPs. ‘Malodour’ was ranked as third and fifth symptoms by 13.2% and 14.4% of the NHPs, respectively. ‘Increase of local skin temperature’ was chosen by 13.3% as the fourth most important symptom (Table 3).
Ranks one and two were not filled by 14 NHPs, and rank three was missed by 15 responders; 16 NHPs did not mention their fourth symptom, whereas 17 failed to rank the fifth symptom.
Chronic diabetic ulcers
Of the NHPs, 34.4% considered ‘pus/abscess' as the most specific symptom, indicating the actual presence of infection and 19.6% choose ‘pus/abscess' also as their number two. ‘Malodour’ was rated as the third, fourth and fifth ranks by 14.0%, 14.2% and 13.2% respectively. ‘Delayed/arrested wound healing despite measures' was also ranked in third position by 14.2% of the NHPs (Table 3).
Do the criteria for chronic wound infection match with those of Cutting et al.?
Chronic PU
The ranking of the sample of Dutch NHPs did not match with the criteria of Cutting et al. Unlike Cutting et al., ‘pus/abscess' and ‘malodour’ were considered as important indicators of chronic pressure ulcer infection (20).
Chronic post‐surgical wounds
The choice of the sample of NHPs matched with the criteria of Cutting et al. (20). The choice for ‘pus/abscess' was in correspondence with the choice of the experts of the Delphi group. The Delphi experts also mentioned ‘cellulitis’ to be a specific symptom of infection. ‘Malodour’ and ‘erythema’ scored less in both groups and seemed mutually comparable between NHPs and the Delphi Panel experts.
Chronic VLU
The ranking of the Dutch NHPs did not match with the criteria of Cutting et al. (20). Unlike the Delphi experts, Dutch NHPs regarded ‘pus/abscess' to be more diagnostic than ‘cellulitis’. For both the Dutch NHPs and Delphi experts, the symptoms ‘delayed/arrested wound healing despite measures' and ‘increase in local skin temperature’ had only additional value in the diagnosis of chronic VLU infection.
Chronic arterial ulcers
For Cutting et al., both the symptoms ‘pus/abscess' and ‘cellulitis’ were equally important in the suspicion of infection of a chronic arterial ulcer. The NHPs held ‘pus/abscess' to be far more important and did not mention ‘cellulitis’ in the ranking. The NHPs scored ‘malodour’ higher than the Delphi experts.
Chronic diabetic ulcers
The NHPs chose both ‘pus/abscess' and ‘malodour’ as the most important symptoms. Besides ‘pus/abscess', the Delphi experts also gave ‘cellulitis’, ‘lymphangitis’, ‘phlegmon’ and ‘purulent exudate’ the highest rank. Unlike the Dutch NHPs, the Delphi experts considered ‘malodour’ less important.
DISCUSSION
Considering the turnover of 44 000 patients in Dutch nursing homes each year, it is striking that no scientific literature is available on chronic wounds and infection for this patient group.
This study gives an indication of the occurrence of chronic PU, chronic post‐traumatic wounds, chronic venous leg ulcers, chronic arterial and chronic diabetic ulcers in Dutch nursing homes.
Except for PU, the prevalence of (chronic) wounds in the Netherlands is unknown. Since 1998, the prevalence of PU is measured in nursing homes by the Dutch National Prevalence Measurement of Care problems (LPZ). In 2008, more than one third (119) of Dutch nursing homes participated. Taking into account the counted prevalence of 6.1% by the LPZ (that is without grade I) for Dutch nursing homes in 2008, the results of this study match with those of the LPZ (22).
In the Netherlands, 26 000 hip prostheses and nearly 19 000 knee prostheses are completed each year. In 2007, 5400 orthopaedic patients were transferred for rehabilitation from Dutch hospitals to nursing homes 6, 23. The indicated small number of chronic post‐surgical wounds is in contradiction to the substantial number of patients who go through a long‐term rehabilitation program in Dutch nursing homes.
From a health district in the United Kingdom and from a metropolitan population in Perth, Western Australia, a prevalence of 1.48–3.3/1000 patients for chronic venous leg ulcers can be derived 24, 25, 26 Given the specificity of the population of frail and disabled elderly in Dutch nursing homes, it may be expected that Dutch NHPs should treat chronic venous leg ulcers more often than is indicated.
The prevalence of diabetes mellitus in the Netherlands was estimated to be over 600 000 patients in 2003 with more than 100/1000 for people over 60 years of age (27). Dutch NHPs and Dutch family physicians may underestimate the problem of diabetic ulcers given that they indicate that they do not treat many chronic diabetic ulcers (28).
The results of this study show a clear difference in suspicion of infection between chronic PU and other types of chronic wounds. Although the cause of this difference is unknown, it is a fact that NHPs are professionally more focussed on PU than on other types of wounds. The Dutch Health Care Inspectorate declared the prevalence of PU as a quality indicator and since 2003, in contrast to other types of wounds, an occupational group guideline for Dutch NHPs on PU exists 29, 30.
Furthermore, Dutch NHPs appear to neglect other criteria in chronic wound types as outlined by Cutting et al. (20). They also tend to hold tight to more ‘traditional’ criteria of chronic wound infection as ‘pus/abcess' and ‘malodour’. It seems that the available scientific literature in wound care has not yet been implemented. Alternatively, the answers given by Cutting et al. may not be indisputable with the ‘right’ answers and reflect the difficulties associated with diagnosing infection in complex wounds (20). The international guideline of the World Union of Wound Healing Society published triggers for suspecting wound infection and focuses merely on the difference between acute and chronic wounds. ‘Purulent discharge’ and ‘malodour’ are especially shown as triggers for suspecting a localised infection in an acute wound (31).
The methodology used in this study is limited by the ambiguity of the definitions used and the term ‘importance’ in relation to ranking of the NHPs. However, the results of the study are representative of Dutch NHPs with regard to the equal distribution of males and females of responders and non responders.
CONCLUSIONS
According to this study, NHPs do not frequently see many chronic wounds. The greater numbers that are treated are PU. For NHPs, the identification of infection of all types of chronic wounds is difficult.
To provide a more accurate measure of the prevalence of (infected) chronic wounds in Dutch nursing homes, further research is needed.
The use of unreliable criteria may lead to ineffective treatment and even seriously damage patients, yet the clinical identification of infection is still dependent on experts' opinion. Further research on triggers for suspicion of wound infection and the development of an evidence‐based guideline is necessary.
CONFLICT OF INTEREST
The authors of this article declare that there is no conflict of interest or any financial and personal relationships with other people or organisations that had an inappropriate influence (bias) on this work.
ACKNOWLEDGEMENTS
This study was completed as part of an MSc in Wound Healing and Tissue Repair at Cardiff University. The authors thank Mrs Vanessa Jones for her help during the initial development of the project.
REFERENCES
- 1. Falanga V, Zitelli JA, Eaglstein WH. Wound healing. J Am Acad Dermatol 1988;19:559–63. [DOI] [PubMed] [Google Scholar]
- 2. Seaman S. Considerations for the global assessment and treatment of patients with recalcitrant wounds. Ostomy Wound Manage 2000;46(Suppl 1A): 10S–29S. [PubMed] [Google Scholar]
- 3. Mostow EN. Diagnosis and classification of chronic wounds. Clin Dermatol 1994;12:3–9. [DOI] [PubMed] [Google Scholar]
- 4. Thomas DW, Harding KG. Wound healing. Br J Surg 2002;89:1203–5. [DOI] [PubMed] [Google Scholar]
- 5. Ribbe MW, Van Mens JT. Characteristics of nursing home patients. Ned Tijdschr Geneeskd 1986;130:642–6 [in Dutch]. [PubMed] [Google Scholar]
- 6. Schols JMGA, Crebolder HFJM, Van Weel C. Nursing home and nursing home physician: the Dutch experience. J Am Med Dir Assoc 2004;5:207–12. [DOI] [PubMed] [Google Scholar]
- 7. De Pijper NF, Ribbe MW, Stoop JA. Nursing home medicine, a new medical specialism. Ned Tijdschr Geneeskd 1995;139:1820–3 (in Dutch). [PubMed] [Google Scholar]
- 8. Edwards R, Harding KG. Bacteria and wound healing. Curr Opin Infect Dis 2004;17:91–6. [DOI] [PubMed] [Google Scholar]
- 9. Robson MC, Stenberg BD, Hegger JP. Wound healing alterations caused by infections. Clin Plast Surg 1990;17:485–92. [PubMed] [Google Scholar]
- 10. Mast BA, Schultz GS. Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Repair Regen 1996;4:411–20. [DOI] [PubMed] [Google Scholar]
- 11. Cooper RA. The contribution of microbial virulence to wound infection. Br J Nurs 2002;11(Suppl): 10–14. 11845487 [Google Scholar]
- 12. Ovington L. Feature: Bacterial toxins and wound healing. Ostomy Wound Manage 2003;49(7A Suppl): 8–12. [PubMed] [Google Scholar]
- 13. Dow G. Bacterial swabs and the chronic wound: when, how and what do they mean. Ostomy Wound Manage 2003;49:8–13. [PubMed] [Google Scholar]
- 14. Bendy RH, Nuccio PA, Wolfe E, Collins B, Tamburro C, Glass W, Martin CM. Relationship of qualitative wound bacterial counts to healing of decubiti. Effects of topical gentamicin. Antimicrob Agents Chemother 1964;4:147–55. [PubMed] [Google Scholar]
- 15. Robson MC, Lea CE, Dalton JB, Heggers JP. Quantitative bacteriology and delayed wound closure. Surg Forum 1968;19:501–2. [PubMed] [Google Scholar]
- 16. Robson MC, Heggers JP. Bacterial quantification of open wounds. Mil Med 1969;134:19–24. [PubMed] [Google Scholar]
- 17. Robson MC, Heggers JP. Delayed wound closures based on bacterial counts. J Surg Oncol 1970;2:379–83. [DOI] [PubMed] [Google Scholar]
- 18. Bowler P. Bacterial growth guideline: reassessing its clinical relevance in wound healing. Ostomy Wound Manage 2003;49:44–53. [PubMed] [Google Scholar]
- 19. Bowler P. Progression towards healing: wound infection and the role of advanced silver‐containing Hydrofiber® dressing. Ostomy Wound Manage 2003;49:2–5. 12856288 [Google Scholar]
- 20. European Wound Management Association. Position document: identifying criteria for wound infection. London: Medical Education Partnership Ltd, 2005. [Google Scholar]
- 21. Capaciteitsplan voor de medische en tandheelkundige vervolgopleidingen. Advies over de initiële opleiding geneeskunde. www.ggd.nl/ggdnl/uploaddb/downl_object.asp?atoom=34314&VolgNr=269 2005.
- 22. Halfens RJG, Schols JMGA, Meijers JMM, Neyens JCL, Offermans MPW. Rapportage resultaten Landelijke Prevalentiemeting Zorgproblemen. Maastricht: Datawyse, Universitaire Pers, 2008. [Google Scholar]
- 23. Peerenboom PBG, Spek J, Zekveld G, Cools HJM, Van Balen R, Hoogenboom MJ. Revalidatie in de AWBZ. Omvang, aard en intensiteit Leusden: ECT Tangram,/PHEG/LUMC Verpleeghuisgeneeskunde, 2008. [Google Scholar]
- 24. Cornwall J, Doré C, Lewis J. Leg ulcers: epidemiology and aetiology. Br J Surg 1986;73:693–6. [DOI] [PubMed] [Google Scholar]
- 25. Baker S, Jopp‐McKay A, Hoskin S, Thompson P. Epidemiology of chronic venous ulcers. Br J Surg 1991;78:864–7. [DOI] [PubMed] [Google Scholar]
- 26. Callam J, Ruckley C, Harper D, Dale J. Chronic ulceration of the leg: extent of the problem and provision of care. BMJ 1985;290:1855–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ubink‐Veltmaat L, Bilo HJG, Groenier KH, Houweling ST, Rischen RO, Meyboom‐de Jong B. Prevalence, incidence and mortality of type 2 diabetes mellitus revisited. A prospective population‐based study in the Netherlands (ZODIAC‐1). Eur J Epidemiol 2003;18:793–800. [DOI] [PubMed] [Google Scholar]
- 28. Statius Muller I, De Grauw WJC, Van Gerwen WHEM, Bartelink ML, Van Den Hoogen HJM, Rutten GEHM. Foot ulceration and lower limb amputation in type 2 diabetic patients in Dutch primary health care. Diabetes Care 2002;25:570–4. [DOI] [PubMed] [Google Scholar]
- 29. Nederlandse Vereniging Van Verpleeghuisartsen, editors. Tripartiete multidisciplinaire richtlijn: Samenwerking en logistiek rond decubitus Duiven: Drukkerij Tamminga, 2003;[in Dutch]. [Google Scholar]
- 30. Rapport Inspectie voor de gezondheidszorg. Kwaliteitsverbetering mogelijk door actief gebruik indicatoren. Resultaten inspectieformulier verpleeghuiszorg, verzorgingshuiszorg en thuiszorg. Den Haag, 2006;[in Dutch].
- 31. WUWHS. Infection: principles of best practice wound infection in clinical practice. An international consensus London: MEP Ltd, 2008. [Google Scholar]


