Abstract
The purpose of this project was to evaluate associations of increasing diabetic foot surgery stage with postoperative outcome. This project, designed as a retrospective cohort model, was conducted at three large, urban referral‐based diabetic foot clinics. The investigators abstracted medical records from 180 patients with diabetes, 76·1% male, aged 57·8 ± 11·2 years, falling equally into four classes of a previously reported diabetic foot surgery classification system. These classes included class 1 (elective), class 2 (prophylactic), class 3 (curative) and class 4 (emergency). There was a significant trend towards increasing risk of ulceration/reulceration (χ2 trend= 17·8, P= 0·0001), peri‐postoperative infection (χ2 trend= 96·9, P= 0·0001), all‐level amputation (χ2 trend= 41·7 P= 0·001) and major amputation (χ2 trend= 8·6, P= 0·003), with increasing class of foot surgery. The results of this study suggest that a non vascular foot surgery classification system including variables such as the presence or absence of neuropathy, an open wound and acute infection may be predictive of peri‐ and postoperative complications. This may assist the surgeon in better identifying risk when determining a rationale for and type of surgery in persons with diabetes.
Keywords: Amputation, Classification, Risk, Surgery, Wound
Introduction
Over the past decade, there have been numerous descriptive studies detailing various surgical techniques in the treatment of the high‐risk diabetic foot (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16). While there has been a relative dearth of studies evaluating specific procedures, relatively recent studies have suggested some potential benefit from judicious intervention in this high‐risk diabetic foot population (17, 18, 19).
In response to the increasing attention to this area of intervention and the lack of a concise nomenclature of indications, a diabetic foot surgery classification system was proposed in 2003 (20). This system divides non vascular diabetic foot surgery into four classes: elective, prophylactic, curative and emergency (Figure 1). There have been no studies in the medical literature evaluating the ability of this system to predict key outcomes (i.e. risk for amputation, peri/postoperative infection, ulceration). Therefore, the purpose of this project was to evaluate the associations of increasing diabetic foot surgery stage with postoperative outcome.
Figure 1. Types of initial surgery performed.
Methods
This project, designed as a retrospective cohort model, was conducted at three large, urban referral‐based diabetic foot clinics. Medical records were abstracted from 180 patients with diabetes, 76·1% male, aged 57·8 ± 11·2 years, who met the following criteria: 1) a diagnosis of diabetes by their primary care physician, 2) the ability to ambulate freely without the assistance of a wheelchair and 3) at least 1 year of reliable follow‐up information. All foot surgeries performed on patients were classified using the aforementioned diabetic foot surgery classification system (Table 1) (20). Of all the procedures done, 45 consecutive patients receiving foot surgery from each of the four classes of surgery who met the criteria for assignment into that specific surgery class in addition to the above‐mentioned entry criteria were selected. These included class 1 (elective), class 2 (prophylactic), class 3 (curative) and class 4 (emergency). Data were abstracted over a 5‐year period for procedures fitting the above criteria.
Table 1.
Classification of non vascular diabetic foot surgery
| Class | Type | Definition |
|---|---|---|
| 1 | Elective | Procedure performed on patient with protective sensation intact to eliminate pain or to improve function |
| 2 | Prophylactic | Procedure performed on patient with protective sensation absent but no open wound to reduce deformity and reduce occurrence/recurrence |
| 3 | Curative | Procedure performed on patient with an open wound with the goal of promoting healing and reducing risk for recurrence |
| 4 | Emergency | Procedure performed with goal of limiting the spread of limb‐ or life‐threatening infection |
Patients were excluded if they had a diagnosis of clinically significant vascular disease. Vascular status was evaluated by pedal pulse palpation. The diagnosis of ischaemia was standardised in the facilities where data were abstracted. This diagnosis was made by the absence of more than one foot pulse or a non audible signal on Doppler ultrasonography of the dorsalis pedis or posterior tibial pulses the affected extremity. This method of evaluation, while arguably not as sensitive as other non invasive methods such as transcutaneous oximetry, segmental extremity pressure studies or laser Doppler flowmetry, has the benefit of having been performed systematically on all patients in this study 21, 22, 23, 24.
Soft tissue or bone infection was a clinical diagnosis made by the treating physician at the time of assessment. As per standard protocol, the diagnosis of infection was consistent with the current criteria described by the International Working Group on the Diabetic Foot (25). This includes the presence of purulence, advancing cellulitis or two or more other local signs of inflammation. Major versus minor amputation was defined as surgery performed above or below the ankle. The period of evaluation for infection included the period from time to surgery to wound healing or amputation.
Patients in all categories were treated in a standardised fashion per the protocol followed in the high‐risk diabetic foot centres where treatment was rendered. All procedures were performed under local anaesthesia and monitored sedation. These procedures were performed by surgeons with similar training. Postoperative care for all patients was identical, with the first dressing change performed at 2 days postoperatively and weekly thereafter. Elective, prophylactic and curative postoperative wounds were dressed with moisture‐retentive gauze and were not disturbed between weekly postoperative visits. On postoperative visit, three (at 2 weeks) sutures were removed, based on clinician’s assessment. Patients were then switched to a daily dressing change regime until the wound healed. Patients were offloaded in a standard fashion using a DH pressure‐relief walker or sandal (Royce Medical, Incorporated, Camarillo, CA, USA). These devices were converted into ‘instant total contact casts’ at the discretion of the attending clinician 26, 27, 28. Patients who underwent emergency (class 4) surgery received daily dressing changes during the immediate postoperative period.
Care following healing was according to the standardised protocols in place at the treating clinics. All healed subjects were assessed for appropriate footgear by the podiatrist and prosthetics/pedorthics team, as required. This footgear consisted of either comfort shoes or prescriptive‐depth inlay shoes with sufficient room in the toebox to accommodate an accommodative pressure‐reducing insole. Patients were followed‐up every 2 months following healing for foot examination and shoe gear checks.
At the aforementioned 1‐year period, several outcomes were analysed, including proportion of infections, reulceration and amputation at 1 year. Foot infection was defined clinically, by criteria consistent with the International Working Group guidelines (25) i.e. the presence of purulence or at least two local signs or symptoms of inflammation. These criteria were, in all cases, evaluated by the attending clinician.
Determination of the sample size to abstract was based on the data accumulated from postoperative infection rates of the previous studies of prophylactic and curative procedures. These rates have ranged from 0 to 14% for prophylactic and up to 40% for curative procedures 3, 18, 29, 30. Therefore, to identify a 35% difference in postoperative infection between prophylactic and curative procedures (classes 2 and 3), a sample size of 41 was required in each group, yielding a power (beta) exceeding 90% and an alpha of 5%. The authors therefore felt comfortable with abstracting data for 45 subjects in each group to allow for the considerable variation in previous reports.
To evaluate the differences in continuous variables among the four foot surgery groups, a Tukey range test for multiple comparisons was used. To evaluate the potential trends towards increasing prevalence of ulceration, infection and amputation based on increasing foot surgery class, a chi‐squared test for trend (χ2 trend) was used (31). All data were reported as mean ± standard deviation unless otherwise stated. For all analyses, the alpha was set at 0·05.
Results
Descriptive characteristics for this population are outlined in Table 2. The types of procedures performed are outlined in Figure 1. There was not a significant difference in age, gender or duration of diabetes between foot surgery classes 2–4. However, persons receiving class 1 procedures were younger than their higher risk counterparts (P= 0·001 for all between‐class associations). Additionally, persons receiving class 1 procedures had significantly lower glycosylated haemoglobin than did persons receiving either class 3 (P= 0·04) or class 4 (P= 0·05).
Table 2.
Population descriptive statistics
| Class 1 | Class 2 | Class 3 | Class 4 | Total | |
|---|---|---|---|---|---|
| N | 45 | 45 | 45 | 45 | 180 |
| Age (years) | 50·9 ± 9·8 | 59·5 ± 11·3 | 59·7 ± 9·7 | 61·1 ± 11·4 | 57·8 ± 11·2 |
| Gender (% male) | 77·8 | 75·6 | 71·1 | 80·0 | 76·1 |
| Glycosylated haemoglobin (%) | 8·4 ± 1·0 | 8·6 ± 0·9 | 9·0 ± 1·0 | 9·0 ± 1·2 | 8·8 ± 1·1 |
| Duration of diabetes mellitus (years) | 11·2 ± 6·4 | 13·4 ± 6·4 | 12·1 ± 6·6 | 12·5 ± 6·3 | 12·3 ± 6·4 |
General outcomes associated with persons falling into the four foot surgery classes are outlined in Table 3 and Figure 2. Analysis of variance suggested tendencies towards poorer outcomes based on increasing foot surgery class. Using specific multiple comparisons between adjacent surgery classes, there was a significant difference in both infection and amputation between classes 3 and 4 (P= 0·001 for both associations). Similar characteristics could be shown when specifically evaluating for trend. There was a significant trend towards increasing risk of ulceration/reulceration (χ2 trend= 17·8, P= 0·0001), peri‐postoperative infection (χ2 trend= 96·9, P= 0·0001), all‐level amputation (χ2 trend= 41·7 P= 0·001) and major amputation (χ2 trend= 8·6, P= 0·003) with increasing class of foot surgery.
Table 3.
Peri‐ and postoperative outcome by diabetic foot surgery class
| Class 1 | Class 2 | Class 3 | Class 4 | Total | |
|---|---|---|---|---|---|
| Ulceration/reulceration (%) | 0 | 2·2 | 11·1 | 24·4 | 9·4 |
| Postoperative infection (%) | 2·2 | 6·7 | 20·0 | 100·0 | 32·2 |
| Amputation (%) | 0 | 2·2 | 6·7 | 48·9 | 14·4 |
| Major amputation (%) | 0 | 0 | 4·4 | 11·1 | 3·9 |
| Major/minor amputation ratio | 0 | 0 | 1·5 | 0·2 | 0·3 |
Figure 2.
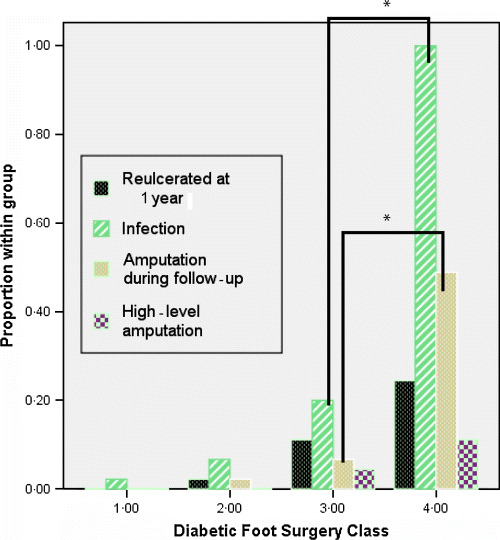
Outcomes by foot surgery classification. There was a significant trend towards reulceration, infection, amputation and high‐level amputation for all classes of surgery (P < 0·05 for all associations). Using specific comparisons between adjacent surgery classes, there was a significant difference in both infection and amputation between classes 3 and 4 (P= 0·0001). *P= 0·0001
Discussion
The results of this study suggest that this diabetic foot surgery classification system may be predictive of postoperative complications. This may assist the surgeon in better assessing risk when determining a rationale for and type of surgery in persons with diabetes. It can further help the surgeon explain the risks of surgery and long‐term complications associated with surgery to patients and their families. This classification systematically eliminated persons with severe peripheral vascular disease. It would therefore be inappropriate to generalise these results to the entire diabetic population.
The trend analysis in this classification for the outcomes of ulceration, infection and amputation is driven by the first and last categories. As expected, there was little or no morbidity associated with risk group 1, elective surgery, and a very high rate of ulceration, infection and amputation in risk group 4, emergency surgery. However, the reulceration rate was at the low end of what other specialty foot centres have reported in high‐risk patients. The lower incidence of recurrent ulceration may be partly a result of eliminating severe peripheral vascular disease from the risk pool or because of a very high level of preventive care including therapeutic shoe, education and frequent access to podiatry care.
The reulceration and amputation rates in risk group 3 were very low compared with those reported in published results 32, 33. The reulceration rates in other published reports range from 19 (34) to 63% (32) in 12–18 months. In the last decade, there has been a growing focus on Achilles tendon lengthening as both an adjunctive and a primary procedure to facilitate ulcer healing 19, 35, 36. The reulceration rate in several published reports at tendo achilles lengthening (TAL) was similar to our findings.
From published studies, postoperative infection rates for prophylactic and curative procedures (risk groups 2 and 3) range from 0 to 14% and up to 40%, respectively 3, 18, 29, 30. Data from this project fall squarely in the middle of the existing work. The prevalence of bone and soft tissue infection in an open diabetic foot ulcer exceeds 50% over the life cycle of a wound (37). Balanced against the prevalence of infection resulting from an open ulceration, infection rates in surgery classes 2 and 3 were much better. Based on the little knowledge of the natural history of ulceration and the risk of recidivism, the short‐term benefits of surgery in appropriately selected high‐risk groups 2 and 3 seem to improve clinical outcomes.
In this study, persons receiving class 1 procedures had significantly lower glycosylated haemoglobin than did persons receiving either class 3 (P= 0·04) or class 4 (P= 0·05). This is an interesting finding as glycosylated haemoglobin levels have not been previously identified as a significant predictor in ulceration risk. Rigid glycaemic control (38) has been shown to be of fundamental importance to help delay the onset and slow the progression of complications associated with diabetes. Future studies may provide insight into the relationship between elevated glycosylated haemoglobin levels and surgical prognosis in this specific population.
There were a number of limitations to the current model. This was a retrospective design and is thus subject to various methodological biases unique to the model. Additionally, it was not possible to assess inter‐ or intrarater variability through this model. Certainly, the definitions of neuropathy, deformity, infection and an open wound are subject to some degree of interpretation. This has been a feature similarly affecting most other classification systems in this milieu. That being said, the application of those definitions as assessed by the clinicians using the classification system nonetheless appeared to lead to an association with poorer outcome based on increasing surgery class. Future studies should continue to enhance operational definitions while also considering variability factors among clinicians of various disciplines.
As mentioned above, there was a trend towards poorer outcomes based on increasing foot surgery class. This trend corresponds well to the foot risk classification system promoted by the International Working Group on the Diabetic Foot (25) as well as similar classification systems described by Rith‐Najarian and coworkers 39, 40 and Armstrong et al. (41). Additional studies by Peters and Lavery (42) and Mayfield et al. (43) seem to corroborate this general line of assessment; that the presence of neuropathy and history of ulcerations are predictors of poor outcomes. The diabetic foot surgery classification is predictive of postoperative complications, whereas the foot‐risk classification systems previously described are predictive of ulcer formation. Despite differences in focus, the trends inferred by these classification systems coincide well and may ultimately prove useful in tandem. The highlight of the diabetic foot surgery classification is that presence of ulcer and infection seems to affect risk for amputation when patients undergo surgery. It is this information that, when collated, can better help the clinician identify and communicate risk to his or her patient.
References
- 1. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217–28. [DOI] [PubMed] [Google Scholar]
- 2. Giurini JM, Basile P, Chrzan JS, Habershaw GM, Rosenblum BI. Panmetatarsal head resection. A viable alternative to the transmetatarsal amputation. J Am Podiatr Med Assoc 1993;83:101–7. [DOI] [PubMed] [Google Scholar]
- 3. Rosenblum BI, Giurini JM, Chrzan JS, Habershaw GM. Preventing loss of the great toe with the hallux interphalangeal arthroplasty. J Foot Ankle Surg 1994;33:557–66. [PubMed] [Google Scholar]
- 4. Petrov O, Pfeifer M, Flood M, Chagares W, Daniele C. Recurrent plantar ulceration following pan metatarsal head resection. J Foot Ankle Surg. 1996;35:573–7; discussion 602. [DOI] [PubMed] [Google Scholar]
- 5. Strauss MB, Bryant BJ, Hart JD. Forefoot narrowing with external fixation for problem cleft wounds. Foot Ankle Int 2002;23:433–9. [DOI] [PubMed] [Google Scholar]
- 6. Fleischli JE, Anderson RB, Davis WH. Dorsiflexion metatarsal osteotomy for treatment of recalcitrant diabetic neuropathic ulcers. Foot Ankle Int 1999;20:80–5. [DOI] [PubMed] [Google Scholar]
- 7. Wetz HH. Orthopedic surgery treatment of diabetic neuropathic osteoarthropathy. Z Orthop Ihre Grenzgeb 1997;135:Oa20–2. (In German) [PubMed] [Google Scholar]
- 8. Rosenblum BI, Pomposelli FB Jr, Giurini JM, Gibbons GW, Freeman DV, Chrzan JS, Campbell DR, Habershaw GM, LoGerfo FW. Maximizing foot salvage by a combined approach to foot ischemia and neuropathic ulceration in patients with diabetes. A 5‐year experience. Diabetes Care 1994;17:983–7. [DOI] [PubMed] [Google Scholar]
- 9. Patel VG, Wieman TJ. Effect of metatarsal head resection for diabetic foot ulcers on the dynamic plantar pressure distribution. Am J Surg 1994;167:297–301. [DOI] [PubMed] [Google Scholar]
- 10. Cohen M, Roman A, Malcolm WG. Panmetatarsal head resection and transmetatarsal amputation versus solitary partial ray resection in the neuropathic foot. J Foot Surg 1991;30:29–33. [PubMed] [Google Scholar]
- 11. Griffiths GD, Wieman TJ. Metatarsal head resection for diabetic foot ulcers. Arch Surg 1990;125:832–5. [DOI] [PubMed] [Google Scholar]
- 12. Tillo TH, Giurini JM, Habershaw GM, Chrzan JS, Rowbotham JL. Review of metatarsal osteotomies for the treatment of neuropathic ulcerations. J Am Podiatr Med Assoc 1990;80:211–7. [DOI] [PubMed] [Google Scholar]
- 13. Iannucci AJ, King PL, Channell RW, Farrell DJ. Spontaneous fractures of the lesser metatarsals secondary to an amputated hallux and peripheral neuropathy. J Foot Surg 1987;26:66–71. [PubMed] [Google Scholar]
- 14. Dannels EG. A preventive metatarsal osteotomy for healing pre‐ulcers in American Indian diabetics. J Am Podiatr Med Assoc 1986;76:33–7. [DOI] [PubMed] [Google Scholar]
- 15. Jacobs RL. Hoffman procedure in the ulcerated diabetic neuropathic foot. Foot Ankle 1982;3:142–9. [DOI] [PubMed] [Google Scholar]
- 16. Singer A. Surgical treatment of mal perforans. Arch Surg 1976;111:964–8. [DOI] [PubMed] [Google Scholar]
- 17. Armstrong DG, Lavery LA, Vazquez JR, Short B, Kimbriel HR, Nixon BP, Boulton AJ. Clinical efficacy of the first metatarsophalangeal joint arthroplasty as a curative procedure for hallux interphalangeal joint wounds in persons with diabetes. Diabetes Care 2003;26:3284–7. [DOI] [PubMed] [Google Scholar]
- 18. Armstrong DG, Rosales MA, Gashi A. Efficacy of fifth metatarsal head resection for treatment of chronic diabetic foot ulceration. J Am Podiatr Med Assoc 2005;95:353–6. [DOI] [PubMed] [Google Scholar]
- 19. Mueller MJ, Sinacore DR, Hastings MK, Strube MJ, Johnson JE. Effect of achilles tendon lengthening on neuropathic plantar ulcers. A randomized clinical trial. J Bone Joint Surg Am 2003;85A:1436–45. [PubMed] [Google Scholar]
- 20. Armstrong DG, Frykberg RG. Classification of diabetic foot surgery: toward a rational definition. Diabet Med 2003;20:329–31. [DOI] [PubMed] [Google Scholar]
- 21. Bongard O, Krahenbuhl B. Predicting amputation in severe ischemia: the value of transcutaneous PO2 measurement. J Bone Joint Surg Br 1988;70B:465. [DOI] [PubMed] [Google Scholar]
- 22. Hauser CJ, Klein SR, Mehringer CM, Appel P, Shoemaker WC. Assessment of perfusion in the diabetic foot by regional transcutaneous oximetry. Diabetes 1984;33:527–31. [DOI] [PubMed] [Google Scholar]
- 23. Forst T, Pfutzner A, Bauersachs R, Avin M, Bach B, Biehlmaier H, Kustner E, Beyer J. Comparison of the microvascular response to transcutaneous electrical nerve stimulation and postocclusive ischemia in the diabetic foot. J Diabetes Complications 1997;11:291–7. [DOI] [PubMed] [Google Scholar]
- 24. Wyss CR, Matsen FA, Simmons CW, Burgess EM. Transcutaneous oxygen tension measurements on limbs of diabetic and nondiabetic patients with peripheral vascular disease. Surgery 1984;95:339–46. [PubMed] [Google Scholar]
- 25. International Working Group on the Diabetic Foot . International consensus on the diabetic foot. Maastricht: International Working Group on the Diabetic Foot, 1999. [Google Scholar]
- 26. Armstrong DG, Lavery LA, Wu SC, Boulton AJM. Evaluation of removable and irremovable cast walkers in the healing of diabetic foot wounds: a randomized controlled trial. Diabetes Care 2005;28:551–4. [DOI] [PubMed] [Google Scholar]
- 27. Katz IA, Harlan A, Miranda‐Palma B, Prieto‐Sanchez L, Armstrong DG, Bowker JH, Mizel MS, Boulton AJ. A randomized trial of two irremovable offloading devices in the management of neuropathic diabetic foot ulcers. Diabetes Care. In press. [DOI] [PubMed] [Google Scholar]
- 28. Armstrong DG, Short B, Nixon BP, Boulton AJM. Technique for fabrication of an “instant” total contact cast for treatment of neuropathic diabetic foot ulcers. J Am Podiatr Med Assoc 2002;92:405–8. [DOI] [PubMed] [Google Scholar]
- 29. Armstrong DG, Lavery LA, Stern S, Harkless LB. Is prophylactic diabetic foot surgery dangerous? J Foot Ankle Surg 1996;35:585–9. [DOI] [PubMed] [Google Scholar]
- 30. Piaggesi A, Schipani E, Campi F, Romanelli M, Baccetti F, Arvia C, Navalesi R. Conservative surgical approach versus non‐surgical management for diabetic neuropathic foot ulcers: a randomized trial. Diabet Med 1998;15:412–7. [DOI] [PubMed] [Google Scholar]
- 31. Kirkwood BR. Essentials of medical statistics. Oxford: Blackwell, 1988. [Google Scholar]
- 32. Edmonds ME, Blundell MP, Morns ME, Thomas EM, Cotton LT, Watkins PJ. Improved survival of the diabetic foot: the role of a specialized foot clinic. Q J Med 1986;60:763–71. [PubMed] [Google Scholar]
- 33. Uccioli L, Faglia E, Monticone G, Favales F, Durola L, Aldeghi A, Quarantiello A, Calia P, Menzinger G. Manufactured shoes in the prevention of diabetic foot ulcers. Diabetes Care 1995;18:1376–8. [DOI] [PubMed] [Google Scholar]
- 34. Helm PA, Walker SC, Pulliam GF. Recurrence of neuropathic ulcerations following healing in a total contact cast. Arch Phys Med Rehabil 1991;72:967–70. [PubMed] [Google Scholar]
- 35. Armstrong DG, Stacpoole‐Shea S, Nguyen HC, Harkless LB. Lengthening of the achilles tendon in diabetic patients who are at high risk for ulceration of the foot. J Bone Joint Surg Am 1999;81A:535–8. [DOI] [PubMed] [Google Scholar]
- 36. Lin SS, Lee TH, Wapner KL. Plantar forefoot ulceration with equinus deformity of the ankle in diabetic patients: the effect of tendo‐achilles lengthening and total contact casting. Orthopedics 1996;19:465–75. [DOI] [PubMed] [Google Scholar]
- 37. Lavery LA, Armstrong DG, Wunderlich RP, Boulton AJM, Tredwell JL. Diabetic foot syndrome: evaluating the prevalence and incidence of foot pathology in Mexican Americans and non‐Hispanic whites from a diabetes disease management cohort. Diabetes Care 2003;26:1435–8. [DOI] [PubMed] [Google Scholar]
- 38. Tesfaye S, Chaturvedi N, Eaton SE, Ward JD, Manes C, Lonescu‐Tirgoviste C, Witte DR, Fuller JH; EURODIAB Prospective Complications Study Group. Vascular risk factors and diabetic neuropathy. N Engl J Med 2005;352:341–50. [DOI] [PubMed] [Google Scholar]
- 39. Smieja M, Hunt DL, Edelman D, Etchells E, Cornuz J, Simel DL. Clinical examination for the detection of protective sensation in the feet of diabetic patients. International Cooperative Group for Clinical Examination Research. J Gen Intern Med 1999;14:418–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rith‐Najarian S, Branchaud C, Beaulieu O, Gohdes D, Simonson G, Mazze R. Reducing lower‐extremity amputations due to diabetes. Application of the staged diabetes management approach in a primary care setting. J Fam Pract 1998;47:127–32. [PubMed] [Google Scholar]
- 41. Armstrong DG, Lavery LA, Harkless LB. Who’s at risk for diabetic foot ulceration? Clin Podiatr Med Surg 1998;15:11–9. [PubMed] [Google Scholar]
- 42. Peters EJ, Lavery LA. Effectiveness of the diabetic foot risk classification system of the International Working Group on the Diabetic Foot. Diabetes Care 2001;24:1442–7. [DOI] [PubMed] [Google Scholar]
- 43. Mayfield JA, Reiber GE, Nelson RG, Greene T. A foot risk classification system to predict diabetic amputation in Pima Indians. Diabetes Care 1996;19:704–9. [DOI] [PubMed] [Google Scholar]


