Abstract
A case study reporting on the successful treatment of a patient affected by a basal cell carcinoma is submitted. Because the carcinoma had infiltrated deeply, a wide excision was necessary, including the removal of bone tissue. The deep tissue defect was treated with PROMOGRAN* Matrix, a protease‐modulating matrix, to promote granulation and ensure that the skin graft do survive and heal successfully. In this case study, a rapid development of granulation tissue on the exposed surface of the bone was observed. The benefits of the dressing enabled a successful split‐thickness skin grafting to be carried out which gave very good aesthetic and functional results.
Keywords: Basal cell carcinoma, Skin transplantation, Tissue defect, Tumour excision, Wounds
Introduction
Basal cell carcinomas are semi‐malignant skin tumours, which, starting from basal keratinocytes, infiltrate at a local level, yet which only rarely become metastatic. Those that rapidly infiltrate and grow destructively (sclerosing basal cell carcinomas) can, if located near the orifices of the body, i.e. the eyes, ears or nose, soon spread into these anatomical areas. In extreme cases, it may result in the loss of the relevant sensory function. Because it is not always possible to evaluate the exact extent of these invasive tumours clinically, one frequently used method is to excise them using an intraoperative marking procedure, so‐called micrographic‐controlled surgery (MCS), first described as Mohs surgery (1). The resulting tissue defect needs to be treated with plastic surgery, and if a skin graft is planned, the wound must be treated to encourage granulation tissue to form. Because of aesthetic considerations, the face presents particular problems, for example around the eyes where, as a result of taut scar tissue, a postoperative ectropion might be caused with significant associated functional impairment (2). Deep defects with exposed bone without periosteum represent a difficult scenario to initiate a stable granulation tissue. For these reasons, it is necessary to use special wound dressings which allow the spreading of grow factors and migration of fibroblasts starting from the wound edges. Promogran* Matrix (Johnson & Johnson, Somerville, NJ, USA) has shown to be highly effective to form granulation tissue, especially in wounds which are difficult to heal (3).
Methodology
Case history
According to the details provided by the 64‐year old patient himself, for the past 6 years, he has had a small scar‐like indentation on his right forehead. It repeatedly bled and then crusted over. For the last months, it has grown progressively bigger and was accompanied by pain.
Clinical findings
On the right‐hand side of the forehead, above the eyebrow, there is a skin‐coloured tumour measuring some 2·5 × 1·5 cm which was scarred, indented and rough to the touch and firmly attached at its base (Figure 1). In order to ensure a correct diagnosis, a diagnostic excision was performed.
Figure 1.
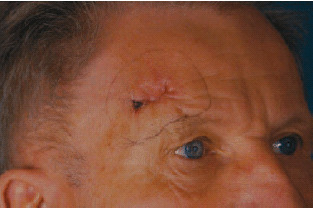
Initial diagnosis of tumour.
Histopathology
The diagnosis was a multi‐focal sclerosing basal cell carcinoma. After the excisions of the tumour region by the MCS in the five o'clock position, extensive residual basal cell carcinoma structures were found across the entire base of the post‐excision area. In the bone shavings, parts (periosteum) of the attached fibrous connective tissue showed structures of multi‐focal basal cell carcinoma (Figure 2).
Figure 2.
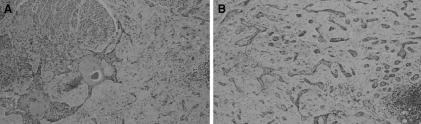
Histopathology [HE‐staining, magnification: (A) 60:1, (B) 200:1].
Treatment and result
A micrographic‐controlled excision of the visible tumour region was made with a safety margin and because clusters of further deep‐seated tumourous cells were found, further surgical procedures were necessary. Using the MCS, it was possible to remove the tumour in its entirety (Figure 3). Sections of the periosteum were needed to be removed, and this resulted in a tissue defect measuring 9 × 8 cm with an exposed section of bone measuring 3 × 3 cm in the middle (Figure 4a).
Figure 3.
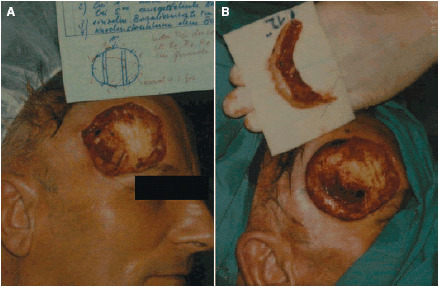
(a and b) Second resection (micrographic‐controlled surgery).
Figure 4.
(a) Clinical finding prior to local treatment. (b) Central areas of granulation tissue (after 3 weeks).
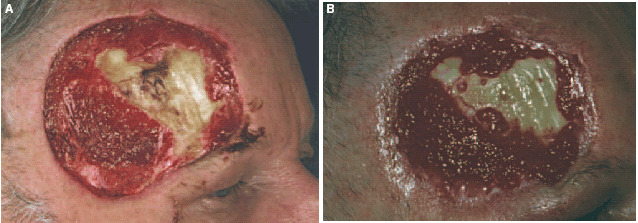
The patient has as a teacher constantly an audience, and because of this, he refused to have a transposition skin flap using skin taken from the capillitium. We therefore planned for secondary wound closure using a split skin graft. In preparation for the graft, it was essential that a well‐granulated wound bed be achieved. This appeared to be of particular importance due to the exposed section of bone, which made treatment particularly difficult. We used PROMOGRAN* Matrix, an innovative wound treatment, which was covered with a hydrocolloid dressing changing every 3 days. After a relatively short time, this therapy resulted in granulation, which started from the edge of the wound and spread inwards. One surprising finding was the formation of new islands of granulation tissue in the area of the exposed bone (Figure 4b). Three months later, there was a well‐granulated residual defect measuring 4 × 3 cm. Stable granulation tissue had been created on the periosteum, and epithelialisation could be observed from the outer edges of the excision (Figure 5a). A mesh graft using skin taken from his right upper arm was carried out. After full healing, the result was pleasing to look at. The patient is very satisfied, because he is able to work as a teacher without restrictions (Figure 5b).
Figure 5.
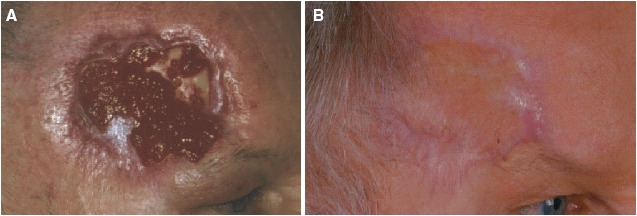
(a) Clinical finding before split skin graft (after 10 weeks). (b) Result after split‐thickness skin graft.
Discussion
For extensive basal cell carcinomas and other destructive growing tumours that appear on the face and which, in this particular patient, also invaded the periosteum, complete removal using MCS is the treatment of choice. This method makes it possible to carry out a continuous histogram of the entire outer edge and basic of the excision area (3‐D histology) and thus an accurate evaluation of the removed specimen (4). This prevents local recurrences and allows a targeted surgical procedure that is nonetheless gentle on the tissue (1). Once the edges of the excision are tumour free, the defect can be primary closed surgical using either skin flaps or free skin grafts (5, 6). For secondary wound closure, after generation of stable granulation tissue, there is the possibility to use skin grafts or autologous epidermal equivalents (5, 7, 8). In our case, the tumour had invaded the periosteum, and sections of bone needed to be removed. Because of the size of the defect, the fact of exposed bone and the anatomical proximity to the upper eyelid, there was no question of a single surgical procedure. We discussed the possibility of covering the defect using an expanded skin flap taking from the left side of the forehead and from the scalp. This the patient rejected, on aesthetic grounds, because an expander would be inserted and of his concerns that hair might subsequently grow in this area.
We decided in association with the patient to carry out wound treatment with the aim of reducing the size of the defect, followed by transplantation of a split‐thickness skin graft. For creating a vascularised granulation tissue and in order to guarantee that the graft do survive with successful take, we used PROMOGRAN* Matrix, a protease‐modulating matrix. This interactive wound treatment comprises a unique combination of oxidised, regenerated cellulose (ORC) and collagen (9, 10). The product is a sterile, freeze‐dried matrix which, once the exudate has been absorbed, forms an adaptable gel which is in turn naturally re‐absorbed by the body. It will bind and inactivate any existing excess proteases (matrix metalloproteinases, plasmin and elastase) and bind and protect growth factors from proteolytical degradation. When the product is re‐absorbed, any growth factors that have been bound will be re‐delivered in their active form (11). It has been demonstrated that the matrix influences the chemotaxis and proliferation of fibroblasts (12). Clinical experience in treating, difficult to heal, chronic (3, 13, 14), traumatic and surgical (15) wounds has shown rapid and increased granulation and epithelialisation under this treatment. We observed after a short time, the creation of well‐vascularised, stable granulation tissue from the edge of the defect. In addition and surprisingly, new granulation islands began to form on the periosteum‐free bone. The composition and structure of bio‐materials used to support bone defects have a major influence on the migration and proliferation of osteoblasts (16). In this particular case, the composition and the matrix structure of PROMOGRAN* Matrix, i.e. the collagen, a major component of the extra‐cellular matrix and the ORC, might have supported the ‘edge effect’, i.e. might have induced the recreation of epithelial cells due to cellular migration and proliferation from the edge of the wound (7, 17). This local treatment was able to be given on an outpatient basis with a view to rapidly reducing the size of the defect, which meant that after an interval of time and under a local anaesthetic, a split skin graft was able to be carried out using skin taken from the upper arm. Using this less‐invasive grafting procedure, a very good aesthetically satisfactory result was achieved for the patient. His duties as a teacher were in no way impaired. Also, by positioning the sutures in the vicinity of the right upper eyelid, no contracting scar tissue was created and the functional results were excellent. We submit this option of wound treatment as one very successful way of promoting granulation, even on bone that has no periosteum.
Acknowledgement
We thank Dr C Welling, Wound Care Consultant, MMSI Consultancy, Germany, for her technical support.
References
- 1. Breuninger H, Schaumburg‐Lever G. Control of excisional margins by conventional histopathological techniques in the treatment of skin tumours. An alternative to Moh's technique. J Pathol 1988;154(2):167–71. [DOI] [PubMed] [Google Scholar]
- 2. Oishi SN, Luce EA. The difficult scalp and skull wound. Clin Plast Surg 1995;22(1):51–9. [PubMed] [Google Scholar]
- 3. Coelho S, Amarelo M, Ryan S, Reddy M, Sibbald RG. Rheumatoid arthritis‐associated inflammatory leg ulcers: a new treatment for recalcitrant wounds. Int Wound J 2004;1(1):81–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kirsner RS, Falanga V, Eaglstein WH. The biology of skin grafts. Skin grafts as pharmacological agents. Arch Dermatol 1993;129(4):481–3. [PubMed] [Google Scholar]
- 5. Dinehart SM, Dodge R, Stanley WE, Franks HH, Pollack SV. Basal cell carcinoma treated with Mohs surgery. A comparism of 54 younger patients with 1050 older patients. J Dermatol Surg Oncol 1992;18: 560–6. [DOI] [PubMed] [Google Scholar]
- 6. Petres J. Pedicled flap‐plasties. Z Hautkr 1988;63 Suppl 2: 30–4. [PubMed] [Google Scholar]
- 7. Tausche AK, Richter‐Huhn G, Sebastian G. Treatment of recalcitrant wounds with autologous epidermal equivalents after excision of multiple cylindromas of the scalp. Hautarzt 2004;55(3):296–300. [DOI] [PubMed] [Google Scholar]
- 8. Tausche AK, Skaria M, Bohlen L, Liebold K, Hafner J, Friedlein H, Meurer M, Goedkoop RJ, Wollina U, Salomon D, Hunziker T. An autologous epidermal equivalent tissue‐engineered from follicular outer root sheath keratinocytes is as effective as split‐thickness skin autograft in recalcitrant vascular leg ulcers. Wound Repair Regen 2003;11(4):248–52. [DOI] [PubMed] [Google Scholar]
- 9. Cullen B, Watt PW, Lundqvist C, Silcock D, Schmidt RJ, Bogan D, Light ND. The role of oxidised regenerated cellulose/collagen in chronic wound repair and its potential mechanism of action. Int J Biochem Cell Biol 2002;34(12):1544–56. [DOI] [PubMed] [Google Scholar]
- 10. Osaki K, Miura A, Koide M, Katakura T, Sugihara T. The effect of a collagenous implant on deep dermo‐peristal defects in the rabbit. J Dermatol 1996;23(2):83–8. [DOI] [PubMed] [Google Scholar]
- 11. Cullen B, Smith R, McCulloch E, Silcock D, Morrison L. Mechanism of action of Promogran, a protease modulating matrix, for the treatment of diabetic foot ulcers. Wound Repair Regen 2002;10(1):16–25. [DOI] [PubMed] [Google Scholar]
- 12. Hart J, Silcock D, Gunnigle S, Cullen B, Light ND, Watt PW. The role of oxidised regenerated cellulose/collagen in wound repair: effects in vitro on fibroblast biology and in vivo in a model of compromised healing. Int J Biochem Cell Biol 2002;34(12):1557–70. [DOI] [PubMed] [Google Scholar]
- 13. Vin F, Teot L, Meaume S. The healing properties of Promogran in venous leg ulcers. J Wound Care 2002;11(9):335–41. [DOI] [PubMed] [Google Scholar]
- 14. Veves A, Sheehan P, Pham H. A randomized controlled trial – Promogran vs standard treatment in the management of diabetic foot ulcers. Arch Surg 2002;137(11):822–7. [DOI] [PubMed] [Google Scholar]
- 15. Guarnera G, Restuccia A. Promogran and complex surgical lesions: a case report. J Wound Care 2004;13(6):237–9. [DOI] [PubMed] [Google Scholar]
- 16. Botticelli D, Berglundh T, Lindhe J. The influence of a biomaterial on the closure of a marginal hard tissue defect adjacent to implants. Clin Oral Implants Res 2004;15(3):285–92. [DOI] [PubMed] [Google Scholar]
- 17. Koizumi M, Matsuzaki T, Ihara S. The subsets of keratinocytes responsible for covering open wounds of neonatal rat skin. Cell Tissue Res 2004;315(2):187–95. [DOI] [PubMed] [Google Scholar]


