Abstract
Fibrous materials in some modern absorbent wound dressings have the ability to sequester and retain bacteria; however, this ability varies according to the nature of the fibres. We studied the bacterial retention capacity of alginate and carboxymethylcellulose dressings, using an infected skin ulcer model on the backs of rats. Wound surfaces were inoculated with either Staphylococcus aureus or Pseudomonas aeruginosa at a concentration of 1·5 × 106 colony‐forming units per wound. AQUACEL®; Hydrofiber®;, Kaltostat®; or Sorbsan®; were applied to the contaminated wounds for 12 h. Each dressing was then divided into two pieces. Total viable bacterial count within the dressing was calculated using one piece, and bacterial count released from the dressing into physiological saline was determined using the other piece, enabling bacterial retention rate to be calculated. Bacterial counts in tissue were also determined. Each dressing was tested on each of 10 wounds contaminated with each bacterium. Statistical analyses were performed using one‐way analysis of variance (ANOVA) for replicated measures combined with Duncan's multiple comparison test. AQUACEL®; Hydrofiber®; dressing was most effective in its ability to retain both Staphylococcus aureus and Pseudomonas aeruginosa (p < 0·05). Bacterial counts in tissue showed no significant change with respect to pathogen or the type of dressing used. It can be concluded that the bacterial retaining ability of AQUACEL®; Hydrofiber®; dressing was found to be significantly higher than that of alginate dressings in an infected animal wound model.
Keywords: Carboxymethylcellulose, Chronic skin ulcer, Staphylococcus aureus, Wound dressing, Wound preparation
Introduction
Patients suffering from chronic skin ulcers, represented by chronic leg ulcers and pressure ulcers, often require hospitalisation when infection occurs. Infection of diabetic ulcers can have serious consequences, posing challenges in terms of high morbidity and medical expenses and sometimes requiring leg amputation. Furthermore, postoperative infection of surgical wounds necessitates prolonged periods of hospitalisation (1).
Several decades have passed since the introduction of modern wound dressings for skin ulcers into the clinical setting. This treatment accelerates wound healing by keeping the wound surface in a moist environment and absorbing exudate (2). Because colonisation by bacteria accompanies almost all cases of chronic skin ulcers, use of modern wound dressings was initially associated with concern about the potential risk of infection caused by sealing the wound tightly and creating a moist environment. However, experimental studies have shown no increase in infection; indeed, they have clarified that the infection rate associated with modern wound dressings is somewhat lower than that observed with ointments and gauze (3, 4). Additional factors such as thermal insulation, maintenance of circulation and activation of leucocytes, suppression of tissue necrosis and change of pH in the closed environment are all related to this lower rate of infection under moisture‐retentive dressings (5).
Many of the modern wound dressings are designed to absorb large volumes of exudate and can absorb an amount of moisture of up to 15–20 times their own weight (6). It has also been clarified that bacteria on the wound surface move into the dressing as wound exudate is absorbed, and that these dressings with high levels of fluid retention function to absorb and retain bacteria, making them useful for wound bed preparation where there is severe colonisation (7). These dressings may help to reduce cross‐infection by wound pathogens. However, the mechanism of this bacterial retention has not yet been clearly elucidated.
Alginate material is said to have high‐level affinity for bacteria (8). Bowler et al. carried out in vitro experiments using Pseudomonas aeruginosa and Staphylococcus aureus and reported that AQUACEL® Hydrofiber® demonstrates superior bacterial retention when compared to alginate (9). They also confirmed by electron microscopy that carboxymethylcellulose (CMCH) confines bacteria inside the dressing due to the decreased gaps between fibres when the fibres absorb moisture and become swollen (10). The purpose of the current experiment was to study the ability of fibrous dressings to retain bacteria using an in vivo model of infected skin ulcers developed on the backs of rats.
Materials and methods
Materials
The following materials were used in the study: polyurethane film (3M Corporation, St Paul, MN, USA), Eakin seal®, Kaltostat®, AQUACEL® Hydrofiber® dressing (ConvaTec, Skillman, NJ, USA), Sorbsan® Flat dressing (Alcare, Tokyo, Japan), 100% woven cotton gauze (Osaki Medical Products Company, Nagoya, Japan), brain‐heart infusion (BHI, Difco Laboratories, Detroit, MI, USA), pentobarbital sodium (Nembutal; Dainippon Pharmaceutical Co., Ltd, Osaka, Japan), Pseudomonas aeruginosa 5142rif (Serotype E, non mucoid), Staphylococcus aureus (clinical isolate MSSA no. 7743114), rats (Saitama Experimental Animals Supply Co., Ltd. Sugito, Saitama, Japan).
Methods
Non mucoid P. aeruginosa was kindly provided as a gift by Dr Ikeda, Department of Bacteriology at Teikyo University School of Medicine. Bacterial suspensions were prepared by the following method. Methicillin‐susceptible S. aureus was a clinical isolate obtained from an infected surgical wound of an orthopaedic surgery patient. Bacteria were grown on blood agar overnight. One colony of each of these bacterial strains was incubated in BHI for 12 hours at 37°C. Cultured cells were centrifuged three times in physiological saline at 980 g and suspended in saline at a concentration of 0·5 × 108 colony‐forming units (CFU)/ml. After the suspension was vortexed, the colony count was determined by measuring absorbance at 600 A.
The method of creation of infected wound was described previously (11). Fifteen male Sprague–Dawley rats aged 12 weeks were used in this study. Rats were anaesthetised by intraperitoneal injection of pentobarbital (Nembutal, 30 mg/kg), the hair on the back of each animal was removed with clippers, and the skin was then sterilised with iodine. 15 mm × 15 mm full‐thickness skin wounds that included the muscular membrane were prepared on the back of each rat. Four wounds were created in each rat and each wound was separated by 25 mm. Gauze that had been soaked in a 5000‐fold dilution of adrenaline in physiological saline was placed on the wound surface for haemostasis. A 15 mm × 15 mm, 7·4 mg piece of fresh gauze was placed on the base of the ulcer, after which the gauze was soaked with bacterial suspension at a concentration of 1·5 × 106 CFU/wound. After bacterial inoculation of the wound surface, the periphery of the wound was sealed with an Eakin® seal, and the entire area was then covered with polyurethane film to achieve a closed environment. We have previously shown that the wound in this condition produced exudate containing 1·0 × 108 bacteria per wound (11). Wounds were assigned to the following six experimental groups, a P. aeruginosa and S. aureus group for each of AQUACEL® Hydrofiber®, Kaltostat® and Sorbsan®. Each dressing was assigned to ten wounds inoculated with each bacterium. All animals were given drinking water containing acetaminophen at a concentration of 0·25 mg/ml.
The test dressings, 15 mm × 15 mm in size, were applied to the individual wounds on the second day after wound preparation, and the dressings were maintained in a closed environment. After 12 hours, the dressings were removed and the weight of each whole dressing was measured. Each dressing was then divided equally into two parts and the weight of each piece was determined. One half of each divided dressing was homogenised for 2 minutes at 37°C in 100 ml of physiological saline with 0·1% Tween 80, to enable determination of total viable bacterial count. The remaining portion of each dressing pieces was soaked in 100 ml of physiological saline with 0·1% Tween 80 at 37°C for 1 minute. After the dressings were removed from the solution, the bacterial count in the solution was measured as follows. One millilitre of the solution was sampled and diluted according to the tenfold dilution assay protocol. One millilitre of this diluted solution was transferred to an empty Petri dish (Funakoshi, Tokyo, Japan), to which 25 ml of Trypticase Soy Agar (TSA) at 50°C was added. The agar was allowed to set and then the plates were incubated for 18 hours, after which colonies were enumerated. The number of bacteria retained by the dressing was calculated by subtracting the count of bacteria leached into solution from the total bacterial count per piece of dressing obtained by homogenisation. Each dressing was changed after 12 h intervals. After three dressing changes, bacterial counts in tissue were measured. After removal of each dressing and removal of the gauze from the base of each wound, the tissue at the base of the wound was surgically removed and weighed. The tissue was placed in a stomacher bag containing 100 ml of physiological saline with 0·1% Tween 80 and washed for 2 minutes to sluice off surface bacteria, and the tissue was then homogenised for 15 seconds in a Polytron homogeniser, (Ishii Laboratory Works, Osaka, Japan) followed by 1 minute in a glass homogeniser. The tissue was then placed in a stomacher bag containing 100 ml of physiological saline with 0·1% Tween 80 and homogenised further for 2 minutes. Subsequently, 1 ml of this homogenate was sampled and diluted according to the tenfold dilution assay protocol. Two tryptone soy agar plates were inoculated with 0·1 ml of this diluted homogenate and incubated for 18 hours, after which the total viable count was determined.
The protocol for animal experimentation described herein was approved by the Animal Research Committee of Teikyo University School of Medicine.
Statistical analysis
The comparison of three dressings was performed using one‐way analysis of variance (anova) for replicated measures combined with Duncan's Multiple Comparison Test.
Results
In both the S. aureus and P. aeruginosa test groups, the bacterial count for tissue to which AQUACEL® Hydrofiber® dressing was applied was lowest, followed in order by the counts in tissue to which Sorbsan® and Kaltostat® dressings were applied; however, these differences did not reach statistical significance (Figure 1 a,b). In each group, the average bacterial count value was high (approximately 2·5 × 108 CFU/g tissue).
Figure 1.
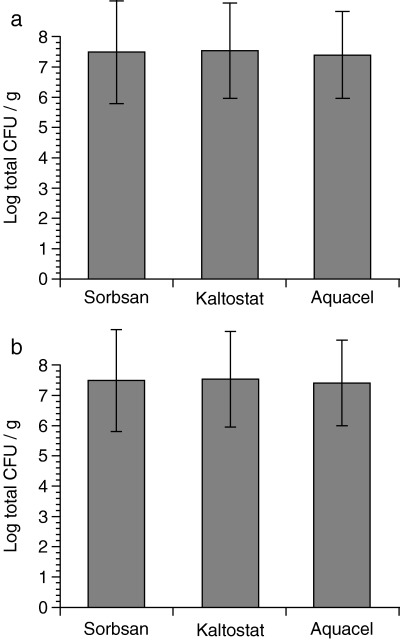
Effect of dressings on bacterial counts in tissue biopsies. Dressings were changed three times to new ones with 12 hours of interval. (a) Staphylococcus aureus; (b) Pseudomonas aeruginosa. Ten biopsies were taken per dressing and logarithmic transformation of the quantitative bacterial count per gram was calculated.
Observation of the amounts of S. aureus trapped in dressings revealed significant differences between groups; retention and immobilisation of S. aureus by AQUACEL® Hydrofiber®, Kaltostat® and Sorbsan® was 87%, 64% and 37%, respectively (Figure 2). Retention and immobilisation of P. aeruginosa by AQUACEL® Hydrofiber®, Kaltostat® and Sorbsan® were 81%, 59% and 29%, respectively (Figure 3).
Figure 2.
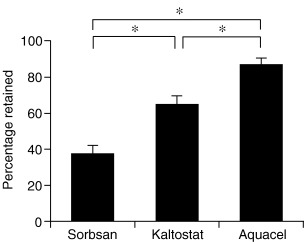
Mean percentage dressing retention using a Staphylococcus aureus challenge. Ventral bar represents the standard error of the mean (SEM). AQUACEL® Hydrofiber® dressing was most effective in its ability to retain S. aureus when comparing with Kaltostat® dressing and Sorbsan® dressing (*p < 0·05).
Figure 3.
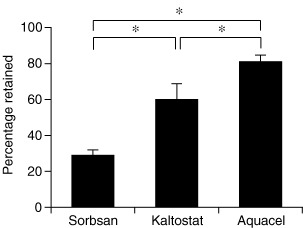
Mean percentage dressing retention using a Pseudomonas aeruginosa challenge. Ventral bar represents the standard error of the mean (SEM). AQUACEL® Hydrofiber® dressing was most effective in its ability to retain Pseudomonas aeruginosa when comparing with Kaltostat® dressing and Sorbsan® dressing (*p < 0·05).
Discussion
Retention rates of both S. aureus and P. aeruginosa by AQUACEL® Hydrofiber® dressings were higher than those by alginates, agreeing with the results of the earlier in vitro load testing by Bowler et al. (9). However, while Bowler et al. reported that the rate of bacterial retention by alginates differed between S. aureus and P. aeruginosa, data from the current study showed very similar retention rates between the two pathogens. While protein and other blood cell components might contribute towards the difference between our results and those of Bowler et al., the cause remains unknown. The present study revealed significant differences in the bacterial trapping rates of two alginate dressings; this was possibly due to differences of fibre thickness and chemical composition between individual alginate dressings.
AQUACEL® Hydrofiber® dressing and alginates are both used widely to absorb exudate in chronic wounds, and these fibrous dressings have been reported to have the ability to retain and immobilise wound‐derived microorganisms (12). Bowler reported marked variation in the ability of dressings to retain bacteria within their matrix and speculated the following mechanism to account for bacterial retention; following hydration, the interstitial space is reduced due to swelling of the fibrous component of hydrofibres, and a gel then forms that blocks further fluid flow along the fibres (9). Recently, Walker observed such gel formation by scanning electron microscopy. The AQUACEL® Hydrofiber® dressing formed a continuous coherent gel, caused by merging of fully hydrated fibres that were then indistinguishable from each other, and the bacteria appeared to be absorbed into this gel. Conversely, the alginate wound dressing did not form a coherent, single structure but instead displayed a patchwork of gelled regions with fibres still identifiable within the gel structure. Some bacterial populations, adherent to the surrounding non hydrated fibres, were visible (10).
Chronic ulcer surfaces are always colonised with mixed microorganisms; however, many factors determine the progression to infection (13, 14). As bacterial burden increases, the colonised wound is transformed into a covert infection that may not involve extensive tissue invasion but is sufficient to inhibit wound healing. Cases in which colonisation proceeds to infection can be predicted in the aged and in immuno‐compromised patients. This concept of critical colonisation was demonstrated recently (15, 16). In such cases, dressings with the ability to trap and retain bacteria and possibly prevent the formation of biofilms in wounds are clinically very advantageous and may also lead to a reduction in cross‐infection by wound pathogens.
When used in the management of chronic ulcers, AQUACEL® Hydrofiber® dressings can play a compensatory role in infection control.
Acknowledgements
This work was supported in part by ConvaTec. The authors thank Dr Michel HE Hermans for the helpful suggestion regarding the calculation methods.
References
- 1. Nichols RL. Preventing surgical site infections: a surgeon's perspective. Emerg Infect Dis 2001; 7: 220–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eaglstein WH. Moist wound healing with occlusive dressings: a clinical focus. Dermatol Surg 2001;27: 175–81.DOI: 10.1046/j.1524-4725.2001.00299.x [DOI] [PubMed] [Google Scholar]
- 3. Hutchinson JJ, Lawrence JC. Wound infection under occlusive dressings. J Hosp Infect 1991;17: 83–94.DOI: 10.1016/0195-6701(91)90172-5 [DOI] [PubMed] [Google Scholar]
- 4. Kannon GA, Garrett AB. Moist wound healing with occlusive dressings. A clinical review. Dermatol Surg 1995;21: 583–90.DOI: 10.1016/1076-0512(94)00114-6 [DOI] [PubMed] [Google Scholar]
- 5. Field FK, Kerstein MD. Overview of wound healing in a moist environment. Am J Surg 1994;167(1A): 2S–6S.DOI: 10.1016/0002-9610(94)90002-7 [DOI] [PubMed] [Google Scholar]
- 6. Williams C. An investigation of the benefits of Aquacel Hydrofibre wound dressing. Br J Nurs 1999;8: 676–7, 680. [DOI] [PubMed] [Google Scholar]
- 7. Wysocki AB. Evaluating and managing open skin wounds: colonization versus infection. AACN Clin Issues 2002;13: 382–97. [DOI] [PubMed] [Google Scholar]
- 8. Thomas S. Alginate dressings in surgery and wound management – Part 1. J Wound Care 2000;9: 56–60. [DOI] [PubMed] [Google Scholar]
- 9. Bowler PG, Jones SA, Davies BJ, Coyle E. Infection control properties of some wound dressings. J Wound Care 1999;8: 499–502. [DOI] [PubMed] [Google Scholar]
- 10. Walker M, Hobot JA, Newman GR, Bowler PG. Scanning electron microscopic examination of bacterial immobilisation in a carboxymethyl cellulose (AQUACEL (R)) and alginate dressings. Biomaterials 2003;24: 883–90. [DOI] [PubMed] [Google Scholar]
- 11. Tachi M, Hirabayashi S, Yonehara Y, Suzuki Y, Bowler PG. Development of an experimental model of infected skin ulcer. International Wound Journal 2004;1: 49–55.DOI: 10.1111/j.1742-481x.2004.00006.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Armsrong SH, Ruckley CV. Use of a fibrous dressing in exudating leg ulcer. J Wound Care 1997;6: 322–4. [DOI] [PubMed] [Google Scholar]
- 13. Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev 2001;14: 244–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bowler PG. Wound pathophysiology, infection and therapeutic options. Ann Med 2002;34: 419–27. [DOI] [PubMed] [Google Scholar]
- 15. Robson MC. Wound infection. A failure of wound healing caused by an imbalance of bacteria. Surg Clin North Am 1997;77: 637–50. [DOI] [PubMed] [Google Scholar]
- 16. Schultz GS, Sibbald RG, Falanga V, Ayello EA, Dowsett C, Harding K, Romanelli M, Stacey MC, Teot L, Vanscheidt W. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003;11 (Suppl. 1):S1–S28.DOI: 10.1046/j.1524-475X.2003.11101.x [DOI] [PubMed] [Google Scholar]


