Abstract
Non blanchable erythema, i.e. stage I pressure ulcer, is common in patients in acute and geriatric care and in nursing homes. Research has shown that this type of lesions is prone to develop into more severe pressure ulcers. The peripheral skin blood perfusion is of major importance for the development of pressure ulcers. The aim of this study was to explore the peripheral skin blood perfusion over time, in areas with non blanchable erythema and in corresponding undamaged areas on the opposite side of the body. A total of 19 measurements were performed, over time, using a laser Doppler perfusion imager. The blood flow distribution profiles over areas with non blanchable erythema and undamaged skin were found to be different. As the area of the non blanchable erythema decreased, the blood perfusion distribution profiles gradually became more heterogeneous; an area of high blood perfusion in the centre of the lesions was seen and the perfusion successively decreased closer to the edge. These results indicate that there are differences in blood perfusion between skin areas of non blanchable erythema and undamaged skin. The results also indicate that the visible redness in areas with non blanchable erythema is related to altered blood perfusion. The skin blood perfusion also seems to increase in relation to the size of the non blanchable erythema.
Keywords: Non blanchable erythema, Pressure relief, Pressure ulcer, Skin blood flow, Skin blood perfusion
Introduction
Non blanchable erythema with intact skin surface, i.e. stage I pressure ulcers (1), is common in patients in acute and geriatric care and in nursing homes. The lesion is caused by unrelieved pressure resulting in damage of the underlying tissue 2, 3. This type of lesion is also frequently observed in patients who have undergone surgery 4, 5. Non blanchable erythema is an important early sign of tissue damage, and this type of lesion is prone to develop into more severe pressure ulcers 6, 7.
The frequency of pressure ulcers increases with advancing age, and elderly patients with pressure ulcers have a significantly lower systolic blood pressure than elderly patients without pressure ulcers 8, 9. Immobility affects the microcirculation in several ways. Impairment of the ability to increase the blood flow with rising temperature was found in older inpatients (10), as well as impaired reactive hyperaemia after bed rest in healthy young men (11). This may lead to a risk of insufficient blood flow, ischaemia in the tissue, which in turn contributes to the development of pressure ulcer (10).
The skin blood perfusion is of major importance for the supply of nutrients and oxygen to the tissue and the removal of waste products. The skin blood perfusion plays a role in the temperature and blood pressure regulation as well as in the distribution of blood flow and blood volume in the body (12). When performing a histological examination of damaged human tissue, such as non blanchable erythema, changes in the blood vessels in the papillary part of the dermis were found, which revealed concentration of red blood cells, dilation of blood vessels and bleeding (13). Changes in the papillae and the papillary layers may be related to impairment of tissue viability and contribute to the development of pressure ulcers (14). Schubert and Fagrell (15), who examined skin blood flow in human skin exposed to pressure, found that the skin perfusion increased in line with increased surface pressure up to 12–50 mmHg. A gradual decrease in the perfusion was then seen with increasing pressure. Herrman et al. found similar results in an animal model; the skin perfusion reached a maximum of about 14 mmHg, after which perfusion decreased with increased pressure and reached zero perfusion at a pressure of 58 mmHg. One explanation for the changes in perfusion could be that it is an artefact related to the laser Doppler technique. The authors concluded that the perfusion changes were probably due to the result of vasodilation as the changes were only seen in unstressed skin and not in stressed skin (16).
Ek et al. (1987) measured the basal skin blood flow, using laser Doppler flowmetry in 11 healthy individuals and 21 patients with partial or total hemiplegia. The measurements were performed on the skin over the sacrum, m. gluteus maximus and heels, and the median skin blood flow in healthy individuals were 0·045, 0·054 and 0·228 V, respectively. In the patients, the blood flow over the sacrum was 0·08 V; in the m. gluteus maximus, and paretic and non paretic sides, the blood flow was 0·024 V; and in the heels, corresponding sides, the blood flow was 0·060 and 0·068 V, respectively. There were no significant differences in blood flow between the paretic and the non paretic sides in patients (10). When measuring blood flow in elderly patients in the early stages of pressure ulcers a significantly increased blood flow was seen at the edge and in the centre of the lesions (17).
No earlier studies were found that examine blood flow in skin areas with non blanchable erythema over a period of time, e.g. weeks. However, experience tells us that when these skin areas are relieved of pressure, the lesions tend to heal quite rapidly. Because there is a lack of knowledge in this area, it is of great importance to explore this problem in order to promote preventive nursing care measures.
Aim
The aim of this study was to explore and compare the peripheral skin blood perfusion over time, in areas with non blanchable erythema and in corresponding undamaged areas on the opposite side of the body.
Method
The Research Ethics Committee of the Faculty of Health Sciences, Linköping University, approved the study.
Patients
The measurements were carried out on five patients, four women and one man. One patient was included when in hospital and four were included when in nursing homes. The mean age of the patients was 76·8 ± 5·5 years, ranging from 68 to 83 years. Four of the patients suffered from stroke and the fifth patient from a knee fracture. Three of the skin damages were situated at the heels, one at the malleolie, and one at the Achilles tendon (Table 1).
Table 1.
Demographic data of each patient included in the study
| Age (in years) | Gender | Localisation of non blanchable erythema | Diagnosis | |
|---|---|---|---|---|
| Patient 1 | 68 | Male | Right Achilles tendon | Knee fracture, hypertension |
| Patient 2 | 78 | Female | Left heel | Stroke, dementia |
| Patient 3 | 79 | Female | Left malleolie | Stroke |
| Patient 4 | 76 | Female | Right heel | Stroke, diabetes mellitus, cardiac infarction, kidney disease |
| Patient 5 | 83 | Female | Left heel | Stroke, cardiac infarction, epilepsy |
The registered nurses on the hospital wards and at the nursing homes were asked to contact a member (M. L.) of the scientific team whenever a patient developed a non blanchable erythema. The patients and/or their next of kin were informed about the study both orally and in writing. After informed consent was obtained, the patients were included in the study.
Procedures and instruments
In this study, the skin blood perfusion in areas with non blanchable erythema, and in non damaged areas on the opposite side of the body, was measured. The patients were thus their own controls. Non blanchable erythema (stage I pressure ulcer) is defined as a red‐coloured or a reddish‐blue‐coloured skin area with an intact skin surface 1, 8, remaining after 2 hours of total pressure relief. The area of non blanchable erythema was then to be kept free of pressure during the entire study period.
The measurements were performed a maximum of four times over 3 weeks, in the patient’s own environment, e.g. the person’s own room on the ward or at the nursing home, with the exception of one patient, where two of the measurements were performed in the patient’s home. The patients were placed in a supine position, with the damaged areas unloaded, that was comfortable for them and were stabilised with pillows to ensure that they would be able to lie still. The measurement areas were marked with a waterproof pen, to ensure measurements at the same area from time to time. The measurements were performed after at least 20 minutes of bed rest 18, 19. The procedure was standardised; in all cases, the measurements were taken in a specific order. Blood perfusion in the areas of skin damages was measured first, followed by measurement in the undamaged areas on the opposite side of the body. Precautions were taken to ensure that the undamaged skin areas would not be loaded before measurements. Afterwards, photographs of the non blanchable erythema were taken, and the local skin temperature, the body temperature, the pulse, the blood pressure, and the ambient temperature were measured.
The skin blood perfusion measurements were performed, using a laser Doppler perfusion imager (LDPI; PIM 2·3 Laser Doppler perfusion imager®; Lisca Development AB, Linköping, Sweden) 10, 20, 21. The LDPI scans the tissue in a stepwise manner, using a low‐power laser (output power <1 mW, wavelength 635 nm) and generates colour‐coded images of the tissue blood flow to a depth of approximately 0·6 mm (<1 mm). The skin blood perfusion is defined as the product of the average speed of the blood cells and the concentration (22). The measurements were performed in a non invasive way without touching the skin, i.e. at a distance of 20 cm. Each measurement lasted approximately 5 minutes and a dark green cloth with a hole for the scanning area covered the skin. The scanning areas were also marked using a black permanent pen to make it possible to scan the same area for the duration of the study.
The skin temperature was measured using a Raynger®ST non contact thermometer (Raytek ST, Santa Cruz, CA, USA). Because there was a margin of measurement error of±1°C, the mean value of five measurements was calculated and registered. The body temperature was measured in the armpit, using a Terumo digital thermometer, according to the routine procedure for such body temperature measurements. The ambient temperature was measured using a Schwille electronic digital thermometer.
The size of the skin area with non blanchable erythema was calculated using the grid tracing method 23, 24 i.e. a transparent film was placed over the damaged area and the area was outlined with a permanent marker pen, and the drawing was then transferred onto another transparent plastic film with a preprinted square grid, where each square had an area of 0·25 cm2. The area was then calculated using the following formula: (N+N C/2) × 0·25 cm2, where N equals the number of whole squares, present in the tracing, and N C the number of partial squares in the tracing.
Photographs of the non blanchable erythema areas were taken using a digital camera.
Statistics
The skin blood perfusion values were presented in terms of mean ± standard deviation. Differences in skin blood perfusion distribution between non blanchable erythema and undamaged skin were compared using Levene’s test for equality of variances and the Kolmogorov–Smirnov test. Because the results from these tests were similar, only the results from Levene’s test are presented in the Results section. P < 0·05 was considered to be significant.
The skin blood perfusion distribution profiles are presented as cumulative relative frequency histograms (21). In these, the cumulative relative frequency of the observations is shown on the y‐axis and the perfusion value (V) on the x‐axis.
Results
Four measurements were taken in four patients, and the measurements were performed in the patient’s own room at the nursing homes. In patient 1, three measurements were made, the first of which was made in the patient’s room in the hospital ward and the remaining two at the patient’s own home.
All the measurements were performed in an ambient temperature ranging from 22·6 ± 0·3 to 24·7 ± 0·3°C. The patients’ skin temperature in damaged and undamaged skin areas ranged from 29·4 ± 0·4 to 33·9 ± 0·5°C and 28·2 ± 1·2 to 33·7 ± 0·9°C, respectively. All patients had a normal blood pressure, systolic, ranging from 132·5 ± 5·0 to 156·7 ± 10·4 mmHg, and diastolic pressure ranging from 67·5 ± 5·0 to 75·0 ± 5·8 mmHg (Table 2).
Table 2.
Overview of measurements for each patient, presented as mean ± SD
| Measurement | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|---|
| Ambient temperature (°C) | 22·6 ± 0·3 | 24·7 ± 0·3 | 23·7 ± 1·2 | 24·2 ± 2·3 | 24·4 ± 0·1 |
| Body temperature (°C) | 36·9 ± 0·2 | 36·7 ± 0·6 | 36·8 ± 0·3 | 36·9 ± 0·6 | 36·2 ± 0·3 |
| Skin temperature of non blanchable erythema (°C) | 33·3 ± 1·9 | 32·8 ± 1·0 | 33·9 ± 0·5 | 29·4 ± 0·4 | 30·1 ± 1·4 |
| Skin temperature of undamaged skin (°C) | 28·2 ± 1·2 | 31·4 ± 4·5 | 33·7 ± 0·9 | 30·9 ± 1·1 | 29·3 ± 0·8 |
| Area of non blanchable erythema (cm2) | 3·1 ± 0·4 | 24·5 ± 2·0 | 2·3 ± 0·8 | 10·6 ± 4·3 | 2·3 ± 3·1 |
| Systolic blood pressure (mmHg) | 156·7 ± 10·4 | 141·3 ± 21·0 | 132·5 ± 5·0 | 135·0 ± 12·9 | 145·0 ± 4·1 |
| Diastolic blood pressure (mmHg) | 73·3 ± 2·9 | 73·6 ± 8·5 | 75·0 ± 5·8 | 67·5 ± 5·0 | 72·5 ± 5·0 |
The blood perfusion was different in all skin areas with non blanchable erythema as compared with the blood flow in undamaged skin with some exceptions. In patient 2, measurement 1 was non significant and, in this patient, the non blanchable erythema was located at the paretic side of the body. The other exception was measurement 3, in patient 5, where no differences were found, and the non blanchable erythema was no longer visible to the naked eye (Table 3).
Table 3.
Skin blood flow in areas with undamaged skin and non blanchable erythema, mean and SD
| Measurement number | Blood flow in undamaged skin (V) | Blood flow in non blanchable erythema (V) | P value* | Area of non blanchable erythema (cm2) | Systolic/diastolic blood pressure (mmHg) |
|---|---|---|---|---|---|
| Patient 1 | |||||
| 1 | 0·85 ± 0·39 | 1·15 ± 0·51 | 0·000 | 2·6 | 145/75 |
| 2 | 0·62 ± 0·16 | 0·83 ± 0·57 | 0·000 | 3·4 | 160/75 |
| 3 | 0·78 ± 0·21 | 0·97 ± 0·35 | 0·000 | 3·3 | 165/70 |
| Patient 2† | |||||
| 1 | 2·28 ± 1·04 | 1·93 ± 0·98 | 0·539 | 22·4 | 135/70 |
| 2 | 2·58 ± 0·96 | 2·43 ± 1·65 | 0·000 | 23·5 | 140/65 |
| 3 | 2·78 ± 1·04 | 3·22 ± 1·35 | 0·000 | 25·0 | 120/75 |
| 4 | 2·01 ± 1·11 | 2·01 ± 0·98 | 0·000 | 27·1 | 178/85 |
| Patient 3 | |||||
| 1 | 1·19 ± 0·47 | 2·22 ± 1·28 | 0·000 | 3·0 | 140/70 |
| 2 | 1·74 ± 1·12 | 1·87 ± 1·29 | 0·002 | 2·8 | 130/80 |
| 3 | 1·44 ± 0·74 | 1·65 ± 1·10 | 0·000 | 1·6 | 130/80 |
| 4 | 1·77 ± 0·85 | 1·45 ± 0·97 | 0·003 | 1·6 | 130/70 |
| Patient 4 | |||||
| 1 | 1·25 ± 0·38 | 1·57 ± 0·49 | 0·000 | 14·0 | 130/70 |
| 2 | 1·06 ± 0·31 | 1·14 ± 0·44 | 0·000 | 14·4 | 120/60 |
| 3 | 0·85 ± 0·17 | 1·10 ± 0·44 | 0·000 | 6·0 | 150/70 |
| 4 | 1·10 ± 0·28 | 1·60 ± 0·39 | 0·000 | 7·9 | 140/70 |
| Patient 5 | |||||
| 1 | 1·19 ± 0·32 | 2·05 ± 0·64 | 0·000 | 6·6 | 145/70 |
| 2 | 1·28 ± 0·32 | 1·72 ± 1·00 | 0·000 | 2·6 | 145/70 |
| 3 | 1·47 ± 0·31 | 0·89 ± 0·30 | 0·067 | 0 | 150/70 |
| 4 | 1·08 ± 0·34 | 1·07 ± 0·29 | 0·000 | 0 | 140/80 |
Levene’s test, F‐test for equality of variance used.
Non blanchable erythema in the paretic side of the body.
There were no significant correlations in blood perfusion between the skin areas with non blanchable erythema or undamaged skin and the remaining variables such as ambient temperature, skin temperature, body temperature and blood pressure.
Observing the blood perfusion distribution profiles, there were no obvious pattern in the differences in the profiles between the skin areas with non blanchable erythema and the undamaged skin in patients 1 and 2. The skin areas of non blanchable erythema, in these patients, increased over time, by 26·9% and 21%, respectively, despite pressure relief. However, a possible small decrease was seen at the last measurement for patient 1 (Figure 1A, 1B, 1C).
Figure 1.
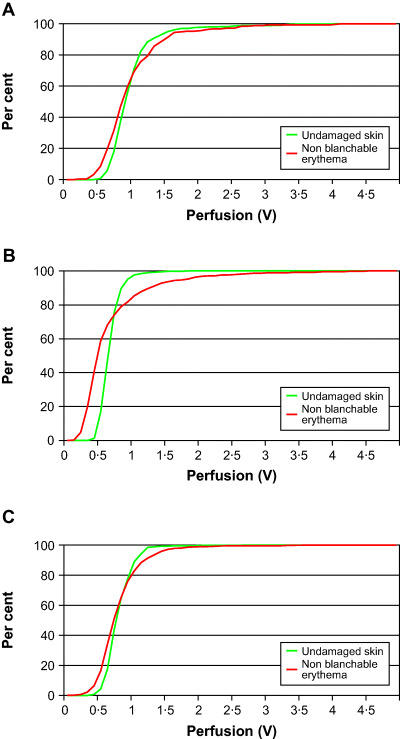
(A, B, C) Cumulative relative frequency line graphs, showing the perfusion in undamaged skin and in areas with non blanchable erythema in patient 1 (21).
In patient 3, the blood perfusion distribution profiles over areas of non blanchable erythema and undamaged skin were found to be separated at the first measurement, compared with the later measurements, becoming more homogeneous over time. The area of the non blanchable erythema decreased gradually, from measurements 1 to 4. The total decrease was 46·7% (Figure 2A, 2B).
Figure 2.
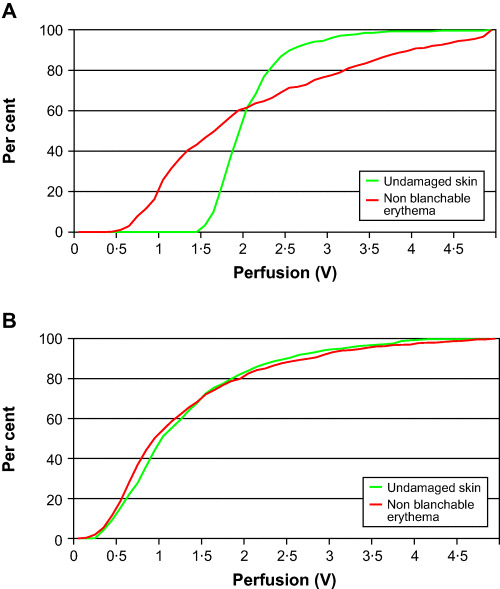
(A, B) Cumulative relative frequency line graphs, showing the perfusion in undamaged skin and in areas with non blanchable erythema in patient 3 (first and fourth measurements) (21).
In patient 4, the blood perfusion distribution profiles were quite similar in areas with non blanchable erythema and in undamaged skin areas in measurements 1 to 3. The area of the non blanchable erythema decreased between measurements 1 and 3 by 57·1%, although a small increase was seen in measurement 4.
In patient 5, the blood perfusion distribution profiles were separated from each other in measurements 1 and 2. In measurements 3 and 4, the distribution profiles were close and almost identical, and the non blanchable erythema was no longer visible by that time (Figure 3A, 3B).
Figure 3.
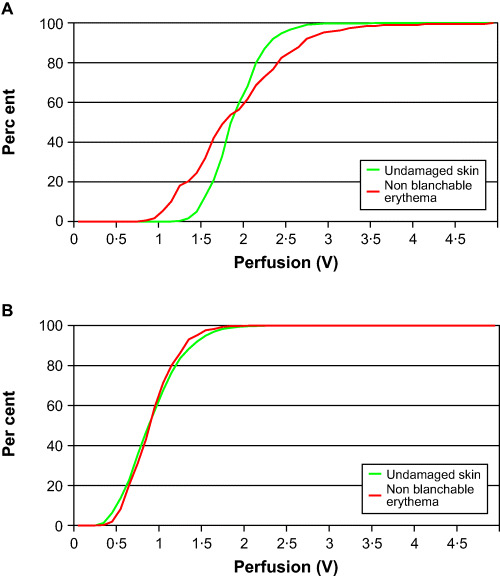
(A, B) Cumulative relative frequency line graphs, showing the perfusion in undamaged skin and in areas with non blanchable erythema in patient 5 (first and fourth measurements) (21).
When the perfusion data of all patients were pooled and corrected for mean, the cumulative perfusion profile showed the same patterns as presented in the individual histograms (21).
A typical illustration of blood perfusion in an area of non blanchable erythema is shown in Figure 4. There is an area of high blood flow in the centre of the lesion and the flow successively decreases closer to the edge.
Figure 4.
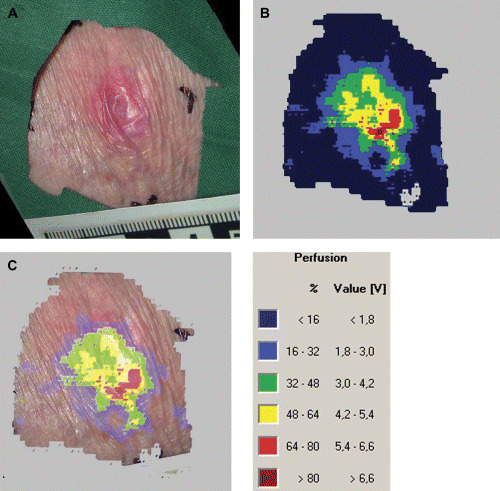
Typical result from a laser Doppler perfusion imager measurement in an area with non blanchable erythema. (A) The digital photograph, (B) the perfusion image and (C) the combined digital photograph and perfusion image are shown.
Discussion
The skin blood perfusion in this study was measured using a laser Doppler perfusion imaging. The laser Doppler technique has been used in a large number of studies for measuring the skin blood flow perfusion with different techniques, however 7, 10, 20, 21. The technique has been developed for about 25 years and has been found to be a reliable method for measuring skin blood perfusion (25).
In this study, we found different blood perfusion distribution profiles in skin areas with non blanchable erythema as compared with undamaged skin. These findings may indicate that the visible redness in areas of non blanchable erythema may be related to an increased blood perfusion in this area. Histological examination of human tissue with non blanchable erythema showed a high concentration of red blood cells in the affected areas (13). Because the LDPI technique measures both the average speed of the blood cells and the concentration of red blood cells, the higher values and the redness could be related also to the large number of red blood cells in the area.
The patients in this study had considerably higher skin blood perfusion values (Table 3) than patients with undamaged skin and healthy individuals in an earlier study (10). Four of the five patients also had partial hemiplegia following stroke. This may have influenced the results to some extent. However, when measuring basal blood perfusion in a previous study, no significant differences were found in skin blood flow between the paretic and the non paretic sides (10). In a future study, it would be of major importance to perform measurements on healthy individuals for comparison.
The distribution profiles in areas of undamaged skin are almost identical to those found in an earlier study on healthy individuals (21). The blood flow profile in areas of non blanchable erythema shows a different pattern compared with undamaged skin areas. In addition, the distribution profiles in areas of non blanchable erythema become increasingly similar to the profile of the undamaged skin as the size of the erythema decreases, the profiles becoming almost identical when the non blanchable erythema is no longer visible.
The areas of non blanchable erythema were supposed to be totally relieved of pressure during the study and the assumption was that the areas would successively decrease after pressure relief. It is however difficult to see any effects of the pressure relief because some of the areas of non blanchable erythema increased while some decreased. Perhaps, these changes are related to the possibility of performing pressure relief for different patients.
Observing the perfusion images, a typical pattern of non blanchable erythema could be observed. There was a concentration of high blood flow in the centre of the damaged area with a successive decrease closer to the edges. Schubert et al. (7)found, however, an increased blood flow both in the centre and at the edge of non blanchable erythema.
This is an explorative study with few patients; hence, it is difficult to generalise from these results. Data concerning movements were not registered. Also, the risk for cooling of the uninjured foot during the measurement procedure was not considered. A random order of measurements could perhaps have minimised this problem. Nevertheless, this study has generated many interesting questions from a methodological point of view as well as questions of interest for further study of skin blood perfusion and pressure sores.
In conclusion, these results indicate that there are differences in blood flow perfusion between skin areas of non blanchable erythema and undamaged skin. The results also indicate that the visible redness in areas with non blanchable erythema is related to altered blood perfusion. The skin blood perfusion also seems to increase in relation to the size of the non blanchable erythema.
Acknowledgements
Grants from Linköping University, the Research fund of the County of Östergötland and ‘Vårdalstiftelsen’ nr V 96‐142 and V 98‐360 are gratefully acknowledged.
References
- 1. AHCPR, Agency for Health Care policy and Research . Pressure sores in adults: prediction and prevention. Clinical Practice Guidelines, No. 3. Rockville, MD: U.S. Department of Health and Human Services, 1992. [Google Scholar]
- 2. Lindgren M, Unosson M, Ek A‐C. Pressure sore prevalence within a public health services area. Int J Nurs Pract 2000;6:333–7. [Google Scholar]
- 3. O’Dea K. The prevalence of pressure damage in acute hospital patients in the UK. J Wound Care 1999;8(4):192–4. [DOI] [PubMed] [Google Scholar]
- 4. Gunningberg L, Lindholm C, Carlsson M, Sjödén P‐O. The development of pressure sores in patients with hip‐fractures: inadequate nursing documentation is still a problem. J Adv Nurs 2000;31:1155–64. [PubMed] [Google Scholar]
- 5. Hoshowsky V, Schramm C. Intra operative pressure sore prevention: an analysis of bedding materials. Res Nurs Health 1994;17:333–9. [DOI] [PubMed] [Google Scholar]
- 6. Ek A‐C. Prevention, treatment and healing of pressure sores in long‐term care patients. Scand J Caring Sci 1987;1:7–13. [DOI] [PubMed] [Google Scholar]
- 7. Schubert V, Héraud J. The effects of pressure and shear on skin microcirculation in elderly stroke patients lying in supine or semi‐recumbent positions. Age Ageing 1994;23:405–10. [DOI] [PubMed] [Google Scholar]
- 8. Ek A‐C, Unosson M, Larsson J, Von Schenk H, Bjurulf P. The development and healing of pressure sores related to the nutritional state. Clin Nutr 1991;10:245–50. [DOI] [PubMed] [Google Scholar]
- 9. Schubert V, Fagrell B. Evaluation of the dynamic cutaneous post‐ischaemic hyperaemia and thermal response in elderly subjects and in area at risk for pressure sores. Clin Physiol 1991;11:169–82. [DOI] [PubMed] [Google Scholar]
- 10. Ek A‐C, Gustavsson G, Lewis DH. Skin blood flow in relation to external pressure and temperature in the supine position on a standard hospital mattress. Scand J Rehabil Med 1987;19:121–6. [PubMed] [Google Scholar]
- 11. Friman G, Hamrin E. Changes in reactive hyperaemia after clinical bed rest for seven days. Ups J Med Sci 1976;81:79–83. [DOI] [PubMed] [Google Scholar]
- 12. Guyton AC. Textbook of medical physiology. Philadelphia: W.B. Saunders Company, 1999. [Google Scholar]
- 13. Witkowski JA, Parish LC. Histopathology of the decubitus ulcer. J Am Acad Dermatol 1982;6:1014–21. [DOI] [PubMed] [Google Scholar]
- 14. Hiromi A, Obata M, Shimada T, Hagisawa S. Morphological characteristics of the dermal papillae in the development of pressure sores. J Tissue Viability 1998;8(3):17–23. [DOI] [PubMed] [Google Scholar]
- 15. Schubert V, Fagrell B. Local skin pressure and its effect on skin microcirculation as evaluated by laser‐Doppler fluxmetry. Clin Physiol 1989;9:535–45. [DOI] [PubMed] [Google Scholar]
- 16. Herrman EC, Knapp CF, Donofrio JC, Salcido R. Skin perfusion response to surface pressure‐induced ischemia: implications for the developing pressure ulcer. J Rehabil Res Dev 1999;36(2):109–20. [PubMed] [Google Scholar]
- 17. Schubert V, Perbeck L, Schubert P‐Å. Skin microcirculatory and thermal changes in elderly subjects with early stage of pressure sores. Clin Physiol 1994;14:1–13. [DOI] [PubMed] [Google Scholar]
- 18. Daly MJ, Henry RE. Quantitative measurement of skin perfusion with xenon‐133. J Nucl Med 1980;21:156–60. [PubMed] [Google Scholar]
- 19. Roberts MF, Wenger CB. Control of skin blood flow during exercise by thermal reflexes and baroreflexes. J Appl Physiol, 1980;48:717–23. [DOI] [PubMed] [Google Scholar]
- 20. Droog EJ, Steenbergen W, Sjöberg F. Measurement of depth of burns by laser Doppler perfusion imaging. Burns 2001;27:561–8. [DOI] [PubMed] [Google Scholar]
- 21. Golster H, Thulesius O, Nilsson GE, Sjöberg F. Heterogeneous blood flow response in the foot on dependency, assessed by laser Doppler perfusion imager. Acta Physiol Scand 1997;159:101–6. [DOI] [PubMed] [Google Scholar]
- 22. Wårdell K, Andersson T, Anderson C. Analysis of laser Doppler perfusion images of experimental irritant skin reactions. Skin Res Technol 1996;2:149–57. [DOI] [PubMed] [Google Scholar]
- 23. Schubert V. Measuring the area of chronic ulcers for consistent documentation in clinical practice. Wounds 1997;9(5):153–9. [Google Scholar]
- 24. Öien RF, Håkansson A, Hansen BU, Bjellerup M. Measuring the size of ulcers by planimetry: a useful method in the clinical setting. J Wound Care 2002;11(5):165–8. [DOI] [PubMed] [Google Scholar]
- 25. Wårdell K, Nilsson GE. Laser Doppler imaging of skin. In: Serup J, Jemec BJ, editors. Non‐invasive methods and the skin London: CRC Press Inc., pp 421–428. (1995). [Google Scholar]


