Abstract
In the wound bed of chronic venous leg ulcers, an imbalance of matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs) may cause excessive proteolysis and impair wound granulation. Soluble mediators in the wound environment may be responsible for this imbalance. The in vitro effect of wound fluid from venous leg ulcers on dermal fibroblast production of MMP‐1, MMP‐3 and TIMP‐1 was compared with the effect of acute wound fluid from two different sources: fluid from post‐mastectomy axillary drains and fluid from skin graft donor sites. Significantly higher MMP‐1 and MMP‐3 levels were induced by chronic venous leg ulcer wound fluid compared with both types of acute wound fluid (P < 0·005). Chronic venous ulcer wound fluid reduced TIMP‐1 protein levels significantly more than acute graft fluid (P < 0·05). Venous ulcer wound fluid significantly increased MMP‐1 and MMP‐3 production in dermal fibroblasts and reduced TIMP‐1 production, confirming that mediators in the leg ulcer microenvironment can potentially induce excessive proteolysis in the ulcer dermis by altering the balance between MMPs and TIMPs. Inflammatory mediators including interleukin‐1β and tumour necrosis factor‐α can induce these MMPs. Further work is required to confirm the factors responsible for the induction of a high MMP and low TIMP profile in fibroblasts by venous ulcer wound fluid.
Keywords: Dermal fibroblasts, Matrix metalloproteinases, Proteolysis, Venous leg ulcers, Wound healing
Introduction
The causes of impaired healing of chronic venous leg ulcers remain uncertain. High levels of proteolytic activity have been demonstrated in chronic leg ulcer exudates and may result from an imbalance in the expression of matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs) 1, 2, 3. In the wound bed, excessive proteolytic activity could be responsible for the degradation of growth factors, cytokines, cell surface receptors and extracellular matrix (ECM) components (4) and impair wound granulation 4, 5, 6.
ECM degradation is largely mediated by MMPs. MMP activity increases in normal wound healing to enable cell migration and tissue remodelling. During normal wound repair, both MMP‐1 and MMP‐3 are expressed in dermal fibroblasts and participate in the formation and removal of granulation tissue and resolution of scar tissue 7, 8. MMP‐3 is synthesised primarily by fibroblasts (8). MMP activity is regulated at the extracellular level by TIMPs produced by fibroblasts and other cell types. TIMP‐1 is temporally and spatially regulated during wound repair 2, 7.
The expression of MMP and TIMP by connective tissue cells in culture has been shown to be regulated by inflammatory cytokines, growth factors, hormones, cell–cell and cell–matrix interactions (9). This study explores the in vitro effect of wound fluid from venous leg ulcers on dermal fibroblast production of MMP‐1, MMP‐3 and TIMP‐1 and compares this with the effect of wound fluid from acute wounds. We hypothesise that venous ulcer wound fluid contains soluble mediators responsible for inducing MMP‐1 and MMP‐3 in fibroblasts. As there is debate over the most appropriate acute wound to use as a comparison for venous leg ulcers, this study compares two sources of acute wound fluid – fluid from post‐mastectomy axillary drains and fluid from donor split‐thickness skin graft sites on the upper thigh.
Materials and methods
Chronic wound fluid
Patients with leg ulcers caused by confirmed venous disease (CEAP Class 6) were recruited into the study (n= 9). Venous photoplethysmography (10) was performed to determine the presence of venous disease. A diagnosis of venous disease was confirmed by a venous refilling time less than 25 seconds. In all cases, patients’ ulcers had failed to respond to outpatient treatment (compression therapy) over the previous 3 months as defined by no reduction in the size of the ulcer in more than 3 months or a continued increase in the size of the ulcer. Patients with ulcers because of arterial disease (ankle brachial index < 0·8), ulcers on the foot, rheumatoid arthritis or diabetes (requiring insulin treatment) were excluded from the study.
Wound fluid was collected by a standardised method (11). The patient was fasted from midnight and a transparent occlusive film (Opsite; Smith & Nephew, Hull, UK) placed over the wound at 8:00 hours. The patient’s leg was placed in a dependant position, and the patient was encouraged to drink 1 l of water to aid in standardising the state of hydration. After 1 hour, the fluid was aspirated from beneath the dressing, centrifuged at 14 000 g for 10 minutes and snap‐frozen in liquid nitrogen for immediate storage at −80°C.
Acute wound fluid – post‐mastectomy
Acute wound fluid was collected from female patients who had a mastectomy for primary breast carcinoma with axillary lymph node clearance (n= 8). Wound fluid that accumulated in the vacuum drain from the axilla during an 8‐hour period on day 3 post‐operation was collected, centrifuged at 14 000 g for 10 minutes and stored at −80°C. Samples that were visibly blood‐tinged were excluded from the study.
Acute wound fluid – skin graft donor site
Acute wound fluid was also collected from the upper thigh split‐thickness donor sites of leg ulcer patients who had a skin graft (n= 3). The wound fluid was collected on day 3 post‐graft by placing a transparent occlusive dressing over the wound for 1 hour. Wound fluid that accumulated was then aspirated, centrifuged at 14 000 g for 10 minutes and stored at −80oC. Samples that were visibly blood‐tinged were excluded from the study.
Consent
All subjects provided their written informed consent. The study was approved by the South Metropolitan Area Health Service Human Research Ethics Committee and was conducted according to Declaration of Helsinki Principles.
Protein assay
Total protein in individual wound fluid samples was quantified using the BCA procedure (Pierce Rockford, IL) and FLUOstar Optima microplate reader (BMG Labtech GmbH, Offenburg, Germany).
Pooled wound fluid
For each category of wound fluid, volumes containing equivalent quantities of protein were pooled from each patient.
Cell culture medium
Minimum essential media (MEM) with Earle’s Salts (Thermo Trace, Melbourne, Australia), 1 mM sodium pyruvate (Thermo Trace), 1× non essential amino acids (Thermo Trace), 2 mM l‐glutamine (Thermo Trace), 100 U penicillin/ml, 0·1 mg/ml streptomycin (Sigma‐Aldrich, St. Louis, MO) and 10% fetal calf serum (FCS) (CSL, Parkville, Australia). This supplemented Earle’s MEM is hereafter referred to as complete medium.
Dermal fibroblasts
Normal human neonatal dermal fibroblasts (Clonetics NHDF 9227, Catalogue number CC‐2509; Lonza, Basel, Switzerland) were maintained in complete medium at 37°C and 5% CO2. Fibroblasts were passaged at a split ratio of 1:3 every 3 days and medium was exchanged every 2–3 days. Fibroblasts from passage 5 to 10 were seeded at 3 × 104 cells/cm2 in tissue culture‐treated 96‐well plates (Microtest III, Falcon; BD Biosciences, Bedford, MA), grown overnight, washed twice with phosphate‐buffered saline and serum starved for 24 hours in FCS‐free medium.
Experimental conditions
Following serum starvation, fibroblasts were incubated for 24 hours with test media:
-
1
5% (v/v) pooled chronic venous ulcer wound fluid in MEM supplemented with 10% (v/v) FCS
-
2
5% (v/v) pooled acute mastectomy wound fluid in MEM supplemented with 10% (v/v) FCS
-
3
5% (v/v) pooled acute graft wound fluid in MEM supplemented with 10% (v/v) FCS
-
4
MEM supplemented with 10% (v/v) FCS
Each test and control condition was replicated in nine wells, and after 24 hours, conditioned supernatants from three wells were pooled, yielding three replicate samples for each treatment. These supernatants were stored frozen at −20°C until assayed for MMP‐1, MMP‐3 and TIMP‐1. Fibroblasts remained adherent to the 96‐well plate and were quantified using an MTT (3‐[4,5‐dimethylthazol‐2‐yl]‐2,5‐diphenyl tetrazolium bromide) assay as described below. The entire experiment was then repeated.
MTT assay
MTT assays were performed on adherent fibroblasts to quantify cell number and provide a means of normalising individual sample wells (12). Immediately following collection of conditioned cell culture supernatants, 100 μl complete medium containing 1 mg/ml MTT (stock solution: 5 mg/ml phosphate‐buffered saline stored at −20°C protected from light) was added immediately to fibroblasts in 96‐well plates. Fibroblasts were then incubated for 4 hours at 37°C. An equivalent volume of extraction buffer (stock solution: 20% w/v sodium dodecyl sulphate and 50% v/v N,N‐dimethylformamide, pH 4·7) was added and incubated overnight at 37°C to dissolve the formazan crystals. Colour generation was quantified spectrophotometrically at 570 nm on a FLUOstar Optima microplate reader (BMG Labtech), 24 hours following the addition of MTT to the fibroblasts.
Human MMP‐1, MMP‐3 and TIMP‐1 enzyme‐linked immunosorbent assays (ELISAs)
Total MMP‐1, MMP‐3 and TIMP‐1 levels in individual wound fluid samples, pooled wound fluid, festal calf serum, MEM and conditioned cell culture supernatants collected from two replicate experiments were each measured in duplicate according to the manufacturers’ instructions [MMP‐1 and TIMP‐1: Biotrak ELISA System; Amersham Biosciences, Piscataway, NJ (MMP‐1: measures free MMP‐1 and MMP‐1/TIMP complexes; TIMP‐1: measures free TIMP‐1 and TIMP‐1/MMP complexes); MMP3 CytoSets; Biosource Invitrogen, Carlsbad, CA (measures pro‐MMP‐3, activated MMP‐3 and MMP‐3/TIMP‐1 and MMP‐3/TIMP‐2 complexes)].
The concentrations of MMP‐1, MMP‐3 and TIMP‐1 in conditioned supernatants were normalised to MTT activity of adherent fibroblasts. Levels present in the 5% (v/v) pooled wound fluid in complete medium before exposure to cells were subtracted from the values obtained from conditioned supernatants.
Results
Patients
The details of each group of patients are summarised in Table 1. The groups were matched for age but not for gender. The size of the acute graft fluid group was smaller than the other groups.
Table 1.
Patient details
| Patients | n | Gender | Ulcer size (cm2): median (interquartile range) | Age (yrs): median (range) |
|---|---|---|---|---|
| Venous leg ulcers | 9 | 3 males; 6 females | 72·2 (39·0–100·3) | 83 (67–90) |
| Post‐mastectomy | 8 | 8 females | N/A | 63·5 (53–80) |
| Skin graft for leg ulcer | 3 | 1 male; 2 females | N/A | 78 (78–79) |
N/A, not applicable.
Wound fluids
The protein concentrations of each wound fluid type (individual and pooled) are summarised in Table 2. The acute graft wound fluids had a significantly higher protein concentration than the acute mastectomy fluids (Mann–Whitney test, P < 0·01) and the chronic venous leg ulcer fluids (Mann–Whitney test, P < 0·05).
Table 2.
Protein concentration of wound fluid samples and fetal calf serum
| Wound fluid | n | Protein concentration of individual samples (mg/ml): range | Protein concentration of pooled wound fluid (mg/ml) |
|---|---|---|---|
| Chronic – venous leg ulcer | 9 | 27–59 | 40·9 |
| Acute – mastectomy | 8 | 30–53 | 41·7 |
| Acute – graft | 3 | 58–74 | 65·0 |
| Fetal calf serum (FCS) | 1 | 40 | N/A |
N/A, not applicable.
MMP and TIMP levels in wound fluid
The type of wound fluid had a significant effect on MMP‐1 levels (Kruskal–Wallis test, P < 0·001) (Figure 1A). There was a significantly higher concentration of MMP‐1 in chronic venous leg ulcer wound fluid compared with that in acute mastectomy wound fluid (Mann–Whitney test, P < 0·0005) but not compared with that in acute graft wound fluid. Acute graft wound fluid had a significantly higher concentration of MMP‐1 than acute mastectomy wound fluid (Mann–Whitney test, P < 0·01).
Figure 1.
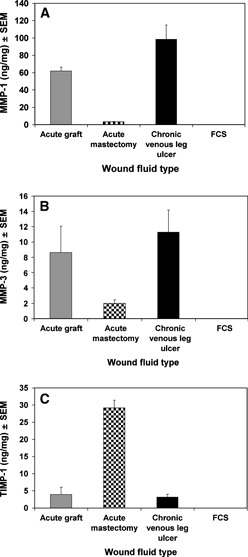
(A) Mean matrix metalloproteinase (MMP)‐1 concentration in wound fluid. Concentrations were determined from 1:25 and 1:50 dilutions of wound fluid. (B) Mean MMP‐3 concentration in wound fluid. Concentrations were determined from 1:25 and 1:100 dilutions of wound fluid. (C) Mean tissue inhibitor of metalloproteinase (TIMP)‐1 concentration in wound fluid. Concentrations were determined from 1:50 and 1:150 dilutions of wound fluid.
The type of wound fluid also had a significant effect on MMP‐3 levels (Kruskal–Wallis test, P= 0·005) (Figure 1B). As observed for MMP‐1, there was a significantly higher concentration of MMP‐3 in chronic venous leg ulcer wound fluid compared with that in acute mastectomy wound fluid (Mann–Whitney test, P < 0·001) but not compared with that in acute graft wound fluid. Acute graft wound fluid had significantly more MMP‐3 than acute mastectomy wound fluid (Mann–Whitney test, P < 0·05).
The type of wound fluid had a significant effect on TIMP‐1 levels (Kruskal–Wallis test, P < 0·001) (Figure 1C). There was approximately fivefold more TIMP‐1 in acute mastectomy wound fluid than in both acute graft wound fluid (Mann–Whitney test, P < 0·01) and chronic venous leg ulcer wound fluid (Mann–Whitney test, P < 0·0005). There was no significance difference between the TIMP‐1 levels in chronic venous leg ulcer wound fluid and acute graft wound fluid (Mann–Whitney test).
The levels of MMP‐1, MMP‐3 and TIMP‐1 were below detectable limits in FCS and MEM (data not shown).
Fibroblast proliferation with wound fluid
The MTT proliferation assay results revealed that the type of wound fluid did not have a significant effect on fibroblast proliferation (Kruskal–Wallis test) (Figure 2A). The addition of 5% wound fluid to the 10% FCS did not significantly alter the proliferation measured with 10% FCS alone (Mann–Whitney test).
Figure 2.
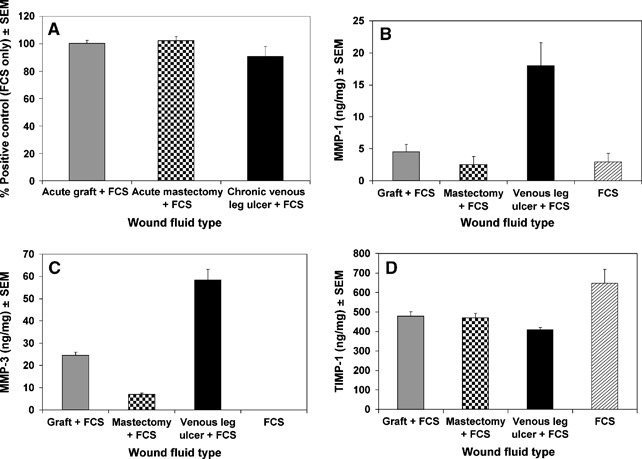
(A) Effect of wound fluid type on neonatal dermal fibroblast proliferation after 24 hours. Mean proliferation determined from MTT activity of fibroblast monolayers. (B) Effect of wound fluid on secretion of total matrix metalloproteinase (MMP)‐1 from neonatal dermal fibroblasts (mean). 1:5 dilutions were used for data analysis. The level of MMP‐1 present in the pooled wound fluid was subtracted from the total level of MMP‐1. (C) Effect of wound fluid on secretion of total MMP‐3 from neonatal dermal fibroblasts (mean). 1:20 dilutions were used for data analysis. The level of MMP‐3 present in the pooled wound fluid was subtracted from the total level of MMP‐3. (D) Effect of wound fluid on secretion of total tissue inhibitor of metalloproteinase (TIMP)‐1 from neonatal dermal fibroblasts (mean). 1:160 dilutions were used for data analysis. The level of TIMP‐1 present in the pooled wound fluid was subtracted from the total level of TIMP‐1.
Effect of wound fluid on MMP and TIMP levels produced by dermal fibroblasts
The type of wound fluid had a significant effect on fibroblast MMP‐1 production (Kruskal–Wallis test, P < 0·01) (Figure 2B). The mean level of MMP‐1 induced by chronic venous leg ulcer wound fluid was approximately fourfold higher than the levels induced by both types of acute wound fluid or the FCS control. Pair‐wise testing (Mann–Whitney test) confirmed a significant increase in the MMP‐1 levels induced by chronic venous leg ulcer wound fluid compared with acute graft wound fluid (P < 0·005), acute mastectomy wound fluid (P < 0·005) or FCS (P < 0·005).
The type of wound fluid had a significant effect on MMP‐3 production (Kruskal–Wallis test, P= 0·001) (Figure 2C). FCS alone did not induce any MMP‐3. The mean level of MMP‐3 induced by chronic venous leg ulcer wound fluid was approximately twofold higher than the level induced by acute graft wound fluid and eightfold higher than the level induced by acute mastectomy wound fluid. Pair‐wise testing (Mann–Whitney test) confirmed that significantly more MMP‐3 was induced by chronic venous leg ulcer wound fluid than acute graft wound fluid (P < 0·005), acute mastectomy wound fluid (P < 0·005) or FCS (P < 0·005). Acute graft wound fluid induced significantly more MMP‐3 production than acute mastectomy wound fluid (Mann–Whitney test, P < 0·005).
Wound fluid type also had a significant effect on TIMP‐1 production (Kruskal–Wallis test, P < 0·05) (Figure 2D). Pair‐wise testing (Mann–Whitney test) confirmed that FCS alone induced significantly more TIMP‐1 than when combined with chronic venous leg ulcer wound fluid (P < 0·05), acute graft wound fluid (P < 0·05) or acute mastectomy wound fluid (P < 0·05). The degree of suppression of TIMP‐1 production was approximately 25% for the acute wounds fluids and 37% for the chronic wound fluid. The difference between chronic venous leg ulcer wound fluid and acute graft wound fluid was significant (Mann–Whitney test, P < 0·05).
Discussion
This study has compared the MMP and TIMP levels in chronic and acute wound fluid and used an in vitro system to test if the extracellular mediators in the venous leg ulcer environment are likely to stimulate excessive production of MMPs in the ulcer dermis. Wound fluid from chronic venous leg ulcers represents extracellular fluid of the wound and is a useful tool for analysing the wound environment (11). It is important to note that wound fluid may contain mediators produced by epidermal and dermal cells and from the circulation, so does not provide any spatial information.
Enhanced MMP‐1 protein levels and activity have been reported in chronic venous ulcer wound fluid compared with acute wound fluid 13, 14, accompanied by a greater number of cells expressing MMP‐1 protein and MMP‐1 messenger RNA (mRNA) in the dermis 15, 16, 17. Elevated levels of MMP‐3 have also been reported in chronic wounds, with MMP‐3 mRNA and protein prominent in dermal fibroblasts in chronic venous ulcers compared with acute wounds 5, 8, 15. Higher levels of MMP‐1 and MMP‐3 in chronic venous leg ulcer wound fluid were confirmed in this study when the leg ulcer fluid and acute mastectomy fluid were compared. However, this difference was not demonstrated when chronic venous leg ulcer fluid was compared with acute wound fluid from the split‐thickness graft site on the upper thigh.
It can be argued that wound fluid from the split‐thickness graft site is the more appropriate acute wound control for leg ulcer wound fluid because the collection protocol and anatomical location of the collection site are more similar to those for the leg ulcer wound fluid than post‐mastectomy axillary wound fluid. The composition of post‐mastectomy wound fluid can also vary considerably between patients in relation to the duration of serosanguinous drainage, but in this study, any day 3 post‐operation samples visibly blood‐tinged were excluded. The fluid collected from the skin graft donor site is nearly always serous on day 3 post‐operation. Importantly, the split‐thickness graft site has a large surface area of dermal and epidermal remnants, similar to chronic leg ulcers.
TIMP‐1 levels in wound fluid were very low in both the chronic leg ulcer wound fluid and the acute graft fluid and high in the acute mastectomy fluid. Previous studies have also shown that wound fluid from non healing venous leg ulcers contains low levels of TIMP‐1 protein 13, 18, 19 compared with acute wound fluid, while abundant TIMP‐1 mRNA expression is detected in the dermis of both chronic venous leg ulcers and acute wounds (20). The reduced wound fluid levels in leg ulcer wound fluid may be because of reduced levels of TIMP‐1 in keratinocytes in chronic leg ulcers (15). Like MMP‐1 and MMP‐3 above, the different TIMP‐1 levels in acute mastectomy and acute graft wound fluid may be due to several variables including the nature of the wounds themselves and the wound fluid collection protocol.
To investigate proteolytic activity in the dermis, the effect of wound fluid on MMP and TIMP production by dermal fibroblasts is a more relevant consideration than wound fluid levels of MMPs and TIMPs. Here, we did see clear differences between the chronic and the acute wounds, with chronic venous ulcer wound fluid inducing significantly more MMP‐1 and MMP‐3 secretion in dermal fibroblasts compared with acute mastectomy wound fluid or acute graft wound fluid.
Expression of MMPs by dermal fibroblasts is induced by a range of soluble mediators. Most studies show that the inflammatory mediators interleukin (IL)‐1β and tumour necrosis factor‐α (TNF‐α) upregulate MMP‐1 protein in human skin fibroblasts 21, 22 and fibroblasts isolated from other tissues 23, 24, 25. Monocyte chemoattractant protein‐1 stimulates the production of MMP‐1 protein in human fibroblasts via IL‐1α(26), basic fibroblast growth factor induces synthesis of proMMP‐1 (22) and interferon‐gamma enhances MMP‐3 gene expression (27). Of these mediators, IL‐1 and TNF‐α protein levels are known to be higher in venous ulcer wound fluid than in acute mastectomy wound fluid (28) and decrease as venous ulcers start to heal 29, 30. Our recent studies have also shown that a polymorphism in the TNF‐α gene (−308A), which may increase the production of TNF‐α, is associated with increased risk of venous ulceration (31).
Interestingly, the addition of all types of wound fluid to FCS suppressed the production of TIMP‐1 by 25–30% compared with the FCS control. The downregulation by chronic leg ulcer wound fluid was significantly greater than that by the acute graft wound fluid, but the difference was modest. The differential regulation of TIMP‐1 in acute versus chronic inflammation is complex and seems to be controlled by multiple factors including regulation of the TIMP‐1 promoter, mRNA stability and repressive elements in intron‐1 32, 33, mediated by molecules such as IL‐1β and transforming growth factor‐β.
In conclusion, significant differences were noted in the MMP and TIMP profile of acute wound fluid from skin graft sites compared with post‐mastectomy wounds, with the acute graft fluid having a profile very similar to that of chronic venous leg ulcer fluid. However, compared with both types of acute wound fluid, venous ulcer wound fluid (5% v/v) significantly increased MMP‐1 and MMP‐3 production in dermal fibroblasts and reduced TIMP‐1 production, providing in vitro evidence that mediators in the leg ulcer microenvironment can potentially induce excessive proteolysis in the ulcer dermis by altering the balance between MMPs and TIMPs. Further work is required to determine the precise mediators responsible for creating a high MMP and low TIMP profile in dermal fibroblasts induced by venous ulcer wound fluid. Possible candidates include the inflammatory cytokines IL‐1β and TNF‐α, which are known to be elevated in chronic venous leg ulcers.
Acknowledgements
Our special thanks go to Dr Sim Yeoh for guidance in the early stages of this study. We also thank research nurses, Ms Yvonne Vandongen and Ms Sue Hoskin, for their assistance with wound fluid collection and clinical assessments. Dr KS was supported by a vacation scholarship from the University of Western Australia, School of Surgery and Pathology.
The work was done at the School of Surgery and Pathology, The University of Western Australia, Fremantle Hospital, Fremantle, Western Australia, Australia.
References
- 1. Saarialho‐Kere UK, Chang ES, Welgus HG, Parks WC. Distinct localization of collagenase and tissue inhibitor of metalloproteinases expression in wound healing associated with ulcerative pyogenic granuloma. J Clin Invest 1992;90:1952–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vaalamo M, Weckroth M, Puolakkainen P, Kere J, Saarinen P, Lauharanta J, Saarialho‐Kere U. Patterns of matrix metalloproteinase and TIMP‐1 expression in chronic and normally healing human cutaneous wounds. Br J Dermatol 1996;135:52–9. [PubMed] [Google Scholar]
- 3. Agren M, Eaglstein W, Ferguson M, Harding K, Moore K, Saarialho‐Kere U, Schultz G. Causes and effects of the chronic inflammation in venous leg ulcers. Acta Derm Venereol Suppl 2000;210:3–17. [PubMed]
- 4. Herouy Y, Trefzer D, Zimpfer U, Schopf E, Wanscheidt W, Norgauer J. Matrix metalloproteinases and venous leg ulceration. Eur J Dermatol 2000;10:173–80. [PubMed] [Google Scholar]
- 5. Yager D, Nwomeh B. The proteolytic environment of chronic wounds. Wound Repair Regen 1999;7:433–41. [DOI] [PubMed] [Google Scholar]
- 6. Nicolaides A. Chronic venous disease and the leukocyte‐endothelium interaction: from symptoms to ulceration. Angiology 2005;56 Suppl. 1:S11–9. [DOI] [PubMed] [Google Scholar]
- 7. Stricklin G, Liying L, Jancic V, Wenczak B, Nanney L. Localization of mRNAs representing collagenase and TIMP in secretions of healing burn wounds. Am J Pathol 1993;143:1657–66. [PMC free article] [PubMed] [Google Scholar]
- 8. Saarialho‐Kere UK, Pentland AP, Birkedal‐Hansen H, Parks WC, Welgus HG. Distinct populations of basal keratinocytes express stromelysin‐1 and stromelysin‐2 in chronic wounds. J Clin Invest 1994;94:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nagase H, Woessner J. Matrix metalloproteinases. J Biol Chem 1999;274:21491–4. [DOI] [PubMed] [Google Scholar]
- 10. Abramowitz HB, Queral LA, Finn WR, Nora PF Jr, Peterson LK, Bergan JJ, Yao JS. The use of photoplethysmography in the assessment of venous insufficiency: a comparison to venous pressure measurements. Surgery 1979;86:434–41. [PubMed] [Google Scholar]
- 11. Trengove N, Langton S, Stacey M. Biochemical analysis of wound fluid from nonhealing and healing chronic leg ulcers. Wound Repair Regen 1996;4:234–9. [DOI] [PubMed] [Google Scholar]
- 12. Hansen MB, Nielsen SE, Berg K. Re‐examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods 1989;119:203–10. [DOI] [PubMed] [Google Scholar]
- 13. Nwomeh BC, Liang HX, Cohen IK, Yager DR. MMP‐8 is the predominant collagenase in healing wounds and nonhealing ulcers. J Surg Res 1999;81:189–95. [DOI] [PubMed] [Google Scholar]
- 14. Weckroth M, Vaheri A, Lauharanta J, Sorsa T, Konttinen Y. Matrix metalloproteinases, gelatinase and collagenase, in chronic leg ulcers. J Invest Dermatol 1996;106:1119–24. [DOI] [PubMed] [Google Scholar]
- 15. Saarialho‐Kere U. Patterns of matrix metalloproteinases and TIMP expression in chronic ulcers. Arch Dermatol Res 1998;290 (Suppl):S47–54. [DOI] [PubMed] [Google Scholar]
- 16. Saarialho‐Kere U, Kovacs S, Pentland A, Olerud J, Welgus H, Parks W. Cell‐matrix interactions modulate interstitial collagenase expression by human keratinocytes actively involved in wound healing. J Clin Invest 1993;92:2858–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vaalamo M, Mattila L, Johansson N, Kariniemi AL, Karjalainen‐Lindsberg ML, Kahari VM, Saarialho‐Kere UK. Distinct populations of stromal cells express collagenase‐3 (MMP‐13) and collagenase‐1 (MMP‐1) in chronic ulcers but not in normally healing wounds. J Invest Dermatol 1997;109:96–101. [DOI] [PubMed] [Google Scholar]
- 18. Bullen E, Longaker M, Updike D, Benton R, Ladin D, Hou Z, Howard E. Tissue inhibitor of metalloproteinases‐1 is decreased and activated gelatinases are increased in chronic wounds. J Invest Dermatol 1995;104:236–40. [DOI] [PubMed] [Google Scholar]
- 19. Trengove NT, Stacey MC, Macauley S, Bennett N, Gibson J, Burslem F, Murphy G, Schultz G. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen 1999;7:442–52. [DOI] [PubMed] [Google Scholar]
- 20. Vaalamo M, Leivo T, Saarialho‐Kere U. Differential expression of tissue inhibitors of metalloproteinases (TIMP‐1, ‐2, ‐3, and ‐4) in normal and aberrant wound healing. Hum Pathol 1999;30:795–802. [DOI] [PubMed] [Google Scholar]
- 21. Duncan M, Berman B. Differential regulation of collagen, glycosaminoglycan, fibronectin, and collagenase activity production in cultured human adult dermal fibroblasts by interleukin 1‐alpha and beta and tumor necrosis factor‐alpha and beta. J Invest Dermatol 1989;92:699–706. [DOI] [PubMed] [Google Scholar]
- 22. Loffek S, Zigrino P, Angel P, Anwald B, Krieg T, Mauch C. High invasive melanoma cells induce matrix metalloproteinase‐1 synthesis in fibroblasts by interleukin‐1alpha and basic fibroblast growth factor‐mediated mechanisms. J Invest Dermatol 2005;124:638–43. [DOI] [PubMed] [Google Scholar]
- 23. Wassenaar A, Verschoor T, Kievits F, Den Hartog M, Kapsenberg M, Everts V, Snijders A. CD40 engagement modulates the production of matrix metalloproteinases by gingival fibroblasts. Clin Exp Immunol 1999;115:161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. MacNaul K, Chartrain N, Lark M, Tocci M, Hutchinson N. Discoordinate expression of stromelysin, collagenase, and tissue inhibitor of metalloproteinases‐1 in rheumatoid human synovial fibroblasts: synergistic effects of interleukin‐1 and tumor necrosis factor‐alpha on stromelysin expression. J Biol Chem 1990;265:17238–45. [PubMed] [Google Scholar]
- 25. Baugh M, Hollander A, Evans G. The regulation of matrix metalloproteinase production in human colonic fibroblasts. Ann N Y Acad Sci 1998;859:175–9. [DOI] [PubMed] [Google Scholar]
- 26. Yamamoto T, Eckes B, Mauch C, Hartmann K, Krieg T. Monocyte chemoattractant protein‐1 enhances gene expression and synthesis of matrix metalloproteinase‐1 in human fibroblasts by an autocrine IL‐1 alpha loop. J Immunol 2000;164:6174–9. [DOI] [PubMed] [Google Scholar]
- 27. Lee K, Ryoo Y, Song J. Interferon‐gamma upregulates the stomelysin‐1 gene expression by human skin fibroblasts in culture. Exp Mol Med 1998;30:59–64. [DOI] [PubMed] [Google Scholar]
- 28. Tarnuzzer R, Schultz G. Biochemical analysis of acute and chronic wound environments. Wound Repair Regen 1996;4:321–5. [DOI] [PubMed] [Google Scholar]
- 29. Wallace H, Stacey M. Levels of tumour necrosis factor‐alpha (TNF‐alpha) and soluble TNF receptors in chronic venous leg ulcers – correlations to healing status. J Invest Dermatol 1998;110:292–6. [DOI] [PubMed] [Google Scholar]
- 30. Trengove NT, Bielefeldt‐Ohmann H, Stacey M. Mitogenic activity and cytokine levels in non‐healing and healing chronic leg ulcers. Wound Repair Regen 2000;8:13–25. [DOI] [PubMed] [Google Scholar]
- 31. Wallace H, Vandongen Y, Stcaey M. Tumor necrosis factor alpha gene polymorphism associated with increased susceptibility to venous leg ulceration. J Invest Dermatol 2006;126:923–6. [DOI] [PubMed] [Google Scholar]
- 32. Gardner J, Borgmann K, Deshpande M, Dhar A, Wu L, Persidsky R, Ghorpade A. Potential mechanisms for astrocyte‐TIMP‐1 downregulation in chronic inflammatory diseases. J Neurosci Res 2006;83:1281–92. [DOI] [PubMed] [Google Scholar]
- 33. Dean G, Young D, Edwards D, Clark I. The human tissue inhibitor of metalloproteinases (TIMP)‐1 gene contains repressive elements within the promoter and intron 1. J Biol Chem 2000;275:32664–71. [DOI] [PubMed] [Google Scholar]


