Abstract
Following severe burn injury, persistent inflammation perpetuated by surface eschar, bacterial colonisation and neutrophil proteolytic activity can impede normal healing and result in further tissue damage. Extracorporeal shock wave treatment (ESWT) has been shown in the clinical setting to promote the healing of burn and difficult‐to‐heal wounds; however, the mechanism is unclear. We investigated the role of ESWT on the early proinflammatory response using a severe, full‐thickness and highly inflammatory cutaneous burn wound in a murine model. Various wound‐healing parameters were measured and leukocyte infiltration quantitated. A panel of 188 candidate genes known to be involved in acute inflammation and wound healing was screened. We show that ESWT of burn wounds 1 hour postwounding significantly blunts polymorphonuclear neutrophil and macrophage infiltration into the wound. ESWT treatment potently attenuates both CC‐ and CXC‐chemokine expression, acute proinflammatory cytokine expression and extracellular matrix proteolytic activity at the wound margin. Given these findings and the clinical success of ESWT, we speculate that ESWT may be a potential therapeutic modality to treat severe wounds wherein excessive inflammatory responses involving increased levels of inflammatory cells, proinflammatory cytokines and proteases may become self‐resolving allowing wound healing to progresses by way of normal physiological repair processes.
Keywords: Burns, Chemokines, Extracorporeal shock wave therapy, Inflammation, Wound healing
Introduction
Wound healing is a dynamic process that progresses in an orderly fashion to restoration of tissue function and integrity. Successful wound healing depends on tightly regulated haemostasis, inflammation, matrix synthesis, proliferation, wound contraction and tissue remodelling. The failure to progress through these physiological phases of wound healing leads to chronicity 1, 2, 3, 4. It is clear that much of the tissue damage to viable tissue in the perfused burn wound subsurface and its margin is caused by toxic inflammatory mediators produced by damaged tissue and/or recruited leukocytes 5, 6, 7, 8, 9, 10. Although an early inflammatory response is required for healing, overproduction of mediators can result in increased capillary permeability and further tissue damage beyond that of the primary insult. Understanding the biology of wound healing and the effects of new therapeutics on normal wound‐healing processes permits the researcher to ascertain the factors that contribute to poor healing. This knowledge is critical to the development of future therapeutic molecular targets and wound‐healing treatment strategies.
Advances in the understanding of wound care biology have led to therapeutic refinements aimed at abrogating the chronic inflammatory state and excessive protease activity and promoting angiogenesis, fibroblast proliferation and keratinocyte migration. Recent developments in wound‐dressing technology, utilisation of topical growth factors and protease inhibitors, application of bioengineered skin equivalents, and administration of therapeutic ultrasound and acoustic pressure waves show promise in treating challenging difficult‐to‐heal as well as chronic wounds 11, 12, 13, 14, 15. Extracorporeal shock wave therapy (ESWT) is one example of acoustic energy being used as a means of improving healing of chronic wounds. We and others have completed clinical trials that show unfocused low‐energy shock wave therapy is a feasible modality for a variety of complicated, non healing soft tissue wounds, particularly posttraumatic and postoperative wounds, decubitus ulcers and burns (13). In our study population, the use of unfocused, low‐energy ESWT on a large population of patients with acute and chronic soft tissue wounds was associated with complete closure of the majority of wounds. Although the precise mechanism of shock wave therapy remains unclear, preliminary findings in both acute and chronic wounds supporting the ability of a mechanical stimulus to exert a biological effect serves as the basis for the present investigation.
Before commencing with these studies, we hypothesised that low‐energy therapeutic acoustic pressure waves may promote wound healing by optimising the cellular and molecular microenvironment, particularly the local chemokine landscape.
With the understanding that a severe burn injury can lead to a persistent inflammatory state perpetuated by surface eschar, bacterial colonisation, increased leukocyte infiltration and heightened protolytic activity, the current investigation was undertaken to evaluate the effects of low‐energy shock waves on early proinflammatory chemokines and cytokines in a murine burn wound model. Our data show significant changes in acute early proinflammatory mediators [chemokines, cytokines and early tissue remodelling matrix metalloproteinases (MMPs)] within the burn wounds, which are related to reduced local leukocyte (neutrophil and macrophage) infiltration.
Materials and methods
Animals
Seven to 8‐week‐old female BALB/c mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained at the Walter Reed Army Institute of Research (WRAIR, Silver Spring, MD) animal facility, which is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International. All procedures were conducted using facilities and protocols approved by the Animal Care and Use Committee of WRAIR (protocol K06‐005). Mice were housed five animals per cage before any treatment and individually caged postburn injury in standard microisolator polycarbonate caging. Mice were used for experimentation at 10–14 weeks of age. Animal rooms were maintained at 21 ± 2°C with 50 ± 10% humidity on a 12‐hours light/dark cycle. Commercial rodent ration (Harlan Teklad Rodent Diet 8604) was available freely, as was acidified (pH = 2·5) water to control opportunistic infections.
Experimental design
At 10 weeks of age, mice received a 15% total body surface area (TSBA) full‐thickness dorsal burn, and the wounds were either untreated or treated with ESWT (200 impulses) 1 hour postwounding. Two sets of experiments were conducted; one (20 experimental and control animals each) was for assessment of survival rate, macroscopic wound closure and immunopathological analysis of the wound site. Photographs of wounds in the presence of a scaled ruler were taken with a digital Fuji Finepix Camera. Images of wounds were imported into Adobe Photoshop CS2 (Adobe Systems, San Jose, CA) for reproduction and wound area determination. For quantification of wound area, raw digital files were imported into NIH ImageJ software (v1·37) for processing and planimetry was used to calculate wound surface area. Mice were euthanized by CO2 inhalation followed by cervical dislocation at days 10, 14, 21 and 28 postwounding for tissue acquisition (n = 5 animals per strain at each time point in both the control and the ESWT treatment group). Wound sites and adjacent normal skin were excised, fixed with 4% formaldehyde in a buffered zinc solution (Z‐fix), embedded in paraffin and sectioned. After deparaffinization and rehydration, 5‐μm sections were washed (3×) with phosphate‐buffered saline and stained with haematoxylin and eosin (H&E) or Mason’s trichrome to visualise blood vessel density per high‐power field at ×200 magnification across the entire wound bed. Planimetry of H&E‐stained sections was used to calculate morphometrical data, such as the distance the epithelium had traversed over the open wound (degree of re‐epithelialisation), the area of open wound (epithelial gap) and the percentage of wound closure. Lateral wound margins were determined by (a) the presence of intact hair follicles and organised epidermis and dermis compared with few or no hair follicles, (b) altered epidermal/dermal organisation and (c) the disorganisation of collagen fibres at wound edges and within the wound.
In the second set of experiments, control and ESWT‐treated animals (n = 8 per group) were terminated at 4 and 24 hours postwounding. Wounds and surrounding normal skin tissue were excised. Immunohistochemistry assessments were performed for leukocyte infiltration at the wound margin and in the wound bed. RNA isolated from the wound margin was screened (TaqMan® RT‐PCR) against a panel of 184 known inflammatory mediators, chemokines, cytokine and MMPs known to play a role in early wound healing.
Burn wound model
Mice were anaesthetised using isoflurane inhalation. After shaving the dorsum, the exposed skin was washed gently with room temperature sterile water and prepped with Betadine (a 10% povidone–iodine solution for skin disinfection). The Betadine solution from the prepared area was wiped off using three series of sponge gauzes containing 70% isopropyl alcohol. Using a surgical skin marker, a 12·5‐mm‐diameter circular area along the paramedian dorsal region was outlined. A full‐thickness burn (∼15% total body) was introduced with electrocautery bovie (370–400°C for 1·5 seconds; Bovie Aaron Medical, St. Petersburg, FL). This protocol causes a well‐demarcated, full‐thickness, anaesthetic injury that is non lethal (<0·5% mortality). Wounds became covered with inflammatory eschar and no evidence of infection was evident macroscopically. Wounds were treated with bacitracin (applied topically) immediately after wounding, left uncovered and allowed to dessicate. Once mice recovered from anaesthesia, they were placed alone in separate cages and maintained under standard conditions in the animal facility (as described above). Buprenorphine (Reckitt Benckiser Pharmaceuticals, Richmond, VA) was given subcutaneously twice a day (0.1 mg/kg) on postoperative days 1 and 2 for pain management. No topical wound care was provided aside from the aforementioned bacitracin.
ESWT treatment
One hour postburn, mice were anaesthetised briefly using isoflurane inhalation. A liberal amount of bacitracin ointment, which we have found serves well as a conductive gel, was applied directly to the burn wound and the adjacent area. The unfocused lens of the DermaGoldTM (Tissue Regeneration Technologies, LLC, Woodstock, Georgia) shock wave applicator, which comprises a parabolic reflector, was gently placed directly on the ointment‐covered wound and treated with 200 impulses (energy level 0·1 mJ/mm2, frequency 5 pulses per second) for all treatments. The parabolic reflector permits a large treatment area to be stimulated by the acoustical field. ESWT treatment lasted approximately 45 seconds. Following treatment, the excess bacitracin was removed carefully using sterile gauze. No dressing was applied. Sham‐treated wounds were treated identically; however, no shock wave impulses were administered.
RNA extraction
Total RNA was extracted from skin excised from the wound margin and stored in RNALater (Ambion, Austin, TX). Briefly, skin tissue was homogenised using Trizol reagent (Invitrogen, Carlsbad, CA) and total RNA was isolated using Qiagen RNeasy Lipid Tissue Mini Kit (QIAGEN Inc., Valencia, CA) according to manufacturer’s instructions. Wound margin RNAs were resuspended in 30 μl of 10 mM Tris buffer, pH 7·5. Sample purity, quantity and quality were assessed by determining the A260/280, A260/230 ratio on an Nanodrop Spectrophotometer (NanoDrop Technologies Inc., Wilmington, DE) and by measuring 28S/18S ribosomal RNA ratio and RNA integrity number using an Agilent 2100 BioAnalyzer (Agilent Technologies Inc., Santa Clara, CA). Agilent RNA integrity values for all sampled wound specimens in this study were greater than equal to 8·5. Reverse transcription was performed with Roche 1st Strand Synthesis Kit (Roche Diagnostics Corporation, Indianapolis, IN). Briefly, 2·5 μg sample RNA was added to a master mix containing 1× reaction buffer, 5 mM MgCl2, 1 mM deoxynucleotide mix, 6·4 μg random primers, 100 units RNase inhibitor and 40 units avian myeloblastosis virus (AMV) reverse transcriptase. Ten millimolars of Tris buffer, pH 7·5 was used to reach 40 μl final reaction volume. Then, final reaction mixture was subjected to a single reverse transcription cycle of 25°C for 10 minutes, 42°C for 60 minutes, 99°C for 5 minutes and 4°C for at least 10 minutes.
Real‐time quantitative polymerase chain reaction gene profiling for proinflammatory transcripts
Quantitative‐real‐time polymerase chain reaction (RT‐PCR) was performed using the ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA). Custom designed ‘Wound Repair’ TaqMan low‐density array (TLDA) cards (Applied Biosystems) were used to assess gene expression. The set of TLDA cards comprise 188 individual target assays [including respective forward and reverse primers and a dual‐labelled probe (5′‐6‐FAM; 3′‐MGB)] in quadruplicate on a 384 well card (96 genes per card). Amplification parameters were as follows: one cycle of 50°C for 2 minutes and 95°C for 10 minutes followed by 40 cycles of 95°C for 30 seconds and 60°C for 1 minute. Two samples were processed on each card.
RT‐PCR data analysis
RT‐PCR data were analysed using the Sequence Detection System version 2·1 included with the ABI Prism 7900HT SDS or using Microsoft Excel. The threshold cycle (C t) for each sample was manually set to 0·2 and the baseline was set between 3 and 15 cycles. 18S ribosomal RNA was used as an endogenous housekeeping control for normalisation, and the comparative C t method was used to calculate the relative fold expression by 2‐ΔΔCt. Assays with Ctvalues greater than 35 cycles were excluded from analysis 16, 17.
Immunohistochemical studies
For histological analysis, the wound area (including the eschar and a 5–10 mm of epithelial margin) was excised. As a control, a similar amount of skin was taken from the dorsum of normal uninjured mice. Skin samples were fixed with 4% buffered formalin, embedded in paraffin and sectioned. For quantification of myeloperoxidase (MPO)‐positive neutrophils and F4/80 macrophages, skin sections were incubated with rabbit anti‐MPO (1:100 dilution, citrate buffer epitope retrieval boiling 20 minutes; Neo Markers, Freemont, CA) or rat antimouse F4/80 antigen (1:50 dilution, proteinase K pre‐treatment, clone CI:A3‐1, Serotec Inc., Raleigh, NC), respectively. Subsequently, the sections were reacted with appropriate biotinylated secondary antibodies and visualised using a streptavidin–biotin–peroxidase staining kit (Vector Laboratories, Burlingame, CA) according to the manufacture’s instructions. The sections were developed using 3, 3′‐diaminobenzidine as a chromogen (Sigma, St. Louis, MO, USA). Slides were counterstained with haematoxylin and cover slipped with a permanent mounting medium (Permount; Fisher Scientific, Waltham, MA, USA). A total of five random fields from three epidermal regions were examined, which included the wound site, the region adjacent to the wound and a region far from the wound site. The number of MPO‐ and F4/80‐labelled cells per field at ×400 was determined. Images were photographed using a BX50 Olympus microscope equipped with an Insight Firewire Spot Color Camera and Spot 4·6 photographic and analysis software (Diagnostic Instruments, Inc., Sterling Heights, MI).
Statistics
Statistical analysis of variance was used to analyse the data and Mann–Whitney U test was used to determine the level of significance of differences in sample means (GraphPad PRISM 4·0). A P value less than 0·05 was considered significant.
Results
A single ESWT treatment has no adverse effect on the rate of severe burn wound closure
The areas of open wounds were measured at 1, 7, 14, 21 and 28 days after wounding to assess macroscopic healing defects (Figure 1A, B). The rate of macroscopic wound closure contraction, the degree of subeschar keratinocyte migration, rate of wound re‐epithelialisation and granulation development did not significantly differ between untreated and ESWT treated following a 15% TBSA severe dorsal burn (data not shown).
Figure 1.
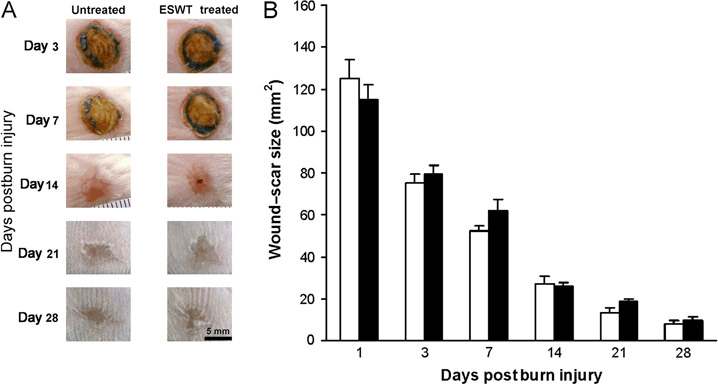
Macroscopic changes in wound healing in untreated and extracorporeal shock wave treatment (ESWT)‐treated severe third‐degree full‐thickness burn wounds. (A) Representative photographs of the wound sites at the indicated time postinjury. (B) Changes in the total wound area during the evaluation interval. Values represent the mean ± standard error of the mean; □ untreated and ▪ ESWT treated (n = 5 animals per group).
ESWT‐treated burn wounds display significantly reduced inflammatory cell infiltrate
Understanding the effects of ESWT on the regulation of leukocyte migration in wound healing is important because neutrophil infiltration particularly in the surface exudate of burn wounds results in increased damage to viable tissue. These deleterious effects are mediated through polymorphonuclear (PMN) proteases and oxidants and PMN consumption of oxygen, which can result in either delayed healing or persistent wounds. The number of infiltrating leukocytes were significantly reduced in ESWT‐treated mice (2, 3).
Figure 2.
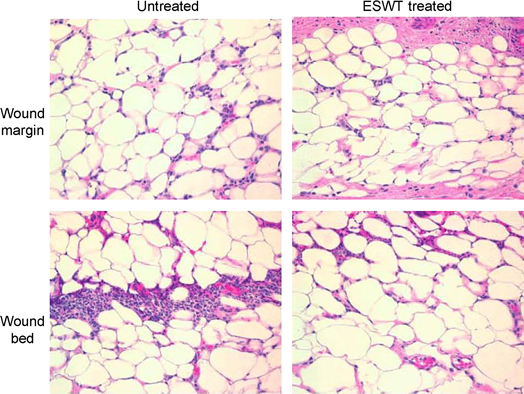
Histological sections of wounded skin from untreated and extracorporeal shock wave treatment‐treated BALB/c mice at (A) 4 hours and (B) 24 hours after full‐thickness third‐degree burn injury. Neutrophils were detected in haematoxylin‐ and eosin‐stained sections (original magnification, ×600).
Figure 3.
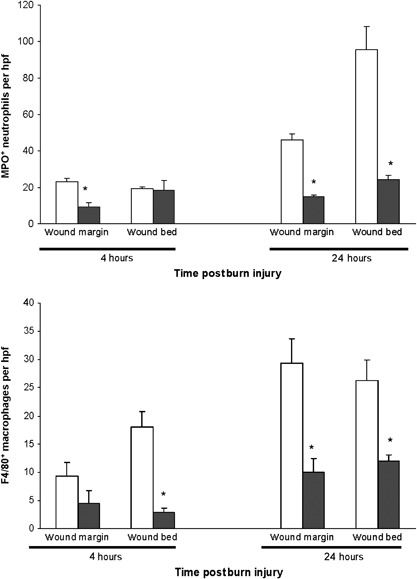
Quantification of (A) MPO+ neutrophil and (B) F4/80+ macrophage inflammatory cell infiltration at 4 hours and 24 hours postburn injury. Values represent the mean ± standard error of the mean number of infiltrating cells per high‐power field (hpf) (×400) at the wound margin and in the central wound bed; □ untreated, ▪ extracorporeal shock wave treatment treated (n = 5 animals per group per time point; three to ten fields in each area on three sections per wound were analysed). Mann–Whitney U test was used to determine the level of significance of differences in sample means. *P < 0·05, ESWT‐treated compared to sham‐treated burn wounds.
Immunohistochemical techniques were used to enumerate the numbers of neutrophils and macrophages that migrated outside the blood vessels into the wound tissues based on membrane expression of MPO and F4/80, respectively, at 4 and 24 hours after burn injury (Figure 3). A significant reduction (60–68%, P < 0·05) of MPO+ neutrophils infiltrating the wound margin and central wound bed was identified at 24 hours following burn injury. Infiltrating F4/80 macrophages within the wound bed were reduced significantly 4 hours postburn (P < 0·05). A significant reduction (55–66%, P < 0·05) of F4/80 macrophages at the wound margin and within the wound bed was shown at 24 hours following burn injury. Thus, ESWT treatment resulted in reduced wound infiltration of both neutrophils and macrophages.
ESWT treatment is associated with global suppression of chemokines, proinflammatory cytokines and MMPs
Neutrophils and macrophages in the initial inflammatory phase of healing are major sources of cytokines, chemokines, growth factors and mediators that can promote as well as impair wound healing. Because inflammatory cell recruitment was markedly reduced in ESWT‐treated wounds, we examined the expression of chemokines, proinflammatory cytokines and MMPs. Skin obtained from the wound margin was collected at 4 and 24 hours postburn injury. Skin from untreated versus ESWT‐treated wounds were examined by quantitative RT‐PCR for the level of 188 gene transcripts, which have been shown to be key factors involved in the early wound reparative response including leukocyte infiltration, acute‐phase cytokines, chemokines, adhesion molecules and MMPs.
A significant greater than fivefold increase in the expression of chemokines and proinflammatory cytokines was shown 4 hours postburn in untreated mice. This increase in CCL2, CCL7, CXCL2, CXCL5, CXCL28, IL‐1β, IL‐6, PGE2s, early growth response gene, NFKβ‐Ras‐like protein‐2, FGF‐1, IFNβ, E‐selectin and P‐selectin gene expression after injury in untreated wounds was significantly decreased in ESWT‐treated wounds (Figure 4, n = 4 per group per time point).
Figure 4.
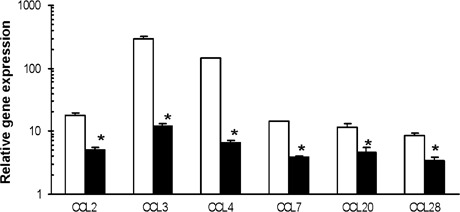
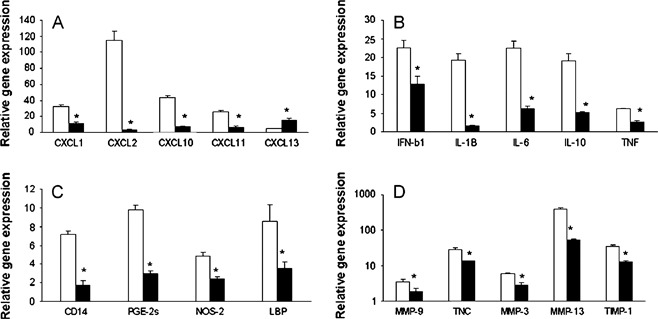
The analysis of chemokine, proinflammatory cytokine and matrix metalloproteinases gene expression at burn wound margins of untreated and extracorporeal shock wave treatment (ESWT)‐treated BALB/c mice. Quantitative RT‐PCR was performed as described in the Material and Methods and representative results from two independents experiments are shown. The ratios of genes to 18S at the wound margin of untreated (□) and ESWT‐treated mice (▪) at 4 hours (panel I) and at 24 hours (panel II) postburn injury were determined. Each value represents the mean ± standard error of the mean (n = 4) versus uninjured skin of BALB/C (*P < 0·05 versus untreated skin).
Expression levels of all other upregulated (greater than threefold expression when compared with naïve skin) gene transcripts (agiopoietin‐4, BMP‐15, CXCL20, procollagen, G‐CSF, GM‐CSF, CXCL1, CXCL13, IL‐10, integrin alpha‐5, IL‐18, LIF, PDGFα, oncostain M‐receptor, signal transducer/activator of transcription‐3, TIMP‐1, TNF‐α and TNFR5) were similar between the untreated and the ESWT‐treated wounds 4 hours postinjury.
We next examined the levels of gene transcripts at 24 hours postinjury (Figure 4, n = 4 per group). CCL and CXCL chemokine transcript levels were significantly reduced in ESWT‐treated wounds. Similarly, transcripts for proinflammatory cytokines IFNβ, IL‐1β, IL‐6, IL‐10 and TNFR5, as well as tissue remodelling wound MMPs (MMP‐3, MMP‐9 and MMP‐13) and the endogenous MMP inhibitor TIMP‐1, were markedly reduced in ESWT‐treated wounds. Reduced CD14, PGE2s, nitric oxide synthase (NOS‐2) and Lipopolysaccharide (LPS)‐binding protein transcript levels in the ESWT‐treated wounds were consistent with our immunohistochemical results showing decreased macrophage infiltration. These results strongly suggest that ESWT treatment after an acute inflammatory burn injury significantly downregulates the expression of potent neutrophil and macrophage chemoattractants, acute‐phase cytokines and several key metalloproteinases involved in basement membrane degradation remodelling. In turn, the decreased chemokine expression may be responsible for the observed decrease in leukocyte infiltration.
Discussion
In this study, we investigated the effect of ESWT on full‐thickness cutaneous open burn wound healing by examining the early proinflammatory response to wounding, the profile of leukocyte infiltration in a highly inflammatory injury and the differential gene expression of proinflammatory cytokines, chemokines and MMPs immediately following severe tissue damage. ESWT treatment significantly reduced the number of both infiltrating neutrophils and macrophages after a 15% TBSA thermal injury. Furthermore, expression of proinflammatory cytokines, chemokines and MMPs produced by cells at the wound site were globally suppressed.
Wound healing is a well‐orchestrated interplay between key effector cells (neutrophils, macrophages, lymphocytes and fibroblasts), soluble mediators which affect various cell types, extracellular matrix synthesis and remodelling in the wound environment. Under normal healing conditions, these dynamic processes are allowed to proceed unperturbed through pivotal phases of healing and complete wound closure ensues in a timely and orderly manner, manifesting typically as a scar in the adult. The inflammatory and subsequent proliferative phase of wound healing occur in a temporal sequence and is regulated precisely by inflammatory cytokines and chemokines (18). When this complex biology is disrupted by co‐existing disease, pharmaceuticals or by the presence of wounds exceeding the ability of these repair mechanisms, the normal pattern of wound repair fails and a chronic inflammatory state is created that further impairs healing. Although the onset of inflammation is a requisite part of healing, excessive production of mediators of inflammation following burn injury, especially proteases and oxidants, can cause additional capillary endothelial and skin damage in otherwise viable tissue 6, 8, 9. Similarly, the failure to suppress early proinflammatory responses is often used to explain how difficult‐to‐heal wounds develop and fail to heal 1, 2, 3, 19, 20, 21, 22, 23. Although this transition from acute to chronic wound are not completely elucidated, it appears that disturbances in the inflammatory and proliferative phases by elevated chemokine and inflammatory cytokine synthesis and dysregulated neutrophil and macrophage migration are contributory to delayed healing in acute wound repair 4, 19. Whether this aberrant inflammatory milieu governs delayed wound healing or is simply an indicator of errant biology remains to be defined; however, there is some evidence to suggest a bystander effect of elevated chemokine expression directly impairing wound repair mechanisms (24).
ESWT has been shown to contribute to complete wound healing in a variety of chronic, large and difficult‐to‐heal wounds including burns, ischaemic, stasis and pressure ulcers, as well as traumatic and postoperative wounds (13). In addition, ESWT is often used at higher dose intensities to treat fracture non unions, which highlights the broad spectrum of biological effects of an applied physical energy produced by this technology. The effect(s) and molecular mechanism(s) of ESWT remain to be determined; however, it is speculated that the acoustic energy generated by ESWT perturbs cell membranes and alters membrane potentials that induce cell‐signalling effects (25). The results reported in the present study are the first to show the effects of ESWT in altering the cellular and molecular environment involved with proinflammatory wound‐healing processes.
In comparison to incisional and excisional wounds, a large full‐thickness burn wound is extremely inflammatory. Early release of inflammatory mediators, oxidants and increased proteolytic activity in a severe burn injury is well established (26). Moreover, the prolonged presence of eschar (without excision of non viable tissue) with a dessicated wound surface impedes delivery of nutrients and immune cells to the wound bed but also markedly retards the ability of keratinocytes to migrate across the wound surface. It has been shown that keratinocytes require a moist environment (occlusive dressing) to migrate across a wound. On the contrary, inflammation and re‐epithelialisation processes are significantly prolonged on a dessicated wound, as migrating keratinocytes must burrow beneath the eschar using controlled release of proteases (27). Collectively, these findings provide a clear rationale for investigations such as ours using a severe burn wound model to evaluate the effects of ESWT on early wound‐healing processes.
The predominant role of leukocyte migration and ensuing inflammatory mediator production is intrinsic to wound healing, which is a balance between inflammatory and counterinflammatory responses 7, 18, 28. Early migration of neutrophils to the wound surface correlates with the expression of CXCL chemokines such as IL‐8 (CXCL8) and GROα (CXCL1). Twenty‐four to 96 hours following wounding expression of CCL chemokines such as MCP‐1 (CCL2) predominate and stimulate local macrophage infiltration. By day 4 of healing wound macrophages begin to diminish and lymphocyte migration predominates, driven initially by CCL2 then by CXCL9 (Mig) and CXCL10 (IP‐10) expression. We show that the early application of a single ESWT treatment impairs leukocyte migration and global expression of proinflammatory cytokines, chemokines and MMPs. The wide ranging and substantial downregulation of both the CCL and the CXCL families of chemokines evident in this study is striking and suggests a generalised antiinflammatory effect of ESWT in severe dry open burn wounds. This is highly relevant as chemokine expression has been shown to be highly interdependent; for example, deletion of MCP‐1 promotes expression of IL‐1β, MIP‐2, MSP, IL‐1ra, CCR5, CCR3, Il‐11, CCR4 and CD3Z both in vivo and in vitro (29).
Self‐resolving inflammation is a necessary prerequisite for fibroblast activation and net wound matrix synthesis. Similarly, protease activity in normal wound healing must be tightly regulated both temporally and spatially 3, 30. A severe burn injury can induce marked wound inflammation, tissue oedema, extensive necrosis, hypoxia, macrophage hyperactivity and immune cell dysfunction 5, 6, 7, 10, 26. In addition to local inflammation, severe dermal burns through the systemic liberation of inflammatory mediators and aberrant neutrophil trafficking are known to induce systemic inflammatory response syndrome, which is associated with multisystem organ dysfunction and high mortality 9, 31. The observed global suppression of proinflammatory factors in the severe burn wound 4 and 24 hours after a single ESWT treatment, which was coupled with impaired neutrophil and macrophage migration, in this experimental model has important clinical implications. The chemokine‐driven, heightened inflammatory nature of severe full‐thickness burn wounds and the favourable healing response to ESWT when applied to such a wound provide a compelling basis for the therapeutic effects of ESWT evident clinically in a variety of difficult‐to‐heal wounds (13). In addition to chemokine overproduction, the suppression of proinflammatory cytokines (IL‐1β, IL‐6 and TNFα) in response to ESWT observed in this study is highly significant given that overexpression of these genes has been associated with impaired wound healing in human non healing wounds and in diabetic mouse models 1, 3, 4, 23. Furthermore, ESWT treatment was associated with downregulation of MMPs and TIMP‐1, remodelling enzymes reported to be markedly elevated in chronic compared with acute wound effluent 22, 32, 33, which are surrogate markers of successful wound healing (34).
In summary, we show a profound effect of ESWT on severe burn wound chemokine–cytokine expression and leukocyte infiltration all of which are key in regulating a wide variety of phase‐specific wound‐healing mechanisms. Although these effects have been shown in a severe inflammatory burn wound‐healing model, clinical evidence suggests that chronic inflammatory events in the wound‐healing cascade set the stage for the development of prolonged wound healing and chronicity. Hence, early interventions such as described herein maybe beneficial. As such, ESWT is a promising treatment modality associated with clinical wound healing (13). Clinical success of ESWT in treating severe burn and difficult‐to‐heal wounds coupled with the mechanistic findings of this study provide the biological basis for a positive therapeutic effect. The temporal relationships, however, between treatment timing, optimal shock wave dosage and the long‐term effects of shock waves on immune and reparative cells within the wound‐healing environment highlight the need for further in depth small animal mechanistic studies followed by preclinical large animal studies which are ongoing and in development.
Authorship
TAD and EAE conceived and designed the research. TAD, KA, MA and SN performed the research and data collection. TAD, EAE, DT, AS and GEP were involved in the data analysis, interpretation and supported the writing of the manuscript. All authors read and approved the final version of the manuscript.
Acknowledgements
We thank Tissue Regenerative Technologies Inc. (Woodstock, GA, USA) for the use of the DermaGold shock wave instrument. This work was supported by the Office of Naval Research (ONR) work unit 601153N.4508.519.A0508.
The authors are employees of the US Government. This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that ‘Copyright protection under this title is not available for any work of the United States Government’. Title 17 U.S.C §101 defined a US Government work as a work prepared by a military service member or employees of the US Government as part of that person’s official duties. The opinions or assertions contained in this article are the private views of the authors and are not to be construed as reflecting the views, policy or positions of the Department of the Navy or Army, Department of Defense nor the US Government. The experiments reported herein were conducted in compliance with the Animal welfare Act and in accordance with the principles set forth in the current edition of the Guide for Care and Use of Laboratory Animals, Institute for Laboratory Animal Resources, National Research Council, National Academy Press, 1996.
References
- 1. Mast BA, Schultz GS. Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Repair Regen 1996;4:411–20. [DOI] [PubMed] [Google Scholar]
- 2. Tarnuzzer RW, Schultz GS. Biochemical analysis of acute and chronic wound environments. Wound Repair Regen 1996;4:321–5. [DOI] [PubMed] [Google Scholar]
- 3. Trengove NJ, Stacey MC, MacAuley S, Bennett N, Gibson J, Burslem F, Murphy G, Schultz G. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen 1999;7:442–52. [DOI] [PubMed] [Google Scholar]
- 4. Wetzler C, Kampfer H, Stallmeyer B, Pfeilschifter J, Frank S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J Invest Dermatol 2000;115:245–53. [DOI] [PubMed] [Google Scholar]
- 5. Baskaran H, Yarmush ML, Berthiaume F. Dynamics of tissue neutrophil sequestration after cutaneous burns in rats. J Surg Res 2000;93:88–96. [DOI] [PubMed] [Google Scholar]
- 6. Piccolo MT, Wang Y, Verbrugge S, Warner RL, Sannomiya P, Piccolo NS, Piccolo MS, Hugli TE, Ward PA, Till GO. Role of chemotactic factors in neutrophil activation after thermal injury in rats. Inflammation 1999;23:371–85. [DOI] [PubMed] [Google Scholar]
- 7. Faunce DE, Llanas JN, Patel PJ, Gregory MS, Duffner LA, Kovacs EJ. Neutrophil chemokine production in the skin following scald injury. Burns 1999;25:403–10. [DOI] [PubMed] [Google Scholar]
- 8. Schwacha MG, Chaudry IH. The cellular basis of post‐burn immunosuppression: macrophages and mediators. Int J Mol Med 2002;10:239–43. [PubMed] [Google Scholar]
- 9. Rodriguez JL, Miller CG, Garner WL, Till GO, Guerrero P, Moore NP, Corridore M, Normolle DP, Smith DJ, Remick DG. Correlation of the local and systemic cytokine response with clinical outcome following thermal injury. J Trauma 1993;34:684–94; discussion 94–5. [DOI] [PubMed] [Google Scholar]
- 10. Alexander M, Chaudry IH, Schwacha MG. Relationships between burn size, immunosuppression, and macrophage hyperactivity in a murine model of thermal injury. Cell Immunol 2002;220:63–9. [DOI] [PubMed] [Google Scholar]
- 11. Ennis WJ, Foremann P, Mozen N, Massey J, Conner‐Kerr T, Meneses P. Ultrasound therapy for recalcitrant diabetic foot ulcers: results of a randomized, double‐blind, controlled, multicenter study. Ostomy Wound Manage 2005;51:24–39. [PubMed] [Google Scholar]
- 12. Moiemen NS, Vlachou E, Staiano JJ, Thawy Y, Frame JD. Reconstructive surgery with Integra dermal regeneration template: histologic study, clinical evaluation, and current practice. Plast Reconstr Surg 2006;117 Suppl 7:160S–74S. [DOI] [PubMed] [Google Scholar]
- 13. Schaden W, Thiele R, Kolpl C, Pusch M, Nissan A, Attinger CE, Maniscalco‐Theberge ME, Peoples GE, Elster EA, Stojadinovic A. Shock wave therapy for acute and chronic soft tissue wounds: a feasibility study. J Surg Res 2007;143:1–12. [DOI] [PubMed] [Google Scholar]
- 14. Steed DL. Clinical evaluation of recombinant human platelet‐derived growth factor for the treatment of lower extremity ulcers. Plast Reconstr Surg 2006;117 Suppl 7:143S–9S; discussion 50S–51S. [DOI] [PubMed] [Google Scholar]
- 15. Veves A, Falanga V, Armstrong DG, Sabolinski ML. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care 2001;24:290–5. [DOI] [PubMed] [Google Scholar]
- 16. Hoffmann SC, Pearl JP, Blair PJ, Kirk AD. Immune profiling: molecular monitoring in renal transplantation. Front Biosci 2003;8:e444–62. [DOI] [PubMed] [Google Scholar]
- 17. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) Method. Methods 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 18. Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol 2001;69:513–21. [PubMed] [Google Scholar]
- 19. Menke NB, Ward KR, Witten TM, Bonchev DG, Diegelmann RF. Impaired wound healing. Clin Dermatol 2007;25:19–25. [DOI] [PubMed] [Google Scholar]
- 20. Falanga V. The chronic wound: impaired healing and solutions in the context of wound bed preparation. Blood Cells Mol Dis 2004;32:88–94. [DOI] [PubMed] [Google Scholar]
- 21. Faria DT, Nickoloff BJ, Poverini PJ, Kunkel S, Burdick M, Strieter RM. Chemokine and inflammatory cytokine changes during chronic wound healing. Wound Repair Regen 1997;5:310–22. [DOI] [PubMed] [Google Scholar]
- 22. Harris IR, Yee KC, Walters CE, Cunliffe WJ, Kearney JN, Wood EJ, Ingham E. Cytokine and protease levels in healing and non‐healing chronic venous leg ulcers. Exp Dermatol 1995;4:342–9. [DOI] [PubMed] [Google Scholar]
- 23. Trengove NJ, Bielefeldt‐Ohmann H, Stacey MC. Mitogenic activity and cytokine levels in non‐healing and healing chronic leg ulcers. Wound Repair Regen 2000;8:13–25. [DOI] [PubMed] [Google Scholar]
- 24. Iocono JA, Colleran KR, Remick DG, Gillespie BW, Ehrlich HP, Garner WL. Interleukin‐8 levels and activity in delayed‐healing human thermal wounds. Wound Repair Regen 2000;8:216–25. [DOI] [PubMed] [Google Scholar]
- 25. Wang FS, Yang KD, Wang CJ, Huang HC, Chio CC, Hsu TY, Ou CY. Shockwave stimulates oxygen radical‐mediated osteogenesis of the mesenchymal cells from human umbilical cord blood. J Bone Miner Res 2004;19:973–82. [DOI] [PubMed] [Google Scholar]
- 26. Linares HA. From wound to scar. Burns 1996;22:339–52. [DOI] [PubMed] [Google Scholar]
- 27. Vogt PM, Andree C, Breuing K, Liu PY, Slama J, Helo G, Eriksson E. Dry, moist, and wet skin wound repair. Ann Plast Surg 1995;34:493–9; discussion 9–500. [DOI] [PubMed] [Google Scholar]
- 28. Engelhardt E, Toksoy A, Goebeler M, Debus S, Brocker EB, Gillitzer R. Chemokines IL‐8, GROalpha, MCP‐1, IP‐10, and Mig are sequentially and differentially expressed during phase‐specific infiltration of leukocyte subsets in human wound healing. Am J Pathol 1998;153:1849–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ferreira AM, Rollins BJ, Faunce DE, Burns AL, Zhu X, Dipietro LA. The effect of MCP‐1 depletion on chemokine and chemokine‐related gene expression: evidence for a complex network in acute inflammation. Cytokine 2005;30:64–71. [DOI] [PubMed] [Google Scholar]
- 30. Parks WC. Matrix metalloproteinases in repair. Wound Repair Regen 1999;7:423–32. [DOI] [PubMed] [Google Scholar]
- 31. Al‐Qattan MM. ‘Late’ multiorgan failure in major burns: a “three‐event” construct rather than a “two‐event” construct. Burns 2007;33:268–70. [DOI] [PubMed] [Google Scholar]
- 32. Yager DR, Zhang LY, Liang HX, Diegelmann RF, Cohen IK. Wound fluids from human pressure ulcers contain elevated matrix metalloproteinase levels and activity compared to surgical wound fluids. J Invest Dermatol 1996;107:743–8. [DOI] [PubMed] [Google Scholar]
- 33. Cook H, Davies KJ, Harding KG, Thomas DW. Defective extracellular matrix reorganization by chronic wound fibroblasts is associated with alterations in TIMP‐1, TIMP‐2, and MMP‐2 activity. J Invest Dermatol 2000;115:225–33. [DOI] [PubMed] [Google Scholar]
- 34. Ladwig GP, Robson MC, Liu R, Kuhn MA, Muir DF, Schultz GS. Ratios of activated matrix metalloproteinase‐9 to tissue inhibitor of matrix metalloproteinase‐1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair Regen 2002;10:26–37. [DOI] [PubMed] [Google Scholar]


