Abstract
The aim of our case report was to analyse the results obtained with the Matriderm® system and autologous skin grafting for the surgical treatment of diabetic ulcers. We recruited one patient with diabetic ulcers admitted at the Department of Plastic and Reconstructive Surgery, University of Rome ‘Tor Vergata’. The patient underwent Matriderm® system and autologous skin grafting for diabetic ulcer treatment. After just a single treatment, we obtained reduction in ulcer after 15 days from the surgical treatment. We achieved a reduction in pain and exudate secretion of the ulcer. We noticed an almost complete restoration of the missing volume and good quality of skin. Matriderm® system and autologous skin grafting is a simple, safe and feasible technique. This method, when compared with other methods of treatment, is simple, cheap, less time consuming and does not require sophisticated laboratory facilities.
Keywords: Autologous skin grafting, Diabetic ulcers, Matriderm® system
INTRODUCTION
Diabetes mellitus constitutes one of the most important public health problems with a worldwide impact due to its high prevalence and enormous social and economic consequences (1). Unfortunately, many diabetic patients over time will develop the chronic complications of diabetes: retinopathy, nephropathy, peripheral neuropathy and atherosclerotic vascular disease; their treatment is difficult, prolonged and often unsuccessful, and the patients are prone to serious complications (2). Diabetic ulcers are among the most prevalent of chronic wounds and along with pressure ulcers comprise a majority of no healing wounds in the ageing population. It is estimated that approximately 15% of the diabetic population will develop diabetic ulcers some time in their life (3) and over half of these patients develop an infection, often osteomyelitis, with 20% requiring some form of amputation during the course of their ulcer treatment (4). Many authors (5) described that up to 85% of the lower limb amputations in diabetic patients are preceded by ulcers that fail to heal.
Factors that affect development and healing of diabetic patients' ulcers include the degree of metabolic control, the presence of ischaemia or infection and continuing trauma to feet from excessive plantar pressure or poorly fitting shoes. In addition, diabetic ulcers are frequently associated with morbidity, pain and decreased quality of life in affected patients. Diabetic ulcer is initially small vessel disease, which interferes with peripheral circulation leading to hypoxia of the tissue, which may result in ulceration after minor or major trauma. Peripheral gangrene may occur as a large single ulcer characteristically situated at the side or at the back of the ankle due to atherosclerosis. Peripheral neuropathy may co‐exist, with cold, swollen and dry feet. Trivial trauma or blister due to any cause may lead to ulceration (6). An understanding of the processes that precipitate and propagate ulcers in diabetic patients should dictate a rational approach to therapy.
Therapy for diabetic patients with ulcers can be difficult and quite often disappointing. Nowadays, diabetic ulcer can be managed using a wide range of therapeutic methods, traditional, new and experimental, each of which has different indications, efficacy and side effects. Healing of diabetic ulcers needs a multifactorial approach. Each diabetic patient must be properly controlled on general state of health. It is important to create a systematic programme that plans both general control and topical medical cares and that has curative and protective aspects. In addition, diabetic ulcer treatment largely depends on the underlying cause, that is, ischaemia, neuropathy or a combination of both. The aim of our case report was to analyse the results obtained with the use of Matriderm® system and autologous skin grafting for the diabetic ulcers' surgical treatment.
CASE REPORT
Patient anonymity was respected and an informed consent was obtained before the surgical procedure and digital image production. The protocol of the study was approved by the research ethics board of our institution. A 65‐year‐old man admitted to our department presented with diabetic ulcer on the lower limb caused by inadequate control of blood sugar (Figure 1). The patient had no knowledge of diabetes, which was diagnosed after a routine check of the analysis, showing a fasting glucose of 310 mg/dl. His medical history showed no medical or surgical treatments. The ulcer has developed on an affected limb, 10 years earlier, by direct flame burns. Regarding the burn, we did not handle this issue; however, the patient reported that he was treated with autologous grafts dermo epidemic network. The patient has no flexion contracture, and the ulcer had been present for at least 3 years or more. Physical examination showed 35‐cm‐long and 25‐cm‐wide diabetic ulcers on the lower limb (the third of the proximal lateral right leg), with a variable depth of up to 1 cm. The echo colour doppler has shown a slight venous incontinence. The bottom of the diabetic ulcer occurred fibrinous, necrotic, malodorous and secreting. For these reasons, we performed a biopsy of the diabetic ulcer that did not demonstrate small vessel vasculitis but detected the presence of Pseudomonas aeruginosa, Proteus mirabilis and Staphylococcus aureus. After the biopsy and the response of the antibiotic susceptibility, the patient was submitted for 30 days to a specific antibiotic therapy (Gentamicin 120 mg). In the meanwhile, the patient was treated with advanced medications three times a week to prepare the bed of the wound. The patient was treated with Hydrofibra‐Ag and Alginate‐Ag. Therefore, we decided to treat the ulcer with Matriderm® system and autologous skin grafting (2, 3, 4). After surgery, the patient was submitted to antibiotic therapy once a week.
Figure 1.
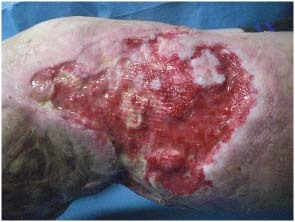
Preoperatory view of diabetic ulcer (35‐cm long and 25‐cm wide) of the lower limb with a variable depth of up to 1 cm.
Figure 2.
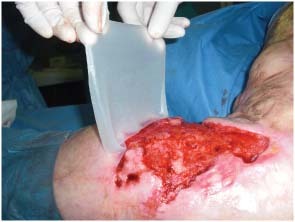
Intraoperatory view of Matriderm® system application.
Figure 3.
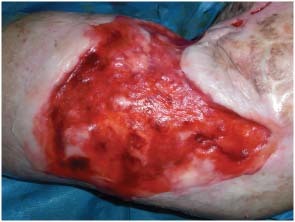
Intraoperatory view of Matriderm® system adherent to the ulcer.
Figure 4.
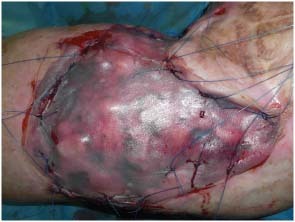
Intraoperatory view of Matriderm® system and skin graft fixation.
Seven days after the surgical treatment, the skin graft did not seem to be completely taking root, instead 15 days later, the epithelial buttons' appearance indicated total integration of the two materials with the diabetic ulcer bottom.
MATERIALS AND METHODS
Matriderm® system
Matriderm® is a single‐use three‐dimensional matrix composed of native structurally intact collagen fibrils and elastin for supporting dermal regeneration. The collagen is obtained from bovine dermis and contains the dermal collagen types I, III and V. The elastin is obtained from bovine nuchal ligament by hydrolysis. Matriderm® serves as a scaffold in the skin reconstitution and modulates scar tissue formation. Moreover, Matriderm® has an excellent haemostatic property and thus reduces the risk of split‐skin sub‐graft haematoma. The non use of chemical cross‐linking of the collagen results in a matrix that is especially biocompatible.
Matriderm® system and autologous skin grafting procedure
With routine aseptic precautions and under local anaesthesia (1% lidocaine without adrenaline), the ulcer is first surgically debrided, until the wound bed is well perfused. Careful haemostasis is carried out before the application of Matriderm®. Inadequate control of the bleeding could lead to the separation of the Matriderm®. Matriderm® may be applied using a single‐stage or a two‐stage procedure, depending on the regimen treatment: in our study, we used a single stage. In this, Matriderm® is immediately covered with split skin through the 1‐mm thick matrix by diffusion. The patient benefits in particular from the fact that a second operation is not necessary. Matriderm® is supplied in sterile double‐bagged packs and these may only be opened under sterile conditions. Before the use, Matriderm® must be rehydrated in ample physiological saline solution and to avoid trapped pockets of air (air pockets can hinder the diffusion and thus jeopardise the attached graft), Matriderm® should be laid on the surface of the water and not immersed. The matrix is ready for use as soon as the appearance of the entire surface has changed from white to translucent. To apply Matriderm®, it should be cut to the exact size of the ulcer. We applied the matrix by hands, and it is crucial that Matriderm® is in complete contact with the whole area of the ulcer bed and adheres to it. Air bubbles between the ulcer bed and the matrix should therefore be carefully removed by smoothing them out the margins of the matrix. A split‐thickness cutaneous was harvested from an uninvolved area (the inguinal region whenever possible) using a Zimmer dermatome (Zimmer, IN, USA). The split skin is grafted into the ulcer area directly on the top of Matriderm®; an additional attachment of Matriderm® with the split skin is achieved by sutures. A slight pricking is recommended to avoid the formation of seromas. To cover the ulcer, we use a non occlusive silicon film or paraffin gauze. Medication was performed with non adhering dressings (Adaptic—Johnson and Johnson Wound Management, Ethicon, Somerville, NJ, USA) and gauzes on the affected area.
Postoperative follow‐up evaluation
The dressing was removed after 7 days; the patient was treated with prophylactic antibiotic twice daily for 1 week starting 1 day prior to the surgical treatment. Postoperative follow‐up consisted of four visits during the first month–one for each week–and two additional visits at the third and sixth month. The patient's active and passive range of motion in a single plane was evaluated with the help of a physical therapist at 1 and 6 months from surgery. Secondary endpoints were the assessment of infections, inflammations or any adverse effects of the Matriderm® system and autologous skin grafting procedure, particular medications assumed and postoperative pain (evaluated with the visual‐analogic scale). The postoperative course was uneventful, and the early postoperative result was very satisfactory to both the surgeon and the patient.
RESULTS
After a single treatment, 15 days after surgery, we detected the ulcer reduction. In the days before the ingrowth, the skin graft seems compromised, but as we have noted in other patients with other types of ulcers, from the second week, epithelial islands and buttons appear from the centre and the margins of the ulcer.
The pain reduction had been a great deal for the patient as well as the decline of wound secretion.
The wound infection had been tested with negative tampon confirmation performed at 2‐week interval four times.
The new skin is renewed, refreshed in volume and texture, even the colour looks good compared with the baseline (Figure 5).
Figure 5.
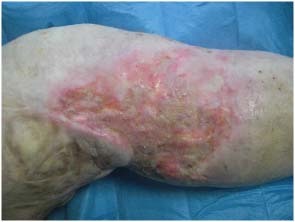
Postoperatory view at 45 days, the new skin is renewed, refreshed in the volume and texture, even the colour looks good compared with the baseline.
DISCUSSION
Diabetes is a chronic and multisystem disease, it needs to be controlled in every own aspect and people with diabetes have a 12–25% lifetime risk of developing an ulcer 6, 7. In nearly every case, lower limb ulcers benefit from evidence‐based therapy aimed at reducing the risk of atherosclerotic vascular disease 8, 9.
First of all, it is important to maintain good hygiene and keep a good blood sugar control (10). Mostly, they result in development of lower limb neuropathy. There is now excellent evidence that improved control of diabetes can markedly reduce the incidence of neuropathy (11). It is, moreover, important to cease smoking, to undergo appropriate treatment of hypertension and dyslipidaemia and in selected cases to use anti‐platelet medication, which is underlying to reduce cardiovascular risk 12, 13. Medical and surgical specialists should work with the patient's primary care provider to achieve these goals 14, 15. In diabetic ulcer treatment, the doctor should immediately take the necessary steps to prevent infectious complications that can result, if neglected, in generalised sepsis. Deep infections require early, aggressive surgical debridement in addition to antibiotics. The choice of antibiotics ultimately depends on the results of bacterial culture 16, 17. Initially, while cultures are being obtained, the spectrum of antibiotics should reflect the broad variety of bacteria isolated from these wounds in diabetic patients. It is important to bear in mind that the aim of antibiotic treatment is to cure the infection and not to heal the wound, which usually takes a much longer time.
Primary principles of good wound care incorporate regular debridement to remove necrotic material from the ulcer site and application of appropriate dressings. The main purpose of surgery is to remove infected and necrotic soft and bony tissue back to a healthy base that will support granulation tissue and allow healing by secondary intention. Advanced surgical techniques, such as free tissue transfers (flap or revascularisation), by skilled hands, could provide excellent results in selected patients 18, 19.
Topical therapy for ulcers should keep the ulcer bed moist, but not excessively wet so as to promote maceration. Moistened gauze dressings are frequently used but have a tendency to dry out unless changed several times daily. This changes the moist dressing to a wet‐to‐dry dressing, which loses the proven benefits of the moist environment. Removal can also damage the healing tissue. A variety of commercially available hydrogels can maintain the moist environment necessary to the wound and can be applied topically every 1–3 days depending on moisture requirements. A non adherent top dressing can then be applied over the hydrogel.
Another therapy is hyperbaric oxygen that has been used as adjunct therapy for diabetic foot ulcers. A series of small studies demonstrated more rapid healing and reduced amputation rates in patients treated with hyperbaric oxygen than in control subjects 20, 21. The current consensus recommendation is that hyperbaric oxygen could be a useful adjunct therapy for standard approaches to wound care in diabetic patients but that this field requires further investigation (22).
Recent advances in technology have produced growth factors (such as platelet‐derived growth factor) and tissue engineering of living skin replacements to replenish components of the wound care process. Dermagraft® and becaplermin are two examples of these new technologies that have a proven benefit in some diabetic patients with no healing ulcers (23).
There are many options to treat ulcer in diabetic patients (24). In the past, in our clinical experience, we used Integra®, a commercially available collagen‐based dermal substitute combined with a silicone top layer that functions as a temporary epidermal replacement (25). Comparison with Matriderm® shows that this dermal substitute, in our opinion, is better because: it is composed of native collagen, it does not contain Glutaraldehyde (the non use of chemical cross‐linking of the collagen results in a matrix that is especially biocompatible) and it allows the procedure in one stage and is cost effective. From a clinical point of view, the benefits that we found are: an immediate integration with the recipient bed, a better dermal architecture, a significantly improved elasticity and a low rate of contraction. Matriderm® system and autologous skin grafting procedure is a particular surgical option. Matriderm® is used for dermis reconstruction in full thickness defects in combination with autologous split‐skin grafts. The aim of this treatment is to develop a dermal substitute in order to avoid excessive scarring and wound contraction. This led to use of this system for the treatment of diabetic ulcers.
CONCLUSION
Foot and lower limb ulceration are common complications of diabetes which has potentially disastrous consequences for patients. Current indications for Matriderm® are extensive cutaneous defects, such as second‐ to third‐degree burn wounds, large decollements or the revision of large adherent scars. Our case demonstrates that it is also possible to use a combination of Matriderm® with autologous split‐skin grafts to cover diabetic ulcers resulting in an excellent functional and cosmetic outcome. Undoubtedly, this cannot be the method of choice, but in selected cases, such as ours, a fast one‐step approach, rather the procedure described, seems to be a good choice or an interesting possibility.
REFERENCES
- 1. King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabet Care. 1998;21:1414–31. [DOI] [PubMed] [Google Scholar]
- 2. Landau Z, Schattner A. Topical hyperbaric oxygen and low energy laser therapy for chronic diabetic foot ulcers resistant to conventional treatment. Yale J Biol Med. 2001;74:95–100. [PMC free article] [PubMed] [Google Scholar]
- 3. Reiber GE. The epidemiology of diabetic foot problems. Diabet Med. 1996;13 Suppl 1:6–11. [PubMed] [Google Scholar]
- 4. Wu S, Armstrong DG. Risk assessment of the diabetic foot and wound. Int Wound J. 2005;2:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation: basis for prevention. Diabet Care. 1990;13:513–21. [DOI] [PubMed] [Google Scholar]
- 6. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217–28. [DOI] [PubMed] [Google Scholar]
- 7. Abbott CA, Garrow AP, Carrington AL, Morris J, Van Ross ER, Boulton AJ. Foot ulcer risk is lower in South‐Asian and African‐Caribbean compared with European diabetic patients in the UK: the north‐west diabetes foot care study. Diabet Care 2005;28:1869–75. [DOI] [PubMed] [Google Scholar]
- 8. Burns P, Lima E, Bradbury AW. What constitutes best medical therapy for peripheral arterial disease? Eur J Vasc Endovasc Surg 2002;24:6–12. [DOI] [PubMed] [Google Scholar]
- 9. Burns P, Gough S, Bradbury AW. Management of peripheral arterial disease in primary care. BMJ 2003;326:584–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jeffcoate WJ, Price P, Harding KG. Wound healing and treatments for people with diabetic foot ulcers. Diabet Metab Res Rev 2004;20 Suppl 1:S78–89. [DOI] [PubMed] [Google Scholar]
- 11. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment on the development and progression of long‐term complications in insulin‐dependent diabetes. N Engl J Med 1993;329:977–86. [DOI] [PubMed] [Google Scholar]
- 12. Hobbs SD, Bradbury AW. Smoking cessation strategies in patients with peripheral arterial disease: an evidence‐based approach. Eur J Vasc Endovasc Surg 2003;26:341–47. [DOI] [PubMed] [Google Scholar]
- 13. Whaley‐Connell A, Sowers JR. Hypertension management in type 2 diabetes mellitus: recommendations of the Joint National Committee VII. Endocrinol Metab Clin North Am 2005;34:63–75. [DOI] [PubMed] [Google Scholar]
- 14. Sumpio BE. Foot ulcers. N Engl J Med 2000;343:787–93. [DOI] [PubMed] [Google Scholar]
- 15. Aronow WS. Management of peripheral arterial disease of the lower extremities in elderly patients. J Gerontol A Biol Sci Med Sci 2004;59:172–77. [DOI] [PubMed] [Google Scholar]
- 16. Cunha BA. Antibiotic selection for diabetic foot infections: a review. J Foot Ankle Surg 2000;39:253–57. [DOI] [PubMed] [Google Scholar]
- 17. Lipsky BA, Berendt AR. Principles and practice of antibiotic therapy of diabetic foot infections. Diabetes Metab Res Rev 2000;16 Suppl 1:S42–6. [DOI] [PubMed] [Google Scholar]
- 18. Illig KA, Moran S, Serletti J, Ouriel K, Orlando G, Smith A, Shortell CK, Green RM. Combined free tissue transfer and infrainguinal bypass graft: an alternative to major amputation in selected patients. J Vasc Surg 2001;33:17–23. [DOI] [PubMed] [Google Scholar]
- 19. Ozkan O, Coskunfirat OK, Ozgentas HE. Reliability of free‐flap coverage in diabetic foot ulcers. Microsurgery 2005;25:107–12. [DOI] [PubMed] [Google Scholar]
- 20. Zamboni WA, Wong HP, Stephenson LL, Pfeiffer MA. Evaluation of hyperbaric oxygen for diabetic wounds: a prospective study. Undersea Hyperb Med 1997;24:175–9. [PubMed] [Google Scholar]
- 21. Faglia E, Favales F, Aldeghi A, Calia P, Quarantiello A, Barbano P, Puttini M, Palmieri B, Brambilla G, Rampoldi A, Mazzola E, Valenti L, Fattori G, Rega V, Cristalli A, Oriani G, Michael M, Morabito A. Adjunctive systemic hyperbaric oxygen therapy in treatment of severe prevalently ischemic diabetic foot ulcer. Diabet Care 1996;19:1338–43. [DOI] [PubMed] [Google Scholar]
- 22. American Diabetes Association. Consensus development conference on diabetic wound care. Diabet Care 1999;22:1354–6. [DOI] [PubMed] [Google Scholar]
- 23. Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet‐derived growth factor‐BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. Diabet Care 1998;21:822–7. [DOI] [PubMed] [Google Scholar]
- 24. Sarkar PK, Ballantyne S. Management of leg ulcers. Post Grad J 2000;76:674–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schneider J, Biedermann T, Widmer D, Montano I, Meuli M, Reichmann E, Schiestl C. Matriderm versus Integra: a comparative experimental study. Burns 2009;35:51–7. [DOI] [PubMed] [Google Scholar]


