Abstract
The purpose of this study was to evaluate and validate the Cardiff Wound Impact Schedule (CWIS), a disease‐specific quality‐of‐life measure, in a diabetic foot ulcer (DFU) population. Patients with DFUs have restrictions as part of their treatment and rehabilitation, which can affect health‐related quality of life (HRQoL). Because of the high number of comorbidities experienced in diabetes, a disease‐specific quality‐of‐life measure is needed to best assess the affect of a foot ulcer on HRQoL. Patients with DFUs completed the CWIS and a World Health Organization generic quality‐of‐life questionnaire. Validity was assessed by comparing domains of the questionnaires. Patients were categorised using the University of Texas wound classification system. Mean CWIS scores were compared between categories to assess the questionnaire's ability to differentiate wound severity. Patients with open ulcers scored significantly lower on the CWIS than those with healed ulcers. Correlations between questionnaire domains were as follows: Social Life with Social Functioning (r = 0·641, P < 0·001); Well‐Being with General Health (r = 0·533, P < 0·01); Physical Symptoms and Daily Living with Physical Functioning (r = 0·631, P < 0·01) and Health‐Related Quality of Life with Vitality (r = 0·425, P < 0·01). However, there was no significant difference in mean CWIS scores between categories of wound severity. We have demonstrated the ability of the CWIS in assessing HRQoL in a DFU population and its ability to differentiate between healed and non healed states.
Keywords: Diabetic foot ulcer, Cardiff wound impact schedule, Quality of life
INTRODUCTION
Chronic foot ulcers are among the many serious complications associated with diabetes mellitus. Lifetime incidence of foot ulcers in patients with type I and type II diabetes has been reported to be as high as 25% (1). This represents a significant challenge to health care systems as diabetic foot ulcers (DFUs) account for 20% of all diabetic admissions to hospital (2). Furthermore, eight out of ten non traumatic amputations are performed on diabetics, 85% of which are preceded by a foot ulcer (1). The significant burden on patients and health care providers is expected to worsen with conservative estimates suggesting that the number of people with diabetes will double between the years 2000 and 2030 (3).
DFUs have been defined as lesions with loss of epithelium, which may extend into the dermis or, in some cases, involve muscle and bone (4). Various predisposing factors lead to the presentation of DFUs, most common are the predisposing factors of neuropathy, deformity and trauma (4).
The presence of DFUs often has profound effects on health‐related quality of life (HRQoL). It is believed that foot ulceration leads to substantial negative effects on emotional, physical and economic functioning for diabetic patients 1, 5. Additionally, patients with DFUs report more depression, less satisfaction with life and poorer psychosocial adjustment to illness than diabetics who do not suffer from foot ulcers (6). The assessment of HRQoL is of importance in establishing evidence‐based treatment protocols. The use of physiological variables such as time to epithilialisation may seem adequate and logical in the assessment of treatment efficacy; however, some wounds may never heal. Thus, the use of HRQoL may be a more complete measure of progress for patients and healthcare providers. Furthermore, as Gordon et al. states, two patients with the same clinical manifestations often have drastically different perceptions of HRQoL (7). Reliably measuring HRQoL is of great importance to patients and clinicians because poor HRQoL has been associated with poor ulcer prognosis (8).
Questionnaires have been developed to quantitatively measure HRQoL in patients with DFUs. The SF‐36v2 is a generic HRQoL instrument, which involves domains such as physical functioning, role limitation because of Emotional and Physical Problems, Bodily Pain, General Health Perceptions, Vitality, Social Functioning and Mental Health. While generic measures of HRQoL are useful, their application to diseases with a high incidence of comorbidities is questionable. For example, when asking generic questions one cannot be sure that a change in HRQoL scores is related to diabetic retinopathy, a DFU or both. On the other hand, disease‐specific questionnaires may have the benefit of being able to more specifically measure the effect of a comorbidity on HRQoL. One such questionnaire is the Cardiff Wound Impact Schedule (CWIS), developed by Price and Harding in 2004 (9). The CWIS was developed through analysis of qualitative data collected from patients with wounds of various etiologies. Through factor analysis following focus‐group discussions, Price and Harding included the following domains in the CWIS: Social Life, Well‐Being, Physical Symptoms and Daily Living, overall HRQoL and satisfaction with overall HRQoL. Price and Harding also used statistical correlation with the SF‐36v2 to display validity when assessing HRQoL in diabetic and leg wounds in a UK population. Questions in the CWIS are directly focussed on the effect of a wound on patient HRQoL.
In caring for patients with DFUs it is also important to assess the severity of their disease in order to gauge treatment efficacy and provide meaningful feedback to patients over the course of wound healing. Traditionally, disease severity has been measured using physical attributes of wounds including size, depth and complications such as infection. While this is meaningful information and may guide treatment, objective measures of wound severity may not accurately reflect the lived experience of people with DFUs. Social scientists often contend that self‐rated health and quality of life may better predict morbidity and mortality than many objective measures (10). Disease‐specific HRQoL measures such as the CWIS need to be validated for use in assessing wound severity as they may be the key to fully understand how the severity of disease is related to the patient's subjective experience of living with a DFU.
At present, further disease‐specific HRQoL instrument research is required to validate this instrument for patients with chronic diabetic foot wounds. Assessing HRQoL is integral to measuring treatment efficacy in clinical trials. A valid and disease‐specific HRQoL measure is also needed for advancement of evidence‐based treatment by assessing patient need and progress in clinical care. In order for a HRQoL measure to be clinically useful it must reliably reproduce results in patients who have not experienced significant changes in their physical condition. The purpose of this study is to further evaluate the validity of the CWIS through comparison with the SF‐36v2 in patients with chronic diabetic foot wounds. A second purpose of this study is to assess the ability of the CWIS to differentiate between levels of wound severity through comparison with a validated objective measure of wound severity, the University of Texas diabetic wound classification system (11).
METHODS
A total of 30 patients over the age of 18 with open DFUs were recruited in an outpatient wound care clinic. Licensure for the SF‐36v2 (acute form) and the CWIS was obtained and the study was approved by the St Michael's Hospital Research Ethics Board. Patients consenting to participate were asked to complete both the CWIS and the SF‐36v2. In order to evaluate test–retest reliability of the CWIS, participants were given a second copy in a postage‐paid and addressed envelope to complete and return in 5–7 days. Twenty‐four patients completed the second administration of the CWIS. Participants were also assessed for severity of their wound according to the University of Texas wound classification system (Table 1) (11). An experienced member of the St Michael's Hospital wound care team performed classification according to the University of Texas wound classification system.
Table 1.
The University of Texas wound classification system
| Grade | |||
|---|---|---|---|
| 0 | I | II | III |
| Completely epithelialised | Superficial Wound | Wound penetrates to tendon or capsule | Wound penetrates to bone or joint |
| Stage | |||
|---|---|---|---|
| A | B | C | D |
| No infection or ischaemia | Infection | Ischaemia | Infection and ischaemia |
Construct validity was assessed through linear regression between relevant domains of the CWIS and the SF‐36v2. Test–retest reliability was assessed by comparing participant CWIS scores between the first and second administration of the questionnaire. Analysis of correlation coefficients was based on the following guidelines: <0·3 insignificant, 0·3–0·45 significant, 0·45–0·6 substantially significant and >0·6 highly significant (12).
The utility of the CWIS in discriminating between healed and unhealed DFUs was assessed by comparing mean scores of patients with healed DFUs from a previous study by Price and Harding and patients with unhealed diabetic foot wounds from the current study. The ability of the CWIS to differentiate between wound severity levels was examined by grouping patients by categories of the University of Texas wound classification system and comparing mean CWIS scores between each category of wound severity.
RESULTS
Study population demographics are summarised in Table 2. Thirty participants completed the CWIS and SF‐36v2. Six participants did not complete the second administration of the CWIS. The average age of participants was 59 years with a standard deviation of 11 years. The male‐to‐female ratio of participants was 5 to 1. Participants had a mean duration of current DFU of 2·3 years.
Table 2.
Participant characteristics
| Number of participants | 30 |
| Number of participants not completing retest | 6 |
| Mean age of participants ± SD (years) | 59 ± 11 |
| Male:Female | 5:1 |
| Mean number of current DFUs ± SD | 1·2 ± 0·4 |
| Mean number of previous DFUs ± SD | 1·1 ± 1·5 |
| Mean duration of current DFU ± SD (years) | 2·3 ± 2·3 |
DFU, diabetic foot ulcer.
Table 3 contains results from a liner correlation between domains of the CWIS and the SF‐36v2. Significant correlations were found between the Social Life domain of the CWIS and the SF‐36v2 domains of Vitality (r = 0·429, P < 0·01), Emotional Role Limitation (r = 0·560, P < 0·01) and Social Functioning (r = 0·641, P < 0·01). The CWIS domain of Well‐Being showed significant correlation with the SF‐36v2 domains of General Health (r = 0·533, P < 0·01) and Social Functioning (r = 0·514, P < 0·01). The CWIS domain of Physical Symptoms and Daily Living showed significant correlation with the SF‐36v2 domains of Physical Functioning (r = 0·631, P < 0·01), Bodily Pain (r = 0·452, P < 0·01) and Vitality (r = 0·489, P < 0·01). The CWIS domain of Total HRQoL showed significant correlations with the SF‐36v2 domains of Physical Functioning (r = 0·566, P < 0·01), Emotional Role Limitation (r = 0·465, P < 0·01), Mental Health (r = 0·472, P < 0·01) and Vitality (r = 0·425, P < 0·01).
Table 3.
Linear regression between domains of the CWIS and the SF‐36v2
| CWIS domain | SF‐36v2 domain | Correlation coefficient |
|---|---|---|
| Social Life | Vitality | 0·429 |
| Social Functioning | 0·641 | |
| Emotional Role | 0·560 | |
| Well‐Being | General Health | 0·533 |
| Social Functioning | 0·514 | |
| Physical Symptoms and Daily Living | Physical Functioning | 0·631 |
| Bodily Pain | 0·452 | |
| Vitality | 0·489 | |
| Total HRQoL | Physical Functioning | 0·566 |
| Vitality | 0·425 | |
| Emotional Role | 0·465 | |
| Mental Health | 0·472 |
All correlations shown were significant (P < 0·01). CWIS, Cardiff Wound Impact Schedule; HRQoL, health‐related quality of life.
Table 4 shows correlation coefficients comparing each domain of the CWIS between the first and second administration of the questionnaire. Highly significant correlations were found between the first and second administration of the CWIS, in all questionnaire domains.
Table 4.
Correlation coefficients for each domain of the CWIS between the first and second administration of the questionnaire
| CWIS domain | Correlation coefficient | |
|---|---|---|
| Experience | Stress | |
| Social Functioning | 0·632 | 0·809 |
| Physical Symptoms and Daily Living | 0·725 | 0·808 |
| Well‐Being | 0·562 | – |
| Global HRQoL | 0·773 | – |
| Satisfaction with HRQoL | 0·828 | – |
‘Experience’ refers to questions related to the experience of the domain. ‘Stress’ refers to questions related to stress associated with the domain. All correlations shown were significant (P < 0·01). CWIS, Cardiff Wound Impact Schedule; HRQoL, health‐related quality of life.
Figure 1 contains mean CWIS scores of participants based on level of wound severity as determined by the University of Texas wound classification system (Table 1). A higher mean CWIS score represents a higher HRQoL. Study participants with greater wound severity did not score significantly lower on the CWIS.
Figure 1.
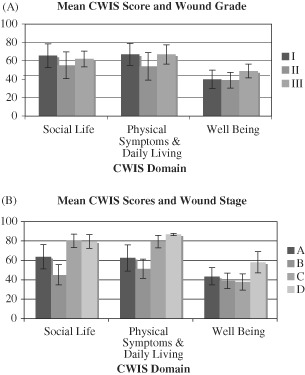
Comparison of mean Cardiff Wound Impact Schedule (CWIS) scores for patients grouped based on the University of Texas diabetic wound classification system for wound grade (A) and wound stage (B). (Refer to Table 1 for descriptions of grades and stages of wound severity.)
CWIS scores of participants with non healed DFUs were compared to CWIS scores of patients with healed DFUs from a previous study in the UK (Figure 2). Participants with healed DFUs scored significantly higher in the CWIS domains of Social Life (P < 0·001), Physical Symptoms and Daily Living (P < 0·01) and Well‐Being (P < 0·001).
Figure 2.
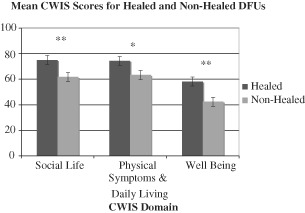
Comparison of mean Cardiff Wound Impact Schedule (CWIS) scores between patients with healed and non healed diabetic foot ulcers (DFUs) (** P < 0·001, * P < 0·01). Mean CWIS scores shown were collected from non healed patients enrolled in the present study and healed patients from a previous study conducted in the UK.
DISCUSSION
The purpose of this study was to determine if the CWIS is a valid and reliable measure of HRQoL in patients with DFUs. Hitherto, many studies examining HRQoL in patients with foot ulcers have relied on generic measures and semi‐structured interviews 13, 14, 15, 16, 17, 18. Similarly, studies comparing wound care practices have used generic measures of HRQoL as outcome measures (19). With a valid, disease‐specific measure of HRQoL, future studies can minimise the confounding effects of diabetic comorbidities on study outcomes. It should be noted that other disease‐specific measures of HRQoL have been developed to assess the affect of DFUs and diabetic neuropathy on HRQoL 20, 21.
In this study, all domains of the CWIS were shown to correlate with relevant domains of the SF‐36v2. This suggests that the CWIS is a valid disease‐specific measure of HRQoL in patients with DFUs. This supports the use of the CWIS in research as an outcome measure that better assesses the specific effect of a DFU on the HRQoL of patients.
The usefulness of the CWIS in assessing HRQoL in this population is further exemplified by the findings of test–retest reliability. Highly significant correlations were found between the first and second administration of the CWIS. This supports the use of the CWIS longitudinally in practice and clinical research as a measure of treatment outcome.
This study was not able to show that patient CWIS scores correspond to a specific level of wound severity, using the University of Texas wound classification system. This may have been related to a small sample size or the nature of the University of Texas wound classification system. Perhaps more importantly, these findings point to the subjective nature of HRQoL. Two persons with the same level of physical illness may experience stark differences in their self‐reported HRQoL (22). It may not be possible to accurately correlate wound severity, based on physical characteristics, and HRQoL. Whether or not one can adequately relate the physical and psychosocial aspects of any disease will remain an area of debate. On the other hand, the CWIS has shown promise in discerning health states in a more rudimentary fashion in that we have shown the ability of the CWIS to differentiate between the healed and non healed states. This lends confidence to the use of the CWIS as a research outcome measure and stresses the importance of measuring outcomes in various domains of the patient's disease experience.
Finally, the lack of significant difference in HRQoL between levels of wound severity may be due to a treatment effect. Regardless of wound severity, patients receive similar treatment modalities such as dressings and off‐loading devices. In the authors' experience, patients likely take the success of their treatment into account when reporting their HRQoL. As there is significant overlap in how patients are treated, this may hinder the ability of the study to discern differences in HRQoL among varying levels of wound severity.
This study was limited by a relatively small sample size. Increasing sample size would allow for sub‐group analysis by comorbidities. Controlling for comorbidities may further prove the validity of the CWIS in specifically measuring wound‐related HRQoL.
The CWIS is reliable and valid, when compared to the World Health Organization's SF‐36v2 in patients with DFUs. More research is needed to determine the ability of the CWIS to differentiate between levels of wound severity. The CWIS may be used to differentiate between the healed and non healed wound states.
ACKNOWLEDGEMENTS
The authors acknowledge the contributions of the Wound Care Team at St Michael's Hospital, Toronto, Canada, for their support in data collection. The authors also acknowledge Professor Patricia Price, University of Wales College of Medicine, Cardiff, UK, for sharing of data.
REFERENCES
- 1. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217–28. [DOI] [PubMed] [Google Scholar]
- 2. Levin M. Foot lesions in patients with diabetes mellitus. Endocrinol Metab Clin North Am 1996;25:447–62. [DOI] [PubMed] [Google Scholar]
- 3. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–53. [DOI] [PubMed] [Google Scholar]
- 4. Rathur HM, Boulton AJM. The neuropathic diabetic foot. Nat Clin Pract Endocrinol Metab 2007;3:14–25. [DOI] [PubMed] [Google Scholar]
- 5. Bann CM, Fehnel SE, Gagnon DD. Development and validation of the diabetic foot ulcer scale‐short form (DFS‐SF). Pharmaoeconomics 2003;21:1277–90. [DOI] [PubMed] [Google Scholar]
- 6. Valensi P, Girod I, Baron F, Moreau‐Defarges T, Guillon P. Quality of life and clinical correlates in patients with diabetic foot ulcers. Diabetes Metab 2005;31:263–71. [DOI] [PubMed] [Google Scholar]
- 7. Guyatt GH, Feeny DH, Patrick DL. Measuring health‐related quality of life. Ann Intern Med 1993;118:622–9. [DOI] [PubMed] [Google Scholar]
- 8. Ribu L, Birkeland K, Hanestad B, Moum T, Rustoen T. A longitudinal study of patients with diabetes and foot ulcers and their health‐related quality of life: wound healing and quality‐of‐life changes. J Diabetes Complications 2008;22:400–7. [DOI] [PubMed] [Google Scholar]
- 9. Price P, Harding K. Cardiff wound impact schedule: the development of a condition‐specific questionnaire to assess health‐related quality of life in patients with chronic wounds of the lower limb. Int Wound J 2004;1:10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Idler EL, Benyamini Y. Self‐rated health and mortality: a review of twenty‐seven community studies. J Health Soc Behav 1997;38:21–37. [PubMed] [Google Scholar]
- 11. Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system. Diabetes Care 1998;21:855–9. [DOI] [PubMed] [Google Scholar]
- 12. Burnand B, Kernan WN, Feinstein AR. Indexes and boundaries for “quantitative significance” in statistical decisions. J Clin Epidemiol 1990;43:1273–84. [DOI] [PubMed] [Google Scholar]
- 13. Nabuurs‐Franssen MH, Huijberts MSP, Nieuwenhuijzen Kruseman AC, Willems J, Schaper NC. Health‐related quality of life of diabetic foot ulcer patients and their caregivers. Diabetologia 2005;48:1906–10. [DOI] [PubMed] [Google Scholar]
- 14. Tennvall GR, Apelqvist J. Health‐related quality of life in patients with diabetes mellitus and foot ulcers. J Diabetes Complications 2005;14:235–41. [DOI] [PubMed] [Google Scholar]
- 15. Walters SJ, Morrell CJ, Dixon S. Measuring health‐related quality of life in patients with venous leg ulcers. Qual Life Res 1999;8:327–36. [DOI] [PubMed] [Google Scholar]
- 16. Willrich A, Pinzur M, McNeil M, Juknelis D, Lavery L. Health related quality of life, cognitive function, and depression in diabetic patients with foot ulcer or amputation. A preliminary study. Foot Ankle Int 2005;26:128–34. [DOI] [PubMed] [Google Scholar]
- 17. Hareendran A, Bradbury A, Budd J, Geroulakos G, Hobbs R, Kenkre J, Symonds T. Measuring the impact of venous leg ulcers on quality of life. J Wound Care 2005;14:53–7. [DOI] [PubMed] [Google Scholar]
- 18. Smith JJ, Guest MG, Greenhalgh RM, Davies AH. Measuring the quality of life in patients with venous ulcers. J Vasc Surg 2000;31:642–9. [DOI] [PubMed] [Google Scholar]
- 19. Charles H. Does leg ulcer treatment improve patients' quality of life?. J Wound Care 2004;13:209–13. [DOI] [PubMed] [Google Scholar]
- 20. Vileiktye L, Peyrot M, Bundy C, Rubin RR, Leventhal H, Mora P, Shaw JE, Baker P, Boulton AJM. The development and validation of a neuropathy‐ and foot ulcer‐specific quality of life instrument. Diabetes Care 2003;26:2549–55. [DOI] [PubMed] [Google Scholar]
- 21. Abetz L, Sutton M, Brady L, McNulty P, Gagnon DD. The diabetic foot ulcer scale (DFS): a quality of life instrument for use in clinical trials. Pract Diab Int 2002;19:167–75. [Google Scholar]
- 22. Cella DF, Bonomi AE. Measuring quality of life: 1995 update. Oncology 1995;9(Suppl):47–60. [PubMed] [Google Scholar]


