Abstract
Purpose:
Active surveillance is an established option for men with low risk prostate cancer. Multiparametric magnetic resonance imaging with magnetic resonance imaging-transrectal ultrasound fusion guided biopsy may better identify patients for active surveillance compared to systematic 12-core biopsy due to improved risk stratification. To our knowledge the performance of multiparametric magnetic resonance imaging in following men on active surveillance with visible lesions is unknown. We evaluated multiparametric magnetic resonance imaging and magnetic resonance imaging-transrectal ultrasound fusion guided biopsy to monitor men on active surveillance.
Materials and Methods:
This retrospective review included men from 2007 to 2015 with prostate cancer on active surveillance in whom magnetic resonance imaging visible lesions were monitored by multiparametric magnetic resonance imaging and fusion guided biopsy. Progression was defined by ISUP (International Society of Urological Pathology) grade group 1 to 2 and ISUP grade group 2 to 3. Significance was considered at p ≤0.05.
Results:
A total of 166 patients on active surveillance with 2 or more fusion guided biopsies were included in analysis. Mean followup was 25.5 months. Of the patients 29.5% had pathological progression. Targeted biopsy alone identified 44.9% of patients who progressed compared to 30.6% identified by systematic 12-core biopsy alone (p = 0.03). Fusion guided biopsy detected 26% more cases of pathological progression on surveillance biopsy compared to systematic 12-core biopsy. Progression on multiparametric magnetic resonance imaging was the sole predictor of pathological progression at surveillance biopsy (p = 0.013). Multiparametric magnetic resonance imaging progression in the entire cohort had 81% negative predictive value, 35% positive predictive value, 77.6% sensitivity and 40.5% specificity in detecting pathological progression.
Conclusions:
Multiparametric magnetic resonance imaging progression predicts the risk of pathological progression. Patients with stable multiparametric magnetic resonance imaging findings have a low rate of progression. Incorporating fusion guided biopsy in active surveillance nearly doubled our detection of pathological progression compared to systematic 12-core biopsy.
Keywords: prostatic neoplasms, watchful waiting, ultrasonography, magnetic resonance imaging, biopsy
Prostate cancer is the most common noncutaneous cancer among American men. ACS (American Cancer Society) estimated that 180,000 new cases would be diagnosed in 2016. USPSTF (United States Preventive Screening Task Force) gave PSA screening a grade of D, finding that screening has an unfavorable harm-to-benefit ratio and recommending against routine use.1 This is due to the high rate of diagnosis of clinically insignificant disease coupled with overtreatment and subsequent morbidity.2,3 The utilization of AS continues to increase to address these issues and large AS series with long-term followup report minimal metastatic disease and low prostate cancer specific mortality.4,5 Monitoring these patients relies on periodic surveillance SBs and serum PSA.
MP-MRI in conjunction with MRI/TRUS FB has emerged as a useful alternative to SB.6,7 FB integrates MP-MRI findings with real-time TRUS, allowing for biopsy of areas suspicious for prostate cancer. FB has demonstrated improved cancer detection and localization, especially for clinically significant disease.8–13
With the improved cancer detection and characterization provided by MP-MRI, imaging may help identify men appropriate for surveillance. Multiple studies have shown that when using FB for the confirmatory biopsy in men who met AS criteria, approximately 30% no longer qualify for AS.14,15 The ability of MP-MRI to detect progression in men already on AS is less understood.
The purpose of this study was to investigate whether changes on MP-MRI correlated with pathological progression in men on AS and also to evaluate the performance of FB vs SB in detecting progression.
METHODS
The institutional review board approved clinical data collection in men with prostate cancer at NCI (National Cancer Institute) from August 2007 to October 2015. Patients who enrolled in AS underwent MRI/TRUS FB (TB plus SB) at study enrollment as well as confirmatory FB between 12 and 24 months on surveillance. Only men with lesions identified on MP-MRI were included in study because at our institution men with negative MP-MRIs are referred back to their community urologist for surveillance systematic biopsies. Thus, patients with negative MP-MRIs were not included. A subset of 58 of these patients was previously reported.11
Patients had semiannual PSA levels measured and annual MP-MRIs performed. Patients were categorized into 2 groups, including NIH low risk, defined as ISUP Grade Group 1, and NIH intermediate risk, defined as ISUP Grade Group 2. All patients had PSA less than 20 ng/ml. Patients at low risk had no exclusion criteria placed on the percent of core involvement or the number of positive grade group. Those at intermediate risk were excluded if more than 33% of biopsy cores were positive and no exclusion criteria were placed on the percent of core involvement. Pathological progression in the low risk group was defined as any ISUP Grade Group 2 identified on surveillance biopsy. Pathological progression in the intermediate risk group was defined as any ISUP Grade Group 3 on surveillance biopsy. Table 1 lists specific AS criteria.
Table 1.
Active surveillance criteria definitions
| AS Criteria | Clinical Stage | PSA (ng/ml) | Gleason Grade | Total Pos Cores | % Single Core Pos | PSAD (ng/ml/cc) |
|---|---|---|---|---|---|---|
| NIH risk: | ||||||
| Low | T2a or less | 20 or Less | 3 + 3 or Less | – | – | – |
| Expanded | T2a or less | 20 or Less | 3 + 4 or Less | 33% or Less | – | – |
| Epstein | T1c or less | – | 3 + 3 or Less | 2 or Less | 50% or Less | 0.15 or Less |
| Toronto | – | 10 or Less | 3 + 3 or Less | – | – | – |
| PRIAS | T2a or less | 10 or Less | 3 + 3 or Less | 2 or Less | – | 0.2 or Less |
| Royal Marsden | T2a or less | 15 or Less | 3 + 4 or Less | 50% or Less | – | – |
MP-MRI progression was defined as an increase in suspicion score, an increase in lesion diameter measured in the axial plane or the appearance of any new lesion regardless of suspicion score during followup imaging compared to initial imaging. These parameters were chosen because they are objective measurements that can be followed serially over time and they have previously been shown to be useful in predicting pathological progression.11 PSAD progression was defined as baseline less than 0.15 ng/ml/cc and an increase to 0.15 ng/ml/cc during subsequent biopsies.
Multiparametric Magnetic Resonance Imaging Data Acquisition
MRI sequences included triplanar (axial, coronal and sagittal) T2-weighted, axial diffusion-weighted imaging with apparent diffusion coefficient, axial precontrast T1-weighted and axial dynamic contrast enhanced. Baseline MP-MRIs were obtained with a 3 Tesla Achieva MRI (Philips®) used in combination with both a BPX-30 endorectal coil (Medrad®) and a 16-channel SENSE cardiac coil (Philips). Followup MP-MRIs were obtained on the same 3 Tesla system using a 32-channel cardiac coil with same pulse sequences. Lesions were assigned a suspicion score categorized as low, moderate and high using a previously validated scoring system.16
This cohort of patients predates the PI-RADS™ scoring system. Thus, the validated scoring system was used for consistency and comparison of MRIs during the study period. The NIH lesion suspicion scores low, moderate and high are analogous to PI-RADS 1–2, 3 and 4–5, respectively.17 MRI scans were reviewed in consensus by 2 experienced uroradiologists (BT and PLC) with 9 and 12 years of experience, respectively.
Magnetic Resonance Imaging/Transrectal Ultrasound Fusion Biopsy
Patients underwent TB followed by SB at the same session. FB (targeted and systematic) was performed using the UroNav™ platform. All TBs were obtained in the axial and sagittal planes from each lesion seen for a minimum 2 cores per lesion.18 All biopsies were examined by a single genitourinary pathologist (MMe).
Statistical Analysis
Multivariable logistic regression was performed using variables with p <0.20 on univariate analysis. Statistical analysis was performed using SAS JMP® with p ≤0.05 considered significant.
RESULTS
Demographics
A total of 166 patients on AS with 2 or more FBs met study inclusion criteria, including 128 at low risk and 38 at intermediate risk. Table 2 presents baseline patient demographics.
Table 2.
Baseline patient characteristics and risk of pathological progression
| Low Risk | Intermediate Risk | |
|---|---|---|
| No. pts | 128 | 38 |
| Mean ± SD age | 61.7 ± 6.6 | 65.7 ± 6.7 |
| Mean ± SD PSA (ng/ml) | 5.69 ± 4.19 | 6.16 ± 3.54 |
| Mean ± SD PSAD (ng/ml/cc) | 0.12 ± 0.09 | 0.13 ± 0.08 |
| Mean ± SD No. MRI lesions | 2.6 ± 1.3 | 2.7 ± 1.4 |
| No. MRI suspicion score (%): | ||
| Low | 36 (28.1) | 4 (10.5) |
| Moderate | 86 (67.2) | 33 (86.8) |
| High | 6 (0.05) | 1 (0.03) |
| No. Gleason 7 cores (%): | Not applicable | |
| 1 | 26 (68) | |
| 2 | 7 (18) | |
| 3 | 2 (7) | |
| 4 | 3 (8) | |
| Pathological progression risk (%): | ||
| No. pts (%) | 37 (29.0) | 12 (31.5) |
| No. systematic biopsy progression only (%) | 12 (32.4) | 3 (25) |
| No. target biopsy progression only (%) | 14 (37.8) | 8 (66.7) |
| No. target + systematic biopsy progression (%) | 11 (29.7) | 1 (8) |
| Mean ± SD time to progression (yrs) | 2.7 ± 1.8 | 1.8 ± 1.1 |
Pathological Progression
Mean followup was 25.5 months (range 3.2 to 96.4). In 31% of patients at intermediate risk pathological progression was identified and 29% of patients at low risk had progression (table 2). Those at intermediate risk had progression-free survival of 1.5 years (IQR 1.2–2.1) and those at low risk had progression-free survival of 2.1 years (IQR 1.2–4.0). Of patients with initial MRI suspicion scores indicating low, moderate and high risk pathological progression was identified in 25%, 33% and 33%, respectively. Patients were stratified according to established AS criteria, including Epstein, Toronto, PRIAS (Prostate Cancer Research International Active Surveillance) and Royal Marsden criteria. Table 3 lists progression rates.
Table 3.
Comparison to other active surveillance criteria
| Criteria | No. Pts (% total cohort) | No. Progression (%) |
|---|---|---|
| NIH low risk cohort | 128 (100) | 37 (29.0) |
| Epstein | 88 (69) | 25 (28.4) |
| Toronto | 111 (87) | 33 (29.7) |
| PRIAS | 95 (75) | 26 (27.3) |
| Royal Marsden | 150 (90) | 46 (27.7) |
FB detected the majority of pathological progression in the low and intermediate risk cohorts (67.6% and 75%, respectively). TB alone identified 22 of 49 patients (44.9%) who progressed in comparison to SB alone, which identified 15 of 49 (30.6%) (p = 0.03). Pathological progression was detected in 24.5% of patients by both biopsy techniques.
We performed a total of 215 followup SBs in 166 men to detect 27 pathological progressions. Had we only performed followup TB when there was MP-MRI progression, we would have performed 107 TBs to detect 34 pathological progressions. The number needed to biopsy to detect 1 pathological progression was 7.96 (215/27) for SB vs 3.14 (107/34) for TB (p <0.001).
Multiparametric Magnetic Resonance Imaging Progression
MP-MRI characteristics were examined for an association with pathological progression. A total of 107 patients (64.5%) had progression by MP-MRI. On univariate analysis men with pathological progression were statistically more likely to have MP-MRI progression while the remainder with pathological progression had stable MP-MRIs (79.1% vs 20.8%, p = 0.013). On multivariate analysis controlling for age and the number of positive cores, MP-MRI progression remained significant in predicting pathological progression (p = 0.04). MP-MRI progression was observed in 78.3% (29/37) of low risk patients who had pathological progression and in 83.3% (10/12) of intermediate risk patients.
MP-MRI progression in the entire cohort had 81% negative predictive value, 35% positive predictive value, 77.6% sensitivity and 40.5% specificity to detect pathological progression. As the number of positive criteria for MP-MRI progression increased, positive predictive value for pathological progression increased (p = 0.014, fig. 1). Of patients who met only 1 MRI progression criterion 33.3% had pathological progression compared to 100% who met all 3 MRI criteria (fig. 2). Subgroup analysis of patients in whom pathological progression was detected only by TB showed that 19 of 22 (86.4%) also had MP-MRI progression while 10 of 15 (66.7%) with progression detected only by SB had MP-MRI progression.
Figure 1.
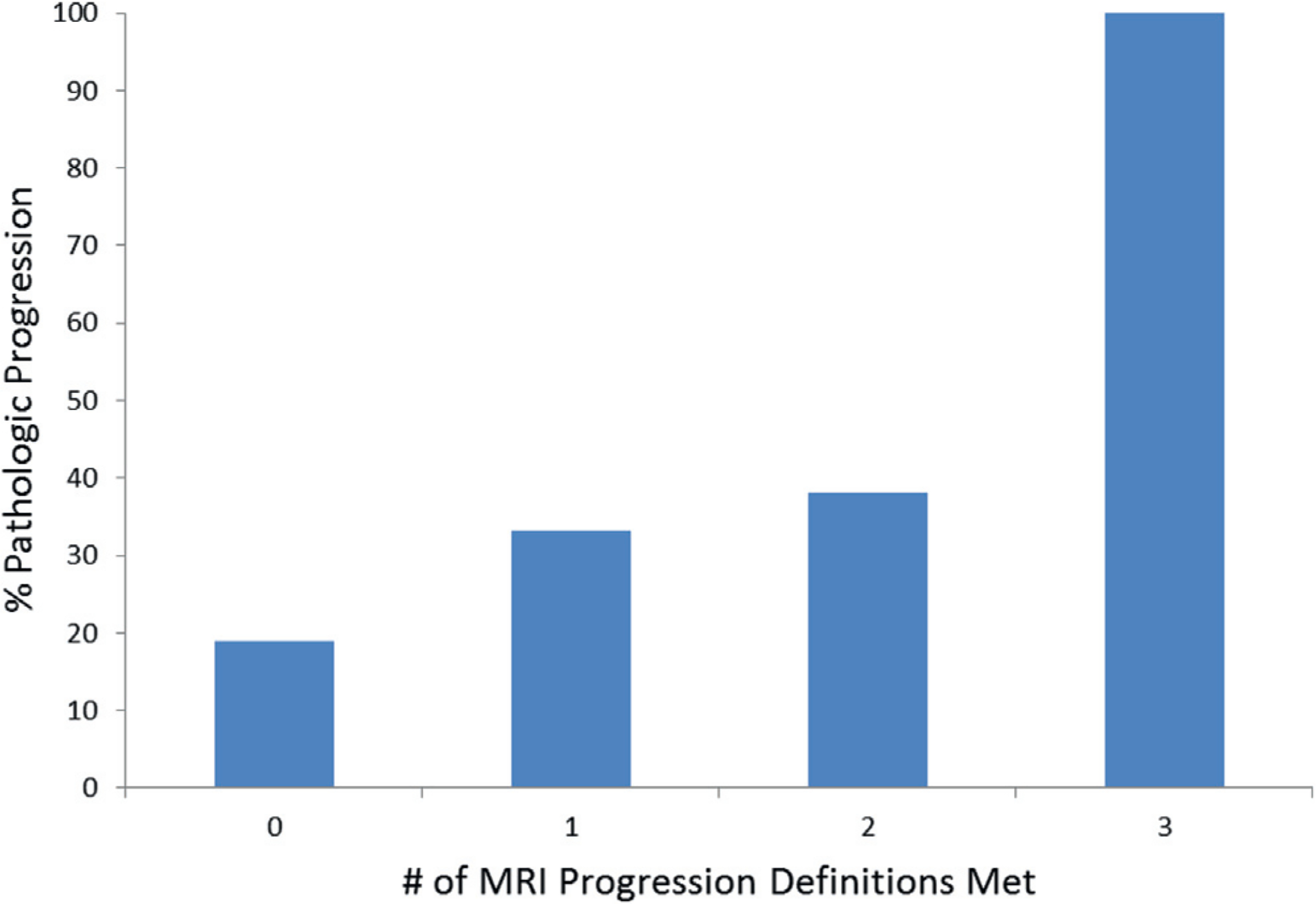
Risk of pathological progression increased with increasing MRI progression.
Figure 2.
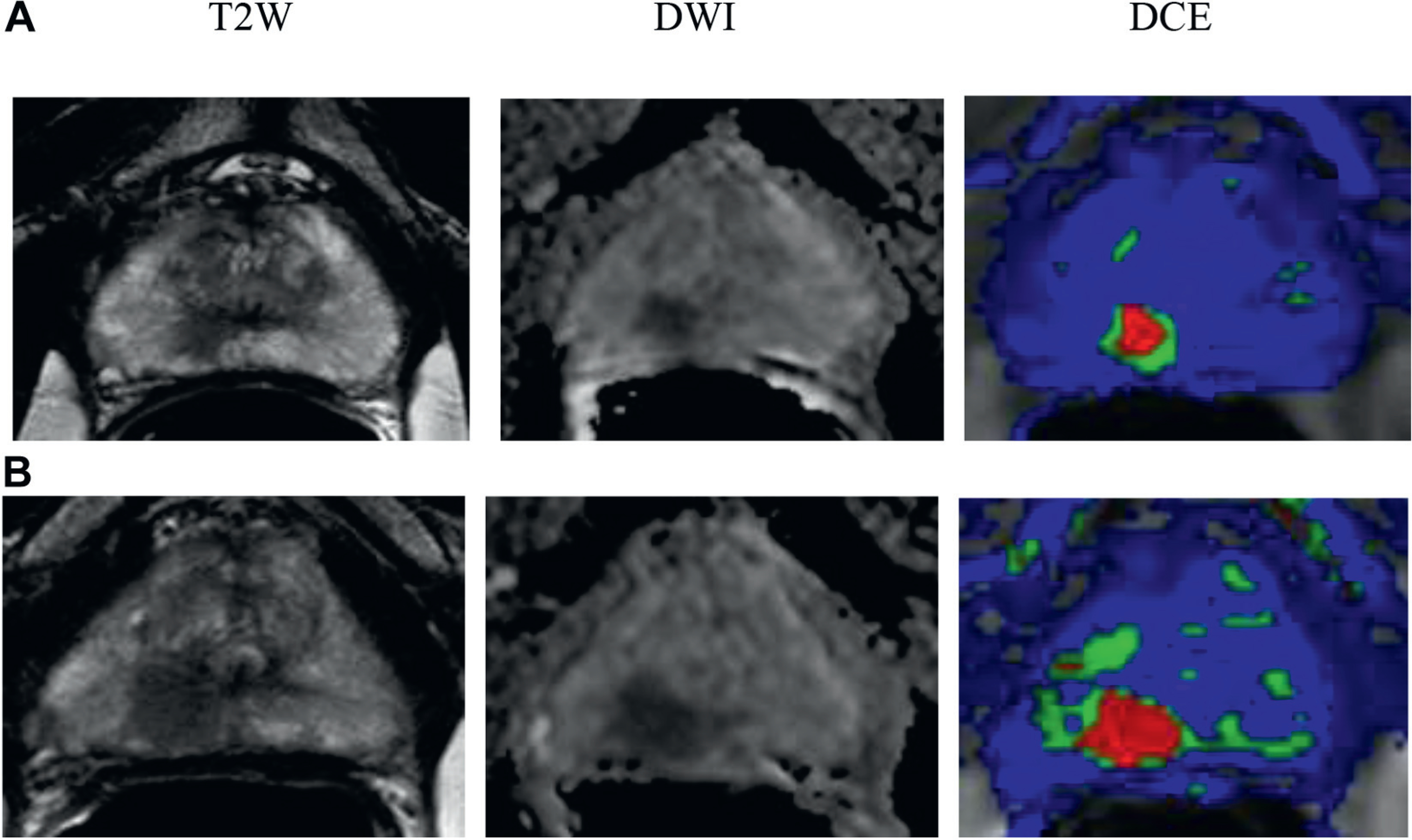
In 61-year-old healthy patient with no comorbidities PSA was 3.04 ng/ml. Initial MP-MRI showed 32 cc prostate and moderate suspicion for prostate cancer with 1 cm lesion in peripheral zone right apex (A). Gleason 3 + 4 = 7 was found by 2 targeted biopsy cores but all systematic biopsies were negative. Followup MP-MRI performed 18 month later showed lesion progression to 1.3 cm (B). MRI was highly suspicious for prostate cancer. Targeted biopsy revealed Gleason 4 + 4 = 8. Radical prostatectomy was done and final pathology findings were Gleason 4 + 4 = 8 and organ confined with negative margins. T2W, T2-weighted. DWI, diffusion-weighted image. DCE, dynamic contrast enhanced.
Clinical variables were evaluated for their performance to predict pathological progression (table 4). A total of 17 patients had surveillance PSAD 0.15 ng/ml/cc or greater and 6 had pathological progression. The performance of PSAD 0.15 ng/ml/cc or greater to predict pathological progression had 35.3% positive predictive value, 70.7% negative predictive value, 12.2% sensitivity and 90.4% specificity.
Table 4.
Predictors of pathological progression
| Pathological Progression |
p Value |
|||
|---|---|---|---|---|
| No | Yes | Univariate | Multivariate | |
| No. PSAD progression 0.15 ng/ml/cc or greater | 11 | 6 | 0.58 | – |
| No. PSA doubling time less than 2 yrs | 11 | 6 | 0.59 | – |
| No. PSA velocity greater than 2 ng/ml/yr | 12 | 8 | 0.29 | – |
| Mean initial PSA (ng/ml) | 5.95 | 5.48 | 0.50 | – |
| Mean initial PSAD (ng/ml/cc) | 0.122 | 0.120 | 0.91 | – |
| Mean age | 62.1 | 64.1 | 0.07 | 0.22 |
| No. highest core involvement greater than 50% | 10 | 8 | 0.34 | – |
| No. greater than 33% pos biopsy cores | 26 | 19 | 0.16 | 0.34 |
| Mean No. prostate biopsies | 3.4 | 3.8 | 0.22 | – |
DISCUSSION
The utilization of AS continues to increase as a viable treatment strategy in patients with favorable histology. However, AS protocols vary considerably, including the triggers for rebiopsy and definitive treatment. The improved detection and risk stratification capabilities of MP-MRI and FB make this approach a useful tool in the AS population.
The established use of MP-MRI and FB in AS has been for confirmatory biopsy with approximately 30% of patients reclassified and upgraded as no longer candidates for AS based on FB.14,15 In a recent study Felker et al similarly looked at the value of serial MRIs in patients on active surveillance using a slightly different definition of MRI progression.19 They found that MRI added incremental but valuable information in predicting which men had pathological progression. Similar to our data, in 47% of men with pathological progression in that study the condition was identified by targeted biopsy alone, again highlighting the added benefit of performing targeted biopsies in patients on AS.
To our knowledge the current study represents the largest AS cohort in the literature with multiple MP-MRI and FB sessions. The use of serial MP-MRIs in men on AS is appealing as it may potentially allow some to delay or forego repeat biopsies based on imaging findings. In this cohort, if repeat biopsy had been restricted to men with MP-MRI progression on serial imaging, 10 with pathological progression would have been missed while 57 would have been spared a biopsy. Tumor heterogeneity of individual lesions and sampling error could possibly account for this.
Although stable MP-MRIs predict favorable repeat biopsy findings, our data suggest that men with stable imaging findings may benefit from surveillance biopsy to minimize the risk of missing pathological progression. Our data also suggest that the combination of SB and TB should be used to follow patients on AS because in 30% pathological progression was identified on SB alone.
Allowing men with Grade Group 2 disease to participate in AS could potentially further reduce the harms of overtreatment. Historically, some groups have allowed patients with low volume Grade Group 2 to enroll in AS.20 Patients at intermediate risk had a 31.5% rate of pathological progression, which was similar to that in men in our low risk group. The use of MP-MRI and FB results in improved risk stratification. There is little doubt that prior to the advent of this technology some men with presumably Grade Group 1 cancer unknowingly harbored Grade Group 2 or greater disease and were followed on AS.6 Tosoian et al found that 21.8% of men with low risk prostate cancer who were eligible for AS had Gleason score upgrading at radical prostatectomy.21 Before imaging, these groups were unknowingly following some patients with Grade Group 2 disease and noting good long-term results, suggesting that some Grade Group 2 cancer behaves in indolent fashion.
For AS eligibility no restrictions were placed on the percent core positive for cancer. Criteria such as this were previously used as a proxy for tumor volume but with imaging we are able to more accurately assess tumor size and volume.22,23 Using more restrictive criteria one may expect to decrease the risk of pathological progression, although this study does not support that hypothesis. Men in our cohort who met strict eligibility for AS based on Epstein criteria had the same overall rate of progression as the entire NIH low risk cohort (28.4% and 29.1%, respectively). Our data suggest that with accurate initial Grade Group classification other AS criteria, such as highest percent core positive and the number of cores positive, may have limited benefit in predicting the risk of progression. Similarly, a study found that increasing the amount of low risk cancer allowable for AS eligibility had no impact on pathological outcomes after surgery, thus, supporting the use of expanded criteria.24 Further, it may not be valid to apply such criteria to patients undergoing FB, given that the criteria were developed in and for patients undergoing SB.
Changes in serial MP-MRI scans were better able to predict men at risk for pathological progression compared to other more conventional clinical variables. The predictive accuracy was dose dependent, in that men who met more than 1 definition of MP-MRI progression had an increased likelihood of pathological progression. Similar to our findings, the use of MRI to predict reclassification in patients on AS was previously shown to be directly related to the degree of suspicion on MP-MRI.25
The ability to monitor lesions over time with MP-MRI resulted in more men being identified with pathological progression. FB likely improves the sampling accuracy of cancerous areas. Thus, one can argue that we were not detecting true biological progression but were more accurately reclassifying these tumors. Regardless of definitions, what these data reveal is improved risk stratification using MP-MRI and FBs. Of men found to have pathological progression on followup biopsy 44.9% were identified solely on a TB. FB identified 34 of all 49 patients (70%) who had pathological progression while SB alone identified the remaining 15 of 49 (30%). MP-MRI is optimal in identifying higher risk and higher volume disease. It appears that SB has a role in surveillance to complement these strengths of MP-MRI.
PSA kinetics have been extensively studied in AS populations as triggers for biopsy or definitive intervention. In this cohort MP-MRI outperformed PSAD, PSA doubling time and PSA velocity. PSAD had only 12.2% sensitivity in identifying patients with pathological progression. A previous study evaluated the usefulness of PSAD and found no correlation with progression on serial biopsy and no correlation with upgrading on final whole mount pathology.26 Other groups have noted that initial PSAD 0.15 ng/ml/cc or greater was predictive of disease progression.27–29 In our cohort 38 patients had initial PSAD greater than 0.15 ng/ml/cc but only 9 had pathological progression (p = 0.37).
Our study has certain limitations. This study is retrospective in nature and represents a select group of men on AS who had MP-MRI identifiable lesions. Thus, results of this study may not be generalizable to an AS cohort in which many have no MP-MRI visible lesions. Previous data suggest that a man with at least 1 MP-MRI visible lesion is at higher risk of clinically significant prostate cancer compared to a patient with a negative MP-MRI.30 The data are also limited by a median followup time of only approximately 2 years, largely due to the only recent investigation of MP-MRI in the AS management scheme. Finally, an institutional MP-MRI scoring system was used since PIRADS was not applied at the time of data collection.
CONCLUSIONS
MP-MRI and FB have important roles in selecting and following men on AS. The incorporation of FB detected 26% more pathological progression on surveillance biopsies compared to SB. Progression on MP-MRI predicts the risk of pathological progression and patients with stable MP-MRIs have a low rate of progression. Ideally, as imaging and FB technology evolve, the number or frequency of biopsies in men on AS may potentially be reduced. Furthermore, MP-MRI may allow for expanded AS criteria, permitting more men to participate in AS with higher confidence in accurate monitoring of disease progression. Larger AS cohorts with longer followup are needed to validate these findings. The optimal use of MP-MRI in AS populations will continue to develop toward the goals of oncologically safe and efficient management of low and intermediate grade cancers.
Abbreviations and Acronyms
- AS
active surveillance
- FB
fusion biopsy
- MP-MRI
multiparametric MRI
- MRI
magnetic resonance imaging
- NIH
National Institutes of Health
- PSA
prostate specific antigen
- PSAD
PSA density
- SB
systematic biopsy
- TB
targeted biopsy
- TRUS
transrectal ultrasound
Footnotes
The corresponding author certifies that, when applicable, a statement(s) has been included in the manuscript documenting institutional review board, ethics committee or ethical review board study approval; principles of Helsinki Declaration were followed in lieu of formal ethics committee approval; institutional animal care and use committee approval; all human subjects provided written informed consent with guarantees of confidentiality; IRB approved protocol number; animal approved project number.
No direct or indirect commercial incentive associated with publishing this article.
REFERENCES
- 1.Moyer VA and U.S. Preventive Services Task Force: Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 157: 120. [DOI] [PubMed] [Google Scholar]
- 2.Cohen MS, Hanley RS, Kurteva T et al. : Comparing the Gleason prostate biopsy and Gleason prostatectomy grading system: the Lahey Clinic Medical Center experience and an international meta-analysis. Eur Urol 2008; 54: 371. [DOI] [PubMed] [Google Scholar]
- 3.Eggener SE, Badani K, Barocas DA et al. : Gleason 6 prostate cancer: translating biology into population health. J Urol 2015; 194: 626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klotz L, Vesprini D, Sethukavalan P et al. : Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol 2015; 33: 272. [DOI] [PubMed] [Google Scholar]
- 5.Tosoian JJ, Trock BJ, Landis P et al. : Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol 2011; 29: 2185. [DOI] [PubMed] [Google Scholar]
- 6.Siddiqui MM, Rais-Bahrami S, Truong H et al. : Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol 2013; 64: 713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frye TP, Pinto PA and George AK: Optimizing patient population for MP-MRI and fusion biopsy for prostate cancer detection. Curr Urol Rep 2015; 16: 521. [DOI] [PubMed] [Google Scholar]
- 8.Turkbey B, Mani H, Shah V et al. : Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol 2011; 186: 1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonn GA, Natarajan S, Margolis DJ et al. : Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. J Urol 2013; 189: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siddiqui MM, Rais-Bahrami S, Turkbey B et al. : Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015; 313: 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walton Diaz A, Shakir NA, George AK et al. : Use of serial multiparametric magnetic resonance imaging in the management of patients with prostate cancer on active surveillance. Urol Oncol 2015; 33: 202.e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kongnyuy M, George AK, Rastinehad AR et al. : Magnetic resonance imaging-ultrasound fusion-guided prostate biopsy: review of technology, techniques, and outcomes. Curr Urol Rep 2016; 17: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chelluri R, Kilchevsky A, George AK et al. : Prostate cancer diagnosis on repeat magnetic resonance imaging-transrectal ultrasound fusion biopsy of benign lesions: recommendations for repeat sampling. J Urol 2016; 196: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Da Rosa MR, Milot L, Sugar L et al. : A prospective comparison of MRI-US fused targeted biopsy versus systematic ultrasound-guided biopsy for detecting clinically significant prostate cancer in patients on active surveillance. J Magn Reson Imaging 2015; 41: 220. [DOI] [PubMed] [Google Scholar]
- 15.Stamatakis L, Siddiqui MM, Nix JW et al. : Accuracy of multiparametric magnetic resonance imaging in confirming eligibility for active surveillance for men with prostate cancer. Cancer 2013; 119: 3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rais-Bahrami S, Siddiqui MM, Turkbey B et al. : Utility of multiparametric magnetic resonance imaging suspicion levels for detecting prostate cancer. J Urol 2013; 190: 1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller BG, Shih JH, Sankineni S et al. : Prostate cancer: interobserver agreement and accuracy with the revised prostate imaging reporting and data system at multiparametric MR imaging. Radiology 2015; 277: 741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong CW, Rais-Bahrami S, Walton-Diaz A et al. : Comparison of MR-US fusion-guided prostate biopsies obtained from axial and sagittal approaches. BJU Int 2015; 115: 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felker E, Wu J, Natarajan S et al. : Magnetic resonance imaging in active surveillance of prostate cancer: incremental value. J Urol 2016; 195: 1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooperberg MR, Carroll PR and Klotz L: Active surveillance for prostate cancer: progress and promise. J Clin Oncol 2011; 29: 3669. [DOI] [PubMed] [Google Scholar]
- 21.Tosoian JJ, JohnBull E, Trock BJ et al. : Pathological outcomes in men with low risk and very low risk prostate cancer: implications on the practice of active surveillance. J Urol 2013; 190: 1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turkbey B, Mani H, Aras O et al. : Correlation of magnetic resonance imaging tumor volume with histopathology. J Urol 2012; 188: 1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okoro C, George AK, Siddiqui MM et al. : Magnetic resonance imaging/transrectal ultrasonography fusion prostate biopsy significantly outperforms systematic 12-core biopsy for prediction of total magnetic resonance imaging tumor volume in active surveillance patients. J Endourol 2015; 29: 1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reese AC, Landis P, Han M et al. : Expanded criteria to identify men eligible for active surveillance of low risk prostate cancer at Johns Hopkins: a preliminary analysis. J Urol 2013; 190: 2033. [DOI] [PubMed] [Google Scholar]
- 25.Hu JC, Chang E, Natarajan S et al. : Targeted prostate biopsy in select men for active surveillance: do the Epstein criteria still apply? J Urol 2014; 192: 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross AE, Loeb S, Landis P et al. : Prostate-specific antigen kinetics during follow-up are an unreliable trigger for intervention in a prostate cancer surveillance program. J Clin Oncol 2010; 28: 2810. [DOI] [PubMed] [Google Scholar]
- 27.Barayan GA, Brimo F, Begin LR et al. : Factors influencing disease progression of prostate cancer under active surveillance: a McGill University Health Center cohort. BJU Int 2014; 114: E99. [DOI] [PubMed] [Google Scholar]
- 28.Dall’Era MA, Konety BR, Cowan JE et al. : Active surveillance for the management of prostate cancer in a contemporary cohort. Cancer 2008; 112: 2664. [DOI] [PubMed] [Google Scholar]
- 29.Loeb S, Bruinsma SM, Nicholson J et al. : Active surveillance for prostate cancer: a systematic review of clinicopathologic variables and biomarkers for risk stratification. Eur Urol 2015; 67: 619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schoots IG, Petrides N, Giganti F et al. : Magnetic resonance imaging in active surveillance of prostate cancer: a systematic review. Eur Urol 2015; 67: 627. [DOI] [PubMed] [Google Scholar]


