Abstract
Persistent (chronic) wound‐related pain is a common experience that requires appropriate assessment and treatment. It is no longer adequate for health care professionals to concentrate on the acute (temporary) pain during dressing change alone. The study provides useful recommendations and statements for assessing and managing total wound‐related pain for patients, health care professionals and other policymakers. The recommendations have been developed with the involvement of an interprofessional panel of health care professionals from around the world.
Introduction
Pain is a subjective and common experience for people living with chronic wounds. Wound‐related pain can be temporary (acute) or persistent (chronic). Acute wound pain can be exacerbated whenever the wound is being handled or manipulated; with dressing removal, wound cleansing or debridement. In contrast, persistent (chronic) wound pain is the background symptom that exists at rest and between wound‐related procedures including dressing changes and turning. Nevertheless, persistent wound pain is just as devastating as temporary acute pain. Accumulating evidence indicates that up to 80% of patients with chronic wounds experience persistent pain between dressing changes 1, 2, 3. The high incidence of pain associated with chronic wounds challenges the erroneous assumptions that venous ulcers, superficial wounds and wounds associated with neuropathy are painless 1, 4.
Although persistent wound pain does not necessarily have a definite trigger, it is often associated with the wound cause and local changes in the wound environment. A sudden emergence of wound‐related pain or an increase in existing pain is often linked to infection or bacterial damage, tissue trauma and other key factors that adversely affect wound healing. Despite the importance of pain as a key clinical indicator, it has traditionally been neglected by health care providers with a lack of documentation and treatment.
The purpose of this study is to increase the health care professional’s awareness of persistent and total wound‐related pain. Both types of pain are common and need to be assessed and treated. It is no longer adequate to concentrate on the pain during dressing change alone. The study provides recommendations and statements for assessing and managing wound‐related pain developed for health care professionals and other policymakers (Figure 1).
Figure 1.
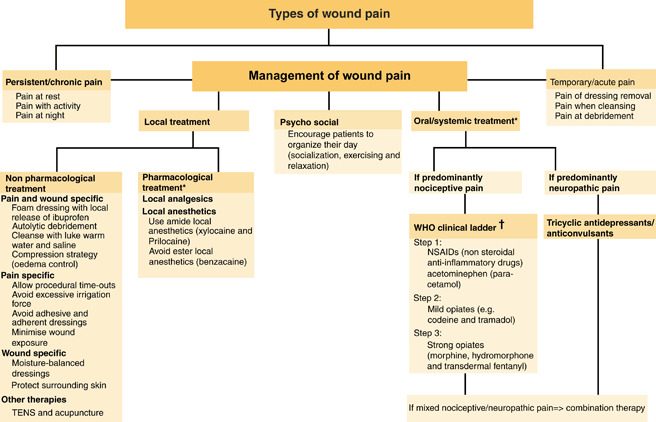
The Wound Pain Management Model – guiding assessment and treatment of wound pain (40). *For all drugs, please refer to individual product monographs.†WHO (41).
A thorough literature search was conducted using Medline, CINAHL and PubMed to identify relevant articles, guidelines and studies related to chronic wound‐associated pain. The literature search results yielded more than 800 articles using terms such as wounds, ulcers, pressure ulcers, venous ulcers, foot ulcers and pain. A total of 170 articles met the criteria of temporary and persistent wound pain. The literature was reviewed and summarised to establish a group of proposed statements. The Wound Pain Management Model© guided the approach (Figure 1).
An expert interprofessional panel was asked to review and rank the statements independently (strongly agree, somewhat agree, somewhat disagree and strongly disagree). The statements were revised until at least 80% of the panel strongly or somewhat agreed. The statements were then posted on a web survey for a larger number of key opinion from health care leaders. Each of the statements in Table 1 will be discussed outlining the rational from the literature and key aspects of expert knowledge.
Table 1.
Statements for the assessment and management of persistent (chronic) and total wound pain
| 1. Assume all chronic wounds are painful until the patient indicates otherwise |
| 2. Assess and study chronic wound pain on a regular basis |
| a. The patient should be assessed with the same standardised tool at every assessment |
| b. Wound pain occurs with activities of daily living, not only at dressing change |
| 3. Discuss with patients and caregivers the options for wound pain management based on assessment results |
| 4. Consider non pharmacological and pharmacological wound pain management options |
| 5. Increased wound pain requires reassessment of underlying conditions or aetiologies for treatable causes |
| a. Increased wound pain may be an important clinical symptom of infection or inflammation |
| 6. Treat the cause and treat the wound pain with the patient’s active participation |
| 7. Wound pain often adversely affects activities of daily living and patient well‐being; effective management may lead to improvement |
| 8. Prevent and/or minimise anticipatory and procedural wound pain by using appropriate pain management techniques |
| 9. An ongoing therapeutic relationship between the interprofessional team and the patient is essential for wound pain management |
| 10. Communicate, educate and implement the wound pain management plan verbally and with documentation to the patients, caregivers and the interprofessional team |
Statement 1: Assume all chronic wounds are painful until the patient indicates otherwise
Although health care providers often consider wound healing to be the primary focus, patients consistently rated pain to be of a higher priority 3, 4, 5. In fact, pain is often expressed to be the worst aspect of having a wound. Several studies indicated that more than 80% of patients affected with a chronic wound reported pain at all times, with half of them rating pain as moderate to the worst possible pain 6, 7.
Given the high prevalence and burden of wound‐related pain, clinicians must maintain a high vigilance on pain assessment. All wounds, no matter how innocuous, are potentially painful during and even after wound healing has occurred. The assessment of wound‐related pain starts with a division into temporary (acute) and persistent (chronic) categories (Figure 1).
How is wound‐related pain generated? Subsequent to tissue injury, persistent inflammation triggers the release of mediators activating local pain receptors, resulting in enhanced sensitivity of the surrounding skin and deeper structures in the wound base. Nociceptive pain is stimulus dependent and usually evoked by tissue damage and described as gnawing, aching, throbbing and tender. In contrast, neuropathic pain is spontaneous occuring as a result of nerve tissue injury and is more likely to be described as burning, stinging, shooting or stabbing.
Most chronic wound pain is a combination of both pain types
In a large study of 758 patients with leg ulcers, Franks and Moffatt (8) showed that the longer the duration of the ulcer, the higher the reported wound‐associated pain (P = 0·022), probably reflecting repeated injury nerve damage. This study reiterates the importance of early and aggressive pain treatment to avoid the potential spiralling effect of chronic pain.
Key messages for practice
-
Patients:
Encourage patients to verbalise their pain experience.
-
Professionals:
Incorporate pain documentation into wound assessment.
-
Policymakers:
Develop standards for pain assessment.
Statement 2: Assess and study chronic wound pain on a regular basis
-
a.
The patient should be assessed with the same standardised tool at every assessment.
-
b.
Wound pain occurs with activities of daily living, not only at dressing change.
Wound pain is influenced by many factors: tissue damage (pressure, friction and shear), chemical irritation, vascular compromise (venous hypertension and arterial insufficiency), infectious agents and abnormal persistent inflammation. To study the changes in pain over time, regular reassessments are needed.
Pain is subjective that is modulated by emotions (e.g. anxiety, fear and depression), expectations and beliefs. It is whatever the patient says it is (9) even if it may seem to others to be out of proportion to the triggering chronic wound. Patients’ self‐report of pain is the most reliable indicator of pain.
A wide array of standardised and validated tools had been developed to measure pain intensity and assess pain characteristics (Table 2).
Table 2.
Tools for assessment of pain intensity and characteristics
| Type of pain assessment | Pain assessment tool | Description |
|---|---|---|
| Quantitative tools | VAS | Patients are asked to indicate their pain intensity by putting a mark along a continuum with one extreme representing no pain and the other end the worst possible pain |
| The Numerical Rating Scale | This is a VAS scale calibrated with numbers from 0 (no pain) to 10 (the worst pain imaginable) either on a continuum scale or in boxes (Numeric Box Scale) | |
| Qualitative and pain characteristics tools | The Verbal Rating Scale | Consists of four adjectives to describe pain severity: none, mild, moderate and severe |
| The Faces Scales (Wong Baker Faces Pain Scale) | A series of faces each depict different facial expressions. The series of faces represent an increasing degree of pain with increasing unhappiness or sadness | |
| Short McGill Pain Questionnaire | Eleven pain‐related words to differentiate neuropathic from nociceptive components of pain |
VAS, Visual Analogue Scale.
The Visual Analogue Scale is a commonly used pain tool, and the Numeric Box Scale has been successfully used with older individuals. Most individuals, even in the presence of cognitive impairment, prefer the Numerical Rating Scale and Verbal Rating Scale for their simplicity, ease of use and directedness.
The Faces Scale may measure the emotional impact of pain instead of pain intensity or pain characteristics. To capture the quality and characteristics of pain, the Short McGill Pain Questionnaire is a popular choice. The 11 pain‐related words in this instrument differentiate neuropathic from nociceptive components of pain. A comprehensive pain assessment tool such as the Brief Pain Inventory provides information about the impact of pain on seven quality‐of‐life issues. However, some patients are not able to communicate their pain verbally because of age, severe cognitive impairment, advanced neurological disease or other disability. Behavioural indicators including facial expressions and body movements are valid surrogate indicators of pain with several validated tools available to measure these non verbal signals.
Patients with chronic wounds constitute a diverse population. The selection of a specific pain scale should consider institutional policy and the patients’ age, language, educational level, the presence of any sensory impairment or decreased cognitive status. Regardless of the tool selected, the same rating scale tool should be used sequentially for a comparison over time.
The frequency of assessment should be determined by the characteristics of pain, the type of treatment interventions, health care system policies and professional standards. As a standard, pain assessment should be considered before and after physical activities and other aspects of patient care, medications or treatment. Frequent assessments are highly recommended if severe pain is present. A patient pain diary is also useful (10) to monitor the intensity and temporal fluctuation of pain. It can also serve as a record to optimise the timing and choice of therapeutic interventions.
Pain assessment must be accompanied with the appropriate documentation to ensure the continuity of care and prompt new symptom treatment. Despite this wide choice of assessment tools, Kammerlander (11) reported that only 16% of the nurses in their three‐country study used a standardised pain scale. Lorimer et al. (12) reviewed 66 nursing records of venous ulcer clients receiving home care. Only 15% of the records contained any documentation of pain, and the assessment was not standardised.
Every health care provider should ask about chronic wound‐related pain at every assessment and obtain essential information on the pain severity and characteristics.
Key messages for practice
-
Patients:
Consider the evaluation tool best suited to the patient’s ability to study the painful experience.
-
Professionals:
The same selected standardised tool should be used for individual patient’s repeated assessments. Consider more frequent assessment if pain is severe or worsening.
-
Policymakers:
Ensure pain measurement tools are available and develop a documentation standard to distinguish nociceptive and neuropathic pain.
Statement 3: Discuss with patients and caregivers the options for wound pain management based on assessment results
There are many misconceptions about wound‐related pain and its treatment. Patients are often reluctant to discuss their wound‐related pain and take an effective dose of pain medication. Major patient concerns include the unsubstantiated fear of addiction or that good patients should not complain about pain and that health care providers should know if they are in pain 13, 14. In the older population, pain is often perceived as unavoidable and integral to the ageing process. It is crucial to explore and discuss their expectations and perceptions of pain through open communication. By developing a therapeutic alliance with patients and their caregivers, clinicians need to foster their active participation in the pain assessment, treatment and coping behaviours (15). The key message is that chronic wound patients do not have to live with wound‐related pain.
Key messages for practice
-
Patients:
Patients with chronic wounds do not have to suffer wound‐related pain.
-
Professionals:
Effective communication develops a therapeutic relationship enhancing patient’s adherence to treatment.
-
Policymakers:
Advocate for patients’ rights and incorporate regular health care team meetings with patients as a standard of care.
Statement 4: Consider non pharmacological and pharmacological wound pain management options
Chronic wound‐related pain is often underestimated and undertreated. There are multiple pharmacological agents available to combat pain. The selection of an appropriate agent should take into account the type and severity of pain based on the recommendations by the World Health Organization (WHO) ladder (16). Nociceptive pain is caused by direct stimulation of pain receptors associated with tissue damage. Most patients with mild to moderate nociceptive pain respond favourably to the regular use of non steroidal anti‐inflammatory drugs (NSAIDs) or acetaminophen. As pain intensifies, the use of mild to strong opioid analgesics is advised.
Neuropathic pain is also common in patients with chronic wounds because of nerve damage and irritation. In one study, Briggs et al. (17) reported that more than 40% of patients with leg ulcers (venous predominant) exhibited neuropathic symptoms. Neuropathic pain responds to a different group of agents including the antidepressants amitriptyline, nortriptyline, desipramine and duloxetine and the anticonvulsants gabapentin, pregabalin and carbamazepine.
The medication should be initiated at a low dose (considering pain levels) and titrated slowly while monitoring therapeutic responses. The exceptions are NSAIDs and acetaminophen; once the recommended dose is reached, higher doses do not confer additional analgesic effect.
Clinicians should be aware of the common side effects. They can also consider a combination of medications with different mechanisms of action to achieve the same analgesic response at lower doses minimising unpleasant side effects. The first through third line treatments (18) for nociceptive and neuropathic pain are outlined in Table 3.
Table 3.
Treatments for nociceptive and neuropathic pain
| Neuropathic pain | Nociceptive pain | |
|---|---|---|
| First line | TCA antidepressant: amitriptyline, nortriptyline, desipramine, SSRIs or DNRIs duloxetine and venlafaxine | Non steroidal anti‐inflammatory drugs or acetaminophen |
| Second line | Anticonvulsants: gabapentin/pregabalin carbamazepine and sodium valproate | Weak opioids (e.g. codeine) |
| Third line | Opioids | Strong opioids (e.g. morphine, hydrocodone and fentanyl) |
Always consider non pharmacological strategies and topical agents in conjunction with systemic medication to achieve optimal wound‐related pain management. Although there is a paucity of scientific evidence available, non pharmacological interventions may take the form of music therapy, natural products, physical activities and rest, repositioning or physical modalities. Topical agents or dressings play a critical role in alleviating wound‐related pain. The use of ‘atraumatic’ dressings should be considered to avoid wound surface trauma and patient distress.
The investigational use of topical morphine has showed positive results in two studies 19, 20. However, this formulation is not commercially available, and the lack of pharmacokinetic data has precluded the use of these compounds in most clinical practices. Topical anaesthetics may be suitable for use prior to local painful procedures or wound manipulations such as debridement. Strong evidence is available to support the use of an anaesthetic, EMLA® (Eutectic Mixture of Local Anaesthetics) creams such as lidocaine and prilocaine prior to the debridement of venous leg ulcers (21). None of the reviewed trials evaluated persistent wound pain.
Since this meta‐analysis was published, new evidence has emerged from randomised controlled trials (RCTs) evaluating persistent and temporary wound pain with an ibuprofen‐releasing foam dressing (Biatain‐Ibu; Coloplast A/S, Humlebaek, Denmark, licenced in Europe and Canada but not in USA at the present time). There were two RCTs involving a total of 146 patients with venous leg ulcers 22, 23. Patients allocated to the foam and ibuprofen group consistently experienced less pain than the control groups. The onset of pain reduction with the ibuprofen foam dressing was rapid occurring within 30 minutes. There was no delay in healing or increase in adverse events with the addition of ibuprofen to the foam dressing.
These studies provide evidence to support the addition of this new dressing class to the local therapeutic toolkit for pain relief.
Key messages for practice
-
Patients:
There are many treatment options to manage wound pain.
-
Professionals:
For optimal pain management, consider the use of non pharmacological approaches and in addition pain medication use: start low dose and go slow.
-
Policymakers:
Develop clinician education programmes to optimise the treatment of wound‐related pain.
Statement 5: Increased wound pain requires reassessment of underlying conditions or aetiologies for treatable causes
Increased wound pain may be an important clinical symptom of infection or inflammation
Pain is an important indicator of the wound status. Cutting and Harding (24) proposed that the presence of unexpected pain or tenderness, along with other criteria, may indicate infection in granulating wounds. Gardner et al. (25) studied that increasing pain was associated with a quantitative biopsy of greater than 105 colony‐forming units per gram of tissue. Their findings confirmed pain as a validated clinical sign of chronic wound infection (specificity value of 100%).
While superficial infection may be treated topically (e.g. silver, cadexomer iodine and chlorhexidine derivative), clinicians must determine if deep or surround tissue infection is present to consider systemic antimicrobial therapy (26). Sibbald et al. created the mnemonic N.E.R.D.S. and S.T.O.N.E.E.S. to separate superficial from deep infection (Figure 2).
Figure 2.
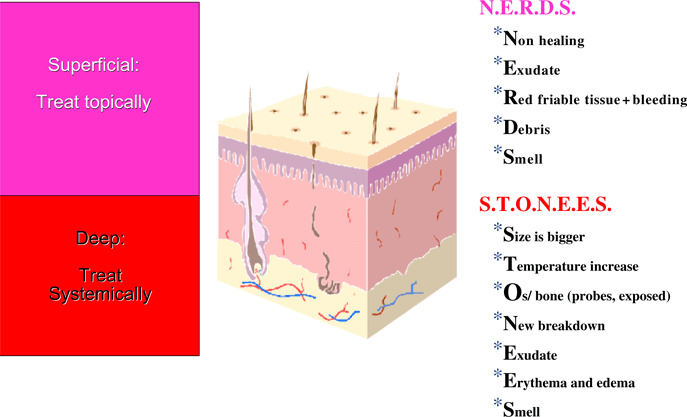
Differences between superficial and deep wounds.
To manage and treat superficial wound infections, ionised silver dressings are increasingly popular. Only two RCTs were published evaluating a moderate level of ionised silver‐released foam (Biatain Ag also known as Contreet; Coloplast A/S). Combined results from more than 700 patients worldwide indicated that the silver foam dressing could significantly reduce selected signs and symptoms of wound infections including increased pain, increased exudate and smell. The 4‐week wound surface area reduction with the silver dressing was impressive (45–50%) compared with the control groups (25–34%) 27, 28. We encourage future RCTs with local wound dressings to monitor the components of wound‐related pain and infection with appropriate surrogate endpoints and the documentation of relevant clinical signs.
While infection is commonly associated with increased wound pain, clinicians must be cognizant of other factors that are associated with inflammation. Pyoderma gangrenosum and vasculitis are inflammatory diseases that are often associated with painful cutaneous manifestations. Recurring trauma and deep structure damage may also perpetuate the inflammatory response and pain.
Key messages for practice
-
Patients:
Report increased pain to their caregivers promptly.
-
Professionals:
It is important to recognise increased pain as a potential sign of infection.
-
Policymakers:
Improving pain is an important wound management quality indicator.
Statement 6: Treat the cause and treat the wound pain with the patient’s active participation
Wound‐related pain may be because of the cause of the chronic wound. It is important to make an accurate wound diagnosis and correct the cause as part of a comprehensive approach to preventing pain. The Wound Pain Management Model provides a framework for considering the cause of wound pain (Figure 3).
Figure 3.
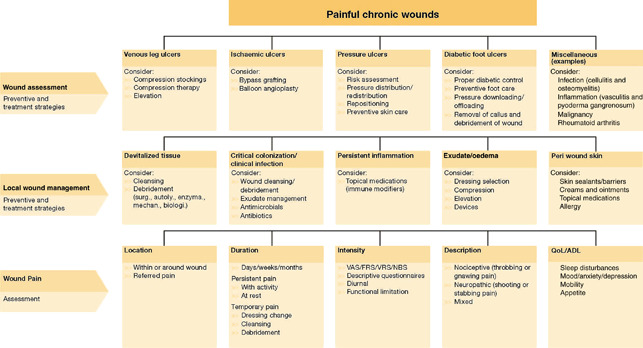
The Wound Pain Management Model – assessing the cause (40). ADL, activities of daily living; FRS, Faces Pain Rating Scale; NBS, Numeric Box Scale; QoL, quality of life; VAS, Visual Analogue Scale; VRS, Verbal Rating Scale.
Pain related to venous oedema may be orthostatic in nature and relieved by leg elevation, compression therapy or exercise that involves the calf muscle pump. Despite solid scientific evidence favouring the use of high compression therapy, patients reported that compression stocking or hosiery was difficult to apply, inconvenient, often too warm and expensive.
To enhance treatment adherence, patients’ preference must be sought to determine the optimal type of compression and exercise regime. Other potential causes of pain that warrant diagnostic consideration include lipodermatosclerosis and infection along with superficial or deep phlebitis. Patients with venous insufficiency have chronic skin‐associated inflammation rendering them more susceptible to contact sensitisation and subsequent itch or occasional painful dermatitis.
The majority of patients with diabetes and loss of protective sensation are afflicted with peripheral neuropathy that may precipitate painful symptoms. Several studies estimated that up to 75% of patients with diabetes experienced pain that is worsened with walking and/or during the night. Of these patients who had pain, as many as 37·9% indicated that their pain was constant (29).
Pressure ulcers may be painful because of persistent and recurrent pressure‐related ischaemia, trauma, shear, friction and skin irritation from incontinence. Optimal pressure redistribution can be achieved by regular repositioning, mobilisation and various support surfaces. Patients must be empowered to participate in determining the plan of care, the type of rehabilitation regime and surface selection.
Key messages for practice
-
Patients:
The treatment of pain should always involve the patients in all decisions.
-
Professionals:
Treatment of pain involves addressing the wound cause and patients’ concerns.
-
Policymakers:
Adequate resources must be allocated to address the wound cause.
Statement 7: Wound pain often adversely affects activities of daily living and patient well‐being; effective management may lead to improvement
Wound‐related pain may restrict many activities of daily living that are important to the patient with a chronic wound and in particular the persistent wound pain. Heinen et al. (30) reported that 75% of the interviewed patients identified pain as the key determinant of reduced physical activities. As a result, patients with chronic wounds are often confined to their home and socially isolated. Many patients experience pain‐associated sleep problems; pain is often exacerbated at night because of muscle spasms, poor circulation or positional changes. It is well studied that sleep disturbance is a major source of patient worry, fatigue and patients’ sense of reduced well‐being. However, pain reduction has been showed to improve patients’ quality of life (31).
Key messages for practice
-
Patients:
Pain can decrease quality of life and restrict activities of daily living.
-
Professionals:
Improvement of wound pain improves patients’ quality of life.
-
Policymaker:
Pain is an important component of an individual’s quality of life.
Statement 8: Prevent and/or minimise anticipatory and procedural wound pain by using appropriate pain management techniques
Pain is an unpleasant sensory and emotional experience contributing to psychological stress and other negative emotional states such as anxiety, fear and depression 32, 33. Heightened anxiety can, in turn, decrease the pain threshold and reduced pain tolerance. In a study of 96 patients with chronic wounds, anxiety levels before dressing change were significantly correlated to anticipatory pain (correlation coefficient r = 0·661), pain at dressing removal (r = 0·527) and pain at cleansing (r = 0·436) (all P values are <0·01) (34).
Pain and its negative psychological impact can impair wound healing (35). To validate the relationship between chronic pain and wound healing, McGuire (36) studied 17 women who underwent gastric bypass surgery. Patient pain ratings over 4 weeks post surgery were significantly associated with delayed healing of a punch biopsy wound site. In a large study of 111 patients with leg and foot ulcers, wound healing was significantly improved in patients with adequate chronic wound pain management (37). Patients who participated in a small pilot study had identified the value of wound care education to alleviate their anxiety and to help them coping with pain (38). The use of touch, music, biofeedback and distraction to reduce pain and associated distress is promising but requires further study with well‐designed clinical trials.
Key messages for practice
-
Patients:
Give patients an opportunity to verbalise their concerns and anxiety about anticipatory and procedural pain.
-
Professionals:
Tailor procedural pain‐related education to meet patient’s psychological needs.
-
Policymakers:
Education of health care professionals to deal with anticipatory and procedural pain reduces the patient’s anxiety and may increase adherence to treatment.
Statement 9: An ongoing therapeutic relationship between the interprofessional team and the patient is essential for wound pain management
A therapeutic relationship between the health care providers and the patient can enhance treatment adherence and optimise patient outcomes. The term adherence connotes a willingness on the patient’s part to actively participate in his or her care rather than the traditional concept of compliance (to obey a health care provider’s order or command). Patients who participated in a social, supportive, information‐sharing environment (Leg Clubs) showed a significant improvement in pain, mood, sleep and ability to work (39).
Recognising the complexity of pain, it is crucial to develop a coordinated team effort with various professional backgrounds to achieve the best possible outcomes. An improvement in wound‐related pain and healing trajectory was studied with a pilot community‐based interprofessional team approach. Wound pain was also significantly improved in patients who achieved complete wound closure.
Key messages for practice
-
Patients:
Encourage patient empowerment with a pain diary with reasonable control over the therapeutic decisions.
-
Professionals:
Optimise collaboration and draw on each other’s expertise.
-
Policymakers:
Establish wound care teams with pain management expertise.
Statement 10: Communicate, educate and implement the wound pain management plan verbally and with documentation to the patients, caregivers and the interprofessional team
The assessment, diagnosis and treatment of wound‐related pain require an integrated collaborative approach with the affected patient. There are several strategies that can be implemented to improve persistent and temporary wound‐related pain. Patients need to be the focus of the care plan with the ability to express their preferences along with the scientific evidence and expert knowledge summarised in this study.
Key messages for practice
-
Patients:
Educate patients to increase their comprehension of wound‐related pain management.
-
Professionals:
Improve patient and team communication and collaboration based on or integrating wound pain assessment documentation.
-
Policymakers:
Implement educational strategies to empower wound care teams with wound‐related pain expertise and foster their collaborative practice.
Recognising the saliency of pain and its adverse effect on patients, the American Nurses Association highlighted pain as the fifth vital sign. In this study, we summarised the key aspects of wound‐related pain and key management strategies. In conclusion, pain is a common problem in patients with chronic wounds. To address this problem, clinicians and policymakers need to work with patients to improve wound‐related pain.
References
- 1. Szor JK, Bourguignon C. Description of pressure ulcer pain at rest and at dressing change. J Wound Ostomy Continence Nurs 1999;26:115–20. [DOI] [PubMed] [Google Scholar]
- 2. Meaume S, Teot. L , Lazareth I, Martini J, Bohbot S. The importance of pain reduction through dressing selection in routine wound management: the MAPP study. J Wound Care 2004;13:409–13. [DOI] [PubMed] [Google Scholar]
- 3. Husband LL. Shaping the trajectory of patients with venous ulceration in primary care. Health Expect 2001;4:189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Phillips TJ, Dover JS. Leg ulcers. J Am Acad Dermatol 1991;25:965–87. [DOI] [PubMed] [Google Scholar]
- 5. Hyland ME, Ley A, Thomson B. Quality of life of leg ulcer patients: questionnaire and preliminary findings. J Wound Care 1994;3:294–8. [DOI] [PubMed] [Google Scholar]
- 6. Pieper B, Rossi R, Templin T. Pain associated with venous ulcers in injecting drug users. Ostomy Wound Manage 1998;44:54–8, 60–7. [PubMed] [Google Scholar]
- 7. Nemeth KA, Harrison MB, Graham ID, Burke S. Understanding venous leg ulcer pain: results of a longitudinal study. Ostomy Wound Manage 2004;50:34–6. [PubMed] [Google Scholar]
- 8. Franks PJ, Moffat CJ. Do clinical and social factors predict quality of life in leg ulceration. Int J Low Extr Wounds 2006;5:236–43. [DOI] [PubMed] [Google Scholar]
- 9. McCaffery M, Pasero C. Pain clinical manual, 2nd edn. 1999: St Louis, Mosby. [Google Scholar]
- 10. Hager KK, Brockopp D. Pilot project: the chronic pain diary – assessing chronic pain in the nursing home population. J Gerontol Nurs 2007;22:14–9. [DOI] [PubMed] [Google Scholar]
- 11. Kammerlander G, Eberlein T. Nurses’ views about pain and trauma at dressing changes: a central European perspective. J Wound Care 2002;11:76–9. [DOI] [PubMed] [Google Scholar]
- 12. Lorimer KR, Harrison MB, Graham ID, Friedberg E, Davies B. Venous leg ulcer care: how evidence‐based is nursing practice? J Wound Ostomy Continence Nurs 2003;30:132–42. [DOI] [PubMed] [Google Scholar]
- 13. Ward S, Hughes S, Donovan H, Serlin RC. Patient education in pain control. Support Care Cancer 2001;9:148–55. [DOI] [PubMed] [Google Scholar]
- 14. Yates P, Dewar A, Edwards H, Fentiman B, Najman J, Nash R, Richardson V, Fraser J. The prevalence and perception of pain amongst hospital in‐patients. J Clin Nurs 1998;7:521–30. [DOI] [PubMed] [Google Scholar]
- 15. Kerns RD, Otis JD, Marcus KS. Cognitive‐behavioural therapy for chronic pain in the elderly. Clin Geriatr Med 2001;17:503–23. [DOI] [PubMed] [Google Scholar]
- 16. World Health Organisation (WHO) pain relief ladder. 2005. URL www.who.int/cancer/palliative//painladder/en/ [accessed on 20 October 2007].
- 17. Briggs M, Bennett MI, Closs SJ. Painful leg ulceration: a prospective, longitudinal cohort study. Wound Repair Regen 2007;15:186–91. [DOI] [PubMed] [Google Scholar]
- 18. Chou R, Huffman LH. Medications for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med 2007;147:505–14. [DOI] [PubMed] [Google Scholar]
- 19. Zeppetella G, Paul J, Ribeiro MDC. Analgesic efficacy of morphine applied topically to painful ulcers. J Pain Symptom Manage 2003;25:555–8. [DOI] [PubMed] [Google Scholar]
- 20. Loitman J, Ward C, Stamm L, Wiersema‐Bryant L. Dulle P. Kirby JP. Improved pain control at dressing changes with topical lidocaine and morphine as part of a multi disciplinary team for comprehensive wound pain management. Wounds: a compendium of clinical research and Practice. Symposium on Advanced Wound Care and Wound Healing Society Meeting; 2007 April 28–May 1; Tampa (FL): Tampa Convention Centre, 2007;19(Suppl 3) 0‐A38 (Abstract). [Google Scholar]
- 21. Briggs M, Nelson EA. Topical agents or dressings for pain in venous leg ulcers. [Update of Cochrane Database of Syst Rev 2000;2:CD001177; PMID: 10796614]. Cochrane Database Syst Rev 2003;1:CD001177. [DOI] [PubMed] [Google Scholar]
- 22. Sibbald RG, Coutts P, Fierheller M. Decreased chronic (persistent) wound pain with a novel sustained release ibuprofen foam dressing. Poster presented at Symposium for Advanced Wound Care and at European Wound Management Association. 2006. [Google Scholar]
- 23. Gottrup F, Jorgensen B, Karlsmark T, Sibbald RG, Rimdeika R, Harding K, Price P, Venning V, Vowden P, Junger M, Wortmann S, Sulcaite R, Vilkevicius G, Ahokas TL, Ettler K, Arenbergerova M. Less pain with Biatain – Ibu: initial findings from a randomised, controlled, double‐blind clinical investigation on painful venous leg ulcers. Int Wound J 2007;4(Suppl 1):24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cutting KF, Harding KG. Criteria for identifying wound infection. J Wound Care 1994;3:198–201. [DOI] [PubMed] [Google Scholar]
- 25. Gardner SE, Frantz RA, Doebbeling BN. The validity of the clinical signs and symptoms used to identify localized chronic wound infection. Wound Repair Regen 2001;9:178–86. [DOI] [PubMed] [Google Scholar]
- 26. Sibbald RG, Woo KY, Ayello EA. Increased bacterial burden and infection: the story of NERDS & STONES. Adv Skin Wound Care 2006;19:447–61. [DOI] [PubMed] [Google Scholar]
- 27. Munter KC, Beele H, Russel L, Crespi A, Grochenig E, Basse P, Alikadic N, Fraulin F, Dahl C, Jemma AP. Effect of a sustained silver‐releasing dressing on ulcers with delayed healing: the CONTOP study. J Wound Care 2006;15:199–206. [DOI] [PubMed] [Google Scholar]
- 28. Jorgensen B, Price P, Andersen KE. The silver‐releasing foam dressing, Contreet foam, promotes faster healing of critically colonised venous leg ulcers: a randomized, controlled trial. Int Wound J 2005;2:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Buttke J. Identifying the Charcot foot. Adv Skin Wound Care 2006;19:189–90. [DOI] [PubMed] [Google Scholar]
- 30. Heinen MM, Evers AWM, Van Uden CJT, Van der Cleuten C, Van de Kerkhof PC, Van Achtenberg T. Sedentary patients with venous or mixed leg ulcers: determinants of physical activity. J Adv Nurs 2007;60:50–7. [DOI] [PubMed] [Google Scholar]
- 31. Jørgensen B, Friis G, Gottrup P. Pain and quality of life for patients with venous leg ulcers: proof of concept of the efficacy of Biatain‐ibu, a new pain reducing wound dressing. Wound Repair Regen 2006;14:233–9. [DOI] [PubMed] [Google Scholar]
- 32. Snyder RJ. Venous leg ulcers in the elderly patient: associated stress, social support and coping. Ostomy Wound Manag 2006;52:58–68. [PubMed] [Google Scholar]
- 33. Vileikyte L. Stress and wound healing. Clin Dermatol 2007;25:49–55. [DOI] [PubMed] [Google Scholar]
- 34. Woo KY Sadavoy J, Sidani S, Maunder R, Sibbald RG. Pain at dressing change. Poster presentation at CAWC London Ontario Canada. 2007. [Google Scholar]
- 35. Kiecolt‐Glaser JK, Marucha PT, Malarkey WB, Mercado AM, Glaser R. Slowing of wound healing by psychological stress. Lancet 1995;346:1194–6. [DOI] [PubMed] [Google Scholar]
- 36. McGuire L, Heffner K, Glaser R, Needleman B, Malarkey W, Dickinson S, Lemeshow S, Cook C, Muscarella P, Melvin WS, Ellison EC, Kiecolt‐Glaser JK. Pain and wound healing in surgical patients. Ann Behav Med 2006;31:165–72. [DOI] [PubMed] [Google Scholar]
- 37. Woo K, Alavi A, Botros M, Kozody LL, Fierheller M, Wiltshire K, Sibbald RG. A transprofessional comprehensive assessment model for persons with lower extremity leg and foot ulcers. Wound Care Canada 2007;5:s34–s47. [Google Scholar]
- 38. Gibson MC, Keast D, Woodbury MG, Black J, Goettl L, Campbell K, O‘Hara S, Houghton P, Borrie M. Educational intervention in the management of acute procedure‐related wound pain: a pilot study. J Wound Care 2004;13:187–90. [DOI] [PubMed] [Google Scholar]
- 39. Edwards H, Courtney M, Finlayson K, Lindsay E, Lewis C, Shuter P, Chang A.. Chronic venous leg ulcers: effect of a community nursing intervention on pain and healing. Nurs Stand 2005;19:47–54. [DOI] [PubMed] [Google Scholar]
- 40. Price P, Fogh K, Glynn C. Managing painful chronic wounds: the Wound Pain Management Model. Int Wound J 2007;4:4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. World Health Organisation . Cancer pain relief, 2nd edn. Geneva: WHO, 1996. [Google Scholar]


