Abstract
Background
Heterogeneity and focalization are the most common epidemiological characteristics of endemic countries in the Americas, where malaria transmission is moderate and low. During malaria elimination, the first step is to perform a risk stratification exercise to prioritize interventions. This study aimed to identify malaria risk strata in the ecoepidemiological regions of Colombia.
Methods
This was a descriptive and retrospective study using cumulative malaria cases in 1,122 municipalities of Colombia from 2010 to 2019. To identify the strata, the criteria proposed by PAHO were adapted. To classify the receptive areas (strata 2, 3, and 4) and nonreceptive areas (stratum 1), 1,600 m above sea level, ecotypes, main malaria vector presence, Plasmodium species prevalence and occurrence of malaria cases were used. The area occupied by the receptive municipalities, the cumulative burden, and the at-risk population in the regions were calculated.
Results
Ninety-one percent of the Colombian territory is receptive to the transmission of malaria and includes 749 municipalities with 9,734,271 (9,514,243–9,954,299) million at-risk inhabitants. Stratum 4 accounted for 96.7% of the malaria burden, and cases were concentrated primarily in the Pacific and Uraba-Bajo Cauca-Sinu-San Jorge regions. Plasmodium vivax predominates in most of the receptive municipalities, except in the municipalities of the Pacific region, where P. falciparum predominates. Anopheles albimanus, An. nuneztovari s.l., and An. darlingi were the main vectors in receptive areas.
Conclusions
In Colombia, 91.2% of the territory is receptive to the transmission of malaria and is characterized by being both heterogeneous and focused. Stratum 4 contains the greatest burden of disease, with a relatively greater proportion of municipalities with a predominance of P. vivax. However, there is a low proportion of municipalities with P. falciparum mainly in the Pacific region. These findings suggest that the latter be prioritized within the malaria elimination plan in Colombia.
Introduction
In the Americas, the major malaria transmission areas are Amazonia (Brazil, Colombia, Peru, Ecuador, Bolivarian Republic of Venezuela, Suriname, Guyana and Guyana Francesa), Mesoamerica (Guatemala, Honduras, Nicaragua, and Panama), Haiti, and the Dominican Republic [1]. In 2019, in the Americas, more than 80% of malaria cases were concentrated in the Bolivarian Republic of Venezuela, Brazil, Colombia, Guyana, Nicaragua, and Panama; however, within each country, there are areas with variable intensities [2]. Heterogeneity and focalization are the most common epidemiological characteristics of these endemic countries in the Americas, where malaria transmission is moderate and low [3,4].
Historically, to address the variability in several malaria transmission scenarios, different typologies based on epidemiology and determining factors of malaria transmission, such as clime and ecotypes, have been used to stratify malaria risks [5]. Application of these methodologies allows the establishment of smaller units or strata geographically defined according to the receptivity, which provides evidence that supports decision-making [5]. The World Health Organization (WHO) suggests malariogenic potential as a method to stratify risk malaria transmission because it is a critical factor in determining strategies to achieve elimination and prevent re-establishment of transmission [1]. Malariogenic potential is defined as the capacity of an ecosystem to favor malaria transmission (receptivity), risk of importation of the parasite (vulnerability), and vector competence (infectivity). Several methods have been proposed for assessing this potential [6,7].
In the Americas, the epidemiological stratification methodology and its application to the study of malaria distribution have been used since 1979 in endemic countries [8]. The stratification was based on different approaches; however, the majority of countries have used the trend of the annual parasite index (API) to stratify risk, which allowed the identification of strata or priority areas for malaria control [8,9]. In this region, there are several examples, such as in the state of Sucre (Venezuela), API was used to identify P. vivax transmission hotspots [10], and in Belize, Brazil and Peru, cumulative burden was used to identify areas where the highest number of cases were concentrated [11–13].
In Colombia, at the beginning of the 1980s, the API was used as a risk criterion to identify critical malaria transmission areas; additionally, this information was complemented with local determinates that contributed to the maintenance of malaria transmission [14]. Previously, it was established that more than 85% of the Colombian territory located below 1,600 m a.s.l. exhibits conditions that favor malaria transmission, and altitude is one of the most important geographical determinants that delimits malaria transmission in endemic areas. There is evidence that at 1,600 m a.s.l., the temperature is approximately 19°C (it changes approximately 0.625°C every 100 m) [15], and these conditions are suitable for the development of the sporogony cycle in malaria vector mosquitoes. Several studies have established that the minimum temperatures required for the development of P. falciparum and P. vivax in mosquitoes are approximately 18°C and 15°C, respectively, and these values delimit malaria at higher elevations [16]. Using these criteria and the ecotypes, six ecoepidemiological regions were identified that allowed elucidation of receptivity and vulnerability in all of them [17]. In most of the Colombian territory, malaria transmission exhibits endemic epidemic behavior that is persistent, variable and of moderate to low transmission [17]. P. vivax infections predominate (55.1%), and complications occur in between 1 and 2% of reported cases [18]. In Colombia 9 Anopheles species are considered as malaria regional and local vectors: Anopheles (Nys.) darlingi, An. (Nys.) nuneztovari s.l., An. (Nys.) albimanus, An. (An.) calderoni, An. (Ker.) neivai s.l., An. (Ker.) pholidotus (as An.lepidotus), An. (An.) pseudopunctipennis s.l., An. (An.) punctimacula s.l. and An. (Nys.) benarrochi B [19–22].
In Colombia, the National Strategic Plan for Malaria 2019–2022 includes strategies for malaria prevention, diagnosis, and treatment and improved surveillance and control proposed by the WHO in Global Technical Strategy for Malaria (2016–2030) to progress toward malaria transmission elimination [23,24]. The goals included in the National Strategic Plan for Malaria are to reduce malaria mortality by 80% by 2021 and morbidity by 40% by 2022 compared to 2017 [24]. The malaria stratification risk is the first step to planning prevention and control and guiding actions to prevent the re-establishment of transmission in areas where it has been interrupted. The objective of the present study was to identify malaria risk strata in the national territory using Pan American Health Organization (PAHO) methodology to generate evidence to prioritize and implement strategies that support the elimination of this disease in Colombia.
Methods
Study setting
Colombia is located in extreme northwestern South America with an area of 1.141.748 km2 and has a population of approximately 48.2 million inhabitants [25]. It is characterized by geographical diversity due to its location and the presence of the Andes Mountains that cross from south to north. These conditions and their interactions with the bioclimatic characteristics have delimited six natural regions: the Caribbean Region, located to the north and corresponding to the coastal zone on the Atlantic Ocean; Pacific Regions, in the eastern part of the country corresponding to the coastal zone on the Pacific Ocean; the Andean Region, situated in the center of this country, comprising Andes Mountain and two inter-Andean valleys; the Orinoco and Amazonia regions to the east of the Andes Mountain; and finally, an insular region composed of islands, cays and islets. Administratively, the country is divided into second-level territorial entities called departments (32) and districts (9) and third-level territorial entities called municipalities (1,122) [26].
Study design and data sources
A descriptive and retrospective study was performed in 1,122 municipalities of Colombia using annual malaria cases from 2010 to 2019, environmental variables and, main malaria vector distributions to establish malaria risk strata according to the methodology proposed by PAHO [27].
Data collection
Information about malaria cases was obtained from the Departmental and District Malaria Prevention and Control Programs and Integrated Information System of Social Protection (SISPRO, in its Spanish acronym; https://www.sispro.gov.co/Pages/Home.aspx). In Colombia, it is mandatory that all malaria cases reported to surveillance systems be confirmed by identification of Plasmodium species using microscopy diagnosis, rapid diagnostic tests or polymerase chain reaction. Microscopic examination of the blood smear is the gold standard for malaria diagnosis. Each case must be classified as indigenous or imported [28]. Records of malaria vectors from the Entomology Group of Instituto Nacional de Salud of Colombia (entomological collection database and literature search about malaria vector occurrence data registered between 2009 to 2020) and Cartographic and geographical information was obtained from the Instituto Geográfico Agustín Codazzi (SIGOT, http://www.sigotn.igac.gov.co/sigotn/). Malaria risk populations were estimated using population projections estimated from the national census conducted in 2005 obtained from the Departamento Administrativo Nacional de Estadística—DANE of Colombia (http://www.dane.gov.co/).
Malaria risk stratification
The analysis unit for malaria risk stratification was the municipality, and to characterize the type of malaria transmission in each municipality, the ecoepidemiological region previously identified in Colombia was used [17]. Additionally, to clarify the receptivity, the ecoepidemiological regions were described using environmental variables, such as altitude, precipitation, temperature, main ecotypes, and the presence of regional and local vectors. The presence of the main malaria vectors was determined by the ecoepidemiological regions because there are no records for all municipalities, especially those with low malaria transmission or nonactive focus. All the malaria cases reported to surveillance systems were revised to confirm origin (indigenous or imported) and parasite species. Mixed infections occurring with low frequency (less than 1% of malaria cases per year) were included as P. falciparum. P. malariae cases are scarce and were not included [29].
The malaria risk population was established by municipalities, taking into account that in Colombia, malaria transmission is primarily rural. All inhabitants estimated in projections of DANE in rural areas in municipalities located below 1,600 m a.s.l. were included; additionally, municipalities located in Pacific and Amazonas regions included urban populations.
Using the methodology proposed in the Manual of Stratification according to the Risk of Malaria and Elimination of Transmission Focuses—Americas Region [27], municipalities were classified according to receptivity (the ability of the ecosystem to allow the transmission of malaria) and vulnerability (the probability that malaria parasites will be imported). To identify the receptivity of municipalities, 1,600 m a. s. l. was used as a cutoff point, and this altitude was established as the maximum altitude that favors development of Plasmodium species in the malaria vectors and the environmental conditions for malaria transmission. Nonreceptive municipalities were those located above this altitude.
To assign a stratum to each municipality, PAHO recommendations for the classification of malaria risk strata were adapted according to the available information in malaria surveillance systems of Colombia, and the exercise was realized step by step, starting from the classification of stratum 1 (S1), followed by stratum 4 (S4, active foci) and stratum 3 (S3, nonactive and residual foci). Finally, the remaining municipalities were included in stratum 2 (receptive only):
Stratum 1 (S1): nonreceptive municipalities located above 1,600 m above sea level in which there is no proven risk of malaria transmission.
Stratum 2 (S2): receptive but not vulnerable municipalities without indigenous or imported cases from endemic areas or border endemic countries.
Stratum 3 (S3): receptive and vulnerable municipalities without indigenous cases in the last four years or residual foci with indigenous cases (≤ 200 cases per year) for at least five years in the last decade.
Stratum 4 (S4): receptive municipalities with indigenous cases from active foci.
Data analysis
All data were stored in a standard format in MS Excel (Microsoft, Redmond, USA) and were analyzed using Stata (release 15, Stata Corporation, College Station, TX, USA), while ArcGIS version 10.5 (ESRI, Redlands, CA) was used to produce maps. Each municipality categorized in the malaria transmission risk strata was verified to meet the criteria for each stratum.
Summary statistics were constructed for the entire dataset by developing absolute frequency measures, such as accumulated cases by region and municipality, territorial extensions (in km2) and the number of inhabitants at risk (confidence interval of projection). To establish the absolute risk of transmission by regions and municipalities in S4, relative frequency measures were constructed as percentages, the proportion of Plasmodium falciparum (PPf), and the median annual parasitic index (APIm) per 1,000 inhabitants in the study period. Classification of the intensity of transmission in S4 was performed from percentile (25th-75th) as follows: very high intensity substratum: APIm 148.1–38.5 x 1,000 inhabitants; high intensity substratum: APIm: 38.4–14.7 x 1000 inhabitants; medium intensity substratum: APIm 14.6–6.1 x 1000 inhabitants and low intensity substratum: APIm 6.0–0.1 x 1,000 inhabitants. Correlations between APIm and cumulative malaria cases were observed, and maps were developed separately for the strata, ecoepidemiological regions, and Anopheles species distributions.
Ethics statement
The present study met the ethical requirements established in Resolution 8430 of 1993 of the Ministry of Health of Colombia, Article 11, which establishes that studies, such as the present one, are risk-free and do not require approval by the Ethics Committee. Confidentiality and anonymity of the data were guaranteed.
Results
Ecoepidemiological region descriptions
In Colombia, malaria transmission primarily occurs between 0 and 1,600 m a.s.l. where the temperature fluctuates between 17 and 34°C and the relative humidity is not greater than 90%. From the natural regions of Colombia, the ecoepidemiological regions of malaria transmission were defined, however it was necessary to define the Uraba-Bajo Cauca-Sinu-San Jorge region considering that the transmission dynamics is different from the natural regions that surround it. The most intense transmission occurs in the Pacific and Uraba-Bajo Cauca-Sinu-San Jorge, where transmission occurs in ecotypes, such as mangroves, floodplains, tropical rainforests, and savannas (Table 1).
Table 1. Description of ecoepidemiological regions for malaria transmission in Colombia.
| Ecoepidemiological region | Environmental variables [15,30] | Climate classification Köppen-Geiger [31] | Ecotypes [32] | Main regional and local vector** [19,20,22,33–37] | |||
|---|---|---|---|---|---|---|---|
| Altitude range (m a.s.l.) | Precipitation (mm/year) | Temperature (°C) | Relative humidity (%) | ||||
| Pacific | 0–1,100 | 3,000–9,000 | 18–30 | 89 | Equatorial rainforest Equatorial monsoon |
Mangroves Coastal rainforest High rainforest Floodplains |
An. albimanus An. darlingi An. nuneztovari s.l. An. neivai s.l. An. calderoni |
| Uraba-Bajo Cauca-Sinú-San Jorge | 0–1,600 | 800–3,600 | 18–30 | 85 | Equatorial monsoon Equatorial savannah Equatorial rainforest |
Flood plains Savannas Rainforest |
An. nuneztovari s.l. An. albimanus An. darlingi |
| Amazonia | 80–400 | 3,000–4,500 | 17–32 | 85 | Equatorial rainforest | Rainforest Flood plains Savannas |
An. darlingi An. benarrochi B |
| Orinoco | 80–500 | 1,500–3,500 | 19–34 | 82 | Equatorial savannah Equatorial monsoon |
Piedmont Flood plains Savannas Gallery forest |
An. nuneztovari s.l. An. darlingi An. albitarsis s.l. |
| Caribbean*** | 0–865 | 500–2,000 | 20–34 | 80 | Equatorial savannah Desert climate |
Mangroves Tropical dry forest |
An. albimanus An. darlingi |
| Andean (Only Tropical region)* | 100–1,100 | 800–5,000 | 18–34 | 82 | Equatorial savannah Equatorial monsoon Equatorial rainforest |
Foothill Rainforest |
An. nuneztovari s.l. An. darlingi An. albimanus |
* Total Andean region 100 ≥ 4,100 m a.s.l.
** Natural infectivity with Plasmodium spp. in the last 15 years.
***Municipalities of the insular region were included in the Caribbean region.
Receptive and nonreceptive areas
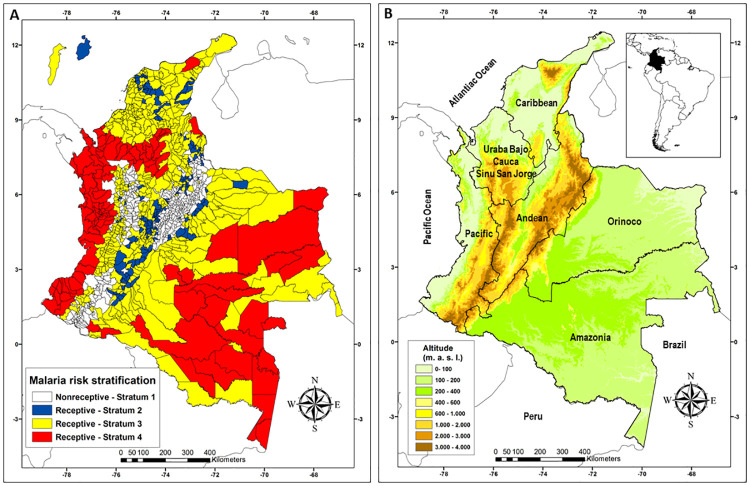
Receptive areas (S4, S3, and S2) with and without indigenous transmission of malaria included 749 municipalities, which accounted for 66.6% of the municipalities in the country (749/1,122). This area encompasses 1,043,003 km2, representing 91.2% of the national territory (Fig 1, Table 2). The at-risk population was approximately 9,734,271 (9,514,243–9,954,299) inhabitants (Table 3), and the cumulative malaria burden between 2010 and 2019 was 607,042 cases (Table 3). Strata with and without indigenous transmission but at risk of importation of the parasite (vulnerable), i.e., S4 and S3, respectively, had a territorial area of 990,712 km2, representing 86.7% of the national territory (Table 2). During the study period, 607,042 cases were reported in these strata that involved 583 municipalities (Table 3).
Fig 1. Risk strata of malaria transmission and ecoepidemiological regions in Colombia, 2010 to 2019.
A) Malaria Risk Strata and B) Eco-epidemiological regions and topography.
Table 2. Municipalities and area by malaria risk stratum and ecoepidemiological regions in Colombia.
| Region | S4 | S3 | S2 | S1 | ||||
|---|---|---|---|---|---|---|---|---|
| Number of municipalities | Area (km2) | Number of municipalities | Area (km2) | Number of municipalities | Area (km2) | Number of municipalities | Area (km2) | |
| Pacific | 43 | 78,226 | 66 | 26,547 | 0 | 0 | 70 | 26,782 |
| Uraba-Bajo Cauca Sinu San Jorge | 27 | 38,757 | 75 | 32,100 | 6 | 1,063 | 47 | 16,071 |
| Amazonia | 20 | 244,375 | 35 | 161,076 | 0 | 0 | 4 | 1,082 |
| Orinoco | 7 | 125,767 | 45 | 123,598 | 5 | 3,872 | 2 | 495 |
| Caribbean | 8 | 12,062 | 121 | 80,138 | 38 | 14,721 | 0 | 0 |
| Andean | 2 | 3,601 | 134 | 64,464 | 117 | 32,636 | 250 | 55,787 |
| 107 | 502,788 | 476 | 487,924 | 166 | 52,291 | 373 | 100,217 | |
Table 3. At-risk population and cumulative burden of malaria by stratum and ecoepidemiological region in Colombia from 2010 to 2019.
| Region | At-risk population | Cumulative cases | ||||
|---|---|---|---|---|---|---|
| S4 | S3 | S2 | S4 | S3 | S2* | |
| Pacific | 1,330,464 | 448,013 | 0 | 319,425 | 1,395 | 0 |
| Uraba-Bajo Cauca Sinu San Jorge | 657,453 | 1,098,113 | 27,092 | 184,760 | 7,072 | 0 |
| Amazonia | 373,887 | 638,079 | 0 | 44,682 | 1,837 | 0 |
| Orinoco | 113,523 | 1,038,168 | 15,514 | 8,357 | 2,155 | 0 |
| Caribbean | 113,347 | 1,609,216 | 178,665 | 18,804 | 4,481 | 0 |
| Andean | 46,170 | 1,389,198 | 657,369 | 10,728 | 3,346 | 0 |
| Total | 2,634,844 | 6,220,787 | 878,640 | 586,756 | 20,286 | 0 |
* Burden malaria cases S2 = 0 in all regions.
In Colombia, areas with indigenous transmission, S4, had a territorial area of 502,788 km2, which was 48.2% of the receptive area, including 107 municipalities (14.3% municipalities of the receptive area) (Table 2) and 2,634,844 (2,575,287–2,694,401) at-risk inhabitants (Table 3). In S4, the cumulative burden of disease between 2010 and 2019 was 586,756 cases, representing 96.7% of the cases registered in the country (Table 3).
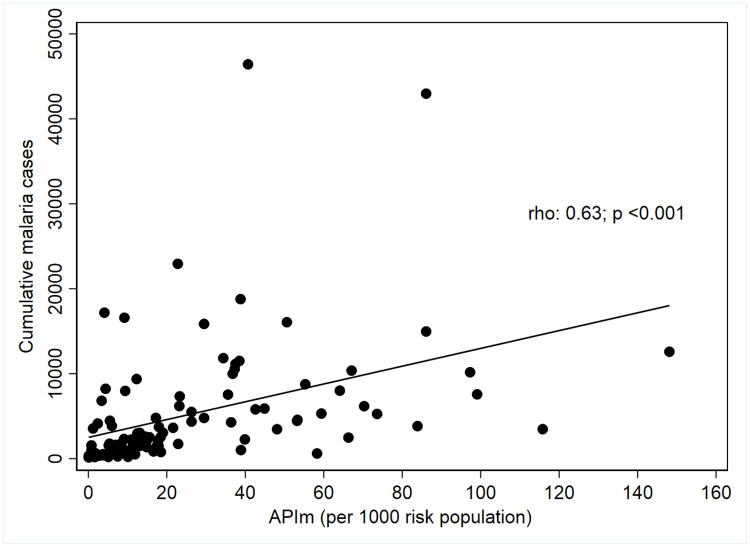
Using variables on the risk and intensity of malaria transmission, S4 was characterized, and the results provide evidence showing the heterogeneity in the municipalities by ecoepidemiological regions. The Uraba-Bajo Cauca-Sinu San Jorge and Pacific regions contribute 92.2% of malaria cases registered in the country, and the absolute risk was 23.2 and 22.4 per 1,000 inhabitants, respectively (Table 4). Furthermore, it was established that in Colombia, there is a statistically significant, moderate, and directly proportional linear relationship between the accumulative burden of malaria cases and the APIm (Rho = 0.63; p <0.001) (Fig 2).
Table 4. Cumulative cases, annual parasite index median (APIm), proportion of Plasmodium falciparum infections (FRIF), and malaria transmission intensity in S4, Colombia from 2010 to 2019.
| Region | Epidemiological variables | Proportion of municipalities by malaria transmission intensity* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cumulative cases 2010–2019 | Proportion cases by country | % variation 2010–2019** | APIm X 1,000 Inh. | PPf (%) | Number of municipalities | % Very high | % High | % Median | % Low | |
| Pacific | 550,450 | 53.7 | 35 (+) | 22.4 | 69 | 43 | 33 (14/43) | 42 (18/43) | 18 (8/43) | 7 (3/43) |
| Uraba-Bajo Cauca Sinu San Jorge | 393,956 | 38.5 | 76 (-) | 23.2 | 18 | 29 | 17 (5/29) | 28 (8/29) | 24 (7/29) | 31 (9/29) |
| Amazonia | 48,577 | 4.7 | 20 (-) | 14.7 | 16 | 20 | 20 (4/20) | 5 (1/20) | 35 (7/20) | 40 (8/20) |
| Orinoco | 4,462 | 0.4 | 18 (-) | 6.7 | 17 | 5 | 0 | 0 | 20 (1/5) | 80 (4/5) |
| Caribbean | 16,283 | 1.6 | 63 (-) | 16.0 | 19 | 8 | 25 (2/8) | 0 | 50 (4/8) | 25 (2/8) |
| Andean | 10,713 | 1.0 | 72 (+) | 20.8 | 3.3 | 2 | 50 (1/2) | 0 | 0 | 50 (1/2) |
| Total | 1,024,441 | 100.0 | 34.2 (-) | 17.8 | 43 | 107 | 24. 1 (26/107) | 25.3 (27/107) | 25.3 (27/107) | 25.3 (27/107) |
*Very high: APIm 148,1–38,5; high: APIm 38,4–14,7; Median: APIm 14,6–6,1; Low: APIm 6,0–0,1.
**(+) increase, (-) decrease.
Fig 2. Univariate correlations of the median annual parasite index (APIm) per 1000 inhabitants with cumulative malaria cases from 2010 to 2019 in Colombia.
In the Pacific region, a PPf of 69% was registered, in contrast to the values of the other regions, which did not exceed 19%, indicating a higher P. vivax infection prevalence. During the study, it was observed that four regions decreased the cumulative malaria cases by 18–76%, but in the Pacific and Andean regions, the increases were 35% and 72%, respectively (Table 4).
Regarding the malaria transmission intensity in the municipalities for each of the ecoepidemiological regions, it was observed that in the Pacific region, 75% belonged to very high and high categories, whereas in the Uraba Bajo Cauca Sinu San Jorge, 45% were between these categories, and 55% were municipalities with median or low intensity. In the Andean region, only two municipalities were classified in S4, one with high intensity and the other with low intensity (Table 4).
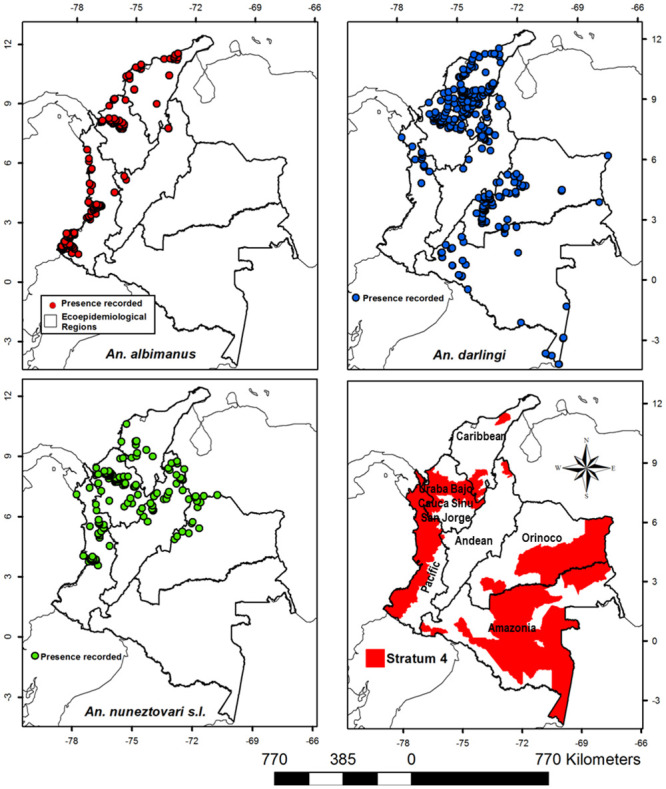
In S4, An. nuneztovari s.l., An. darlingi and An. albimanus, the primary malaria vectors in Colombia, were present. In the Uraba-Bajo Cauca-Sinu-San Jorge region, 46,000 km2 was a suitable habitat for An. nuneztovari s.l., 36,150 km2 was a suitable habitat for An. darlingi, and 35,200 km2 was suitable habitat for An. albimanus, indicating that over 80% of this region area had suitable environmental conditions for these species to sustain malaria transmission. In the Pacific region, the distribution of An. albimanus (55,660 km2), An. nuneztovari s.l., (28,560 km2), and An. darlingi (14,500 km2), showed that 80% of this region had habitat suitability to allow at least one of the primary vectors to be present. In S4 of the Orinoco and Amazonian regions, the main malaria vector was An. darlingi (Fig 3).
Fig 3. Distribution of Anopheles albimanus, An. darlingi, and An. nuneztovari s.l., the primary malaria vectors in Colombia by ecoepidemiological region and stratum 4.
S3 contained 476 municipalities (42.4%) with a territorial area of 487,924 km2 (46.8% of the receptive area) (Table 2) and an at-risk population of 6,220,787 (6,080,176–6,361,398) inhabitants (Table 3). During the study period, 20,286 imported malaria cases were reported in this stratum. In contrast, S2, which includes 166 receptive municipalities without a risk of the importation of parasites, had a territorial area of 52,292 km2 (Table 2) and an at-risk population of 878,640 (858,780–898,500) inhabitants (Table 3), which was the smallest of the strata. Finally, the nonreceptive area (S1) was located at an altitude greater than 1,600 m a.s.l. This area contained 373 municipalities (33.2%) and had a territorial area of 100,217 km2 (Table 2).
Strata distribution by ecoepidemiological regions
Pacific and Uraba-Bajo Cauca-Sinu-San Jorge ecoepidemiological regions accounted for 84.5% of the cases (512,652) registered in Colombia between 2010 and 2019. The major cumulative burden of malaria of these regions was registered in the receptive area with indigenous transmission (S4) and with 504,185 cases (98,3%) compared to the 8,467 cases (1,7%) in the receptive and vulnerable areas (S3) (Table 3). Malaria transmission was concentrated in 72 municipalities, 43 in the Pacific region, and 29 in the Uraba-Bajo Cauca-Sinu-San Jorge region (Table 2). The remaining 15.5% of the cases registered in the country were distributed in the other five ecoepidemiological regions: Amazonia, 7.7%, Caribbean, 3.8%, Andean, 2.3%, and Orinoco, 1.7% (Table 3).
In the Pacific region, S4 had an area of 78,226 km2 and an at-risk population of 1,330,464 (1,300,391–1,360,537) inhabitants, corresponding to a population density of 17 inhabitants per km2, distributed in 43 endemic municipalities (Tables 2 and 3). In the Uraba-Bajo Cauca-Sinu-San Jorge region, S4 had an area of 38,757 km2 and an at-risk population of 657,453 (642,592–672,314) inhabitants, corresponding to a population density of 16.7 inhabitants per km2 distributed in 29 endemic municipalities (Tables 2 and 3).
S3 consisted of 476 municipalities distributed in all ecoepidemiological regions and represented 42,7% of the national territory. This indicated that Colombia has a large receptive area with high vulnerability throughout the country (Table 2, Fig 1). Amazonas, Orinoco, and the Caribbean were the regions that most contributed to this stratum, with 364,812 km2 or 74.8% of their area (Table 2). S2 extended throughout 4.6% of the Colombian territory and was distributed among different ecoepidemiological malaria transmission regions; however, the Andean and Caribbean regions accounted for 90.6% of the total area classified as S2 (Table 2, Fig 1).
Discussion
In this study, four strata were established for the risk of malaria transmission in Colombia, confirming that there are areas where a greater burden of the disease is concentrated. This stratification exercise demonstrated that more than 90% of the Colombian territory has receptivity and vulnerability conditions that favor the maintenance of malaria transmission (1,043,003 km2). This territory includes areas of active and heterogeneous transmission with variable intensity. Despite the large extension, the greatest burden of the disease was concentrated in 107 (14%) municipalities of the receptive area, and the remaining 86% (642 municipalities) currently register low transmission (≤ 200 cases per year) or do not register autochthonous cases. However, they present a high vulnerability given that they present environmental conditions and the presence of vector malaria that would favor the occurrence of cases as a consequence of the importation of cases from sources of active transmission. Additionally, the use of the two approaches to characterize transmission in S4, burden malaria cases and API made it possible to identify the municipalities with a higher burden of the disease within the epidemiological regions, and with the API, the absolute risk was established in each ecoregion. The two approaches are complementary because they allow evidence of heterogeneity within regions and between regions. This malaria transmission pattern has been observed in regions with moderate to low transmission intensities in the Americas, as described in the state of Bolívar (Venezuela) and the Amazonian region (Brazil) [38–40].
In Colombia, the receptive areas S4, S3, and S2 are predominantly rural, and they are continually exposed to environmental changes caused by human influences. These changes are associated principally with deforestation, intensification of mining activities, and illicit crops that have contributed substantially to modifying the distribution and incidence of malaria [29,41,42]. Additionally, the high mobility of the population, immigration from endemic border countries, susceptibility of the human population to infection, and access to health services favor an increase in vulnerability in several risk strata of malaria transmission, especially S3, which is receptive and has a high risk of importing parasites from endemic areas [3,5,17].
Another important characteristic of the receptive area is the presence of several ecotypes where there is high mobility of the population and increased vulnerability, which explains the diversity of the epidemiological pattern of malaria transmission in Colombia. These epidemiological patterns can be explained using the ecoregional approach proposed by Rubio-Palis and Zimmerman [43], who identified five ecoregions using malaria vector distributions and environmental variables: coastal, piedmont, savanna, interior lowland forest and high valley [5,17,43,44].
The malaria risk stratification showed that S4 (presence of indigenous cases) occurs on the coast, floodplains, alluvium, rainforest and in the western Andean piedmont in the Pacific regions and savannas, valleys and floodplains in the Uraba-Bajo Cauca-Sinu-San Jorge region. The main malaria vectors, An. albimanus, An. nuneztovari s.l. and An. darlingi, can be sympatric in many of these regions [20,33,34,45,46]; however, the ecotype diversity in S4 favors the occurrence of local vectors such as An. neivai s.l. along the coast of the Pacific region [35,47], and An. calderoni in the southwestern area of the same region (coastal ecoregion) [22,33,37], and this diversity contributes to sustaining malaria transmission. In the Orinoco and Amazonian regions, S4 corresponds to the interior lowland forest ecoregion, savannas, floodplain and, rainforest and the main malaria vector is An. darlingi [19,48,49]. Furthermore, recent studies have identified An. benarrochi B as a local malaria vector in S4 in southwestern Amazonia (Andean Piedmont, Putumayo department) [21].
S3 (receptive and vulnerable areas) is present in coastal, piedmont, savanna, and interior lowland forest ecoregions [43]. The coastal ecoregions contain the Caribbean and the northern part of the Uraba-Bajo Cauca-Alto San Jorge-Sinu ecoepidemiological regions, and in these areas, An. albimanus and An. darlingi are the main vectors that contribute to malaria transmission [19,22,34]. The Piedmont ecoregion includes Eastern Andean municipalities located in the lowlands of the Inter-Andean valleys where the main malaria vector is An. nuneztovari s.l. [50,51], although An. pholidotus has also been incriminated in the southeast of this region as a local malaria vector [52]. On the other hand, S3 is found in the Orinoco and Amazonian regions, which include savanna, rainforest and interior lowland forest ecoregions. In the savanna, located north of the Orinoco region, the main vector is An. nuneztovari s.l., and An. darlingi is the main malaria vector in the remaining municipalities of these regions [19,36,53].
The stratification and description of S4 showed that there are few municipalities with a predominance of P. falciparum transmission that are located in the Pacific region. This is an opportunity to prioritize these areas to implement strategies to start eliminating transmission of this parasite in Colombia [54]. On the other hand, municipalities with P. vivax transmission are distributed throughout ecoepidemiological regions, and the elimination of this parasite species represents a challenge to malaria elimination due to the presence of mature gametocytes at an early stage of infection, resulting in greater transmissibility and relapse by the activation of dormant hypnozoites [55–58].
The major limitation of this study was information bias in the data and secondary sources.
In the surveillance system, only cases that come to official health services for a diagnosis of malaria are reported, and there is little information on the entities that perform diagnoses outside the system, which could lead to under registration. Furthermore, it is likely that in some registries, there is a misclassification of indigenous and imported cases. To control this bias, the data were cleaned to improve analysis efficiency and ensure correct municipality strata classification according to the criteria defined in the study. Due to available information on malaria cases by municipalities, it was necessary to adjust the strata criteria, especially for S3, because the information did not allow us to clearly define the conditions of active and nonactive residual foci when the municipalities had a low burden of disease. Another limitation was that malaria vector information to confirm receptivity in low malaria transmission municipalities or currently residual nonactive foci was scarce.
This study is the first malaria risk stratification exercise in Colombia that follows the methodology proposed by PAHO adjusted to the current situation of malaria transmission in the country [27]. Although it was only possible to obtain general information up to down to third-level administrative division (municipalities), this allowed us to classify all municipalities in their corresponding strata and select priority municipalities that should be included in the strategic plans for the elimination of malaria. In these municipalities, it is necessary to continue conducting microstratification exercises for the design and implementation of sustainable operational plans to provide solid evidence for the appropriate selection of specific, cost-effective, and sustainable interventions for developing plans to eliminate and prevent the re-establishment of malaria transmission in Colombia. In these areas there is also the presence of other prevalent infectious diseases [59], for which health policies must be implemented that address as many of the social determinants of health as possible in order to contain social costs [60]. Knowledge of the different potential patterns of transmission of the disease in the strata with a predominance of P. falciparum facilitates the definition of the specific goals, objectives and targets according to the priorities established in the elimination plans. However, continuously updating the malaria risk strata is required due to the presence of municipalities where it is mandatory to regularly characterize the transmission dynamics to confirm the presence of nonactive residual foci and register imported cases to implement surveillance actions and prevent reintroduction.
Conclusions
Ninety-one percent of the Colombian territory is receptive and vulnerable to malaria transmission. The transmission risk was heterogeneous and classified into four strata adapted to local transmission dynamics from indigenous transmission strata to nonreceptive strata without indigenous cases and no vulnerability. It was also established that the transmission of P. falciparum mainly occurs in Pacific regions, and these areas could be considered a starting point to implement malaria elimination plans in Colombia.
Acknowledgments
The authors would like to thank the knowledge management, research and innovation network in malaria of the Ministry of Health and Social Protection and the National Institute of Health.
Data Availability
Research data contains sensitive information and the protection of sensitive data is required for legal reasons. This data are available from the Intellectual Property Committee of the National Institute of Health (contact through Maritza Ordoñez mordonezm@ins.gov.co) for researchers who meet the criteria for access to confidential data.
Funding Statement
This study was supported by the National Institute of Health of Colombia. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. World malaria report 2019. Geneva, Switzerland; 2019. https://www.who.int/publications/i/item/world-malaria-report-2019.
- 2.Organización Panamericana de la Salud. Actualización epidemiológica malaria en las Américas. Washington DC; 2019. https://iris.paho.org/bitstream/handle/10665.2/51849/EpiUpdate18November2019_spa.pdf?sequence2&isAllowed=y.
- 3.Beales P, Goriup S, Litsios S, Molineaux L, Onori E, Pull J. The planning of malaria control. In: Wernsdorfer W.H., Malaria. McGregor I (Eds), editor. Churchill Livingstone, Edinburgh; 1988. [Google Scholar]
- 4.Bousema T, Griffin JT, Sauerwein RW, Smith DL, Churcher TS, Takken W, et al. Hitting hotspots: spatial targeting of malaria for control and elimination. PLoS Med. 2012;9: e1001165. 10.1371/journal.pmed.1001165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schapira A, Boutsika K. Malaria Ecotypes and Stratification. Adv Parasitol. 2012;78: 97–167. 10.1016/B978-0-12-394303-3.00001-3 [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Meeting report of the WHO Evidence Review Group on the assessment of malariogenic potential to inform elimination strategies and plans to prevent re-establishment of malaria. Geneva, Switzerland; 2018. https://www.who.int/malaria/mpac/mpac-april2019-session7-report-erg-malariogenic-potential.pdf?ua=1.
- 7.Snow RW, Gilles HM. The epidemiology of malaria. 4th ed. In: Warrel DA, Gilles HM, editors. Essential Malariology. 4th ed. London: CRC Press; 2002. pp. 85–90. [Google Scholar]
- 8.Organización Panamericana de la Salud. Estratificación epidemiológica de la malaria en la Región de las Américas. 1991 [cited 10 Jun 2020]. https://iris.paho.org/handle/10665.2/38971.
- 9.Najera J, Liese B, Hammer J. Malaria: new patterns and perspectives. 1992 [cited 10 Jun 2020]. http://documents.worldbank.org/curated/en/933151468740676854/Malaria-new-patterns-and-perspectives.
- 10.Grillet M, Martínez J, Barrera R. Focos calientes de transmisión de malaria: Implicaciones para un control orientado y efectivo en Venezuela. Bol Mal Salud Amb. 2009;49: 193–207. [Google Scholar]
- 11.Carter R, Mendis KN, Roberts D. Spatial targeting of interventions against malaria. Bull World Health Organ. 2000;78: 1401–1411. [PMC free article] [PubMed] [Google Scholar]
- 12.de Castro MC, Monte-Mór RL, Sawyer DO, Singer BH. Malaria risk on the Amazon frontier. Proc Natl Acad Sci U S A. 2006;103: 2452–2457. 10.1073/pnas.0510576103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosas-Aguirre A, Ponce OJ, Carrasco-Escobar G, Speybroeck N, Contreras-Mancilla J, Gamboa D, et al. Plasmodium vivax malaria at households: spatial clustering and risk factors in a low endemicity urban area of the northwestern Peruvian coast. Malar J. 2015;14: 176. 10.1186/s12936-015-0670-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Organización Panamericana de la Salud. Situación de los programas de malaria en las Américas: XXIX informe. Washington DC; 1981. https://iris.paho.org/handle/10665.2/26493.
- 15.Pabón J, Zea J, León G, Montealegre J, Hurtado G, González O. El Medio Ambiente en Colombia. La atmósfera, el tiempo y el clima. In: Leyva, P., Ed., El medio ambiente en Colombia, Bogotá IDEAM; 1998, pp. 35–91.
- 16.Brower V. Vector-borne diseases and global warming: are both on an upward swing? Scientists are still debating whether global warming will lead to a further spread of mosquitoes and the diseases they transmit. EMBO Rep. 2001;2: 755–757. 10.1093/embo-reports/kve193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Padilla JC, Álvarez G, Montoya R, Chaparro P, Herrera S. Epidemiology and control of malaria in Colombia. Mem Inst Oswaldo Cruz. 2011;106: 114–122. 10.1590/s0074-02762011000900015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Instituto Nacional de Salud. Informe evento malaria. Bogotá, Colombia; 2019. https://www.ins.gov.co/buscador-eventos/Informesdeevento/MALARIASEMESTREI2019.pdf.
- 19.Olano VA, Brochero HL, Sáenz R, Quiñones ML, Molina JA. Mapas preliminares de la distribución de especies de Anopheles vectores de malaria en Colombia. Biomédica. 2001;21: 402–408. [Google Scholar]
- 20.Montoya-Lerma J, Solarte YA, Giraldo-Calderón GI, Quiñones ML, Ruiz-López F, Wilkerson RC, et al. Malaria vector species in Colombia: a review. Mem Inst Oswaldo Cruz. 2011;106: 223–238. 10.1590/s0074-02762011000900028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orjuela LI, Herrera M, Erazo H, Quiñones ML. Especies de Anopheles presentes en el departamento del Putumayo y su infección natural con Plasmodium. Biomédica. 2013;33: 42–52. 10.1590/S0120-41572013000100006 [DOI] [PubMed] [Google Scholar]
- 22.Naranjo-Díaz N, Altamiranda M, Luckhart S, Conn JE, Correa MM. Malaria Vectors in Ecologically Heterogeneous Localities of the Colombian Pacific Region. PLoS One. 2014;9: e103769. 10.1371/journal.pone.0103769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Global Technical Strategy for Malaria 2016–2030. Geneva, Switzerland; 2015. https://www.who.int/malaria/publications/atoz/9789241564991/en/.
- 24.Ministerio de Salud y Protección Social. Plan estrategico nacional de malaria en Colombia, 2019–2022. Bogotá, Colombia; 2020. https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/VS/PP/PAI/plan-estrategico-malaria.pdf.
- 25.DANE. Censo Nacional de Población y Vivienda 2018 Colombia. 2018. https://www.dane.gov.co/index.php/estadisticas-por-tema/demografia-y-poblacion/censo-nacional-de-poblacion-y-vivenda-2018.
- 26.Instituto Geográfico Agustin Codazzi (IGAC). Fronteras y Límites de Entidades Territoriales. 2018, pp. 1. https://www.igac.gov.co/es/contenido/areas-estrategicas/fronteras-y-limites-de-entidades-territoriales.
- 27.Organización Panamericana de la Salud. Manual de estratificación según el riesgo de malaria y eliminacion de focos de transmisión: región de las Américas (Draft). Washington DC; 2019. https://www.paho.org/hq/index.php?optioncom_docman&view=download&slug=malaria-technical-advisory-group-session-8-2019-only-in-spanish&Itemid=270&lang=en.
- 28.Instituto Nacional de Salud. Protocolo de Vigilancia en Salud Pública. 2017. https://www.ins.gov.co/buscador-eventos/Lineamientos/PROMalaria.pdf.
- 29.Instituto Nacional de Salud. Boletín epidemiológico semanal, semana 52 de 2019. 2019 [cited 10 Jun 2020]. http://www.ins.gov.co/buscador-eventos/BoletinEpidemiologico/2019_Boletin_epidemiologico_semana_52.pdf.
- 30.Rangel-Ch J, Aguilar-P M. Una aproximación sobre la diversidad climática en la Regiones Naturales de Colombia. In: Colombia Diversidad Biótica I. Instituto de Ciencias Naturales-Universidad Nacional de Colombia-Inderena, Bogotá. 1995, pp. 25–77. https://issuu.com/diversidadbiotica/docs/dbi.-cap2.diversidad-climatica.
- 31.Kottek M, Grieser J, Beck C, Rudolf B, Rubel F. World map of the Köppen-Geiger climate classification updated. Meteorol Z. 2006;15: 259–263. [Google Scholar]
- 32.Rangel-Ch J, Lowy-C P, Aguilar-P M. Distribucion de los tipos de vegetacion en las regiones naturales de Colombia. In: Colombia Diversidad Biótica II. Tipos de Vegetación en Colombia. 1997, pp. 383–402. https://issuu.com/diversidadbiotica/docs/dbiicap4.distribuci_n_vegetaci_n.
- 33.Ahumada ML, Orjuela LI, Pareja PX, Conde M, Cabarcas DM, Cubillos EFG, et al. Spatial distributions of Anopheles species in relation to malaria incidence at 70 localities in the highly endemic Northwest and South Pacific coast regions of Colombia. Malar J. 2016;15: 407. 10.1186/s12936-016-1421-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gutiérrez LA, González JJ, Gómez GF, Castro MI, Rosero DA, Luckhart S, et al. Species composition and natural infectivity of anthropophilic Anopheles (Diptera: Culicidae) in the states of Córdoba and Antioquia, Northwestern Colombia. Mem Inst Oswaldo Cruz. 2009;104: 1117–1124. 10.1590/s0074-02762009000800008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Escovar JE, Gonzalez R, Quinones ML. Anthropophilic biting behaviour of Anopheles (Kerteszia) neivai Howard, Dyar & Knab associated with Fishermen’s activities in a malaria-endemic area in the Colombian Pacific. Mem Inst Oswaldo Cruz. 2013;108: 1057–1064. 10.1590/0074-0276130256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiménez P, Conn JE, Wirtz R, Brochero H. Anopheles (Díptera: Culicidae) malaria vectors in the municipality of Puerto Carreno, Vichada, Colombia. Biomédica. 2012;32: 13–21. 10.1590/S0120-41572012000500003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orjuela LI, Ahumada ML, Avila I, Herrera S, Beier JC, Quiñones ML. Human biting activity, spatial–temporal distribution and malaria vector role of Anopheles calderoni in the southwest of Colombia. Malar J. 2015;14: 256. 10.1186/s12936-015-0764-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woolhouse ME, Dye C, Etard JF, Smith T, Charlwood JD, Garnett GP, et al. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc Natl Acad Sci U S A. 1997;94: 338–342. 10.1073/pnas.94.1.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sociedad Venezolana de Salud Pública. Análisis del reporte mundial de malaria 2018, y la grave epidemia de malaria en Venezuela. 2018 [cited 10 Jun 2020]. http://icaso.org/wp-content/uploads/2018/11/Anaělisis-del-reporte-mundial-de-malaria-2018-y-la-grave-epidemia-de-malaria-en-Venezuela.pdf.
- 40.Organización Panamericana de la Salud. Actualización epidemiológica. Aumento de malaria en las Américas 2018. 2018 [cited 10 Jun 2020]. https://www.paho.org/hq/index.php?option=com_docman&view=download&category_slug=2018-9582&alias=43437-30-enero-2018-malaria-actualizacion-epidemiologica-437&Itemid=270&lang=en.
- 41.Instituto Nacional de Salud. Boletín epidemiológico semanal, semana 52 de 2017. Bogotá DC; 2017. http://www.ins.gov.co/buscador-eventos/BoletinEpidemiologico/2017Boletínepidemiológicosemana52.pdf.
- 42.Instituto Nacional de Salud. Boletín epidemiológico semanal, semana 52 de 2018. 2018 [cited 10 Jun 2020]. http://www.ins.gov.co/buscador-eventos/BoletinEpidemiologico/2018Boletínepidemiológicosemana52.pdf.
- 43.Rubio-Palis Y, Zimmerman RH. Ecoregional Classification of Malaria Vectors in the Neotropics. J Med Entomol. 1997;34: 499–510. 10.1093/jmedent/34.5.499 [DOI] [PubMed] [Google Scholar]
- 44.Martens WJ, Niessen LW, Rotmans J, Jetten TH, McMichael AJ. Potential impact of global climate change on malaria risk. Environ Health Perspect. 1995;103: 458–464. 10.1289/ehp.95103458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naranjo-Diaz N, Rosero DA, Rua-Uribe G, Luckhart S, Correa MM. Abundance, behavior and entomological inoculation rates of anthropophilic anophelines from a primary Colombian malaria endemic area. Parasit Vectors. 2013;6: 61. 10.1186/1756-3305-6-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Espinosa‐Vélez Y, Altamiranda‐Saavedra M, Correa MM. Potential distribution of main malaria vector species in the endemic Colombian Pacific region. Trop Med Int Heal. 2020;25:861–873. 10.1111/tmi.13399 [DOI] [PubMed] [Google Scholar]
- 47.Gutiérrez LA, Naranjo N, Jaramillo LM, Muskus C, Luckhart S, Conn JE, et al. Natural infectivity of Anopheles species from the Pacific and Atlantic Regions of Colombia. Acta Trop. 2008;107: 99–105. 10.1016/j.actatropica.2008.04.019 [DOI] [PubMed] [Google Scholar]
- 48.Jiménez IP, Conn JE, Brochero H. Preliminary Biological Studies on Larvae and Adult Anopheles Mosquitoes (Diptera: Culicidae) in Miraflores, a Malaria Endemic Locality in Guaviare Department, Amazonian Colombia. J Med Entomol. 2014;51: 1002–1009. 10.1603/me13146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiménez IP, Conn JE, Brochero H. Malaria Vectors in San José del Guaviare, Orinoquia, Colombia. J Am Mosq Control Assoc. 2014;30: 91–98. 10.2987/13-6382.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brochero H, Pareja PX, Ortiz G, Olano VA. Sitios de cría y actividad de picadura de especies de anopheles en el municipio de Cimitarra, Santander, Colombia. Biomédica. 2006;26: 269–277. [PubMed] [Google Scholar]
- 51.Rondón S, León C, Link A, González C. Prevalence of Plasmodium parasites in non-human primates and mosquitoes in areas with different degrees of fragmentation in Colombia. Malar J. 2019;18: 276. 10.1186/s12936-019-2910-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Escovar JE, González R, Quiñones ML, Wilkerson RC, Ruiz F, Harrison BA. Morphology of the larvae, male genitalia and DNA sequences of Anopheles (Kerteszia) pholidotus (Diptera: Culicidae) from Colombia. Mem Inst Oswaldo Cruz. 2014;109: 473–479. 10.1590/0074-0276130596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brochero HL, Rey G, Buitrago LS, Olano VA. Biting activity and breeding sites of Anopheles species in the municipality Villavicencio, Meta, Colombia. J Am Mosq Control Assoc. 2005;21: 182–186. 10.2987/8756-971X(2005)21[182:BAABSO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 54.Olivera MJ, Guerra AP, Cortes LJ, Horth RZ, Padilla J, Novoa J, et al. Artemether-Lumefantrine Efficacy for the Treatment of Uncomplicated Plasmodium falciparum Malaria in Choco, Colombia after 8 Years as First-Line Treatment. Am J Trop Med Hyg. 2020;102: 1056–1063. 10.4269/ajtmh.19-0954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wells TNC, Burrows JN, Baird JK. Targeting the hypnozoite reservoir of Plasmodium vivax: the hidden obstacle to malaria elimination. Trends Parasitol. 2010;26: 145–151. 10.1016/j.pt.2009.12.005 [DOI] [PubMed] [Google Scholar]
- 56.World Health Organization. Control and elimination of Plasmodium vivax malaria–A technical brief. Geneva, Switzerland; 2015. https://www.who.int/malaria/publications/atoz/9789241509244/en/.
- 57.Padilla-Rodríguez JC, Olivera MJ, Guevara-García BD. Parasite density in severe malaria in Colombia. PLoS One. 2020;15: e0235119. 10.1371/journal.pone.0235119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodríguez JAI, Rodríguez SNI, Olivera MJ. Plasmodium vivax malaria across South America: management guidelines and their quality assessment. Rev Soc Bras Med Trop. 2020;53: e20200179. 10.1590/0037-8682-0179-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olivera MJ, Fory JA, Porras JF, Buitrago G. Prevalence of Chagas disease in Colombia: A systematic review and meta-analysis. PLoS One. 2019;14: e0210156. 10.1371/journal.pone.0210156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olivera MJ, Buitrago G. Economic costs of Chagas disease in Colombia in 2017: A social perspective. Int J Infect Dis. 2020;91: 196–201. 10.1016/j.ijid.2019.11.022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data contains sensitive information and the protection of sensitive data is required for legal reasons. This data are available from the Intellectual Property Committee of the National Institute of Health (contact through Maritza Ordoñez mordonezm@ins.gov.co) for researchers who meet the criteria for access to confidential data.