Abstract
Introduction
The detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA by quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) is the standard method for the diagnosis of coronavirus disease 2019 (COVID-19). This PCR test can be positive even in patients who have recovered from the disease, and the duration for achieving viral clearance has not been clarified yet.
Methods
This study was conducted between April 3, 2020, and June 17, 2020, at the Toyama University Hospital and the Toyama Rehabilitation Home. We collected the data of patients with COVID-19, analyzing the duration until twice-consecutive negative qRT-PCR test.
Results
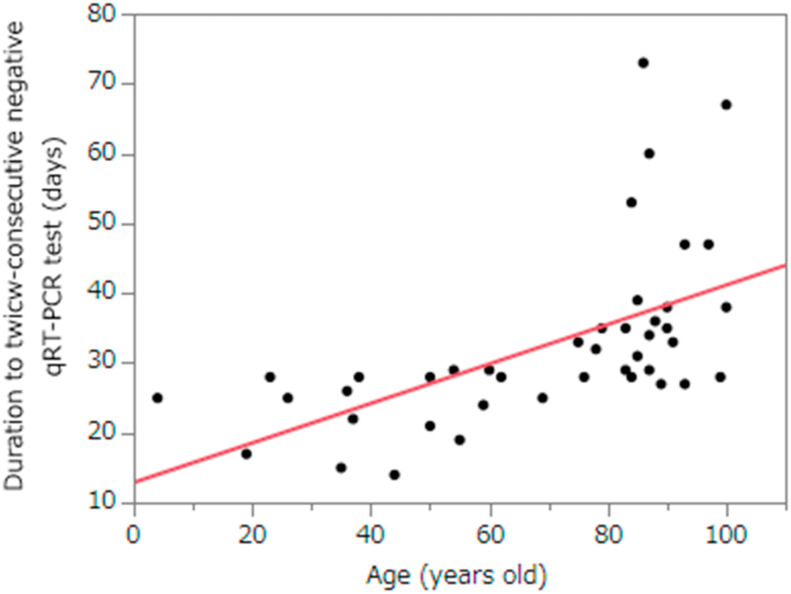
A total of 42 patients were enrolled. The median duration of the twice-consecutive negative qRT-PCR test was 29.0 d (interquartile range: 25.75–35.25). The longest duration of viral shedding was 73 d. The duration of viral clearance was significantly longer in the older (>65 years) group than in the younger group (34.5 d vs. 25.0 d, P < 0.0001).
Conclusion
This study demonstrated that viral clearance tends to be sustained in the older adults.
Keywords: COVID-19, qRT-PCR, Viral clearance
The coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first reported in Wuhan, China, in December 2019 [1]. It has dramatically spread worldwide in 2020. The detection of SARS-CoV-2 by quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) testing of respiratory specimens has been used for diagnosis [2]. However, qRT-PCR tests may continue being positive even after patients are cured of COVID-19. Although a negative qRT-PCR test has been the condition for permission to discharge patients hospitalized due to COVID-19, data on viral clearance in patients recovering from the disease are limited.
We carried out a retrospective study to identify the role of viral clearance in the hospitalized population by measuring the qRT-PCR-positive duration. All patients were hospitalized either at the Toyama University Hospital, which is a 612-bed tertiary hospital in Toyama city, Toyama, Japan, or at the Toyama Rehabilitation Home, which is a 79-bed nursing home located in central Toyama city, Toyama, Japan. They were diagnosed with COVID-19 through qRT-PCR between April 3, 2020, and June 17, 2020. All samples were taken from the nasopharynx using swabs and sent to the Department of Microbiology, Graduate School of Medicine and Pharmaceutical Sciences, University of Toyama, or the Toyama Institute of Health for qRT-PCR assays.
The data collected from the medical records included age, sex, disease severity, medication, outcome, and the duration from presenting symptoms to twice-consecutive negative qRT-PCR test. Disease severity classified mild, moderate, and severe. Mild group did not receive supplemental oxygen therapy, and moderate group received supplemental oxygen therapy. Severe group received mechanical ventilation or extracorporeal membrane oxygenation. Continuous variables were represented as the mean and standard deviation (SD) or as the median and interquartile range (IQR). Continuous data were analyzed using the Wilcoxon rank-sum test. The threshold for statistical significance was set at P < 0.05.
Data were analyzed using JMP Pro version 15.0.0 software (SAS Institute Inc., Cary, NC, USA). This study was approved by the Ethics Committee of the Toyama University Hospital (No. R2020083).
Forty-two patients were included in the study. The median age was 81.0 years (IQR: 50.0–88.3). The proportion of men and women recruited in this study was 14 (33.3%) and 28 (66.7%), respectively. There were 26 patients (61.9%) over 65 years of age and 16 patients (38.1%) under the age of 65. A total of 12 patients was transferred other hospitals after diagnosis. Disease severity was 18 patients (60.0%) with mild illness, 11 patients (36.7%) with moderate illness, and 1 patient (3.3%) with severe illness. Nineteen patients (63.3%) did not receive medical treatment. All patient recovered from COVID-19 and they survived (Table 1 ). The median duration of the twice-consecutive negative qRT-PCR test was 29.0 d (IQR: 25.75–35.25). The longest duration of viral shedding was 73 d. The duration of viral clearance was significantly longer in the older age (>65 years) group than in the younger group (34.5 d vs. 25.0 d, P < 0.0001) (Fig. 1 ).
Table 1.
Characteristics of patients diagnosed COVID-19 and clinical course.
| Total (n = 42) |
|
|---|---|
| NO. (%) | |
| Age | |
| <65 yr | 16 (38.1) |
| ≥65 yr | 26 (61.9) |
| Sex | |
| Men | 14 (33.3) |
| Women | 28 (66.7) |
| Severity (n = 30) ∗ | |
| Mild | 18 (60.0) |
| Moderate | 11 (36.7) |
| Severe | 1 (3.3) |
| Medication (n = 30) ∗ | |
| No medication | 19 (63.3) |
| Favipiravir | 11 (36.7) |
| Remdesivir | 0 (0.0) |
| Dexamethasone | 0 (0.0) |
| Outcome (n = 30) ∗ | |
| Survival | 30 (100.0) |
| Non-survival | 0 (0.0) |
∗Excepted the patients who had been transported other hospitals because their clinical course was unknown.
Fig. 1.
Relationship between the duration to twice-consecutive negative qRT-PCR test and age.
qRT-PCR: quantitative real-time reverse transcription polymerase chain reaction.
The relationship between virus detection by PCR and infectivity is not entirely clear. qRT-PCR may also detect non-infectious fragments of the viral RNA. Singanayagam et al. reported that culture-positive virus was hardly detected within 10 d after symptom onset [3]. Therefore, it is recommended by World Health Organization that symptomatic patients are released from isolation 10 d after symptom onset, plus at least an additional 3 d without symptoms [4]. In Japan, discharge criteria for symptomatic patients are after 10 d symptom onset and 72 h from symptom resolution, or negative two PCR tests at intervals of at least 24 h after 24 h of symptom resolution [5].
At the time of admission, two consecutive negative PCR tests, at least 24 h apart, were required to discharge the patient. As we found in our study, older aged patients can be PCR-positive even after healing. In some patients older than 80 years, it took more than 40 d to become negative PCR tests. These patients were not immunocompromised status but needed assistance for daily living and had diseases related aging such as dementia, hypertension, and osteoporosis. Hattori et al. showed that older age was associated with prolonged viral clearance of SARS-CoV-2 [6]. Rydynski et al. reported that the elderly COVID-19 patients have reduced naïve T cell and incoordination of immune response against SARS-CoV-2 [7]. This may be the reason for persistent viral elimination in the elderly. For the older aged patients, viral detection by qRT-PCR is sustained, resulting in prolonged hospitalization, promoting frailty in this population. In addition, some rehabilitation facilities or nursing home require the result of negative-PCR test at the time of transfer, even though the patient meets the criteria for discharge, which inhibits the recovery of COVID-19 patients and interferes with their quality of life because they cannot be rehabilitated or moved to a facility where they are accustomed to living. We suggest complying with the criteria for discharge regardless of the PCR results in patients recovered from COVID-19.
There are limitations to the small sample size and measurement bias of obtaining samples. Moreover, patients who were in an outbreak institution were included in the study.
Ethical approvase
This study was approved by the Ethics Committee of the Toyama University Hospital (No. R2020083).
Funding source
This study was supported by the Research Program on Emerging and Re-emerging Infectious Diseases from AMED Grant No. JP20he0622035.
ICMJE statement
All authors meet the ICMJE authorship criteria.
Author contributions
AU, HK, Y Miyajima, YF, IS, MS and SY contributed to data acquisition, patient care, analyzed data, and drafted the manuscript. Y Morinaga and KO contributed to the PCR assay. IS and YY were responsible for the medical care of the participants. YY was responsible for the overall organization of this study. All authors contributed to the final manuscript.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgments
We would like to thank all the hospital staff supporting this study.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Diagnostic testing for SARS-CoV-2 interim guidance 11 September 2020. https://www.who.int/publications/i/item/diagnostic-testing-for-sars-cov-212
- 3.Singanayagam A., Patel M., Charlett A., Lopez Bernall J., Saliba V., Ellis J., et al. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917. Pubmed: 2001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization Criteria for releasing COVID-19 patients from isolation Scientific. 2020. https://www.who.int/publications/i/item/criteria-for-releasing-covid-19-patients-from-isolation Brief.
- 5.Hattori T., Amishima M., Morinaga D., Kamada K., Nakakubo S., Yamashita Y., et al. Older age is associated with sustained detection of SARS-CoV-2 in nasopharyngeal swab samples. Journal of Infecton. 2020;S0163–4453:30428–X. doi: 10.1016/j.jinf.2020.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rydynski M.C., Ramirez S.I., Dan J.M., Grifoni A., Hastie K.M., Weiskopf D., et al. Antigen-specific adaptive immunity to SRS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183(4):996–1012. doi: 10.1016/j.cell.2020.09.038. Nov 12. e19, Epub 2020 Sep. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]