Abstract
Background
We examined the safety and efficacy of a treatment protocol containing Favipiravir for the treatment of SARS-CoV-2.
Methods
We did a multicenter randomized open-labeled clinical trial on moderate to severe cases infections of SARS-CoV-2. Patients with typical ground glass appearance on chest computerized tomography scan (CT scan) and oxygen saturation (SpO2) of less than 93% were enrolled. They were randomly allocated into Favipiravir (1.6 gr loading, 1.8 gr daily) and Lopinavir/Ritonavir (800/200 mg daily) treatment regimens in addition to standard care. In-hospital mortality, ICU admission, intubation, time to clinical recovery, changes in daily SpO2 after 5 min discontinuation of supplemental oxygen, and length of hospital stay were quantified and compared in the two groups.
Results
380 patients were randomly allocated into Favipiravir (1 9 3) and Lopinavir/Ritonavir (1 8 7) groups in 13 centers. The number of deaths, intubations, and ICU admissions were not significantly different (26, 27, 31 and 21, 17, 25 respectively). Mean hospital stay was also not different (7.9 days [SD = 6] in the Favipiravir and 8.1 [SD = 6.5] days in Lopinavir/Ritonavir groups) (p = 0.61). Time to clinical recovery in the Favipiravir group was similar to Lopinavir/Ritonavir group (HR = 0.94, 95% CI 0.75 – 1.17) and likewise the changes in the daily SpO2 after discontinuation of supplemental oxygen (p = 0.46)
Conclusion
Adding Favipiravir to the treatment protocol did not reduce the number of ICU admissions or intubations or In-hospital mortality compared to Lopinavir/Ritonavir regimen. It also did not shorten time to clinical recovery and length of hospital stay.
Keywords: Covid19, Favipiravir, SARS-CoV-2, Lopinavir, Ritonavir, Hydroxychloroquine, Clinical trial
1. Introduction
In the SARS-CoV-2 pandemic, the search for effective antiviral therapy resulted in trying existing antiviral drugs used previously for the treatment of many RNA virus-mediated diseases such as SARS, MERS, AIDS, and Ebola [1], [2], [3].
Favipiravir (Avigan) chemically known as 6-fluoro-3-hydroxy-2-pyrazine carboxamide utilized in Japan for the treatment of influenza in 2002 [4], has been used for the treatment of SARS-CoV-2. It selectively inhibits RNA polymerase activity of viruses by binding to RNA-dependent RNA polymerase (RdRp) [5], [6]. It has been recognized as a safe and effective drug treatment for influenza and Ebola [7], [8] and has been suggested frequently as a treatment modality for SARS-CoV-2 patients [1], [2], [6], [7], [9], [10], [11], [12]
We therefore explored the safety and efficacy of a treatment protocol containing this drug in moderate to severe cases of SARS-CoV-2 compared to an alternative regimen containing Lopinavir/Ritonavir in a multicenter, randomized, open-labeled study.
2. Methods
2.1. Patients
Patients with the diagnosis of SARS-CoV-2 based on either a positive real-time polymerase chain reaction (RT-PCR) test or typical ground glass appearance on chest CT scan in need of hospital admission due to a SpO2 reduction of 93% or less were eligible to enter this study. The age of the patients was between 16 and 100 years old from whom an informed and written consent was obtained. Exclusion criteria comprised a history of receiving any antiviral drug such as Ribavirin, Oseltamivir, and Lopinavir/Ritonavir for current illness; a history of chronic renal or liver failure, or gastrointestinal bleeding; being too ill with less than 48 h life expectancy. Also, pregnancy and lactation; known patient of HIV infection/AIDS; and QT interval above 500 ms in Electrocardiogram were excluded.
2.2. Intervention
We compared a treatment protocol that consisted of Favipiravir (made by ZHEJIANG HISUN PHARMACEUTICAL Co., LTD.) and hydroxychloroquine, with Lopinavir/Ritonavir and hydroxychloroquine. At the time hydroxychloroquine was regarded as the standard of care in Iran and skipping it in the treatment protocol was considered unethical. The intervention group received Favipiravir 1600 mg stat and then 600 mg every 8 h plus hydroxychloroquine 200 mg twice a day for 1 week. The control group received a single dose of hydroxychloroquine 400 mg followed by 100 + 400 Lopinavir/Ritonavir twice a day for 1 week. Based on concerns regarding the effect of combination therapy of hydroxychloroquine and Lopinavir/Ritonavir on QT interval, the control group received a single dose of hydroxychloroquine on admission. Later on, during the trial (31 May 2020), in light of emerging evidence, daily hydroxychloroquine in the Favipiravir group was also reduced to a single dose of 400 mg for both treatment and control groups. Both treatment regimens could be continued up to 10 days if needed and physicians could use identical protocols to prescribe other necessary drugs especially for patients in perilous conditions.
2.3. Trial design
We did a multicenter randomized open-labeled clinical trial in 20 centers. Study groups were concurrently registered and patients were randomly allocated into both intervention and control groups. Recruitment started on the first of April and ended on the 3rd of August 2020. (Details of study centers are available in the Iranian Registry of Clinical Trial).
2.4. Randomization
We used stratified block randomization with variable block size of 4 and 6 to create the random sequence for each center, having an equal ratio of distribution of the subjects to both treatment and control groups. A 4-digit special code was allocated to every patient to preserve their identity, and was used to recognize the subjects on the filled CRF forms. Sealed envelopes were used to protect the randomization sequence. We used a central allocation mechanism in which participating centers called a central randomization unit, and registered their eligible patients to receive the 4-digit unique code for assigned group identification.
2.5. Conduct and monitoring
The trial protocol was endorsed by the research committees of the two main universities cooperating in this study. Ethical approval was received from Independent Ethics Committees of the two leading universities (References: IR.IUMS.REC.1399.065 and IR.BMSU.REC.1399.017). Participating centers were approached and invited through national professional bodies and by the chief investigator. Initiation and progress of the study at each participating center were monitored via on-site visits by monitoring teams, including remote central monitoring using specifically designed software intended for this. Discrepancies and errors were detected, recorded, and reported back to the clinical unit for amendments. A five-member Data and Safety Monitoring Board was set up to scrutinize the reported adverse events.
2.6. Outcome definition and measurement
The primary endpoint was the number of admissions to the intensive care unit. We also considered the intubation of patients. Subsidiary endpoints were duration in hospital, In-hospital mortality, time to clinical recovery, and changes in SpO2 after five minutes interval of supplemental discontinuation of the supplemental oxygen for 5 min. Length of hospital stay was defined as the number of days between admissions and discharge on the physician’s advice. Clinical recovery (as an event) was defined as the persistent return of oxygen saturation on ambient air to above 93%, and/or absence of supplemental oxygen requirement and/or medical discharge. This depended on whichever occurred earlier. Treatment side effects were also assessed daily. (Supplemental document-Study protocol)
2.7. Safety and efficacy population
Patients who had been randomly allocated to the Favipiravir and Lopinavir/Ritonavir groups comprised our “intention to treat” (ITT) population. We could not gather any information from the participating centers for 7 patients and therefore did not include them in the modified ITT population. The per-protocol population was defined as those patients who had received their 7 days treatment regimens. Efficacy outcomes were assessed in both modified ITT and per-protocol populations. The safety population was defined as those in the study who had received at least one dose of Favipiravir or Lopinavir/Ritonavir which corresponded to the modified ITT population.
2.8. Data management
A software was developed to manage data collection and transfer. Both the software and paper CRF forms were identical. Clinical units had a local client of the software and locally stored their data. Data were then transferred at will to the main server and stored in a central database as the latest version. An application was also developed to show all the contents of CRF forms in the central database used for remote monitoring.
2.9. Sample size
We calculated our sample size to have 80% power to detect a reduction from 20% to 10% in ICU admissions.
2.10. Statistical analysis
We used Chi-squared to compare proportions of death, ICU admission, and intubation between the two groups. Length of stay in hospital was compared by Man Whitney U test. Time to clinical recovery between the two groups was compared using Survival analysis. Cox proportional hazard model was used to estimate hazard ratios and their 95% confidence intervals. Proportional hazard assumption was checked for each model using proportional hazard and Log minus log survival plots including formal testing. Changes (from admission) in SpO2 after discontinuation of the supplemental oxygen for 5 min were calculated. Their daily values were compared between the treatment groups using the Generalized Estimation Equation (GEE) method considering the time (days), treatment, and their interaction in the model. It was also examined as a binary outcome at beneath and beyond 93% saturation level employing the logit link function. As a sensitivity analysis, multilevel models were built to examine the possible effects of multiple centers. The possible effect of reduction of hydroxychloroquine treatment dose in Favipiravir group following DSMB decision on all analyses was checked. Stata 11 (STATA Corporation) statistical software was used for the analysis.
3. Results
3.1. Study population and baseline comparison
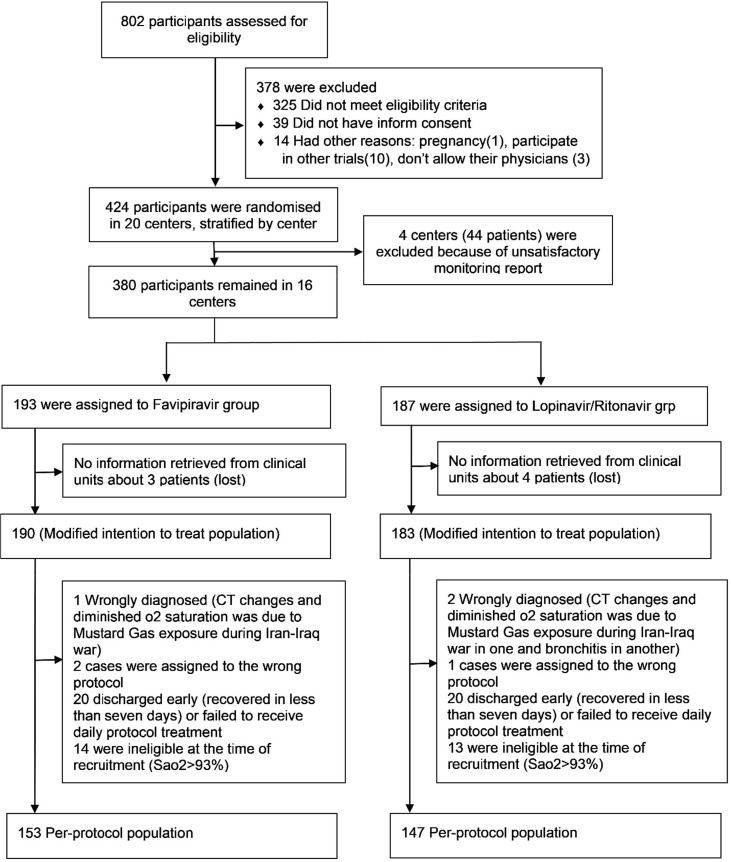
Between 2/4/2020–3/8/2020, 424 eligible SARS-CoV-2 patients were recruited and randomly allocated to the Favipiravir (2 1 6) and Lopinavir/Ritonavir (2 0 8) groups in 17 centers. Four centers (44 patients) were omitted from the investigation due to inadequate monitoring reports and data for 7 patients could not be retrieved. The flow of participants is outlined in Fig. 1 . The remaining 373 patients were used for analysis as a modified ITT population, of which 300 completed the 7-day therapy (per-protocol population) (Fig. 1). Results presented in this paper are based on the modified ITT population unless otherwise specified. Characteristics of the study participants and their baseline comparison in the two study groups are summarized in Table 1 . Table 2 displays the additional drugs received during the study period. Fig. 2 .
Fig. 1.
Participants flow diagram.
Table 1.
Participants characteristics and baseline comparison in the modified ITT population by treatment regimens.
| Favipiravir (N = 190) | Lopinavir/Ritonavir (N = 183) | Total (N = 373) | ||
|---|---|---|---|---|
| Age - mean (SD)* | 58.6 (17.5) | 56.6 (17.1) | 57.6 (17.3) | |
| Sex (Male) - no. (%) | 115 (60.5) | 90 (49.2) | 205 (55.0) | |
| BMI - mean (SD) | 27.2 (4.8) | 27.7 (4.5) | 27.4 (4.6) | |
| Education - no. (%) | ||||
| Elementary | 110 (57.9) | 107 (58.5) | 217 (58.2) | |
| Diploma | 39 (20.5) | 36 (19.7) | 75 (20.1) | |
| Bachelor | 33 (17.4) | 26 (14.2) | 59 (15.8) | |
| Master | 3 (1.6) | 5 (2.7) | 8 (2.1) | |
| Doctoral and above | 5 (2.7) | 9 (4.9) | 14 (3.8) | |
| Comorbidities - no. (%) | ||||
| Hypertension | 64 (33.7) | 66 (36.1) | 130 (34.9) | |
| Diabetes | 57 (30.0) | 39 (21.3) | 96 (25.7) | |
| Chronic heart disease | 19 (10.0) | 21 (11.5) | 40 (10.7) | |
| Chronic lung disease, not asthma | 6 (3.2) | 7 (3.8) | 13 (3.5) | |
| Asthma | 7 (3.7) | 7 (3.8) | 14 (3.8) | |
| Mild liver disease | 1 (0.5) | 4 (2.2) | 5 (1.3) | |
| Kidney disease | 3 (1.6) | 3 (1.6) | 6 (1.6) | |
| Rheumatologic disease | 2 (1.1) | 4 (2.2) | 6 (1.6) | |
| Chronic neurologic disease | 6 (3.2) | 9 (4.9) | 15 (4.0) | |
| Vital signs on admission - no. (%) | ||||
| Fever °C - mean (SD) | 37.2 (0.7) | 37.2 (0.8) | 37.2 (0.8) | |
| Heart rate - mean (SD) | 90.7 (14.5) | 92.0 (14.4) | 91.3 (14.4) | |
| Respiratory rate - median (IQR) | 20 (8) | 22 (8) | 21 (8) | |
| SpO2 % - median (IQR) | 89 (5) | 89 (7) | 89 (6) | |
| Systolic BP mmHg - mean (SD) | 122.8 (16.6) | 124.6 (14.8) | 123.7 (15.8) | |
| Diastolic BP mmHg - mean (SD) | 77.3 (10.1) | 78.1 (9.8) | 77.7 (9.9) | |
| Symptoms - no. (%) | ||||
| Respiratory distress | 35 (18.9) | 40 (22.6) | 75 (20.7) | |
| Chill | 17 (9.2) | 15 (8.5) | 32 (8.8) | |
| Cough | 52 (28.1) | 48 (27.1) | 100 (27.6) | |
| Dyspnea | 19 (10.3) | 24 (13.6) | 43 (11.9) | |
| Chest pain | 127 (68.7) | 133 (75.1) | 260 (71.8) | |
| Anorexia | 47 (25.4) | 47 (26.6) | 94 (26.0) | |
| Diarrhea | 131 (70.8) | 123 (69.5) | 254 (70.2) | |
| Vomiting | 60 (32.4) | 66 (37.3) | 126 (34.8) | |
| Abdominal pain | 42 (22.7) | 58 (32.7) | 100 (27.6) | |
| Sore throat | 32 (17.3) | 35 (19.8) | 67 (18.5) | |
| Myalgia | 98 (53.0) | 88 (49.7) | 186 (51.4) | |
| Arthralgia | 101 (54.6) | 115 (65.0) | 216 (59.7) | |
| Fatigue | 24 (13.0) | 31 (17.5) | 55 (15.2) | |
| Headache | 41 (22.2) | 44 (24.9) | 85 (23.5) | |
| Lung CT scan findings – no. (%) | ||||
| Ground-glass pattern | 163 (85.8) | 153 (83.6) | 316 (84.7) | |
| Consolidation | 29 (15.3) | 37 (20.2) | 66 (17.7) | |
| Bilateral lesions | 172 (90.5) | 160 (87.4) | 332 (89.0) | |
| Multi-lobar lesions | 173 (91.0) | 160 (87.4) | 333 (89.3) | |
| Lab assessment – no. (median)(IQR) | ||||
| WBC (x1000/µl) | 184; 6.9 (5.1–8.9) | 169; 6.3 (4.9–9.1) | 353; 6.5 (5.0–8.9) | |
| Lymphocyte (x1000/µl) | 157; 1.1 (0.9–1.6) | 143; 1.2 (0.8–1.7) | 300; 1.1 (0.9–1.6) | |
| Neutrophil (x1000/µl) | 157; 5.3 (3.6–7.1) | 142; 4.8 (3.3–7.1) | 299; 4.9 (3.4–7.1) | |
| Platelet (x1000/µl) | 180; 195.5 (157.0–251.5) | 166; 194.5 (150–240) | 346; 195 (153.0–249.0) | |
| Hb (g/dl) | 183; 13.2 (12.1–14.1) | 166; 13.15 (11.6–14.3) | 349; 13.2 (11.9–14.2) | |
| HCT (%) | 174; 40.0 (37.3–42.7) | 161; 39.2 (35.6–43.0) | 335; 39.8 (36.3–42.7) | |
| ALT (iu/l) | 137; 27 (18.0–39.0) | 132; 27 (18.0–48.0) | 269; 27 (18.0–42.0) | |
| AST (iu/l) | 137; 33 (25.0–45.0) | 131; 36 (26.0–51.0) | 268; 34 (25.0–48.0) | |
| Alk-Ph (iu/l) | 124; 176 (139.5–212.5) | 118; 183 (153.0–244.0) | 242; 179.5 (146.0–220.0) | |
| PT (sec.) | 133; 13 (12.0–15.0) | 136; 13 (12.0–14.8) | 269; 13 (12.0–14.8) | |
| PTT (sec.) | 136; 34 (30.0–40.0) | 135; 34 (30.0–38.0) | 271; 34 (30.0–39.0) | |
| INR | 135; 1.1 (1.0–1.2) | 132; 1.0 (1.0–1.2) | 267; 1.06 (1.0–1.2) | |
| BUN (mg/dl) | 167; 17 (12.0–26.0) | 158; 16 (11.0–24.0) | 325; 17 (12.0–25.0) | |
| Cr (mg/dl) | 173; 1.1 (0.9–1.3) | 165; 1 (0.9–1.2) | 338; 1.1 (0.9–1.3) | |
| Uric Acid (mg/dl) | 29; 6.2 (4.5–7.7) | 35; 6.6 (4.6–10.1) | 64; 6.35 (4.6–8.9) | |
| BS (mg/dl) | 126; 117.0 (98.0–168.0) | 117; 122 (97.0–156.0) | 243; 118 (98.0–164.0) | |
| Na (mEq/l) | 175; 138 (135.0–140.0) | 165; 138 (135.0–140.0) | 340; 138 (135.0–140.0) | |
| K (mEq/l) | 174; 4.1 (3.8–4.5) | 163; 4.1 (3.8–4.4) | 337; 4.1 (3.8–4.5) | |
| Ca (mg/dl) | 128; 8.7 (8.3–9.1) | 124; 9 (8.3–9.4) | 252; 8.8 (8.3–9.2) | |
| Mg (mEq/l) | 121; 2 (1.9–2.2) | 117; 2 (1.9–2.2) | 238; 2 (1.9–2.2) | |
| Phosphorus (mg/dl) | 102; 3.1 (2.5–3.9) | 106; 3.3 (2.4–4.0) | 208; 3.2 (2.5–4.0) | |
| CRP (mg/l) | 111; 22 (3.4–43.0) | 92; 24.5 (4.3–45.5) | 203; 23 (4.0–44.0) | |
| ESR (sec.) | 135; 40 (27.0–63.0) | 123; 46 (25.0–62.0) | 258; 45.5 (26.0–62.0) | |
| LDH (iu/l) | 120; 551.5 (450.0–714.0) | 124; 546.5 (427.5–763) | 244; 548.5 (441.0–750.5) | |
Mean age was calculated in ITT population
Table 2.
Additional treatments used in study groups in modified ITT population.
| Additional Treatments received during study | Favipiravir (N = 190) | Lopinavir/Ritonavir (N = 183) | Total (N = 373) |
|---|---|---|---|
| Oral steroid | 8 (4.4) | 15 (8.5) | 23 (6.4) |
| Intravenous steroid | 41 (22.5) | 35 (19.8) | 76 (21.2) |
| Interferon | 23 (12.6) | 23 (12.9) | 46 (12.8) |
| Remdesivir | 2 (1.1) | 2 (1.1) | 4 (1.1) |
| Other antivirals | 9 (5.0) | 1 (0.6) | 10 (2.8) |
| Plasmapheresis | 1 (0.5) | 4 (2.3) | 5 (1.4) |
| Intravenous Immunoglobulin (IVIG) | 2 (1.1) | 4 (2.3) | 6 (1.7) |
| NSAIDa | 28 (15.4) | 25 (14.1) | 53 (14.8) |
| Oral Antibiotics | 76 (40.0) | 62 (33.9) | 138 (37.0) |
| IV Antibiotics | 99 (53.5) | 112 (58.1) | 211 (58.1) |
| Oral Anticoagulant | 15 (8.1) | 18 (10.1) | 33 (9.1) |
| IV Anticoagulant | 139 (75.1) | 128 (71.9) | 267 (73.6) |
| Proton Pump Inhibitors | 129 (69.7) | 114 (64.0) | 243 (6.9) |
| ACEI/ARBb | 26 (14.0) | 23 (12.9) | 49 (13.5) |
| Famotidine | 16 (8.6) | 21 (11.8) | 37 (10.2) |
| Statins | 26 (14.0) | 21 (11.8) | 47 (13.0) |
Nonsteroidal anti-inflammatory drugs;
Angiotensin-converting enzyme inhibitor/Angiotensin receptor blocker
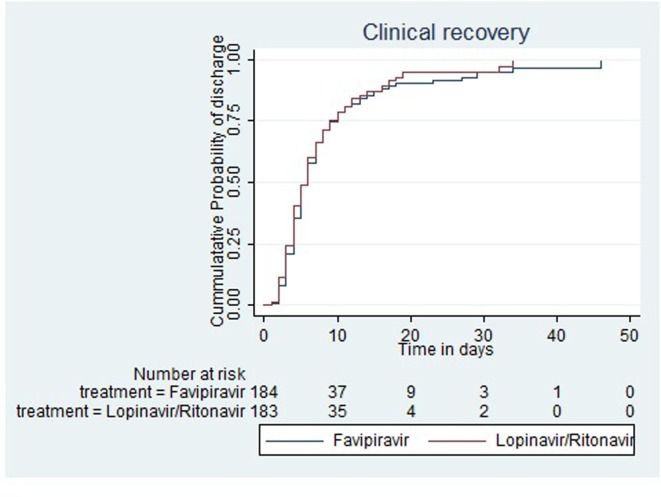
Fig. 2.
Kaplan Meier Failure plot showing time to clinical recovery by treatment groups in modified ITT population.
3.2. Outcomes
3.2.1. Death and intubation
Overall, there were 47 deaths in the modified ITT population, 26 in the Favipiravir group, and 21 in the Lopinavir/Ritonavir group (P = 0.49). 56 people were transferred to the intensive care unit of whom 44 were intubated. Their corresponding figures were 31 and 26 in the Favipiravir, and 25 and 21 in the Lopinavir/Ritonavir groups respectively. The difference between these groups was not statistically significant (see Table 3 ). Table 4 .
Table 3.
Comparison of the study outcomes in Favipiravir and Lopinavir/Ritonavir treatment regimens.
| Modified ITT population |
Per-protocol population |
|||||
|---|---|---|---|---|---|---|
| Favipiravir(N = 190) | Lopinavir/Ritonavir (N = 183) | Total (N = 373) | Favipiravir(N = 153) | Lopinavir/Ritonavir (N = 147) | Total (N = 300) | |
| In-hospital mortality - no. (%) | 26 (13.7) | 21 (11.5) | 47 (12.6) Χ2 = 0.41; p = 0.52 | 22 (14.4) | 17 (11.6) | 39 (13) Χ2 = 0.53; p = 0.47 |
| Intubation - no. (%) | 27 (14.2) | 17 (9.3) | 44 (11.8) Χ2 = 2.17; p = 0.14 | 23 (15.0) | 13 (8.8) | 36 (12) Χ2 = 2.72; p = 0.1 |
| ICU admission - no. (%) | 31 (16.3) | 25 (13.7) | 56 (15.0) Χ2 = 0.51; p = 0.47 | 26 (17.0) | 18 (12.2) | 44 (14.7) Χ2 = 1.35; p = 0.25 |
| Median length of hospital stay - days (IQR)a | 7 (4–9); n = 153 | 6 (4–10); n = 150 | 6 (4–10); n = 303 Mann-Whitney U test: p = 0.85 | 6 (4–9); n = 124 | 6 (4–9); n = 122 | 6 (4–9); n = 246 Mann-Whitney U test: p = 0.92 |
| Median survival time till clinical recovery - days (IQRb) | 6 (4–10); n = 185 | 6 (4–10); n = 182 | Log-rank Χ2 = 0.38; p = 0.54 | 6 (4–9); n = 150 | 5 (3–9); n = 147 | Log-rank Χ2 = 0.38; p = 0.54 |
patients discharged against medical advice were excluded
Inter Quartile Range
Table 4.
Hazard ratios and their 95% CI for clinical recovery estimated from Cox proportional Hazard modelling.
| Modified ITT pop |
Per protocol pop |
|||
|---|---|---|---|---|
| No. of events | Hazard Ratio (95% CI) | No. of events | Hazard Ratio (95% CI) | |
| Lopinavir/Ritonavir | 152 | 1.00 | 124 | 1.00 |
| Favipiravir Group | 156 | 0.94 (0.75–1.17) | 127 | 0.93 (0.73–1.19) |
3.2.2. Length of stay in hospital
The median length of stay in hospital among those medically discharged (on physician’s advice) in the modified ITT population was 7 days (IQR = 4–9) in the Favipiravir, and 6 days (IQR = 4–10) in the Lopinavir/Ritonavir groups (Mann Whitney U test, p = 0.85) (see Table 3).
3.2.3. Clinical recovery
Median survival time to clinical recovery was 6 days (IQR = 4–10) in the Favipiravir group and 6 days (IQR = 4–10) in the Lopinavir/Ritonavir group. Also, the difference in the clinical recovery experience was not statistically significant (Log-Rank test: χ2 = 0.38; p = 0.53) (see Table 3). The hazard ratio for clinical recovery in Favipiravir versus Lopinavir/Ritonavir group was 0.94 (95% CI: 0.75–1.17) using Cox proportional hazard modeling. There were no serious violations of the proportional hazard assumption in all models used.
3.2.4. SpO2 change over-hospitalization
The results from the GEE model showed that the changes in daily SpO2 after 5 min discontinuation of supplemental oxygen at the beginning of admission in un-intubated patients had no significant difference between the two study groups during hospitalization (p = 0.46). SpO2 was also assessed as a binary outcome at beneath and beyond 93% saturation level. SpO2 in patients receiving Favipiravir treatment regimen was equally likely to have a remittance beyond 93% compared with the Lopinavir/Ritonavir treatment group after considering SpO2 on admission (odds ratio = 1.00 95% CI 0.71–1.42; p = 0.997). Multilevel modeling had no effect the results.
3.2.5. Adverse events
Observed adverse drug reactions have been summarized in Table 5 . Overall, more adverse effects were associated with Lopinavir/Ritonavir treatment regimen than with the Favipiravir regimen. Specifically, the adverse effects significantly observed in the Lopinavir/Ritonavir treatment regimen were gastrointestinal, allergic, and respiratory (see Table 5). Reduction of hydroxychloroquine treatment dose in Favipiravir group following DSMB decision did not affect the results.
Table 5.
Frequency of Adverse drug effect in Safety population by study groups.
| Adverse drug effect | Favipiravir (N = 190) | Lopinavir/Ritonavir (N = 183) | Total (N = 373) | P-value | |
|---|---|---|---|---|---|
| Anaphylaxis | 1(0.53) | 9(4.92) | 10(2.68) | 0.009 | |
| Itching | 1(0.53) | 4(2.19) | 5(1.34) | ||
| Vertigo | 0(0.00) | 2(1.09) | 2(0.54) | ||
| Nausea | 0(0.00) | 5(2.73) | 5(1.34) | ||
| Dyspnea | 0(0.00) | 3(1.64) | 3(0.80) | ||
| Diarrhea | 0(0.00) | 3(1.64) | 3(0.80) | ||
| Abdominal pain | 0(0.00) | 1(0.55) | 1(0.27) | ||
| Gastrointestinal | 29(15.26) | 54(29.51) | 83(22.25) | 0.001 | |
| Anorexia | 6(3.16) | 18(9.84) | 24(6.43) | ||
| Nausea | 9(4.74) | 24(13.11) | 33(8.85) | ||
| Vomiting | 0(0.00) | 6(3.28) | 6(1.61) | ||
| Diarrhea | 5(2.63) | 19(10.38) | 24(6.43) | ||
| Abdominal pain | 5(2.63) | 10(5.46) | 15(4.02) | ||
| Dry mouth | 9(4.74) | 16(8.74) | 25(6.70) | ||
| Neurologic | 15(7.89) | 22(12.02) | 37(9.92) | 0.2 | |
| Fatigue | 5(2.63) | 10(5.46) | 15(4.02) | ||
| Headache | 4(2.11) | 8(4.37) | 12(3.22) | ||
| Lethargy | 5(2.63) | 11(6.01) | 16(4.29) | ||
| Loss of balance | 2(1.05) | 4(2.19) | 6(1.61) | ||
| Low mood | 2(1.05) | 1(0.55) | 3(0.80) | ||
| Paresthesia | 1(0.53) | 3(1.64) | 4(1.07) | ||
| Non-specific pain | 0 (0) | 2 (2.56) | 2 (1.25) | ||
| Stupor | 1(0.53) | 1(0.55) | 2(0.54) | ||
| Ophthalmologic | 1(0.53) | 4(2.19) | 5(1.34) | 0.17 | |
| Blurred vision | 0(0.00) | 2(1.09) | 2(0.54) | ||
| Lacrimal gland dysfunction | 1(0.53) | 2(1.09) | 3(0.80) | ||
| Endocrine | 1(0.53) | 2(1.09) | 3(0.80) | 0.55 | |
| Hypothyroidism | 1(0.53) | 0(0.00) | 1(0.27) | ||
| Cardiac | 6(3.16) | 8(4.37) | 14(3.75) | 0.55 | |
| Cardiomyopathy | 3(1.58) | 2(1.09) | 5(1.34) | ||
| Arrhythmia | 1(0.53) | 1(0.55) | 2(0.54) | ||
| Respiratory | 4(2.11) | 12(6.56) | 16(4.29) | 0.04 | |
| Dyspnea | 3(1.58) | 10(5.46) | 13(3.49) | ||
| Cough | 2(1.05) | 9(4.92) | 11(2.95) | ||
| Pharyngitis | 1(0.53) | 0(0.00) | 1(0.27) | ||
| Dermatologic | 2(1.05) | 3(1.64) | 5(1.34) | 0.63 | |
| Skin rash | 0(0.00) | 2(1.09) | 2(0.54) | ||
| Hair loss | 1(0.53) | 1(0.55) | 2(0.54) | ||
| Nephrologic | 5(2.63) | 3(1.64) | 8(2.14) | 0.609 | |
| Polyuria | 2(1.05) | 1(0.55) | 3(0.80) | ||
| Hematuria | 0(0.00) | 1(0.55) | 1(0.27) | ||
| BUN rises | 2(1.05) | 0(0.00) | 2(0.54) | ||
| Creatinine rise | 2(1.05) | 0(0.00) | 2(0.54) | ||
| Hematologic | 1(0.53) | 1(0.55) | 2(0.54) | 0.5 | |
| Anemia | 1(0.53) | 1(0.55) | 2(0.54) | ||
3.2.6. Discussion
In our observation, the Favipiravir therapy had no influence on ICU admission in comparison with Lopinavir/Ritonavir (31 admissions to ICU versus 25). It also did not reduce the need for intubations (27 intubations versus 17) or in-hospital mortality (26 deaths versus 21). Length of hospital stay was analogous in the two therapies, respectively 7 and 6 days. The overall clinical recovery of patients receiving Favipiravir was no better than those receiving Lopinavir/Ritonavir regimen (log-rank test: p = 0.53). Besides, the probability of clinical recovery during the study period was not higher in the Favipiravir compared with Lopinavir/Ritonavir group (HR = 0.94; 95% CI: 0.75–1.17). Change in SpO2 after 5 min discontinuation of supplemental oxygen from SpO2 on admission also followed similar patterns in the two groups (p = 0.46).
Ethical concerns on treatment efficacy to ensure patients receive the best treatment prevented us from comparing the use of Favipiravir only versus placebo. Hydroxychloroquine and Lopinavir/Ritonavir had been listed as recommended treatment options in Iranian and some foreign guidelines [6], [12], [13], [14], [15], hence depriving patients of these two treatment modalities was considered unethical at the time. Emerging works of literature [16], [17], [18] have raised doubts on the efficacy and safety of these treatments. As a result, we can reasonably claim that the design we used in this study should be capable of showing any potential treatment benefit from Favipiravir if it ever existed. Furthermore, difference in the duration of hydroxychloroquine treatment in early trial (up until 31st of May 2020) in the two regimens due to QT interval prolongation concerns in those who received Lopinavir/Ritonavir [19], is unlikely to change our interpretation of current findings. After that date, DSMB decided to make the doses equal. We also could not avoid using additional treatments and supplements in patients entering our study, particularly when their illness worsened. Instead, we followed equal treatment change rules for all patients regardless of their assigned groups. A comparison of other treatments received by patients is recorded in Table 2. Generally, additional treatment modalities were equally used.
Our centralized allocation system protected the randomization sequence and provided assurances against the introduction of bias, despite the open-labelled design. Patients’ national identification and hospital admission reference numbers together with other personal information were registered in the central randomization unit before allocating treatment regimens to the patients. This information was subsequently used in data monitoring. Stratification by center enabled us to exclude data gathered by centers that were regarded as substandard without jeopardizing the random allocation principle.
Patients who received the Favipiravir regimen had poor recovery according to some outcomes. Although this was not statistically significant, it may be an noteworthy. Inferentially, this could result from the compensatory treatment in patients receiving the Lopinavir/Ritonavir treatment regimen, given the open-labelled design. At the time this study was performed, some anecdotal evidence in the social media from clinicians suggested that Favipiravir may be the effective treatment against SARS-CoV-2 disease. This complicated the recruitment process (some patients refused to give consent) and provided additional incentive for extra care by hospital staff in patients not receiving Favipiravir.
One possible explanation for our negative findings regarding the efficacy of Favipiravir in SARS-CoV-2 pneumonia could be the proposed mechanisms for the pathogenicity of this virus. The disease usually has an early infection phase with mild non-specific symptoms, a pulmonary involvement phase with or without hypoxia, and a late phase involving a surge in inflammatory mediators called “cytokine storm” leading to ARDS which is associated with high mortality [20], [21]. It seems that those who are hospitalized because of pneumonia and low SpO2 has already passed the viral replication phase, and therefore are unlikely to benefit from antiviral treatment [21], [22], [23], [24]. Accordingly, some studies suggested early prescription of the drug even in the asymptomatic or ambulatory phase of the disease. [25], [26], [27]. However, a newly published open-labelled randomized trial did not find any benefit from the early prescription of the drug compared with the late prescription, in patients with mild to moderate SARS-CoV-2 [28].
Our patients also included many with moderate cases and the absence of any evidence of rapid clinical recovery in the Favipiravir group may suggest that the severity of the disease is independent of viral replication, even if the findings of reduction in viral load by Favipiravir treatment are true [25], [29], [30]. This explanation is in line with the existing evidence showing that these patients benefited from the suppression of their exaggerated immune response by steroids [31] rather than by any antiviral treatment.
Our study has the strength of exploring hard outcomes such as mortality, ICU admission, and intubation that are less prone to differences in definitions and interpretation. In summary, we found no clinical benefit from a treatment regimen based on Favipiravir in moderate to severe cases of SARS-CoV-2 over a treatment regimen based on Lopinavir/Ritonavir.
Authors’ contributionsRole of the funding source
All authors drafted and drafted the manuscript, revised the manuscript, supervised the treatment and the and wrote the manuscript; and all authors approved the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The study team report grants from Iran University of Medical Science, personal fees, and non-financial support from Baqiyatallah University of Medical Sciences (Trial registration number: IRCT20200318046812N1), during the study. There is no other conflict of interest.
References
- 1.Venkatasubbaiah M., Dwarakanadha Reddy P., Satyanarayana S.V. Literature-based review of the drugs used for the treatment of COVID-19. Curr Med Res Pract. 2020;10(3):100–109. doi: 10.1016/j.cmrp.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Şimşek Yavuz S., Ünal S. Antiviral treatment of COVID-19. Turk. J Med Sci. 2020;50(SI-1):611–619. doi: 10.3906/sag-2004-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuccori M., Convertino I., Ferraro S., et al. The Impact of the COVID-19 “Infodemic” on Drug-Utilization Behaviors: Implications for Pharmacovigilance. Drug Safety. 2020 doi: 10.1007/s40264-020-00965-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furuta Y., Takahashi K., Fukuda Y., et al. In Vitro and In Vivo Activities of Anti-Influenza Virus Compound T-705. Antimicrobial Agents and Chemotherapy. 2002;46(4):977–981. doi: 10.1128/AAC.46.4.977-981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furuta Y., Komeno T., Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proceedings of the Japan Academy Series B, Physical and biological sciences. 2017;93(7):449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shannon A, Selisko B, Le N, et al. Favipiravir strikes the SARS-CoV-2 at its Achilles heel, the RNA polymerase. bioRxiv 2020: 2020.05.15.098731.
- 7.Du Y.X., Chen X.P. Favipiravir: Pharmacokinetics and Concerns About Clinical Trials for 2019-nCoV Infection. Clinical pharmacology and therapeutics. 2020 doi: 10.1002/cpt.1844. [DOI] [PubMed] [Google Scholar]
- 8.Sissoko D., Laouenan C., Folkesson E., et al. Experimental Treatment with Favipiravir for Ebola Virus Disease (the JIKI Trial): A Historically Controlled, Single-Arm Proof-of-Concept Trial in Guinea. PLoS medicine. 2016;13(3) doi: 10.1371/journal.pmed.1001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. Jama. 2020;323(18):1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 10.Vijayvargiya P., Esquer Garrigos Z., Castillo Almeida N.E., Gurram P.R., Stevens R.W., Razonable R.R. Treatment Considerations for COVID-19: A Critical Review of the Evidence (or Lack Thereof) Mayo Clinic proceedings. 2020;95(7):1454–1466. doi: 10.1016/j.mayocp.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolarin J.A., Oluwatoyosi M.A., Orege J.I., et al. Therapeutic drugs for SARS-CoV-2 treatment: Current state and perspective. International Immunopharmacology. 2021;90 doi: 10.1016/j.intimp.2020.107228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kivrak A., Ulaş B., Kivrak H. A comparative analysis for anti-viral drugs: Their efficiency against SARS-CoV-2. International immunopharmacology. 2021;90:107232-. doi: 10.1016/j.intimp.2020.107232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin Y.-H., Cai L., Cheng Z.-S., et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Military Medical Research. 2020;7(1):4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meini S., Pagotto A., Longo B., Vendramin I., Pecori D., Tascini C. Role of Lopinavir/Ritonavir in the Treatment of Covid-19: A Review of Current Evidence, Guideline Recommendations, and Perspectives. Journal of. Clinical Medicine. 2020;9(7) doi: 10.3390/jcm9072050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scientific Bureau of National COVID-19 Management Committee. Iranian National Guideline for dignosis and treatment of COVID-19. MOHME, Ministry of Health and Medical Education 2020. p. 13-4.
- 16.Cao B., Wang Y., Wen D., et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. The New England journal of medicine. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang W., Cao Z., Han M., et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ (Clinical research ed) 2020;369 doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geleris J., Sun Y., Platt J., et al. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. New England Journal of Medicine. 2020;382(25):2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moschini L., Loffi M., Regazzoni V., Di Tano G., Gherbesi E., Danzi G.B. Effects on QT interval of hydroxychloroquine associated with ritonavir/darunavir or azithromycin in patients with SARS-CoV-2 infection. 2020::1–6. doi: 10.1007/s00380-020-01671-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Catanzaro M., Fagiani F., Racchi M., Corsini E., Govoni S., Lanni C. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct Target Ther. 2020;5(1):84-. doi: 10.1038/s41392-020-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu J., Li W., Shi X., et al. Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19) J Intern Med. 2020;288(1):128–138. doi: 10.1111/joim.13063. [DOI] [PubMed] [Google Scholar]
- 23.Mason R.J. Pathogenesis of COVID-19 from a cell biology perspective. European Respiratory Journal. 2020;55(4):2000607. doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giammaria D., Pajewski A. Can early treatment of patients with risk factors contribute to managing the COVID-19 pandemic? Journal of global health. 2020;10(1) doi: 10.7189/jogh.10.010377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu D., Cao R., Zhao L., Li W., Zhong W., Wen J. Oral favipiravir for patients with delayed SARS-CoV-2 viral RNA clearance: a case series. Crit Care. 2020;24(1):578-. doi: 10.1186/s13054-020-03288-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCullough PA. Favipiravir and the Need for Early Ambulatory Treatment of SARS-CoV2 Infection (COVID-19). Antimicrobial Agents and Chemotherapy 2020: AAC.02017-20. [DOI] [PMC free article] [PubMed]
- 27.Dabbous H.M., Abd-Elsalam S., El-Sayed M.H., et al. Efficacy of favipiravir in COVID-19 treatment: a multi-center randomized study. Arch Virol. 2021:1–6. doi: 10.1007/s00705-021-04956-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Doi Y., Hibino M., Hase R., et al. A prospective, randomized, open-label trial of early versus late favipiravir in hospitalized patients with COVID-19. Antimicrob Agents Chemother. 2020 doi: 10.1128/AAC.01897-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai Q, Yang M, Liu D, et al. Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study. Engineering (Beijing, China) 2020. [DOI] [PMC free article] [PubMed]
- 30.Ivashchenko AA, Dmitriev KA, Vostokova NV, et al. AVIFAVIR for Treatment of Patients with Moderate COVID-19: Interim Results of a Phase II/III Multicenter Randomized Clinical Trial. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2020. [DOI] [PMC free article] [PubMed]
- 31.Dexamethasone in Hospitalized Patients with Covid-19 — Preliminary Report. New England Journal of Medicine 2020.