Abstract
Background
Minimally invasive surgery (MIS) provides a new approach for patients with hilar cholangiocarcinoma (HCCA). However, whether it can achieve similar outcomes to traditional open surgery (OS) remains controversial.
Methods
To assess the safety and feasibility of MIS for HCCA, a systematic review and meta-analysis was performed to compare the outcomes of MIS with OS. Seventeen outcomes were assessed.
Results
Nine studies involving 382 patients were included. MIS was comparable in blood transfusion rate, R0 resection rate, lymph nodes received, overall morbidity, severe morbidity (Clavien–Dindo classification > = 3), bile leakage rate, wound infection rate, intra-abdominal infection rate, days until oral feeding, 1-year overall survival, 2-year overall survival and postoperative mortality with OS. Although operation time was longer (mean difference (MD) = 93.51, 95% confidence interval (CI) = 64.10 to 122.91, P < 0.00001) and hospital cost (MD = 0.68, 95% CI = 0.03 to 1.33, P = 0.04) was higher in MIS, MIS was associated with advantages of minimal invasiveness, that was less blood loss (MD = -81.85, 95% CI = -92.09 to -71.62, P < 0.00001), less postoperative pain (MD = -1.21, 95% CI = -1.63 to -0.79, P < 0.00001), and shorter hospital stay (MD = -4.22, 95% CI = -5.65 to -2.80, P < 0.00001).
Conclusions
The safety and feasibility of MIS for HCCA is acceptable in selected patients. MIS is a remarkable alternative to OS for providing comparable outcomes associated with a benefit of minimal invasiveness and its application should be considered more.
Introduction
Hilar cholangiocarcinoma (HCCA) (also called Klatskin’s tumor), which originates from the biliary epithelium at the confluence of the right and left hepatic ducts, is the most common malignancy of biliary tracts and is a devastating malignant disease with a poor prognosis [1–2]. Owing to its aggressiveness, late presentation, and refractory nature, the prognosis of patient with unresectable tumor is quite poor with a median survival of less than 1 year. Radical resection for HCCA is considered to be the only potential curative treatment, which can provide possible long-term survival with a 5-year overall survival ranging from 20–40% [3–5]. This tumor always tends to invade the vascular system, the perineural tissue, and major liver parenchyma [6–7]. Therefore, standard radical resection for HCCA includes extrahepatic bile duct resection, major hepatectomy, radical lymphadenectomy, biliary reconstruction, and even vascular resection and reconstruction [8–9]. With the surgical strategy changing from limited bile duct resections to resections including hepatectomy in the past decades, rate of R0 resection and 5-year survival improved a lot [10–12]. However, the complex anatomic features largely increase the difficulty of the surgery, high postoperative morbidity and mortality remain the issues. On the other hand, for the benefits of smaller incision, less intra-operative blood loss, less pain, earlier oral intake, shorter hospital stay, and faster recovery, minimally invasive surgery (MIS), including laparoscopic surgery and robotic surgery, developed rapidly recent years in managing gastrointestinal carcinomas and liver cancers [13]. In the field of liver resection, laparoscopic approach has showed improved short-term outcomes without compromising long-term oncological outcomes compared with open surgery (OS) [14–15]. Thus, laparoscopic hepatectomy has been accepted to be an alternative to conventional open hepatectomy currently [16]. In 2010, the first case of robotic radical resection for hilar cholangiocarcinoma was reported [17]. Robotic system, which features the dexterous EndoWrist® instruments, magnified stereoscopic view, ability of scale motions, and the elimination of surgeon’s physiologic tremors [18–21], offers notable advantages over traditional laparoscope. It enables operators to perform various complicated manoeuvres more stably and precisely than traditional laparoscopic instruments within a limited space and is an innovative MIS approach [22–23]. However, due to the difficulty of oncological resection and biological characteristics of HCCA, MIS for HCCA has been attempted only in limited and highly selected cases worldwide [24–25]. This approach for HCCA is still in its infancy and its efficiency is controversial. Although several studies comparing the clinical outcomes of MIS with OS for HCCA were carried out in the past years, the issues remained for the limited experience in each single center. Therefore, with the aim of comparing the outcomes of MIS with OS and investigate the efficiency of MIS for HCCA further, we systematically summarized the current available data and performed a meta-analysis.
Materials and methods
Literature search and study selection
We adhered to the 2009 preferred reporting items for systematic reviews and meta-analysis statement [26]. To provide an adequate overview of the current literature, databases of Medline, Embase, and the Cochrane Library from inception to 18 July 2020 were chosen for screening, an additional search with Google Scholar was performed to supplement the primary search. A combination of the following terms was used as a strategy of literature search: hilar cholangiocarcinoma, perihilar cholangiocarcinoma, Klatskin’s tumor, robotic, laparoscopic, and open (search strategy for Medline is shown in S1 Table). Two authors (Deng and Liu) carried out the search independently and any discrepancies regarding to the study selection were resolved by them. No restriction of language or publication type was set in the search. Title and abstract of each identified publication were screened, and only publications that reported the clinical outcomes of interest were further retrieved. The study protocol was approved by the Science and Research Office of the First Affiliated Hospital of Chongqing Medical University. This study protocol was also registered with the Open Science Framework platform and was available at osf.io/ntmh4.
Inclusion and exclusion criteria
The inclusion criteria were: (1) published prospective or retrospective cohort studies and randomized controlled trials; (2) studies comparing MIS with OS; (3) studies reported at least 1 of the undermentioned outcomes of interest. The exclusion criteria were: (1) case reports, reviews, letters, editorials and conference reports; (2) studies lacking a control group; (3) studies without available data; (4) studies without a clear description of methods or baseline characteristics.
Outcomes of interest and definitions
We assessed 17 outcomes of radical resection surgery for hilar cholangiocarcinoma in this meta-analysis, including: (1) Operative parameters: operation time, blood loss, blood transfusion rate, R0 resection rate, and lymph nodes received. (2) Postoperative complications: overall morbidity, severe morbidity (Clavien–Dindo classification > = 3), bile leakage rate, wound infection rate, and intra-abdominal infection rate. (3) Postoperative outcomes: days until oral feeding, length of postoperative analgesia, length of hospital stay, hospital cost, 1-year overall survival, and 2-year overall survival. (4) Postoperative mortality.
Postoperative severe complications were defined according to the Clavien–Dindo classification of surgical complications [27]. Biliary fistula was defined as bile contents in the abdominal drains or leakage found at relaparotomy [28]. Wound infection was diagnosed by the clinical signs (redness, swelling, heat, and pain) of the surgical site and the microbiological analysis of wound samples. Intra-abdominal infection was defined as clinical signs (body temperature higher than 38.5°C combined with leukocyte count higher than 12*109 /L) and the presence of intra-abdominal abscess [29].
Quality assessment and data extraction
Two reviewers (Deng and Liu) examined the studies independently and extracted data according to the predefined criteria. The extracted data included general information (first author, year of publication, source journal, country, study design, sample size, diagnoses of patients, follow-up period, gender, and the source of clinical data) and 17 outcomes (operation time, blood loss, blood transfusion rate, R0 resection rate, lymph nodes received, overall morbidity, severe morbidity, bile leakage, wound infection, intra-abdominal infection, days until oral feeding, length of postoperative analgesia, length of hospital stay, hospital cost, 1-year overall survival, 2-year overall survival, and postoperative mortality). The obtained data were then compared by the reviewers, inconsistencies were discussed and a third reviewer (Du) was consulted to reach a consensus if necessary. The methodological quality of each included study was assessed using the Newcastle-Ottawa quality assessment scale by Deng and Liu independently [30].
Statistical analysis
The meta-analysis was carried out in accordance with the Cochrane Reviewer’s Handbook and statistical analyses were performed with the Review Manager Software (Version 5.3, The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark). The results were presented by odds ratio (OR) or risk difference (RD) with 95% confidence interval (CI) for dichotomous data and mean difference (MD) with 95% CI for continuous data. Heterogeneity among studies was estimated using chi-square test (p < 0.10 represented statistically significant heterogeneity) and I2 test (I2 > 50% represented statistically significant heterogeneity). When indicating no significant heterogeneity, a fix-effect model was used. Otherwise, a random-effect model was used and a subgroup analysis was performed to explore the discrepancy. Funnel plots were performed to assess the publication bias, and the bias was excluded if a symmetrical distribution was showed. Moreover, a sensitivity analysis was performed by removing each study in turn to evaluate the stability of pooled estimate. Pooled analyses were visualized with forest plots and statistical significance was considered at p < 0.05.
Results
Study characteristics and quality assessment
The PRISMA flow diagram of literature search strategies is shown in Fig 1. Initially, 285 articles were found using combination of search terms and 253 irrelevant articles were excluded according to the inclusion and exclusion criteria after screening titles and abstracts. After assessment of full text, 23 studies were removed for the following reasons: 7 studies failed to meet the inclusion criteria, 15 studies were single-arm studies, available data were lacked in 1 study. Finally, 9 studies (all were retrospective cohorts) were included in the meta-analysis [31–39].
Fig 1. Flow diagram showing process of literature search and study selection.
The baseline characteristics of the 9 included studies were summarized in Table 1. All studies were well designed to compare two arms: MIS (robotic or laparoscopic) and OS for HCCA. The studies period ranged from 2014 to 2020. Analyses were performed on 382 patients, of whom 164 (42.9%) underwent MIS and 218 (57.1%) underwent OS. Three studies investigated patients with Bismuth–Corlette type I hilar cholangiocarcinoma, 1 study focused on type IIIb, and 5 studies were about hilar cholangiocarcinoma of all Bismuth–Corlette types. Most of the included studies showed satisfactory quality with selection criteria, comparability of patient characteristics, and adequate follow-up. All cohorts got 7 or more stars as shown in S2 Table.
Table 1. Characteristics of included studies.
| Reference | Year | Region | Study design | Sample size | Bismuth–Corlette classification | Laparoscopic or robotic surgery | Age: median(range)/mean±SD | Gender: male/female | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MIS | OS | MIS | OS | MIS | OS | ||||||
| Xu et al. [31] | 2016 | China | Retrospective cohort | 10 | 32 | Mixed | Robotic | 54(36–77) | 59(37–77) | 8/2 | 20/12 |
| Zhang et al. [32] | 2019 | China | Retrospective cohort | 14 | 9 | Mixed | Laparoscopic | 65.4±8.9 | 65.4±6.9 | 7/7 | 3/6 |
| Jiang et al. [33] | 2020 | China | Retrospective cohort | 54 | 54 | Bismuth type I | Laparoscopic | 67.7±2.3 | 67.7±2.2 | 29/25 | 28/26 |
| Zhu et al. [34] | 2018 | China | Retrospective cohort | 10 | 24 | Bismuth type I | Laparoscopic | 62.0±7.4 | 59.2±8.1 | 6/4 | 13/11 |
| Gong et al. [35] | 2014 | China | Retrospective cohort | 14 | 5 | Mixed | Laparoscopic | 58.5±3.6 | 54.9±4.5 | 8/6 | 3/2 |
| Chou et al. [36] | 2020 | China | Retrospective cohort | 16 | 31 | Mixed | Robotic | 68.0±7.0 | 60.0±9.0 | 11/5 | 20/11 |
| Duan et al. [37] | 2019 | China | Retrospective cohort | 13 | 14 | Bismuth type I | Laparoscopic | 59.2±8.0 | 62.0±7.4 | N/A | 8/6 |
| Chai et al. [38] | 2019 | China | Retrospective cohort | 17 | 17 | Bismuth type IIIb | Laparoscopic | N/A | N/A | N/A | N/A |
| Ratti et al. [39] | 2020 | Italy | Retrospective cohort | 16 | 32 | Mixed | Laparoscopic | 61(48–81) | 63(43–80) | 8/8 | 17/15 |
Meta-analysis of operative parameters
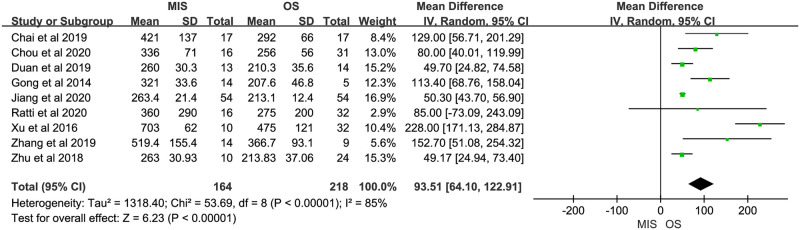
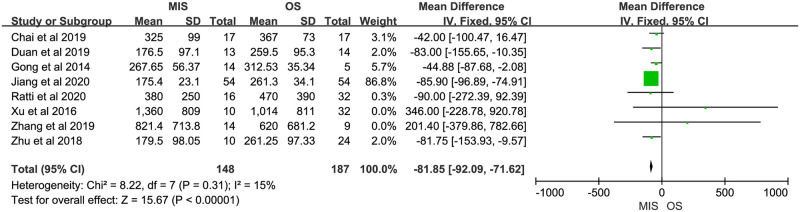
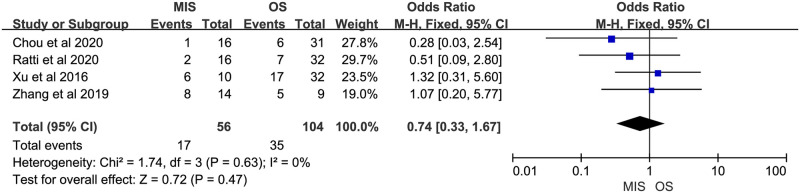
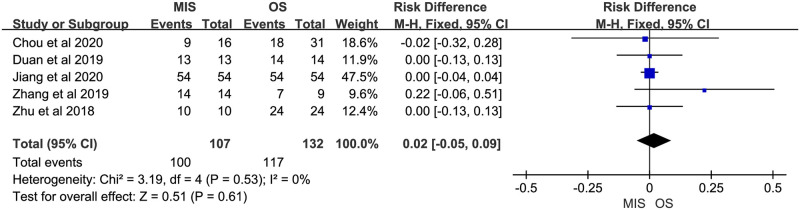
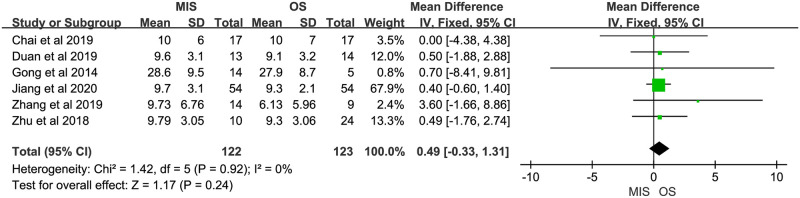
Nine studies reported operation time, all of them suggested it was longer in MIS. There was significant heterogeneity (P < 0.00001, I2 = 85%). A random-effect model indicated a significant difference between MIS and OS, operation time of MIS was much longer than OS (MD = 93.51, 95% CI = 64.10 to 122.91, P < 0.00001; Fig 2). Eight studies reported blood loss, with 5 of them indicating no significant difference between MIS and OS and 3 indicating less blood loss in MIS. No significant heterogeneity was observed (P = 0.31, I2 = 15%). A fixed-effect model was used and pooled results showed blood loss was much less in MIS than OS (MD = -81.85, 95% CI = -92.09 to -71.62, P < 0.00001; Fig 3). Data on blood transfusion rate were available in 4 of the 9 studies. All studies suggested there was no significant difference between MIS and OS. No significant heterogeneity was observed (P = 0.63, I2 = 0%). A fixed-effect model showed comparable blood transfusion rate between MIS and OS (OR = 0.74, 95% CI = 0.33 to 1.67, P = 0.47; Fig 4). Five studies compared the R0 resection rate of MIS with OS groups, the results of 5 studies indicated no significant difference between MIS and OS patients, there was no significant heterogeneity in the R0 resection rates of the 5 studies (P = 0.53, I2 = 0%), and a fixed-effect model showed that the R0 resection rate of MIS group was similar to the rate of OS group (RD = 0.02, 95% CI = -0.05 to 0.09, P = 0.61; Fig 5). Five studies provided data on the lymph nodes received, with all of them suggesting no significant difference between MIS and OS, no significant heterogeneity among the 5 studies was observed (P = 0.92, I2 = 0%), and pooled results of a fixed-effect model were found to be equivalent between MIS and OS for the lymph nodes received (MD = 0.49, 95% CI = -0.33 to 1.31, P = 0.24; Fig 6).
Fig 2. Meta-analysis of studies comparing operation time between minimally invasive surgery and open surgery groups based on a random-effect model.
Fig 3. Meta-analysis of studies comparing blood loss between minimally invasive surgery and open surgery groups based on a fixed-effect model.
Fig 4. Meta-analysis of studies comparing blood transfusion rate between minimally invasive surgery and open surgery groups based on a fixed-effect model.
Fig 5. Meta-analysis of studies comparing R0 resection rate between minimally invasive surgery and open surgery groups based on a fixed-effect model.
Fig 6. Meta-analysis of studies comparing lymph node received between minimally invasive surgery and open surgery groups based on a fixed-effect model.
Meta-analysis of postoperative complications
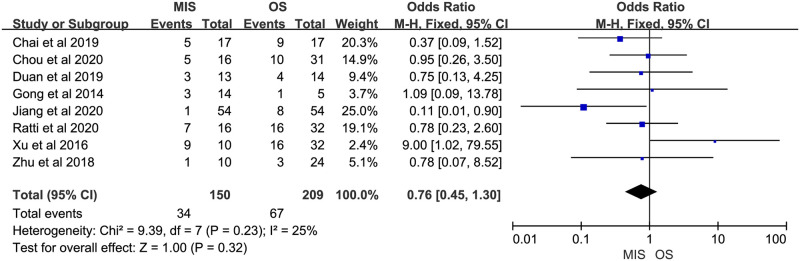
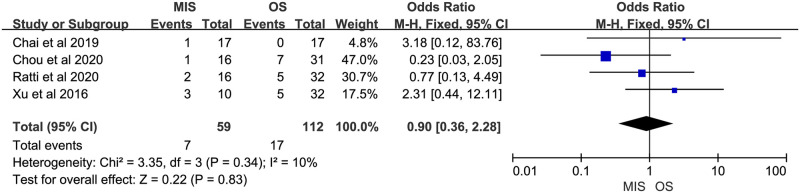
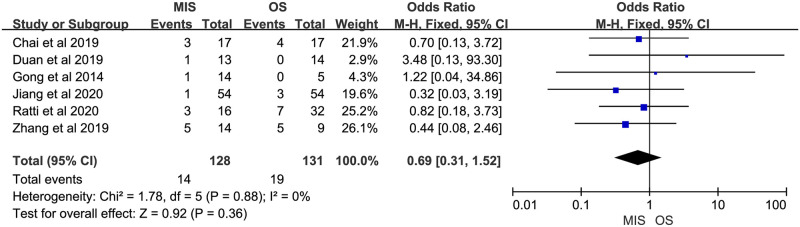
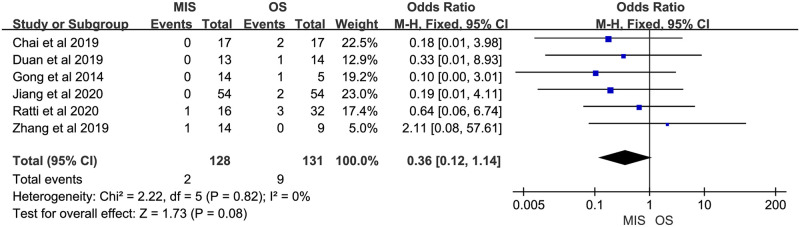
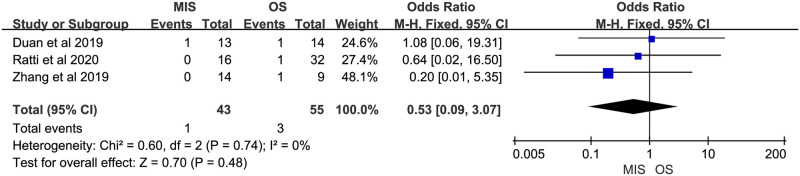
Eight studies reported overall morbidity. Seven of them suggested no significant difference between MIS and OS, 1 study showed a significant higher overall morbidity in MIS group. There was no significant heterogeneity (P = 0.23, I2 = 25%). A fixed-effect model indicated there was no significant difference between MIS and OS for overall morbidity (OR = 0.76, 95% CI = 0.45 to 1.30, P = 0.32; Fig 7). Four studies provided data on severe morbidity, with all of them suggesting no significant difference between MIS and OS patients. Insignificant heterogeneity among the 4 studies was found (P = 0.34, I2 = 10%). A fixed-effect model revealed similar severe morbidity between MIS and OS (OR = 0.90, 95% CI = 0.36 to 2.28, P = 0.83; Fig 8). Six studies reported bile leakage rate, all showed no significant difference existed between MIS and OS groups. A fixed-effect model revealed similar bile leakage rate between MIS and OS (OR = 0.69, 95% CI = 0.31 to 1.52, P = 0.36; Fig 9). There was no significant heterogeneity observed (P = 0.88, I2 = 0%). Six studies reported wound infection rate, all studies suggested there was no significant difference between MIS and OS. No significant heterogeneity was observed (P = 0.82, I2 = 0%). A fixed-effect model revealed a lower but insignificant wound infection rate in MIS than OS group (OR = 0.36, 95% CI = 0.12 to 1.14, P = 0.08; Fig 10). Three studies reported intra-abdominal infection rate. All suggested no significant difference between MIS and OS. No significant heterogeneity was observed (P = 0.74, I2 = 0%). A fixed-effect model revealed comparable intra-abdominal infection rate between MIS and OS group (OR = 0.53, 95% CI = 0.09 to 3.07, P = 0.48; Fig 11).
Fig 7. Meta-analysis of studies comparing overall morbidity between minimally invasive surgery and open surgery groups based on a fixed-effect model.
Fig 8. Meta-analysis of studies comparing severe morbidity between minimally invasive surgery and open surgery groups based on a fixed-effect model.
Fig 9. Meta-analysis of studies comparing bile leakage rate between minimally invasive surgery and open surgery groups based on a fixed-effect model.
Fig 10. Meta-analysis of studies comparing wound infection rate between minimally invasive surgery and open surgery groups based on a fixed-effect model.
Fig 11. Meta-analysis of studies comparing intra-abdominal infection rate between minimally invasive surgery and open surgery groups based on a fixed-effect model.
Meta-analysis of postoperative outcomes
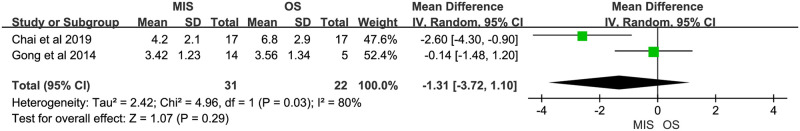
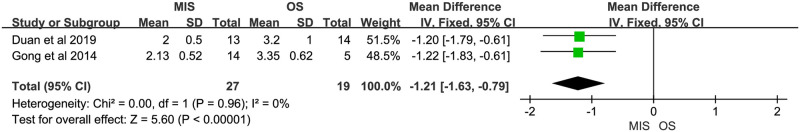
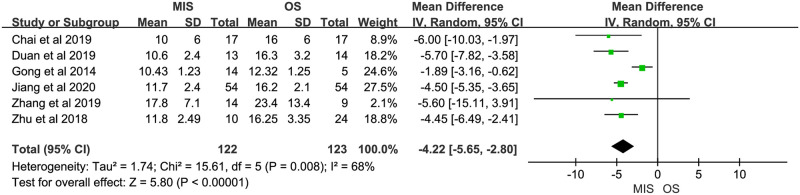
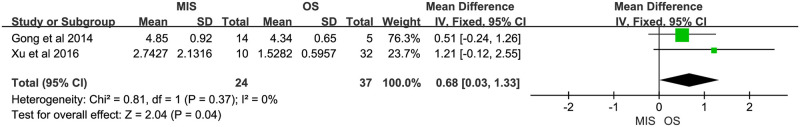
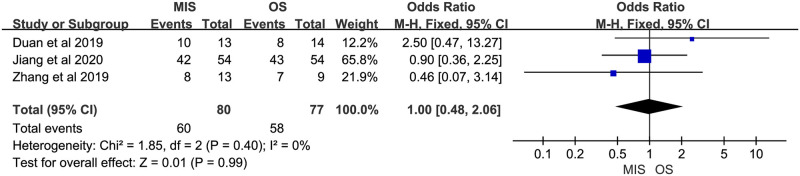
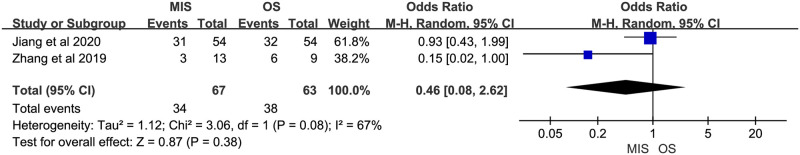
Two studies reported days until oral feeding. One study suggested no significant difference between MIS and OS, another showed a significant less days until oral feeding in MIS group. Significant heterogeneity was observed (P = 0.03, I2 = 80%). A random-effect model suggested no significant difference between MIS and OS (MD = -1.31, 95% CI = -3.72 to 1.10, P = 0.29; Fig 12). Two studies reported length of postoperative analgesia. Both indicated a significant shorter length of postoperative analgesia in MIS. No significant heterogeneity was observed (P = 0.96, I2 = 0%). A fix-effect model showed a significant shorter length of postoperative analgesia in MIS compared with OS (MD = -1.21, 95% CI = -1.63 to -0.79, P < 0.00001; Fig 13). Six studies reported length of hospital stay. Five of them suggested a significant shorter length of hospital stay in MIS patients, and the rest one showed comparable length of hospital stay between MIS and OS groups. Significant heterogeneity was observed (P = 0.008, I2 = 68%). A random-effect model revealed a significantly shorter length of hospital stay in MIS compared with OS (MD = -4.22, 95% CI = -5.65 to -2.80, P < 0.00001; Fig 14). Two studies provided data about hospital cost. Both suggested no significant difference between MIS and OS. There was no significant heterogeneity (P = 0.37, I2 = 0%). A fixed-effect model showed significant higher hospital cost in MIS group (MD = 0.68, 95% CI = 0.03 to 1.33, P = 0.04; Fig 15). Three studies reported 1-year overall survival. All suggested there was no significant difference between MIS and OS. No significant heterogeneity was observed (P = 0.40, I2 = 0%). A fixed-effect model revealed comparable 1-year overall survival between MIS and OS (OR = 1.00, 95% CI = 0.48 to 2.06, P = 0.99; Fig 16). Two studies reported 2-year overall survival. Both studies suggested comparable 2-year overall survival between MIS and OS. Significant heterogeneity was observed (P = 0.08, I2 = 67%). A random-effect model revealed there was no significant difference between MIS and OS groups (OR = 0.46, 95% CI = 0.08 to 2.62, P = 0.38; Fig 17).
Fig 12. Meta-analysis of studies comparing days until oral feeding between minimally invasive surgery and open surgery groups based on a random-effect model.
Fig 13. Meta-analysis of studies comparing length of postoperative analgesia between minimally invasive surgery and open surgery groups based on a fixed-effect model.
Fig 14. Meta-analysis of studies comparing length of hospital stay between minimally invasive surgery and open surgery groups based on a random-effect model.
Fig 15. Meta-analysis of studies comparing hospital cost between minimally invasive surgery and open surgery groups based on a fixed-effect model.
Fig 16. Meta-analysis of studies comparing 1-year overall survival between minimally invasive surgery and open surgery groups based on a fixed-effect model.
Fig 17. Meta-analysis of studies comparing 2-year overall survival between minimally invasive surgery and open surgery groups based on a random-effect model.
Meta-analysis of postoperative mortality
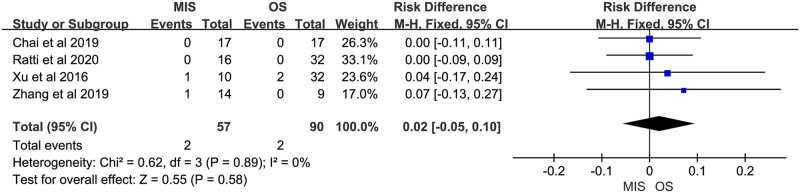
Four studies reported postoperative mortality, all suggested no significant difference between MIS and OS. No significant heterogeneity was observed (P = 0.89, I2 = 0%). A fixed-effect model suggested comparable postoperative mortality between MIS and OS (RD = 0.02, 95% CI = -0.05 to 0.10, P = 0.58; Fig 18).
Fig 18. Meta-analysis of studies comparing postoperative mortality between minimally invasive surgery and open surgery groups based on a fixed-effect model.
Publication bias assessment, sensitivity analysis, and subgroup analysis
There was no evidence of publication bias for blood loss, blood transfusion rate, R0 resection rate, lymph nodes received, overall morbidity, severe morbidity, bile leakage rate, intra-abdominal infection rate, days until oral feeding, length of postoperative analgesia, hospital cost, 1-year overall survival, 2-year overall survival, and postoperative mortality, with a symmetrical appearance on funnel plots. For operation time, wound infection rate, and length of hospital stay, funnel plots showed an asymmetry which suggested negative studies might be less reported. According to the sensitivity analysis, most of overall results did not change after the exclusion of a single study except overall morbidity and wound infection. To investigate the source of heterogeneity among studies, a subgroup analysis was carried out by stratifying the analysis according to several important factors, including: sample sizes, types of Bismuth–Corlette classification, and types of MIS (Table 2). All subgroup results were in line with the main results.
Table 2. Subgroup analysis.
| Outcomes | Subgroup | Studies (n) | Effect estimate (95% CI) | P value | Heterogeneity | Inconsistency with the overall result |
|---|---|---|---|---|---|---|
| Operation time | Sample size < 30 | 3 | 93.41 (34.69, 152.14) | P = 0.002 | P = 0.01, I2 = 77% | |
| Sample size > = 30 | 6 | 97.43 (56.10, 138.75) | P < 0.00001 | P < 0.00001, I2 = 88% | ||
| Bismuth I | 3 | 50.19 (44.02, 56.36) | P < 0.00001 | P = 1.00, I2 = 0% | ||
| Mixed | 6 | 133.80 (82.97, 184.63) | P < 0.00001 | P = 0.003, I2 = 73% | ||
| Robotic surgery | 2 | 152.56 (7.55, 297.57) | P = 0.04 | P < 0.0001, I2 = 94% | ||
| Laparoscopic surgery | 7 | 67.47 (46.81, 88.14) | P < 0.00001 | P = 0.01, I2 = 62% | ||
| Overall | 9 | 93.51 (64.10, 122.91) | P < 0.00001 | P < 0.00001, I2 = 85% | ||
| length of hospital | Sample size < 30 | 3 | -3.87 (-7.20, -0.55) | P = 0.02 | P = 0.009, I2 = 79% | |
| stay | Sample size > = 30 | 3 | -4.55 (-5.32, -3.78) | P < 0.00001 | P = 0.77, I2 = 0% | |
| Bismuth I | 3 | -4.64 (-5.37, -3.90) | P < 0.00001 | P = 0.58, I2 = 0% | ||
| Mixed | 3 | -3.56 (-6.76, -0.36) | P = 0.03 | P = 0.13, I2 = 51% | ||
| Robotic surgery | 0 | N/A | N/A | N/A | N/A | |
| Laparoscopic surgery | 6 | -4.22 (-5.65, -2.80) | P < 0.00001 | P = 0.008, I2 = 68% | ||
| Overall | 6 | -4.22 (-5.65, -2.80) | P < 0.00001 | P = 0.008, I2 = 68% |
Discussion
MIS features smaller incision, less pain, faster recovery, and earlier discharge. It has revolutionized the treatment concept of hepatobiliary surgery [40, 41]. Minimally invasive major hepatectomy and pancreatoduodenectomy have been accepted and widely performed by more and more surgeons with no inferiority to OS [42–44]. Furthermore, in the fields of treatment for gallbladder stone, minimally invasive procedure has been even viewed as a standard treatment method over the years. In this circumstances, the idea of MIS for HCCA formed spontaneously. Recently, the adoption of MIS in treating Bismuth type-I to type-IV HCCA increased for the rapid development of surgical methods and equipment and the accumulation of MIS experience [17]. Compared to laparoscopic procedure, robotic techniques provide fine dissection, endoscopic suturing, and microanastomosis better, and most importantly, enable operating in a delicate space [45–47]. With the increased experience in robotic surgery, biliary reconstruction was no longer a contraindication for MIS [48–50]. However, the complexity of portal dissection and biliary reconstruction is still a big concern that restricts the development of minimally invasive approach and most studies were limited in highly selected cases. MIS for HCCA is still in its infancy [51, 52]. The lack of large volume studies and the uncertainty of the clinical outcomes of MIS for HCCA restricted the progress of minimally invasive concept in HCCA severely. Thus, at these early stages, further studies comparing the outcomes of MIS with OS are crucial in expanding the application of minimally invasive method in HCCA. With the aim of assessing the safety and feasibility of laparoscopic surgery and robotic surgery for HCCA, we conducted this meta-analysis.
R0 resection, which means no residual tumor in the resection margins of the bile ducts, is viewed as the most important factor affecting long-term survival in the surgical therapy for HCCA. The positive rate of resection margins of bile ducts directly affected the prognosis of patient and patient without R0 resection was demonstrated to have a dismal survival [53–55]. Thus, to achieve R0 margins is crucial to curative treatment in HCCA resectional surgery [8]. However, R0 resection was considered to be hard to guarantee for the following 3 reasons. First, numerous vital structures around the tumor in the liver hilum. Second, the difficulty in determining the exact length and width of microscopic tumor extension pre-operatively and intra-operatively for the microscopic biological extending nature along the bile ducts [56]. Third, the accuracy, sensitivity, and specificity of intra-operative frozen-section examination of ductal margins were only 56.5%, 75.0%, and 46.7%, respectively [57]. The uncertainty of R0 resection of MIS for HCCA was a big concern. In this meta-analysis, our result indicated that, compared with traditional OS, patients undergoing MIS for HCCA experienced comparable R0 resection rate. This result is quite crucial and encouraging for the further development of MIS.
The main histopathologic type of HCCA was adenocarcinoma and the lymph node metastasis rate ranged from 30.0% to 60.0% [35]. Lymphadenectomy is an essential component of radical resection for HCCA and is another factor affecting long-term survival. However, the scope of lymphadenectomy is controversial. In most cases, the regional lymph nodes ranging from the hepatoduodenal ligament to the superior border of the pancreatic head should be routinely dissected [58, 59]. In our study, the pooled result indicated that there was no statistical difference regarding the lymph nodes received between the two groups, with relatively lower in OS group. Furthermore, it is worth to note here that excessive dissection of lymph nodes around the hepatic artery might result in the mechanical injury of vessel and increase the incidence rate of hepatic artery pseudoaneurysm. In Zhang’s cases [32], one patient suffered from postoperative bleeding, and the reason was confirmed to be the rupture of hepatic artery pseudoaneurysm during reoperation.
Whether postoperative morbidity is more frequent in MIS remains uncertain. Liver failure was one of the most common cause of death after surgery for HCCA [31, 60]. Previous related studies considered pre-operative embolism of the portal vein might increase postoperative residual liver volume to prevent perioperative death due to acute liver failure [61]. However, some other studies believed the functional reserve of liver was more essential in affecting post-hepatectomy liver failure and postoperative mortality than the residual liver volume preserved [62, 63]. In Xu’s experience [31], percutaneous transhepatic biliary drainage should be considered preoperatively in reducing postoperative liver failure and mortality. Bile leakage was thought to be one of the most common complications that might prolong hospital stay following radical resection for HCCA [64]. Improvement of the surgeon’s skill in suturing the bile-duct wall was the key factor in reducing bile leakage rate, while early detection and timely conservative therapy (including abdominal drainage) were essential to shorten hospital stay [60, 64, 65]. Hepatic arterial pseudoaneurysm was an uncommon complication after surgery for HCCA, in robotic surgery, Xu et al. [31] thought an imperceptible mechanical injury might do some damage to the endothelium by the frequent grasping of the vessels due to the lack of tactile feedback from the instrument. And one patient received reoperation for the rupture of hepatic arterial pseudoaneurysm in their cases. Besides, potential risk factors of high morbidity included prolonged operative time and the physical and hemodynamic stress placed by prolonged duration of pneumoperitoneum over 15 mmHg [66]. Our pooled results indicated that the postoperative morbidity and mortality in MIS were comparable with OS for HCCA.
In addition, patients undergoing MIS for HCCA experienced prolonged operative time compared with traditional OS. This was thought to be due to the surgeons’ inexperience in the early phase of learning curve, the technical limitations of minimally invasive approach in liver mobilization and retraction, and the forced use of anterior approach for vascular management [31]. This phenomenon is commonly observed in the development process of a laparoscopic surgery, and the prolonged operative time will gradually improved with the accumulation of MIS experience and the surgical techniques.
However, we have to acknowledge some limitations in our study. First, all studies included in this systematic review were small retrospective cohort studies, no high volume study or well-designed randomized controlled trial was included. Further studies with larger patient cohorts and sufficient follow-up duration are required to demonstrate the results. Second, the existence of significant heterogeneity in some outcomes was not explained well enough by subgroup analysis. Third, definitions of some outcomes were not clear or uniform in different studies. Fourth, under the current circumstances, MIS for HCCA was only performed for highly selected patients at very limited institutions. In this review, most of the included studies were conducted in China, the scarcity of worldwide report might cause some potential bias.
Conclusions
In conclusion, this is to date the first meta-analysis comparing MIS with OS for HCCA worldwide. Our initial results demonstrated that, in selected patients, MIS was not inferior to OS in consideration of blood transfusion rate, R0 resection rate, lymph nodes received, overall morbidity, severe morbidity, bile leakage rate, wound infection rate, intra-abdominal infection rate, days until oral feeding, patent overall survival, and postoperative mortality. Although the expenditure for MIS was higher due to the instrumental cost, it obtained a benefit of minimal invasiveness, that was less blood loss, less postoperative pain, and shorter hospital stay. The cost-effectiveness of MIS for HCCA is acceptable. Therefore, MIS is a valuable option for patients with HCCA for providing a remarkable alternative to OS, the application of MIS for HCCA should be considered more worldwide.
Supporting information
(PDF)
(PDF)
(PDF)
Acknowledgments
The authors would like to thank the staffs in the library of the First Affiliated Hospital of Chongqing Medical University for literature support.
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
Funding sources: Basic Research and Frontier Exploration Project of Chongqing Science and Technology Commission (cstc2018jcyjAX0825); Basic Research and Frontier Exploration Project of Chongqing Science and Technology Commission (cstc2017jcyjBX0010); Basic Research and Frontier Exploration Project of Chongqing Science and Technology Commission (cstc2018jscx-msybX0133); National Natural Science Foundation of China (81702408).
References
- 1.Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg. 1992;215(1):31–8. 10.1097/00000658-199201000-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu HJ, Mao H, Shrestha A, Tan YQ, Ma WJ, Yang Q, et al. Prognostic factors and long-term outcomes of hilar cholangiocarcinoma: a single-institution experience in China. World J Gastroenterol. 2016;22(8):2601–10. 10.3748/wjg.v22.i8.2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kosuge T, Yamamoto J, Shimada K, Yamasaki S, Makuuchi M. Improved surgical results for hilar cholangiocarcinoma with procedures including major hepatic resection. Ann Surg. 1999;230(5):663–71. 10.1097/00000658-199911000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rea DJ, Munoz-Juarez M, Farnell MB, Donohue JH, Que FG, Crownhart B, et al. Major hepatic resection for hilar cholangiocarcinoma: analysis of 46 patients. Arch Surg. 2004;139(5):514–23. 10.1001/archsurg.139.5.514 [DOI] [PubMed] [Google Scholar]
- 5.Pichlmayr R, Weimann A, Klempnauer J, Oldhafer KJ, Maschek H, Tusch G, et al. Surgical treatment in proximal bile duct cancer. A single-center experience. Ann Surg. 1996;224(5):628–38. 10.1097/00000658-199611000-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsao JI, Nimura Y, Kamiya J, Hayakawa N, Kondo S, Nagino M, et al. Management of hilar cholangiocarcinoma: comparison of an American and a Japanese experience. Ann Surg. 2000;232(2):166–74. 10.1097/00000658-200008000-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Gulik TM, Gouma DJ. Changing perspectives in the assessment of resectability of hilar cholangiocarcinoma. Ann Surg Oncol. 2007;14(7):1969–71. 10.1245/s10434-007-9394-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito F, Cho CS, Rikkers LF, Weber SM. Hilar cholangiocarcinoma: current management. Ann Surg. 2009;250(2):210–8. 10.1097/SLA.0b013e3181afe0ab [DOI] [PubMed] [Google Scholar]
- 9.Mansour JC, Aloia TA, Crane CH, Heimbach JK, Nagino M, Vauthey JN. Hilar cholangiocarcinoma: expert consensus statement. HPB (Oxford). 2015;17(8):691–9. 10.1111/hpb.12450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagino M, Ebata T, Yokoyama Y, Igami T, Sugawara G, Takahashi Y, et al. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg. 2013;258(1):129–40. 10.1097/SLA.0b013e3182708b57 [DOI] [PubMed] [Google Scholar]
- 11.van Gulik TM, Kloek JJ, Ruys AT, Busch OR, van Tienhoven GJ, Lameris JS, et al. Multidisciplinary management of hilar cholangiocarcinoma (Klatskin tumor): extended resection is associated with improved survival. Eur J Surg Oncol. 2011;37(1):65–71. 10.1016/j.ejso.2010.11.008 [DOI] [PubMed] [Google Scholar]
- 12.Nishio H, Nagino M, Nimura Y. Surgical management of hilar cholangiocarcinoma: the Nagoya experience. HPB (Oxford). 2005;7(4):259–62. 10.1080/13651820500373010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim C, Salloum C, Tudisco A, Ricci C, Osseis M, Napoli N, et al. Short- and long-term outcomes after robotic and laparoscopic liver resection for malignancies: a propensity score-matched study. World J Surg. 2019;43(6):1594–1603. 10.1007/s00268-019-04927-x [DOI] [PubMed] [Google Scholar]
- 14.Ciria R, Cherqui D, Geller DA, Briceno J, Wakabayashi G. Comparative short-term benefits of laparoscopic liver resection: 9000 cases and climbing. Ann Surg. 2016;263(4):761–77. 10.1097/SLA.0000000000001413 [DOI] [PubMed] [Google Scholar]
- 15.Fretland ÅA, Dagenborg VJ, Bjørnelv GMW, Kazaryan AM, Kristiansen R, Fagerland MW, et al. Laparoscopic versus open resection for colorectal liver metastases: the OSLO-COMET randomized controlled trial. Ann Surg. 2018;267(2):199–207. 10.1097/SLA.0000000000002353 [DOI] [PubMed] [Google Scholar]
- 16.Bryant R, Laurent A, Tayar C, Cherqui D. Laparoscopic liver resection-understanding its role in current practice: the Henri Mondor Hospital experience. Ann Surg. 2009;250(1):103–11. 10.1097/SLA.0b013e3181ad6660 [DOI] [PubMed] [Google Scholar]
- 17.Giulianotti PC, Sbrana F, Bianco FM, Addeo P. Robot-assisted laparoscopic extended right hepatectomy with biliary reconstruction. J Laparoendosc Adv Surg Tech A. 2010;20(2):159–63. 10.1089/lap.2009.0383 [DOI] [PubMed] [Google Scholar]
- 18.Antoniou SA, Antoniou GA, Antoniou AI, Granderath FA. Past, present, and future of minimally invasive abdominal surgery. JSLS. 2015;19(3):e2015.00052. 10.4293/JSLS.2015.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berber E, Akyildiz HY, Aucejo F, Gunasekaran G, Chalikonda S, Fung J. Robotic versus laparoscopic resection of liver tumours. HPB (Oxford). 2010;12(8):583–6. 10.1111/j.1477-2574.2010.00234.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu R, Zhang T, Zhao ZM, Tan XL, Zhao GD, Zhang X, et al. The surgical outcomes of robot-assisted laparoscopic pancreaticoduodenectomy versus laparoscopic pancreaticoduodenectomy for periampullary neoplasms: a comparative study of a single center. Surg Endosc. 2017;31(6):2380–2386. 10.1007/s00464-016-5238-6 [DOI] [PubMed] [Google Scholar]
- 21.Zhang T, Zhao ZM, Gao YX, Lau WY, Liu R. The learning curve for a surgeon in robot-assisted laparoscopic pancreaticoduodenectomy: a retrospective study in a high-volume pancreatic center. Surg Endosc. 2019;33(9):2927–2933. 10.1007/s00464-018-6595-0 [DOI] [PubMed] [Google Scholar]
- 22.Ocuin LM, Tsung A. Robotic liver resection for malignancy: current status, oncologic outcomes, comparison to laparoscopy, and future applications. J Surg Oncol. 2015;112(3):295–301. 10.1002/jso.23901 [DOI] [PubMed] [Google Scholar]
- 23.Magistri P, Tarantino G, Assirati G, Olivieri T, Catellani B, Guerrini GP, et al. Robotic liver resection for hepatocellular carcinoma: a systematic review. Int J Med Robot. 2019;15(4):e2004. 10.1002/rcs.2004 [DOI] [PubMed] [Google Scholar]
- 24.Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2804 patients. Ann Surg. 2009;250(5):831–41. 10.1097/SLA.0b013e3181b0c4df [DOI] [PubMed] [Google Scholar]
- 25.Koffron AJ, Auffenberg G, Kung R, Abecassis M. Evaluation of 300 minimally invasive liver resections at a single institution: less is more. Ann Surg. 2007;246(3):385–92. 10.1097/SLA.0b013e318146996c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Reprint—preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther. 2009;89(9):873–80. [PubMed] [Google Scholar]
- 27.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–96. 10.1097/SLA.0b013e3181b13ca2 [DOI] [PubMed] [Google Scholar]
- 28.Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149(5):680–8. 10.1016/j.surg.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 29.Grobmyer SR, Rivadeneira DE, Goodman CA, Mackrell P, Lieberman MD, Daly JM. Pancreatic anastomotic failure after pancreaticoduodenectomy. Am J Surg. 2000;180(2):117–20. 10.1016/s0002-9610(00)00423-2 [DOI] [PubMed] [Google Scholar]
- 30.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 31.Xu Y, Wang H, Ji W, Tang M, Li H, Leng J, et al. Robotic radical resection for hilar cholangiocarcinoma: perioperative and long-term outcomes of an initial series. Surg Endosc. 2016;30(7):3060–70. 10.1007/s00464-016-4925-7 [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Dou C, Wu W, Liu J, Jin L, Hu Z, et al. Total laparoscopic versus open radical resection for hilar cholangiocarcinoma. Surg Endosc. 2020;34(10):4382–4387. 10.1007/s00464-019-07211-0 [DOI] [PubMed] [Google Scholar]
- 33.Jiang WX, Zhou L, Hu YJ. Comparison of different surgical procedures for Bismuth-hilar cholangiocarcinoma. Chin J Oper Proc Gen Surg (Electronic Edition). 2020;14(1):78–80. 10.3877/cma.j.issn.1674-3946.2020.01.023 [DOI] [Google Scholar]
- 34.Zhu HC, Zhou LF, Liu TD, Liu HL, Luo DL, Huang MW. Analysis of laparoscopic and open radical resection for hilar cholangiocarcinoma of type Bismuth-I. Chin J of Endosc. 2018;24(2):10–15. 10.3969/j.issn.1007-1989.2018.02.003 [DOI] [Google Scholar]
- 35.Gong JW, Liu CF. The comparative study of laparoscopic and open radical operation for hilar cholangiocarcinoma. J Laparosc Surg. 2014;19(5):321–324. [Google Scholar]
- 36.Chou S, Chang ZY, Zhao GD, Song DD, Zhang X, Hu MG, er al. Robotic hilar cholangiocarcinoma radical resection compared with laparotomy in prognosis. Chin J Surg. 2020;58(3):230–234. 10.3760/cma.j.issn.0529-5815.2020.03.012 [DOI] [PubMed] [Google Scholar]
- 37.Duan C, Zhu QS, Wang MZ, Niu YF, Zhang WL, Li SJ. Clinical investigation of two surgical methods in radical resection of Bismuth-I hilar cholangiocarcinoma. Chin J Oper Proc Gen Surg (Electronic Edition). 2019;13(6):552–555. [Google Scholar]
- 38.Chai W, Zhang ZQ, Lei B, Meng Y, Zhao XL, Zhang L, et al. A case matched study on laparoscopic versus open radical resection for Bismuth-type IIIb hilar cholangiocarcinoma. Chin J Gen Surg. 2019;34(5):377–380. [Google Scholar]
- 39.Ratti F, Fiorentini G, Cipriani F, Catena M, Paganelli M, Aldrighetti L. Perihilar cholangiocarcinoma: are we ready to step towards minimally invasiveness? Updates Surg. 2020;72(2):423–433. 10.1007/s13304-020-00752-3 [DOI] [PubMed] [Google Scholar]
- 40.Buell JF, Cherqui D, Geller DA, O’Rourke N, Iannitti D, Dagher I, et al. The international position on laparoscopic liver surgery: the Louisville Statement, 2008. Ann Surg. 2009;250(5):825–30. 10.1097/sla.0b013e3181b3b2d8 [DOI] [PubMed] [Google Scholar]
- 41.Chana P, Burns EM, Arora S, Darzi AW, Faiz OD. A systematic review of the impact of dedicated emergency surgical services on patient outcomes. Ann Surg. 2016;263(1):20–7. 10.1097/SLA.0000000000001180 [DOI] [PubMed] [Google Scholar]
- 42.Boggi U, Signori S, De Lio N, Perrone VG, Vistoli F, Belluomini M, et al. Feasibility of robotic pancreaticoduodenectomy. Br J Surg. 2013;100(7):917–25. 10.1002/bjs.9135 [DOI] [PubMed] [Google Scholar]
- 43.Coppola A, Stauffer JA, Asbun HJ. Laparoscopic pancreatoduodenectomy: current status and future directions. Updates Surg. 2016;68(3):217–224. 10.1007/s13304-016-0402-z [DOI] [PubMed] [Google Scholar]
- 44.Dagher I, O’Rourke N, Geller DA, Cherqui D, Belli G, Gamblin TC, et al. Laparoscopic major hepatectomy: an evolution in standard of care. Ann Surg. 2009;250(5):856–60. 10.1097/SLA.0b013e3181bcaf46 [DOI] [PubMed] [Google Scholar]
- 45.Ji WB, Wang HG, Zhao ZM, Duan WD, Lu F, Dong JH. Robotic-assisted laparoscopic anatomic hepatectomy in China: initial experience. Ann Surg. 2011;253(2):342–8. 10.1097/SLA.0b013e3181ff4601 [DOI] [PubMed] [Google Scholar]
- 46.Giulianotti PC, Coratti A, Angelini M, Sbrana F, Cecconi S, Balestracci T, et al. Robotics in general surgery: personal experience in a large community hospital. Arch Surg. 2003;138(7):777–84. 10.1001/archsurg.138.7.777 [DOI] [PubMed] [Google Scholar]
- 47.Talamini MA, Chapman S, Horgan S, Melvin WS; Academic Robotics Group. A prospective analysis of 211 robotic-assisted surgical procedures. Surg Endosc. 2003;17(10):1521–4. 10.1007/s00464-002-8853-3 [DOI] [PubMed] [Google Scholar]
- 48.Giulianotti PC, Coratti A, Sbrana F, Addeo P, Bianco FM, Buchs NC, et al. Robotic liver surgery: results for 70 resections. Surgery. 2011;149(1):29–39. 10.1016/j.surg.2010.04.002 [DOI] [PubMed] [Google Scholar]
- 49.Lai EC, Tang CN. Robot-assisted laparoscopic hepaticojejunostomy for advanced malignant biliary obstruction. Asian J Surg. 2015;38(4):210–3. 10.1016/j.asjsur.2015.01.010 [DOI] [PubMed] [Google Scholar]
- 50.Giulianotti PC, Sbrana F, Bianco FM, Elli EF, Shah G, Addeo P, et al. Robot-assisted laparoscopic pancreatic surgery: single-surgeon experience. Surg Endosc. 2010;24(7):1646–57. 10.1007/s00464-009-0825-4 [DOI] [PubMed] [Google Scholar]
- 51.Hu HJ, Wu ZR, Jin YW, Ma WJ, Yang Q, Wang JK, et al. Minimally invasive surgery for hilar cholangiocarcinoma: state of art and future perspectives. ANZ J Surg. 2019;89(5):476–480. 10.1111/ans.14765 [DOI] [PubMed] [Google Scholar]
- 52.Franken LC, van der Poel MJ, Latenstein AEJ, Zwart MJ, Roos E, Busch OR, et al. Minimally invasive surgery for perihilar cholangiocarcinoma: a systematic review. J Robot Surg. 2019;13(6):717–727. 10.1007/s11701-019-00964-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buettner S, Margonis GA, Kim Y, Gani F, Ethun CG, Poultsides G, et al. Conditional probability of long-term survival after resection of hilar cholangiocarcinoma. HPB (Oxford). 2016;18(6):510–7. 10.1016/j.hpb.2016.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hemming AW, Reed AI, Fujita S, Foley DP, Howard RJ. Surgical management of hilar cholangiocarcinoma. Ann Surg. 2005;241(5):693–9. 10.1097/01.sla.0000160701.38945.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ribero D, Amisano M, Lo Tesoriere R, Rosso S, Ferrero A, Capussotti L. Additional resection of an intraoperative margin-positive proximal bile duct improves survival in patients with hilar cholangiocarcinoma. Ann Surg. 2011;254(5):776–81. 10.1097/SLA.0b013e3182368f85 [DOI] [PubMed] [Google Scholar]
- 56.Xiang S, Lau WY, Chen XP. Hilar cholangiocarcinoma: controversies on the extent of surgical resection aiming at cure. Int J Colorectal Dis. 2015;30(2):159–71. 10.1007/s00384-014-2063-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Okazaki Y, Horimi T, Kotaka M, Morita S, Takasaki M. Study of the intrahepatic surgical margin of hilar bile duct carcinoma. Hepatogastroenterology. 2002;49(45):625–7. [PubMed] [Google Scholar]
- 58.Ito K, Ito H, Allen PJ, Gonen M, Klimstra D, D’Angelica MI, et al. Adequate lymph node assessment for extrahepatic bile duct adenocarcinoma. Ann Surg. 2010;251(4):675–81. 10.1097/SLA.0b013e3181d3d2b2 [DOI] [PubMed] [Google Scholar]
- 59.Schwarz RE, Smith DD. Lymph node dissection impact on staging and survival of extrahepatic cholangiocarcinomas, based on U.S. population data. J Gastrointest Surg. 2007;11(2):158–65. 10.1007/s11605-006-0018-6 [DOI] [PubMed] [Google Scholar]
- 60.Feng F, Cao X, Liu X, Qin J, Zhang S, Li Q, et al. Laparoscopic resection for Bismuth type III and IV hilar cholangiocarcinoma: how to improve the radicality without direct palpation. J Surg Oncol. 2019;120(8):1379–1385. 10.1002/jso.25739 [DOI] [PubMed] [Google Scholar]
- 61.Okabe H, Beppu T, Ishiko T, Masuda T, Hayashi H, Otao R, et al. Preoperative portal vein embolization (PVE) for patients with hepatocellular carcinoma can improve resectability and may improve disease-free survival. J Surg Oncol. 2011;104(6):641–6. 10.1002/jso.21928 [DOI] [PubMed] [Google Scholar]
- 62.Kubota K, Makuuchi M, Kusaka K, Kobayashi T, Miki K, Hasegawa K, et al. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997;26(5):1176–81. 10.1053/jhep.1997.v26.pm0009362359 [DOI] [PubMed] [Google Scholar]
- 63.Shirabe K, Shimada M, Gion T, Hasegawa H, Takenaka K, Utsunomiya T, et al. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg. 1999;188(3):304–9. 10.1016/s1072-7515(98)00301-9 [DOI] [PubMed] [Google Scholar]
- 64.Yu H, Wu SD, Chen DX, Zhu G. Laparoscopic resection of Bismuth type I and II hilar cholangiocarcinoma: an audit of 14 cases from two institutions. Dig Surg. 2011;28(1):44–9. 10.1159/000322398 [DOI] [PubMed] [Google Scholar]
- 65.Yu H, Wu SD, Tian Y, Su Y, Li YN. Single-incision laparoscopic resection of Bismuth I hilar cholangiocarcinoma. Surg Innov. 2013;20(3):209–13. 10.1177/1553350612438414 [DOI] [PubMed] [Google Scholar]
- 66.Özdemir-van Brunschot DM, van Laarhoven KC, Scheffer GJ, Pouwels S, Wever KE, Warlé MC. What is the evidence for the use of low-pressure pneumoperitoneum? A systematic review. Surg Endosc. 2016;30(5):2049–65. 10.1007/s00464-015-4454-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting information files.