Abstract
Purpose of Review:
This narrative review synthesizes research from the last two decades on the modulatory role of intranasal OT administration (IN-OT) on social cognition in early life, young/middle adulthood, and older adulthood. Advances and knowledge gaps are identified, and future research directions are discussed within an integrative human lifespan framework to guide novel research on IN-OT and social cognition.
Recent Findings:
Current evidence regarding IN-OT modulation of social-cognitive processes, behavior, and related neurocircuitry is mixed, with some studies suggesting benefits (e.g., improved social perception/interactions, emotion processing) depending on contextual (e.g., social stimuli) and interindividual factors (e.g., age, sex, clinical status). Current research, however, is limited by a focus on isolated life phases, males, and select clinical populations as well as a lack of standardized protocols.
Summary:
This literature-based reflection proposes that greater generalizability of findings and scientific advancement on social-cognitive modulation via IN-OT require standardized, multi-method, longitudinal, and cross-sequential assessments in well-powered, well-controlled, and representative samples in line with an integrative lifespan approach, which considers development as a lifelong dynamic process involving both change and stability characterized by the interplay between genetic, neurobiological, and socio-behavioral factors.
Keywords: Oxytocin, Intranasal Administration, Neuropeptide, Hormone, Lifespan Development, Social Cognition
1. Introduction
Oxytocin (OT) is a nine-amino-acid neuropeptide that is mainly synthesized in the hypothalamus and serves various evolutionarily conserved physiological, behavioral, and cognitive functions vital for survival in both humans and animals (e.g., metabolic and cardiovascular regulation, parental and other adaptive social behaviors) [1,2]. Though not exclusively a “social” neuropeptide, in recent years, OT has garnered particular interest as a modulator of human social cognition [2,3]. In early life as well as in adulthood, unimpaired social-cognitive abilities (e.g., social perception, processing, and related decision-making) are critical for accurate interpretation of social cues and facilitation of social interactions. The need for preserved, adaptive social cognition may also be particularly relevant in advanced age when declines are more evident across functional domains, such as in source memory [4], emotion identification [5], and deception detection [6]. Though the literature has attributed age-related social-cognitive declines to motivational and brain changes, the role of the OT system, and related neurocircuitry, in these declines is not well understood yet [7,8].
Intranasal OT (IN-OT) is currently the most practical, commonly used, and non-invasive route of exogenous OT delivery to the human brain [9,10]. IN-OT can be self-administered via a hand-held spray (most common) or through other specialized methods (e.g., breath-powered devices, nebulizers) [9,11] and has been increasingly utilized to investigate the modulation of social cognition in healthy and clinical populations [12]. IN-OT can impact social and emotional cue processing (e.g., facial emotion identification [13], social reward [14]) and has demonstrated potential to counter social-cognitive dysfunction in different phases of the lifespan (e.g., early development [15], adulthood [16], and aging [17]). Plasma OT concentrations have shown to peak at 15 minutes after IN-OT administration and decline after 75 minutes [18]. Whereas, peak OT response corresponding to regional cerebral blood flow has been reported to be between 39 and 51 minutes after intranasal administration in healthy men [19]. Alteration of regional cerebral blood flow by IN-OT has also been observed for up to two hours after administration [20]. Longer-term IN-OT effects would likely depend on repeated administration in therapeutic contexts [9,12].
It is suspected that IN-OT exerts its modulatory effects through different mechanisms, such as by increasing central OT concentrations via nose-to-brain pathways (e.g., olfactory and trigeminal nerves) [10] and/or altering cerebral blood flow (e.g., in the amygdala) [20]. As researchers continue to investigate IN-OT’s therapeutic potential, more research is needed regarding its pharmacokinetic mechanisms of action towards the development of more precise methods for non-invasive delivery to the brain.
Consistency in the efficacy of IN-OT in modulating social-cognitive processes and behaviors [21] and ameliorating social-cognitive dysfunction [22] also needs to be demonstrated, including across development, before broader therapeutic use is possible. Currently, the replicability of IN-OT effects on specific social-cognitive processes and behaviors (e.g., emotion processing [23]; trust [24]) remains in question. To this end, there is an urgent need for more direct, rather than conceptual, replication studies [25]. Also, the majority of IN-OT studies on human social cognition, to date, is limited by a focus on distinct/isolated age groups without an attempt to integrate findings across the human lifespan.
Development is a lifelong dynamic process involving both change and stability characterized by the interplay between genetic, neurobiological, and socio-behavioral factors. Integrating IN-OT effects on social cognition across the lifespan has the potential to broaden and generalize understanding of OT mechanisms and modulation across different social contexts, individuals, and developmental phases. OT response is not uniform across individuals [9], but rather varies by social situations (e.g., social/emotional memory, perception, recognition, sharing; Bartz et al., 2011) and interindividual factors (e.g., sex and age; [27–29]. We propose that an integrative lifespan approach to IN-OT research offers a more comprehensive understanding of OT mechanisms and modulation of social cognition, which can inform the design of efficacious treatment plans across developmental phases [9,30].
Towards such an integrative perspective, in this narrative review, we synthesize evidence accumulated over the last two decades from studies on IN-OT and social cognition in early life, young/middle adulthood, and older adulthood (see Table 1 for an overview of studies). Advances and current limitations of these still largely parallel lines of research are discussed and developed into constructive suggestions for future investigation within the proposed integrative lifespan framework (see Fig. 1).
Table 1.
Overview of IN-OT research on social cognition in humans (organized by life phase)
| Reference | Age (range and/or mean; in years) | Sex | Dosage & Frequency | Regimen & Duration | Design | Main Finding(s) |
|---|---|---|---|---|---|---|
| EARLY LIFE | ||||||
| PWS (Includes some mixed-age samples) | ||||||
| Einfeld et al., 2014 | 12–29; 17.8 | M/F | 24 and 40 IU (for 16+ year-olds); 18 and 32 IU (for 13–15 year-olds), BID | Repeated, 8 weeks | Double-blind, randomized, placebo-controlled, crossover | IN-OT increased temper outbursts with higher doses; no IN-OT effects on social cognition |
| Kuppens et al., 2016 | 6–13.7; 9.3 (median age) | M/F | 12, 16, 20, or 24 IU, BID | Repeated, 4 weeks | Double-blind, randomized, placebo-controlled, crossover | IN-OT reduced anger, sadness, conflicts, and improved social behavior in children younger than 11 years; no IN-OT effects in children older than 11 years |
| Miller et al., 2017 | 5–11; 8.2 | M/F | 16 IU, once a day | Repeated, 5 days | Double-blind, randomized, placebo-controlled, crossover | IN-OT improved socialization, anxiety, and repetitive behaviors |
| Tauber et al., 2017 | 0.8–5.7 months; 3.9 months | M/F | Step 1: 4 IU, every other day; Step 2: 4 IU, every day; Step 3: 4 IU, BID | Repeated, 7 days | Proof-of-concept, monocentric phase 2 escalating dose | IN-OT improved social withdrawal behavior and mother-infant interactions; increased neural network connectivity correlated with changes in social behavior |
| ASD in Youth | ||||||
| Guastella et al., 2010 | 12–19; 14.88 | M | 18 IU (for 12–15 year-olds); 24 IU (for 16–19 year-olds), once | Acute | Double-blind, randomized, placebo-controlled, crossover | IN-OT improved emotion recognition |
| Gordon et al., 2013 | 8–16.5; 13.2 | M/F | 12 IU (for 7–11 year-olds); 18 IU (for 12–15 year-olds); 24 IU (for 16+ year-olds), once | Acute | Double-blind, randomized, placebo-controlled, crossover | IN-OT increased activity for social judgments, but decreased activity for non-social judgments, in regions of the striatum, middle frontal gyrus, mPFC, orbitofrontal cortex, and superior temporal sulcus |
| Tachibana et al., 2013 | 10–14.5; 11.93 | M | 8, 16, 24 IU, BID (increased every 2 months) | Repeated, ~7 months | Open-label trial | IN-OT improved communication and social interactions; no IN-OT effects on other behavioral outcomes |
| Anagnostou et al., 2014 | 10–17 | M/F | 0.2, 0.26, 0.33, 0.4 IU/kg, BID | Repeated, 12 weeks | Modified Maximum Tolerated Dose Open-label trial | IN-OT improved scores and performance in some reassures of social function and social cognition |
| Dadds et al., 2014 | 7–16; 11.79 (OT), 10.74 (P) | M | 12 IU (for under 40 kg); 24 IU (for over 40 kg), once a day | Repeated, 4 days | Double-blind, randomized, placebo-controlled, between-groups | No IN-OT effects on emotion recognition, social interaction skills, or general behavioral adjustment |
| Guastella et al., 2015 | 12–18; 13.85 (OT), 14 (P) | M | 18 IU (for 12–15 year-olds); 24 IU (for 16–18 year-olds), BID | Repeated, 8 weeks | Double-blind, randomized, placebo‐controlled, between-groups | No IN-OT effects on social behavior |
| Gordon et al., 2016 | 8–16.5; 13.16 | M/F | 24 IU (for 16–19 years); 18 IU (for 12–15 year-olds); 12 IU once (for 7–11 year-olds), once a day | Repeated, 21 days | Double-blind, randomized, placebo-controlled, crossover | IN-OT enhanced connectivity between reward and socioemotional processing related regions especially for social (vs. non-social) stimuli |
| Munesue et al., 2016 | 15–45; 22.5 | M | 8 IU, BID | Repeated, 8 weeks | Double-blind, randomized, placebo-controlled, crossover | IN-OT increased reciprocal social interactions; no IN-OT effects on core social symptoms |
| Yatawara et al., 2016 | 3–8; 6.2 | M/F | 12 IU, BID | Repeated, 5 weeks | Double-blind, randomized, placebo-controlled, crossover | IN-OT increased caregiver-rated social responsiveness |
| Parker et al., 2017 | 6–12; 9.35 (OT), 8.13 (P) | M/F | 24 IU, BID | Repeated, 4 weeks | Double-blind, randomized, placebo-controlled, parallel-groups | IN-OT enhanced social abilities, especially among those with the lowest levels of pre-treatment endogenous OT concentrations; no IN-OT effects on in repetitive behaviors or anxiety |
| YOUNG/MIDDLE ADULTHOOD | ||||||
| Healthy Populations | ||||||
| Kosfeld et al., 2005 | 22 | M | 24 IU, once | Acute | Double-blind, randomized, placebo-controlled, between-groups | IN-OT increased trust in others |
| Kirsch et al., 2005 | 18–40; 26.7 | M | 27 IU; once | Acute | Double-blind, randomized, placebo-controlled, crossover | IN-OT reduced amygdala activation to fearful faces |
| Baumgartner et al., 2008 | 21.7 | M | 24 IU, once | Acute | Double-blind, randomized, placebo-controlled, between-groups | IN-OT reduced fear of social betrayal and activation in regions involved in fear processing (amygdala and midbrain regions) and post-feedback adaptations (dorsal striatum) |
| Bos et al., 2018 | 19–24; 20.2 | F | 24 IU, once | Acute | Placebo-controlled, within-groups | IN-OT was associated with activation in reward and salience processing regions in response to viewing infants’ faces |
| Bartz et al., 2019 | 18–40; 22.7 (M), 21.6 (F) | M/F | 24 IU, once | Acute | Double-blind, randomized, placebo-controlled, crossover | IN-OT selectively improved empathic accuracy for less socially proficient males (vs. more proficient); no IN-OT effects on empathic accuracy for females |
| Quintana et al., 2019 | 20–30; 23.81 | M | 8 IU intranasally; 24 IU intranasally; 1 IU intravenously, once | Acute | Double-blind, randomized, double-dummy, crossover | 8 IU IN-OT reduced pupil dilation and resulted in a positive association with pupil dilation and right amygdala activation when processing social (faces) and non-social (shapes) stimuli; no OT-related effects were obtained for other doses |
| Tabak et al., 2019 | 18–31; 20.88 | M/F | 24 IU, once | Acute | Double-blind, randomized, placebo-controlled, between-groups | No IN-OT effects on various tasks tapping into different constructs (e.g., deception detection, empathy, working memory) and involving various stimuli (e.g., social and non-social) |
| Sheng et al. (2013) | 18–26; 21.88 | M | 32 IU, once | Acute | Double-blind, randomized, placebo-controlled, within-groups | OT elicited larger ERPs (P2) to painful than neutral faces of racial in-group but not racial out-group faces. OT resulted in positive correlation between P2 amplitudes and implicit attitude toward in-group members. |
| Xue et al., 2020 | 21.7 | M | 24 IU, once | Acute | Double-blind, randomized, placebo-controlled, between-groups | IN-OT resulted in faster perception and larger pupil dilation for social (vs. non-social) stimuli in an interocular suppression task tapping into conscious visual awareness |
| Clinical Populations | ||||||
| ASD | ||||||
| Anagnostou et al., 2012 | 18–60; 33 | M | 24 IU, BID | Repeated, 6 weeks | Double-blind, randomized, placebo-controlled, parallel-groups | IN-OT improved social perception measured by RMET, repetitive behaviors, and emotional well-being |
| Domes et al., 2013 | 24 (ASD); 24.3 (HC) | M | 24 IU, once | Acute | Double-blind, randomized, placebo-controlled, between-groups | IN-OT increased right amygdala activity to faces |
| Aoki et al., 2014 | 22–41, 30.8 | M | 24 IU, once | Acute | Double-blind, placebo-controlled, crossover | IN-OT increased accuracy for inferring others’ social emotions and increased right anterior insula activation |
| Watanabe et al., 2015 | 24–43; 32.2 | M | 24 IU, BID | Repeated, 6 weeks | Double-blind, randomized, placebo-controlled, crossover | IN-OT reduced symptoms relating to social reciprocity and increased resting-state functional connectivity between anterior cingulate cortex and dorsomedial prefrontal cortex |
| Kruppa et al., 2019 | 18–26, 21.79 (ASD); 18–25, 22.09 (HC) | M | 20 IU, once | Acute | Double-blind, randomized, placebo-controlled, crossover | IN-OT selectively enhanced reinforcement learning associated with social (vs. non-social) stimuli along with increased brain activity in the nucleus accumbens during social (vs. non-social) feedback |
| Bernaerts et al., 2020 | 18–35; 25 (OT), 24 (P) | M | 24 IU, once a day | Repeated, 4 weeks | Double-blind, randomized, placebo-controlled, parallel-groups | IN-OT was associated with a reduction in self-reported repetitive behaviors and feelings of avoidance towards others; no IN-OT effects on core social symptoms |
| SCZ | ||||||
| Guastella et al., 2015 | 22–57; 37.42 | M | 24 IU, once | Acute | Double-blind, randomized, placebo-controlled, within-groups | IN-OT enhanced higher-order social cognition performance; no IN-OT effects on general neurocognition |
| Jarskog et al., 2017 | 18–65; 41.9 (OT), 36.1 (P) | M/F | 24 IU, BID | Repeated, 12 weeks | Double-blind, randomized, placebo-controlled, between-groups | IN-OT improved negative symptoms only in individuals with SCZ but not schizoaffective disorder; no IN-OT effects on social cognition |
| Bradley et al., 2019 | 40.3 (SCZ); 39.8 (HC) | M | 40 IU, once | Acute | Double-blind, randomized, placebo-controlled, crossover | IN-OT increased fixation time on the eyes (eye gaze); higher attachment anxiety and greater symptom severity were predictive of greater eye fixation time |
| De Coster et al., 2019 | 35.3 (SCZ); 27.96 (HC) | M | 40 IU, once | Acute | Double-blind, randomized, placebo-controlled, crossover | IN-OT increased ToM accuracy and right TPJ activation during ToM tasks |
| Lee et al., 2019 | 44.74 (SCZ); 35.07 (HC) | M/F | 20 IU, BID | Repeated, 3 weeks | Double-blind, randomized, placebo-controlled, parallel-groups | No IN-OT effects on social cognition and functioning |
| PWS | ||||||
| Tauber et al., 2011 | 18.7–43.6; 28.5 (median age) | M/F | 24 IU, once | Acute | Double-blind, randomized, placebo-controlled, between-groups | IN-OT increased trust in others and lowered sadness and disruptive behavior; no IN-OT effects on other social skill tests |
| OLDER ADULTHOOD | ||||||
| Healthy Populations | ||||||
| Barraza et al., 2013 | 60–95; 80.33 | M/F | 40 IU, once a day | Repeated, 10 days | Double-blind, randomized, placebo-controlled, between-groups | IN-OT increased dispositional gratitude and reduced self-reported physical decline and fatigue; no IN-OT effects on social activities/engagement or other affective measures |
| Campbell et al., 2014 | 19.68 (YA), 72.07 (OA) | M/F | 20 IU, once | Acute | Double-blind, randomized, placebo-controlled, between-groups | IN-OT improved emotion recognition in older males but not in older females |
| Ebner et al., 2015 | 22.4 (YA), 71.2 (OA) | M/F | 24 IU, once | Acute | Double-blind, randomized, placebo-controlled, between-groups | IN-OT increased self-reported attention to own feelings only in older males |
| Ebner et al., 2016 | 22.7 (YA), 71.2 (OA) | M/F | 24 IU, once | Acute | Double-blind, randomized, placebo-controlled, between-groups | IN-OT increased resting-state amygdala-mPFC connectivity (trend-wise) in older males |
| Grainger et al., 2018 | 18–26, 20.18 (YA); 65–90, 73.25 (OA) | M/F | 24 IU, once | Acute | Double-blind, randomized, placebo-controlled, within-groups | IN-OT improved ToM ability irrespective of age |
| Grainger et al. 2019 | 18–26, 20.18 (YA); 65–90, 73.37 (OA) | M/F | 24 IU, once | Acute | Double-blind, randomized, placebo-controlled, within-groups | No IN-OT effects on judgments of facial trustworthiness |
| Horta et al., 2019 | 18–31, 22.4 (YA); 63–81, 71.2 (OA) | M/F | 24 IU, once | Acute | Double-blind, randomized, placebo-controlled, between-groups | IN-OT was associated with a large scale amygdalar network in OA for anger, fear, and happiness |
| Clinical Populations | ||||||
| Jesso et al., 2011 | 64.40 | M/F | 24 IU, once | Acute | Double-blind, randomized, placebo-controlled, crossover | IN-OT associated with reduced recognition of angry faces in patients with bvFTD; IN-OT-related improvement in scores on NPI |
| Finger et al., 2015 | 62.1–69.9, 66 (OT); 53.4–69.9, 61.1 (P) | M/F | 24, 48, or 72 IU, BID | Repeated, 1 week | Double-blind, randomized, placebo-controlled, parallel-groups | Trends of improvement were observed for the IN-OT group on the measures of apathy and empathy in patients with bvFTD |
Abbreviations: ASD: Autism Spectrum Disorder, BID: Twice-a-day, bvFTD: Behavioral Variant of Frontotemporal Dementia, ERPs=Event-related potentials; F: Female, HC: Healthy controls, IN-OT: Intranasal Oxytocin, IU: International Units, M: Male, mPFC: Medial Prefrontal Cortex, NPI: Neuropsychiatric Inventory, OA: Older Adults, OT: Oxytocin, P: Placebo, PWS: Prader-Willi Syndrome, RMET: Reading the Mind in the Eyes Test, SCZ: Schizophrenia, ToM: Theory of mind, TPJ: Temporoparietal Junction, YA: Younger adults.
Note: For regimen, “Acute” refers to the administration of IN-OT as a single dose; “Repeated” refers to the administration of IN-OT in chronic (e.g., daily administration) or intermittent (e.g., administration once every other day or once every week) fashion.
Fig. 1:
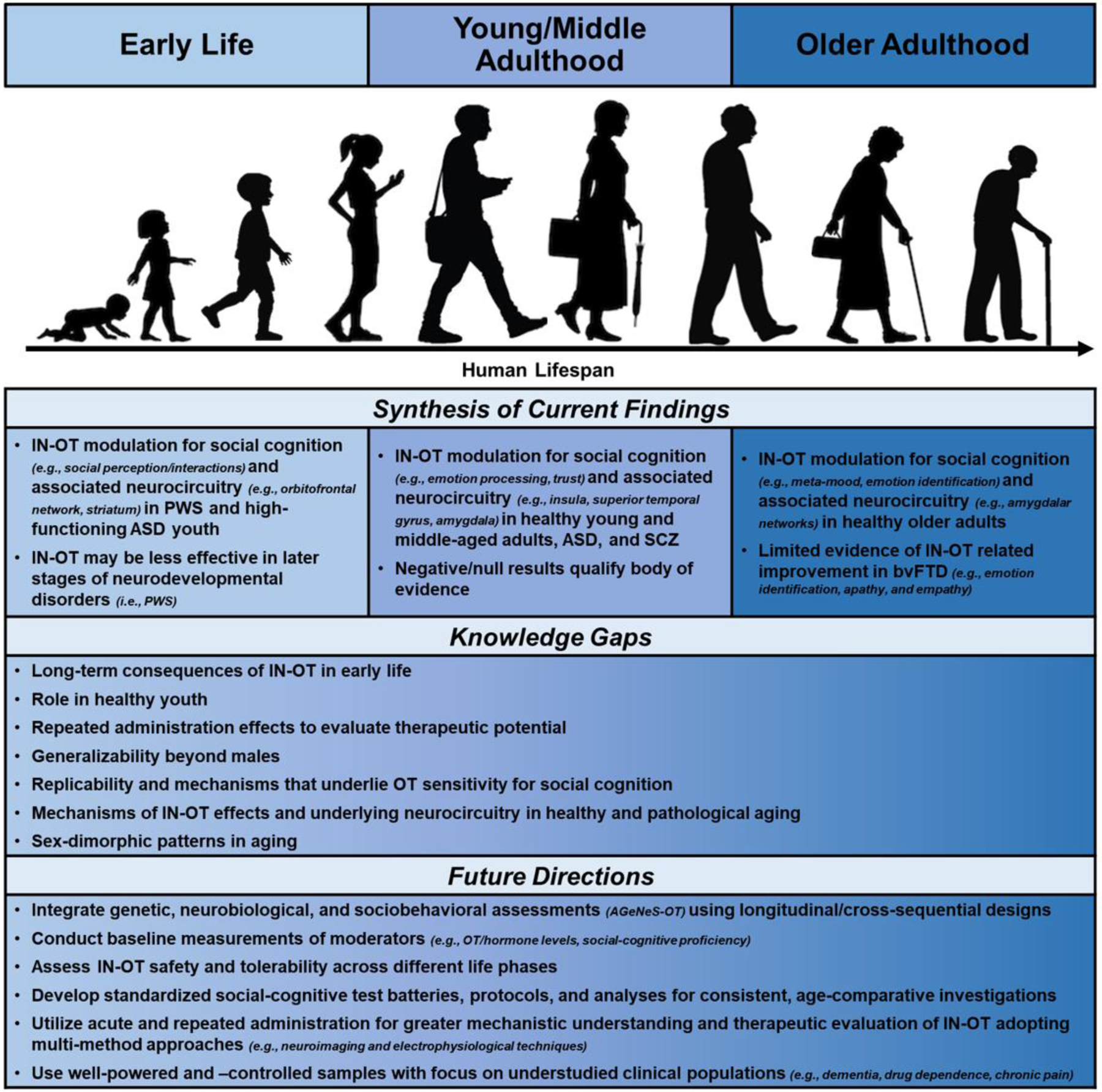
Literature Synthesis, Knowledge Gaps, and Future Directions in IN-OT Research on Social Cognition
Note: IN-OT: Intranasal Oxytocin, PWS: Prader-Willi Syndrome, ASD: Autism Spectrum Disorder, OT: Oxytocin, SCZ: Schizophrenia, bvFTD: Behavioral variant of Frontotemporal Dementia, AGeNeS-OT: Age-Related Genetic, Neurobiological, Sociobehavioral Model of Oxytocin
2. IN-OT Research on Social Cognition in Early Life, Young/Middle Adulthood, and Older Adulthood
Most investigative work on IN-OT modulation of social cognition has been reported in isolated lines of work on early life, young/middle adulthood, and older adulthood without integration across the human lifespan. Evidence from these distinct investigative fields is briefly summarized and reflected on next.
2.1. Early Life
OT is involved in critical functions that support early life including uterine contractions, lactation, and parental behavior [31]. The endogenous OT system is highly plastic during early development [32]. In humans, OT gene expression occurs within 15 weeks of gestation. By 25–26 weeks, the supraoptic and paraventricular nuclei of the hypothalamus have fully developed, with OT neurons comparable to adults [33]. Birth is especially critical for the release of mature OT and thus the propagation of the endogenous OT system. Also, there is an elevated level of OT receptor (OXTR) expression at birth, which undergoes up- and down-regulation throughout development [1].
The expansion of socioemotional and cognitive abilities (e.g., bonding, emotion identification, perspective-taking) is evident across early development. However, certain factors that impact the OT system (e.g., brain/behavioral disorders, early life adversity) may disrupt these abilities [34,35]. For example, developmental defects of the hypothalamus and related deficiencies in OT signaling can contribute to communication and bonding difficulties [1,36].
The majority of IN-OT research on social and emotional function in early development has been conducted in individuals with neurodevelopmental disorders [32], including Prader-Willi Syndrome (PWS; though often as outcomes secondary to feeding impairments) [15,37,38]. PWS is a genetic disorder that severely impairs feeding behaviors and the ability to appropriately interpret social information and interact with others [39]. Individuals with PWS have reduced paraventricular nuclei volume and OT-expressing neurons [40,41].1 IN-OT research on PWS has frequently leveraged third-party observation (e.g., parent, clinician) to assess social behavior and is limited to assessments of social and emotional information processing in youth.
Current evidence supports some benefits from IN-OT on social behaviors in PWS during early development (but see [41] for a discussion of methodological limitations). For example, 7 days of IN-OT on infants with PWS was associated with improved experimenter-blinded scores on infant–parent withdrawal behavior and social interactions (e.g., eye contact, facial expressions, social engagement) [15] and increased functional connectivity of an orbitofrontal network which was correlated with improved social engagement across three dosing schedules.
OT-enhanced social behavior and emotional experience in individuals with PWS, however, appear to vary by age and therapeutic benefits may not be apparent until several weeks of administration [9,12]. For example, IN-OT over 4 weeks was associated with improved parental evaluations of anger and sadness as well as social behavior in children under 11 years but not older [37]. In contrast, 5 days IN-OT did not improve socialization in children aged 11 and younger [38]. Similarly, evidence of IN-OT’s efficacy in modulating social behaviors and emotions is mixed in adolescents and younger adults. For example, in adults with PWS, acute (i.e., single-dose) IN-OT resulted in increased trust in others, decreased disruptive behavior, and less sadness as scored by caretaking staff [42]. This study, however, found no OT-related improvement for validated social-cognitive measures such as the Reading the Mind in the Eyes Test (RMET). Similarly, no RMET improvement was reported after 8 weeks of IN-OT in 12 to 30 year-olds [39].
The stabilization of neuronal deficiencies in the endogenous OT system with age may contribute to mixed findings among individuals with PWS who have aged out of early development [39]. Inconsistent findings may also be due to variations in OT dosage, with lower doses potentially reducing the possibility of binding to closely-related vasopressin receptors, yielding more favorable results than higher doses [38]. Additionally, variations in administration frequency and/or outcome measures across studies may underlie mixed findings [9]. In particular, it appears that studies that used behavioral measures of social cognition, like the RMET, did not observe benefits from IN-OT while studies that utilized third-party observation reported improvements.
Currently, it is difficult to draw definitive conclusions as to whether IN-OT has practical utility for the treatment of social-cognitive deficits in PWS. Moving forward, a combination of behavioral experimental tasks with clinical measures of daily social functioning will inform OT’s social-cognitive benefits in everyday interactions [41]. Furthermore, future research is needed to determine if early, compared to later, IN-OT intervention is particularly beneficial in PWS. Finally, identification of the neural processes underlying social-cognitive changes resulting from IN-OT across early development will advance mechanistic understanding of OT’s role in social cognition in PWS.
Another neurodevelopmental disorder in which IN-OT effects on social cognition have been studied is Autism Spectrum Disorder (ASD) [43–47]. Similar to PWS, individuals with ASD experience difficulties processing social information and communicating with others among other symptoms (e.g., repetitive behavior). Most work has focused on males and those with high-functioning ASD (i.e., no intellectual disability) [48] and, in sum, has pointed to IN-OT related improvement in children and adolescents [49] (e.g., enhanced emotion identification [43]).2
Work in this domain has also explored IN-OT effects on the brain during social and emotional information processing. For example, acute IN-OT was associated with greater activation to social stimuli, and attenuated activation for non-social stimuli, in regions of the “social brain” (e.g., striatum, nucleus accumbens, superior temporal sulcus, premotor cortex) [44]. Similarly, acute OT resulted in greater neural activity and connectivity in these brain regions during social perception [50]. Findings indicate that acute IN-OT benefits social cognition in children and adolescents with ASD, possibly via neuromodulation in social brain regions.
Utilizing repeated OT administration, ranging from several weeks to months, has also gained traction in this research arena. For example, several weeks of IN-OT was linked to improved social responsiveness in children with ASD [51,52], especially among those with the lowest levels of pre-treatment endogenous OT [52]. Similarly, in children and adolescents, longer-term IN-OT improved social interaction/communication [45,53] as well as other social-cognitive processes (i.e., face recognition, theory of mind (ToM), empathic accuracy) [54].
Together these findings indicate that acute and repeated IN-OT modulate brain and behavior in youth with ASD (but see [46,47]). Of note, high-functioning males constitute the typical subject profile in these clinical investigations, while not representative of all individuals impacted by this disorder. The inclusion of females and lower-functioning individuals is warranted for a comprehensive evaluation of therapeutic efficacy. In this context, special attention to biomarkers associated with the endogenous OT system (i.e., peripheral OT levels, OT receptor distribution, and OXTR polymorphisms) has the potential to enhance our understanding of the role of OT on social cognition in ASD [52].
2.2. Adulthood
Most work on IN-OT modulation of social-cognition has been conducted in younger adults, with some samples extending into middle age. Initially, OT-related enhancement of social-cognitive capacities, including ToM [55] and trust [56], was reported for healthy adult males. In further support of IN-OT’s beneficial effects on social-cognitive processes, more recent meta-analytic findings demonstrated positive IN-OT effects on emotion processing [13,23] and trust [57], in healthy men and women. The literature discussed these effects as a potential result from IN-OT enhancing social cue salience [58], perhaps by modulating early attentional resources [59] and possibly without perceptual awareness [60].
Neuroimaging (e.g., fMRI) and electrophysiological (e.g., ERP/EEG) methods have been increasingly used to delineate the neural substrates of OT function in adulthood. The amygdala, midbrain, and striatum were initially proposed as key targets of OT’s social-cognitive action [61,62]. A recent meta-analysis extended understanding of brain mechanisms by indicating that acute IN-OT alters functional activity across various brain regions and networks, and most robustly in the left insula, across a variety of social-cognitive tasks in young men and women [63]. Additional meta-analytic evidence supports that IN-OT increased activation of the superior temporal gyrus [64,65] and caudate during social and emotion processing while reducing bilateral amygdala activity, particularly during negative information processing [65].
During adulthood, IN-OT has also received particular attention for use in neurodevelopmental and psychiatric disorders characterized by social-cognitive impairments, including ASD and schizophrenia (SCZ) [49,66]. In this context, IN-OT has emerged as a potential pharmacological treatment for social-cognitive dysfunction, based on endogenous OT’s roles in social cognition and behavior and given initial promising findings of IN-OT administration in these clinical populations [54,67]. For example, a recent meta-analysis using data from randomized controlled trials showed a significant, yet small, effect of IN-OT on ToM (while no effect on empathy or emotion recognition was observed) in adult clinical populations, including ASD and SCZ [68].3
As noted above, ASD is characterized by impaired social functioning and communication. IN-OT research has shown promise in ameliorating social-cognitive dysfunction for adults with ASD [54]. For example, acute IN-OT increased amygdala activity during face processing [69] and was associated with enhanced ToM and insula activity in men with ASD [70]. IN-OT also improved social reinforcement learning among men with ASD and modulated reward prediction error-related activation of the nucleus accumbens, supporting IN-OT as a candidate for adjunctive treatment to behavioral interventions in social learning [71]. Longer-term (6 weeks) IN-OT, furthermore, resulted in improved ToM, life quality, as well as functional activity in and connectivity of the medial prefrontal cortex (mPFC) [72,73]; it also improved social-cognitive performance and social reciprocity in men with ASD (but see [74] for null effects on social responsiveness from 4-weeks of IN-OT). In sum, both acute and repeated IN-OT studies largely point to beneficial social-cognitive effects in men with ASD.
SCZ and related disorders are characterized by positive (e.g., hallucinations, delusions) and negative (e.g., blunted affect, asociality) symptoms as well as cognitive impairments (e.g., working memory, processing speed), which contribute to deficits across multiple social-cognitive capacities [75]. IN-OT benefits for treating social-cognitive dysfunction in SCZ have been demonstrated in social contexts that involve higher-level processing (e.g., recognition of social faux pas), compared to lower-level processing (e.g., social cue perception), which is inferred quickly and largely without contextual integration [76,77]. For example, acute IN-OT increased eye gaze to faces [78], ToM accuracy, temporoparietal junction (TPJ) activity, and TPJ-mPFC connectivity in men with SCZ and schizoaffective disorders [79]. In contrast, IN-OT over several weeks was not associated with improved social cognition [80,81], suggesting acute, but not long-term, IN-OT benefits on SCZ-related social-cognitive dysfunction.4
In sum, though there is supporting evidence across healthy and clinical populations that IN-OT in modulates certain social-cognitive processes and related behavior, mixed and null findings also indicate that broader therapeutic efficacy of IN-OT in adulthood has yet to be determined.
2.3. Older Adulthood
Previous work on IN-OT effects in social cognition has almost exclusively examined younger populations. This limited approach, combined with evidence that aging is characterized by decline in some social-cognitive abilities (e.g., emotion perception and attribution; [82–84]) that are known to be affected by IN-OT [8], as well as growing support that the OT system may change with age5, has resulted in recent calls for the examination of IN-OT’s social-cognitive effects among older adults [7,8,85].
As summarized in reviews, preclinical evidence regarding age-related change in the endogenous OT system is mixed [8,85] and still rather limited in humans [1]. However, there is first evidence indicating that advanced age, particularly in males, may be associated with a (numerical) decline in plasma OT levels [86,87], OT-immunoreactive neurons [88,89], and receptor binding [90]. Hormonal systems that interact with the OT system [91,92], such as cortisol [93] and estrogen [94], have also shown to affect social cognition in aging [85].
The impact of advanced age on OT signaling with relevance to social-cognitive capacities is unknown [1]. It is possible, however, that age-related changes to these coordinated endogenous systems contribute to interindividual variation in social cognition and response to IN-OT. For example, there is emerging evidence of age- and/or age-by-sex-related differences in IN-OT modulation of emotion recognition and meta-mood [27,28]. It is possible that because older adults differ from younger adults in physiological, cognitive, and socioemotional domains they may experience greater benefits from IN-OT [1,8]. Moving forward, a better understanding of the links between age-related change in endogenous systems and IN-OT social-cognitive response is needed [85,95].
The few existing IN-OT studies to date have generated some encouraging results on social-cognitive benefits in healthy older adults. In particular, acute IN-OT enhanced facial emotion recognition in older men; with no effects among older women [27]. Similarly, IN-OT enhanced self-reported meta-mood [28] and (trend-wise) functional amygdala-mPFC coupling in older men (but again not in older women) [29]. Furthermore, supporting IN-OT effects on brain response in aging, acute IN-OT resulted in a large-scale amygdalar network in older adults during angry, fearful, and happy dynamic face identification, while older adults in the placebo group recruited a different and smaller amygdalar network [96]. However, there also is work that does not support this pattern of age- and age-by-sex differential findings. In particular, acute IN-OT improved ToM comparably in young and older adults [97] and did not improve perceived facial trustworthiness in either age group [98]. These previous studies focused on acute IN-OT administration. Only one study to date has examined prolonged IN-OT effects (over 10 days) in healthy older adults and found that individuals in the OT group reported less physical functioning decline and fatigue as well as increased gratitude, while no changes were found for mood, cardiovascular states, or social activity [99].
Of note, prior work included generally healthy older adults. Although it is currently unclear if/how the OT system in humans is impacted by pathological aging [1], IN-OT may be a valuable, symptomatic treatment option in dementia [17]. This speculation is based on evidence that prefrontal [100] and medial temporal brain regions (e.g., hippocampus and amygdala) [101] are not only structurally altered [102] and related to social-cognitive impairments in various forms of dementia [103], but also overlap with OT-related projection [104,105] and receptor sites [106,107].
However, to date, only one acute IN-OT study on social cognition comprised older individuals with the behavioral variant of frontotemporal dementia (bvFTD) and reported OT-related reduction in facial anger and fear recognition [17]. Repeated IN-OT furthermore resulted in dose-dependent improvement in a subset of behavioral bvFTD symptoms (i.e., apathy and expressions of empathy) and interactions with caregivers [108]. Future research may benefit from extending investigations to other subtypes of dementia towards the determination of IN-OT effects on social-cognitive decline in pathological aging [9].
Taken together, research on IN-OT and social cognition in older adults is limited and results are mixed, with some promising first findings in healthy and pathological aging. Also, in line with work in younger adults, emerging sex-dimorphic patterns of results suggest possible benefits in older men, but not older women, and support the possibility that gonadal hormones (e.g., estrogen, testosterone) and/or other interindividual variables (e.g., clinical status, social proficiency) are at work.
3. Discussion
3.1. Contextual Factors, Interindividual Differences, and Clinical Status Moderate IN-OT Effects
In summary, the current literature on IN-OT’s social-cognitive effects is not yet conclusive, but rather has generated mixed evidence [22] and also several null findings [21]. Meta-analyses furthermore suggest that the magnitude of IN-OT effects are often small [13,23,57,68]. Based on our literature review, it is evident that the observed potential benefits of IN-OT on social cognition across the lifespan are often qualified by moderating contextual factors and interindividual differences.6
In particular, contextual factors, such as the specific social-cognitive processes being examined or the stimuli included in experimental tasks, can contribute to variations in findings. For example, OT has been shown to impact prosocial behavior (e.g., trust and empathic behavior) [109] as well as related neurocircuitry in favor of in- vs. out-group members [57,59,110]. An emerging body of null findings and conflicting evidence across social-cognitive domains in both healthy [21] and clinical [23,68] populations indicates that IN-OT response may be more variable than previously speculated [21,25,49]. The use of standardized, validated social-cognitive measures in future studies may ameliorate methodological shortcomings that could have contributed to these variable/null findings and would facilitate direct replication [25,111]. Additionally, extending investigations to include naturalistic stimuli that are more representative of real-life social interactions (e.g., dynamic, multimodal emotion, and social cue displays; [112]) could constitute another crucial step towards determining more generalizable, socially relevant IN-OT modulatory effects. These stimuli would also allow to improve the determination of IN-OT’s temporal dynamics during social processing and decision-making [59,113].
Importantly, there is growing acknowledgment in the field, that IN-OT related physiological (e.g., pupil diameter) and neural (e.g., amygdala activation, ERPs) modulation occurs not only for social but also for non-social stimuli [59,114], supporting theories proposing that OT’s modulatory effects extend beyond social domains [2,3]. To test these predictions, future experimental tasks can systematically vary stimuli and/or contexts to better assess and distinguish dimensions of salience (e.g., social vs. non-social stimuli) as well as motivational aspects (e.g., threatening vs. non-threatening stimuli) of IN-OT-related processing.
Similarly, the summary of the literature suggests that interindividual differences determine who benefits from IN-OT. For example, IN-OT may be particularly effective in adults with lower social-cognitive proficiency [25] and/or lower endogenous OT levels [8]. Sex may further qualify the modulatory effects of IN-OT on social-cognitive processes and behaviors (e.g., IN-OT related improvements in less socially proficient men [25] and older men [27,28]). Gonadal hormones are known to have co-evolved and interact with the endogenous OT system throughout development [30,85] and the field has recently become more inclusive of women, including investigations on IN-OT modulation of the brain [115,116] and behavior [25].
To better capture interindividual variation in IN-OT effects, studies should measure baseline and post-administration levels of endogenous OT and other hormones (e.g., gonadal hormones, cortisol). In particular, the use of unextracted (vs. extracted) samples in plasma OT assays has received growing support as a reliable measurement [117,118].7 Along these lines, systematic assessment of baseline levels of social-cognitive proficiency will also be beneficial for adequately assessing change after IN-OT and identifying therapeutically relevant effects [25].
Our review further identified that clinical status is another factor that impacts IN-OT modulation of social cognition and its therapeutic efficacy. For example, in PWS, IN-OT administration earlier in development may be more beneficial than later [37,39]. This is perhaps due to the endogenous OT system being more malleable in early life [32] or that certain clinical conditions are associated with greater hypothalamic deficiencies (e.g., decreased OT receptors/neurons) in adulthood [37]. This is in line with the notion of potential critical periods for OT-related brain and behavioral modulation [22], but long-term impacts of IN-OT administration in early life, including safety and tolerability, need to be carefully considered. In particular, concerns have been raised, based largely on preclinical evidence, that repeated OT treatment in early development may lead to long-term social impairment in adulthood (e.g., bonding and parental behaviors), potentially in a sex-dimorphic pattern [119].
Taken together, given that there is a dearth of efficacious pharmacological treatments for social-cognitive dysfunction with little to no side effects [66,67], further exploration of IN-OT as an effective and safe treatment is worthwhile. Additionally, because there is evidence of variations in OT response across contexts and individuals/clinical groups, the effectiveness of IN-OT in modulating social cognition needs to be established with special attention to moderating factors towards the development of tailored treatment plans [9,12]. This will require systematic investigations in well-powered, well-controlled, representative samples, covering a wider age range and using rigorous methodology with greater efforts toward preregistration and direct replication [111].
3.2. Moving IN-OT Research Towards an Integrative Human Lifespan Approach
IN-OT research has proliferated largely due to the popularity of initial promising findings on its use to enhance social cognition. However, this initial excitement was tempered as more research brought attention to methodological and conceptual concerns that limited understanding of OT function and modulation of social cognition, especially across development [2,9,49].
As the field evolved, several theories were put forth to increase conceptually driven, mechanistic OT research. For example, the prosocial hypothesis [36,120] stated that IN-OT promotes affiliative prosocial behaviors (e.g., trust, empathy) by increasing attention to positive emotions. This view was challenged by the social salience hypothesis, which argued that OT increases social salience perception in a context-specific manner, regardless of valence [58,121]. Kemp and Guastella [122,123] put forth the social approach/withdrawal hypothesis, which proposes that IN-OT increases salience of both positive and negative stimuli, eliciting approach-related behavior and inhibiting withdrawal-related behavior, respectively. More interactionist conceptualizations of OT signaling and exogenous modulation then highlighted contextual, interindividual, and developmental dynamics [1,2,26,113,124].
Finally, very recent frameworks conceptualized IN-OT brain and behavioral modulation to account for social and non-social contexts [3,59]. Of these latter more holistic approaches, the allostatic theory [2] most recently proposed that OT is involved in maintaining stability in dynamic environments. According to this framework, OT is involved in the processing of social and non-social cues throughout development, depending on actual and anticipated contextual demands that align with the promotion of survival. These recent perspectives propose that OT function across social and non-social contexts may be mediated by approach–avoidance motivation [3,59].
Although these recent theoretical conceptualizations of IN-OT effects highlighted the importance of focusing on the impact of IN-OT in a broader range of contexts (not only social contexts), as our review shows, current empirical IN-OT research is still limited in its investigative focus on distinct, isolated phases of the lifespan without considering the dynamic and interrelated nature of human development under various contextual influences [125]. However, importantly, our review supports that IN-OT effects are not unilateral or universal across development, populations, and/or social-cognitive processes and behaviors [9]. We propose that an integrative human lifespan approach (Fig. 1) is warranted to capture the complex, dynamic nature of developmental processes that can impact OT modulation of social cognition [126].
Adopting an integrative human lifespan approach will permit researchers to gain unique knowledge about the role of IN-OT on social cognition within and across life phases, accounting for multi-directionality of effects throughout development. The suggestions for future work proposed next, in the context of our integrative human lifespan approach, has the potential to advance etiological understanding of OT throughout development [2] and push the field towards broader generalization [2,9,127]. Fig. 1 synthesizes current research findings and identifies pressing knowledge gaps across the three distinct lifespan segments reviewed. Based on the observations made in this narrative review, we discuss what we have identified as promising future directions for novel research on IN-OT and social cognition specifically within the context of the proposed integrative human lifespan approach.
Future IN-OT research would benefit from consideration of coordinated physiological, cognitive, and behavioral functions of OT as well as potential interactions of IN-OT with the endogenous OT system throughout development. In particular, in line with the Age-Related Genetic, Neurobiological, Sociobehavioral Model of Oxytocin (AGeNeS-OT) [8], using a combination of genetic, neurobiological, and socio-behavioral experimental and clinical assessments will allow for a more comprehensive understanding of IN-OT effects on social cognition across the human lifespan. This includes investigation of baseline physiological (e.g., endogenous OT/hormone levels, genetic profiles, neurobiological responses) and social-cognitive moderators (e.g., emotion identification, ToM, trust) [26,30].
As summarized in Fig. 1, going beyond current practice that examines distinct, isolated age groups outside of their broader developmental contexts, it will be important to design longitudinal (and cross-sequential; [128]) studies that capture inter- and intra-individual (age/cohort) differences in IN-OT effects across the full spectrum of the lifespan, instead of comparisons solely focused on isolated, extreme age groups. This approach will allow for cross-linking throughout the ages, regarding mechanisms of action, efficacy, as well as tolerability/safety. Additionally, it will be crucial to investigate developmentally relevant moderators that can have long-term impacts on endogenous OT levels and sensitivity to IN-OT, like early life adversity [35] and attachment history [30].
To facilitate consistent, age-comparative investigations, moving forward it will be useful to develop validated social-cognitive test batteries; follow standardized OT administration protocols (e.g., dosage, frequency, and duration)8; and periodically synthesize existing research following standardized guidelines (e.g., PRISMA; [129]). Future work will also benefit from the implementation of equivalence and Bayesian hypothesis testing to determine the sensitivity and stability of detecting the absence of significant OT-related effects within age groups [21,130].
More research has moved from using acute to repeated administration over time, which better reflects long-term medical treatment and the prolonged capacity often needed to observe pharmacologically inducted change [9]. Thus, in addition to well-controlled, standardized acute studies to determine basic mechanistic processes, more prolonged clinical trials will help determine OT’s therapeutic potential for improving social cognition, under consideration of developmental dynamics.
Current research largely examines male individuals, while there is growing evidence suggesting sex- and possibly sex-by-age dimorphism in endogenous OT levels [86,131] and IN-OT effects [25]. This investigative focus on males is indicative of a broader bias of excluding females in clinical/drug response trials [132] due to various factors ranging from sex bias in diagnostic and recruitment criteria (e.g., the primary use of males in ASD-related research [49]) to concern over potential hormonal confounds [133]. Increasing the representation of females in IN-OT research is necessary for producing generalizable findings as well as to allow tailoring of treatment plans. Thus, we propose the use of a-priori powered samples with well-balanced sex ratios to explicitly address sex differences in OT response across the lifespan. Careful tracking of baseline levels of gonadal hormones and endogenous OT will be essential in this context [30], as well as strict control and monitoring of factors that interact with OT levels and function (e.g., use of hormonal treatment, stage of the menstruation cycle, medication intake, etc.).
Also, clinical IN-OT research is limited by a focus on high-functioning individuals, primarily with ASD and SCZ. Efforts to summarize IN-OT findings in clinical contexts typically suffer from the heterogeneity of the studied samples that do not differentiate between diverse clinical diagnoses (e.g., ASD, SCZ, borderline personality disorder, posttraumatic stress disorder, etc.; [23,65,68]). These clinical investigations largely address earlier life phases without much consideration of older samples or developmental dynamics. Extending investigative efforts to less researched clinical populations (e.g., dementia, drug dependence, chronic pain; e.g., [134]), while capturing a wider age range to adequately reflect developmental processes, has potential to clarify IN-OT social-cognitive effects within and across diagnoses over the lifespan [22]. Future work in this context should seek to employ larger placebo-controlled trials and consider investigating a broader range of social-cognitive processes and behaviors (e.g., social decision-making, dynamic emotion identification).
In addition to evidence that IN-OT can modulate physiology in brain regions via nasal pathways [20], recent studies have identified multiple projection sites of OT in the brain [2] along with OT-related connectivity among specific brain regions (e.g., amygdala-prefrontal connectivity [29]; cortico-striatal connectivity [115]; resting-state networks [135,136]; and allocation of attention early in the processing stream [59]). However, the neural underpinnings of OT effects are still poorly understood and largely unexplored in older adulthood and concerning developmental dynamics. Thus, we propose greater incorporation of neuroimaging and electrophysiological methodology to allow for increased multi-method investigations and delineation of brain mechanisms underlying OT’s modulation of social-cognitive processes across development.
4. Conclusions
In this narrative review, we synthesized recent evidence on IN-OT and social cognition across the three life phases of early life, young/middle adulthood, and older adulthood, towards our proposal of an integrative human lifespan approach for future IN-OT research on social cognition. We propose that scientific advancement in IN-OT and social cognition research requires an integrative lifespan approach that systematically uses standardized, multi-method, longitudinal, and cross-sequential assessments in well-powered, well-controlled, representative samples. We believe that this approach has the promise to move the field forward by capturing the complex and dynamic nature of IN-OT’s modulation of social cognition across the human lifespan, offering more a comprehensive understanding and broader generalizability.
Acknowledgments and Disclosures
This work was supported by the National Institute on Aging Pre-Doctoral Fellowship on Physical, Cognitive, and Mental Health in Social Context [T32AG020499] to M.H., the University of Florida Substance Abuse Training Center in Public Health [NIH/NIDA, T32DA035167] to M.H., a University of Florida Clinical and Translational Science pilot award [NIH/NCATS, UL1 TR000064] to N.C.E., a University of Florida Claude D. Pepper Older Americans Independence Center pilot award [NIH/NIA, P30AG028740] to N.C.E., a National Institute on Aging grant [R01AG059809] to N.C.E., the Department of Psychology, the College of Liberal Arts and Sciences, Institute on Aging, the Center for Cognitive Aging and Memory, and the McKnight Brain Institute at the University of Florida. The authors would like to thank Kevin Chi for his help with formatting.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
The authors have no conflict of interest to declare.
For a review on OT and social cognition in PWS see [41].
For similar findings regarding emotion recognition in clinical populations see [23].
For a review on IN-OT in SCZ see [137].
See [138] for a discussion on moderating factors of OT’s social-cognitive effects.
See [139] for a recent review that discusses the challenges surrounding current methods for OT measurement.
References List
Recently published papers of interest have been highlighted as * (Important) and ** (Very Important).
- **1.Sannino S, Chini B, Grinevich V. Lifespan oxytocin signaling: Maturation, flexibility, and stability in newborn, adolescent, and aged brain. Dev Neurobiol. Wiley Online Library; 2017;77:158–68. [DOI] [PubMed] [Google Scholar]; A review on OT system development and activity across three distinct life phases: early postnatal period, puberty/adolescence, and aging. This review discusses novel research avenues for use of IN-OT in neurodevelopmental disorders in early life, substance-use disorders among adolescents, and neurodegenerative disease in older populations.
- **2.Quintana DS, Guastella AJ. An Allostatic Theory of Oxytocin. Trends Cogn Sci. Elsevier Current Trends; 2020;24:515–28. [DOI] [PubMed] [Google Scholar]; A review of the OT system and its role in behavior from etiological and developmental perspectives. The paper reconceptualizes OT as an allostatic hormone that modulates both social and non-social behavior to maintain stability across changing environments.
- 3.Harari-Dahan O, Bernstein A. A general approach-avoidance hypothesis of Oxytocin: Accounting for social and non-social effects of oxytocin. Neurosci Biobehav Rev. Elsevier Ltd; 2014;47:506–19. [DOI] [PubMed] [Google Scholar]
- 4.Strickland-Hughes CM, Dillon KE, West RL, Ebner NC. Own-age bias in face-name associations: Evidence from memory and visual attention in younger and older adults. Cognition. Elsevier B.V.; 2020;200:104253. [DOI] [PubMed] [Google Scholar]
- 5.Gonçalves AR, Fernandes C, Pasion R, Ferreira-Santos F, Barbosa F, Marques-Teixeira J. Effects of age on the identification of emotions in facial expressions: a meta-analysis. PeerJ. PeerJ Inc.; 2018;6:e5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruffman T, Murray J, Halberstadt J, Vater T. Age-related differences in deception. Psychol Aging. 2012;27:543–9. [DOI] [PubMed] [Google Scholar]
- 7.Huffmeijer R, van Ijzendoorn MH, Bakermans-Kranenburg MJ. Ageing and oxytocin: A call for extending human oxytocin research to ageing populations--a mini-review. Gerontology. Karger Publishers; 2013;59:32–9. [DOI] [PubMed] [Google Scholar]
- 8.Ebner NC, Maura GM, MacDonald K, Westberg L, Fischer H. Oxytocin and socioemotional aging: Current knowledge and future trends. Front Hum Neurosci. 2013;7:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *9.Horta M, Kaylor K, Feifel D, Ebner NC. Chronic oxytocin administration as a tool for investigation and treatment: A cross-disciplinary systematic review. Neurosci Biobehav Rev. Elsevier Ltd; 2020;108:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]; systematic review of chronic (i.e., repeated, daily administration) IN-OT studies in animals and humans to determine treatment efficacy across a variety of functional domains. This review concludes that while there is growing evidence supporting IN-OT as a treatment for certain clinical conditions, its therapeutic application outside of research is still limited. The paper discusses conceptual and methodological factors that impact the generalizability of findings and offers recommendations for future chronic IN-OT research.
- 10.Quintana DS, Smerud KT, Andreassen OA, Djupesland PG. Evidence for intranasal oxytocin delivery to the brain: recent advances and future perspectives. Ther Deliv. Future Science Ltd; London, UK; 2018;9:515–25. [DOI] [PubMed] [Google Scholar]
- 11.Quintana DS, Westlye LT, Rustan ØG, Tesli N, Poppy CL, Smevik H, et al. Low-dose oxytocin delivered intranasally with Breath Powered device affects social-cognitive behavior: a randomized four-way crossover trial with nasal cavity dimension assessment. Transl Psychiatry. 2015;5:e602–e602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacDonald K, Feifel D. Helping oxytocin deliver: considerations in the development of oxytocin-based therapeutics for brain disorders. Front Neurosci. Frontiers Media SA; 2013;7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shahrestani S, Kemp AH, Guastella AJ. The Impact of a Single Administration of Intranasal Oxytocin on the Recognition of Basic Emotions in Humans: A Meta-Analysis. Neuropsychopharmacology. American College of Neuropsychopharmacology; 2013;38:1929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groppe SE, Gossen A, Rademacher L, Hahn A, Westphal L, Gründer G, et al. Oxytocin Influences Processing of Socially Relevant Cues in the Ventral Tegmental Area of the Human Brain. Biol Psychiatry. 2013;74:172–9. [DOI] [PubMed] [Google Scholar]
- 15.Tauber M, Boulanouar K, Diene G, Çabal-Berthoumieu S, Ehlinger V, Fichaux-Bourin P, et al. The Use of Oxytocin to Improve Feeding and Social Skills in Infants With Prader-Willi Syndrome. Pediatrics. 2017;139:e20162976. [DOI] [PubMed] [Google Scholar]
- 16.Andari E, Duhamel J-R, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci. Proc Natl Acad Sci U S A; 2010;107:4389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jesso S, Morlog D, Ross S, Pell MD, Pasternak SH, Mitchell DG V, et al. The effects of oxytocin on social cognition and behaviour in frontotemporal dementia. Brain. 2011;134:2493–501. [DOI] [PubMed] [Google Scholar]
- 18.Striepens N, Kendrick KM, Hanking V, Landgraf R, Wüllner U, Maier W, et al. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci Rep. Nature Publishing Group; 2013;3:3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paloyelis Y, Doyle OM, Zelaya FO, Maltezos S, Williams SC, Fotopoulou A, et al. A Spatiotemporal Profile of In Vivo Cerebral Blood Flow Changes Following Intranasal Oxytocin in Humans. Biol Psychiatry. Elsevier; USA; 2016;79:693–705. [DOI] [PubMed] [Google Scholar]
- **20.Martins DA, Mazibuko N, Zelaya F, Vasilakopoulou S, Loveridge J, Oates A, et al. Effects of route of administration on oxytocin-induced changes in regional cerebral blood flow in humans. Nat Commun. Nature Research; 2020;11:1160. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper compares the efficiency of different OT administration routes (i.e., intravenous, intranasal spray, and nebulizer) in inducing regional cerebral blood flow change in humans. Findings support the involvement of nasal pathways in modulating the physiology of specific brain regions (e.g., amygdala, anterior cingulate cortex) after IN-OT administration.
- **21.Tabak BA, Teed AR, Castle E, Dutcher JM, Meyer ML, Bryan R, et al. Null results of oxytocin and vasopressin administration across a range of social cognitive and behavioral paradigms: Evidence from a randomized controlled trial. Psychoneuroendocrinology. Elsevier Ltd; 2019;107:124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper reports on a series of experiments testing IN-OT main effects on various processes involving both social and non-social elements. Findings support the notion that IN-OT may have limited direct effects on social and non-social processes and the paper discusses the importance of well-powered studies in this field.
- 22.Erdozain AM, Peñagarikano O. Oxytocin as Treatment for Social Cognition, Not There Yet. Front Psychiatry. Frontiers Media S.A.; 2020;10:930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *23.Leppanen J, Ng KW, Tchanturia K, Treasure J. Meta-analysis of the effects of intranasal oxytocin on interpretation and expression of emotions. Neurosci Biobehav Rev. Elsevier Ltd; 2017;78:125–44. [DOI] [PubMed] [Google Scholar]; A systematic meta-analysis on the effects of single-dose IN-OT on various aspects of social-emotional functioning among healthy and clinical populations (i.e., ASD, SCZ, bvFTD, eating disorders, posttraumatic stress disorder, depression, borderline personality disorder, and drug dependence). Findings provide some support for improved emotion processing in healthy individuals, while IN-OT did not appear to significantly influence emotion processing across clinical populations.
- 24.Nave G, Camerer C, McCullough M. Does Oxytocin Increase Trust in Humans? A Critical Review of Research. Perspect Psychol Sci. 2015;10:772–89. [DOI] [PubMed] [Google Scholar]
- *25.Bartz JA, Nitschke JP, Krol SA, Tellier P-P. Oxytocin Selectively Improves Empathic Accuracy: A Replication in Men and Novel Insights in Women. Biol Psychiatry Cogn Neurosci Neuroimaging. Elsevier Inc; 2019;4:1042–8. [DOI] [PubMed] [Google Scholar]; An empirical replication on IN-OT effects on empathic accuracy in males and extension of investigation to females. This paper replicates the previous finding that IN-OT selectively improves empathic accuracy for less socially proficient males but no significant IN-OT effects on empathic accuracy in females was observed.
- 26.Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci. Elsevier Ltd; 2011;15:301–9. [DOI] [PubMed] [Google Scholar]
- 27.Rash JA, Aguirre-Camacho A, Campbell TS. Oxytocin and Pain. Clin J Pain. 2013;30:1. [DOI] [PubMed] [Google Scholar]
- 28.Ebner NC, Horta M, Lin T, Feifel D, Fischer H, Cohen RA. Oxytocin modulates meta-mood as a function of age and sex. Front Aging Neurosci. Frontiers Research Foundation; 2015;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebner NC, Chen H, Porges E, Lin T, Fischer H, Feifel D, et al. Oxytocin’s effect on resting-state functional connectivity varies by age and sex. Psychoneuroendocrinology. Elsevier; 2016;69:50–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacDonald KS. Sex, Receptors, and Attachment: A Review of Individual Factors Influencing Response to Oxytocin. Front Neurosci. Frontiers; 2013;6:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rilling JK, Young LJ. The biology of mammalian parenting and its effect on offspring social development. Science (80-). American Association for the Advancement of Science; 2014;345:771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor AE, Lee H, Buisman-Pijlman FTA. Oxytocin treatment in pediatric populations. Front Behav Neurosci. Frontiers Media S.A.; 2014;8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grinevich V, Desarménien MG, Chini B, Tauber M, Muscatelli F. Ontogenesis of oxytocin pathways in the mammalian brain: late maturation and psychosocial disorders. Front Neuroanat. Frontiers Media S.A.; 2015;8:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muscatelli F, Desarménien MG, Matarazzo V, Grinevich V. Oxytocin Signaling in the Early Life of Mammals: Link to Neurodevelopmental Disorders Associated with ASD. Curr Top Behav Neurosci. 2017. p. 239–68. [DOI] [PubMed] [Google Scholar]
- 35.Londono Tobon A, Newport DJ, Nemeroff CB. The Role of Oxytocin in Early Life Adversity and Later Psychopathology: a Review of Preclinical and Clinical Studies. Curr Treat Options Psychiatry. Springer; 2018;5:401–15. [Google Scholar]
- 36.MacDonald K, Macdonald TM. The peptide that binds: a systematic review of oxytocin and its prosocial effects in humans. Harv Rev Psychiatry. 2010;18:1–21. [DOI] [PubMed] [Google Scholar]
- 37.Kuppens RJ, Donze SH, Hokken-Koelega ACS. Promising effects of oxytocin on social and food-related behaviour in young children with Prader-Willi syndrome: a randomized, double-blind, controlled crossover trial. Clin Endocrinol (Oxf). 2016;85:979–87. [DOI] [PubMed] [Google Scholar]
- 38.Miller JL, Tamura R, Butler MG, Kimonis V, Sulsona C, Gold J-A, et al. Oxytocin treatment in children with Prader-Willi syndrome: A double-blind, placebo-controlled, crossover study. Am J Med Genet Part A. 2017;173:1243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Einfeld SL, Smith E, McGregor IS, Steinbeck K, Taffe J, Rice LJ, et al. A double-blind randomized controlled trial of oxytocin nasal spray in Prader Willi syndrome. Am J Med Genet Part A. 2014;164:2232–9. [DOI] [PubMed] [Google Scholar]
- 40.Swaab DF, Purba JS, Hofman MA. Alterations in the hypothalamic paraventricular nucleus and its oxytocin neurons (putative satiety cells) in Prader-Willi syndrome: a study of five cases. J Clin Endocrinol Metab. Oxford Academic; 1995;80:573–9. [DOI] [PubMed] [Google Scholar]
- *41.Rice LJ, Einfeld SL, Hu N, Carter CS. A review of clinical trials of oxytocin in Prader–Willi syndrome. Curr Opin Psychiatry. Lippincott Williams and Wilkins; 2018;31:123–7. [DOI] [PubMed] [Google Scholar]; A review of IN-OT efficacy in PWS that concludes there is currently no robust evidence that IN-OT is effective for improving PWS symptoms (e.g., behavioral problems, social skills) largely due to methodological limitations. The paper also underlines the importance of rigorously investigating OT dysfunction in PWS.
- 42.Tauber M, Mantoulan C, Copet P, Jauregui J, Demeer G, Diene G, et al. Oxytocin may be useful to increase trust in others and decrease disruptive behaviours in patients with Prader-Willi syndrome: a randomised placebo-controlled trial in 24 patients. Orphanet J Rare Dis. 2011;6:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, et al. Intranasal Oxytocin Improves Emotion Recognition for Youth with Autism Spectrum Disorders. Biol Psychiatry. 2010;67:692–4. [DOI] [PubMed] [Google Scholar]
- 44.Gordon I, Vander Wyk BC, Bennett RH, Cordeaux C, Lucas MV, Eilbott JA, et al. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci. 2013;110:20953–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tachibana M, Kagitani-Shimono K, Mohri I, Yamamoto T, Sanefuji W, Nakamura A, et al. Long-Term Administration of Intranasal Oxytocin Is a Safe and Promising Therapy for Early Adolescent Boys with Autism Spectrum Disorders. J Child Adolesc Psychopharmacol. 2013;23:123–7. [DOI] [PubMed] [Google Scholar]
- 46.Dadds MR, MacDonald E, Cauchi A, Williams K, Levy F, Brennan J. Nasal Oxytocin for Social Deficits in Childhood Autism: A Randomized Controlled Trial. J Autism Dev Disord. 2014;44:521–31. [DOI] [PubMed] [Google Scholar]
- 47.Guastella AJ, Gray KM, Rinehart NJ, Alvares GA, Tonge BJ, Hickie IB, et al. The effects of a course of intranasal oxytocin on social behaviors in youth diagnosed with autism spectrum disorders: A randomized controlled trial. J Child Psychol Psychiatry. 2015;56:444–52. [DOI] [PubMed] [Google Scholar]
- 48.Ooi Y, Weng S-J, Kossowsky J, Gerger H, Sung M. Oxytocin and Autism Spectrum Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pharmacopsychiatry. Georg Thieme Verlag; 2016;50:5–13. [DOI] [PubMed] [Google Scholar]
- *49.Alvares GA, Quintana DS, Whitehouse AJO. Beyond the hype and hope: Critical considerations for intranasal oxytocin research in autism spectrum disorder. Autism Res. 2017;10:25–41. [DOI] [PubMed] [Google Scholar]; A review of IN-OT studies in ASD that presents a theoretical rationale for IN-OT as a novel treatment and highlights key issues for improvement of research standards in this field.
- 50.Gordon I, Jack A, Pretzsch CM, Vander Wyk B, Leckman JF, Feldman R, et al. Intranasal Oxytocin Enhances Connectivity in the Neural Circuitry Supporting Social Motivation and Social Perception in Children with Autism. Sci Rep. Nature Publishing Group; 2016;6:35054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yatawara CJ, Einfeld SL, Hickie IB, Davenport TA, Guastella AJ. The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: a randomized clinical crossover trial. Mol Psychiatry. Nature Publishing Group; 2016;21:1225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parker KJ, Oztan O, Libove RA, Sumiyoshi RD, Jackson LP, Karhson DS, et al. Intranasal oxytocin treatment for social deficits and biomarkers of response in children with autism. Proc Natl Acad Sci. 2017;114:8119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Munesue T, Nakamura H, Kikuchi M, Miura Y, Takeuchi N, Anme T, et al. Oxytocin for Male Subjects with Autism Spectrum Disorder and Comorbid Intellectual Disabilities: A Randomized Pilot Study. Front Psychiatry. 2016;7:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anagnostou E, Soorya L, Brian J, Dupuis A, Mankad D, Smile S, et al. Intranasal oxytocin in the treatment of autism spectrum disorders: a review of literature and early safety and efficacy data in youth. Brain Res. Elsevier; 2014;1580:188–98. [DOI] [PubMed] [Google Scholar]
- 55.Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biol Psychiatry. 2007;61:731–3. [DOI] [PubMed] [Google Scholar]
- 56.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–6. [DOI] [PubMed] [Google Scholar]
- 57.Van IJzendoorn MH, Bakermans-Kranenburg MJ. A sniff of trust: Meta-analysis of the effects of intranasal oxytocin administration on face recognition, trust to in-group, and trust to out-group. Psychoneuroendocrinology. Elsevier Ltd; 2012;37:438–43. [DOI] [PubMed] [Google Scholar]
- 58.Shamay-Tsoory SG, Abu-Akel A. The social salience hypothesis of oxytocin. Biol Psychiatry. Elsevier; 2016;79:194–202. [DOI] [PubMed] [Google Scholar]
- *59.Pehlivanoglu D, Myers E, Ebner NC. Tri-Phasic Model ofOxytocin (TRIO): A systematic conceptual review of oxytocin-related ERP research. Biol Psychol. Elsevier B.V.; 2020;154:107917. [DOI] [PMC free article] [PubMed] [Google Scholar]; A systematic review of studies that examined ERP to elucidate the temporal dynamics of IN-OT in the brain. Findings conclude that OT modulation occurs in three processing stages ─ perception, selection, and evaluation. The paper provides a novel conceptual framework for the study of OT effects on attentional processes, starting early in the processing stream.
- 60.Xue S-W, Wu H-B, Zhang L, Zhang D-X. Intranasal Oxytocin Increases Perceptual Salience of Faces in the Absence of Awareness. Psychiatry Investig. Korean Neuropsychiatric Association; 2020;17:292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin Shapes the Neural Circuitry of Trust and Trust Adaptation in Humans. Neuron. 2008;58:639–50. [DOI] [PubMed] [Google Scholar]
- 62.Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wigton R, Radua J, Allen P, Averbeck B, Meyer-Lindenberg A, Mcguire P, et al. Neurophysiological effects of acute oxytocin administration: systematic review and meta-analysis of placebo-controlled imaging studies. J Psychiatry Neurosci. Canadian Medical Association; 2015;40:E1–E22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *64.Grace SA, Rossell SL, Heinrichs M, Kordsachia C, Labuschagne I. Oxytocin and brain activity in humans: A systematic review and coordinate-based meta-analysis of functional MRI studies. Psychoneuroendocrinology. Elsevier Ltd; 2018;96:6–24. [DOI] [PubMed] [Google Scholar]; A systematic review and meta-analysis on the neural correlates of IN-OT in healthy and clinical populations (i.e., ASD, SCZ, borderline personality disorder, generalized social anxiety disorder, posttraumatic stress disorder, and depression). Findings suggest that IN-OT exerts its socio-behavioral and cognitive effects through modulation of brain regions, including the superior temporal gyrus.
- 65.Wang D, Yan X, Li M, Ma Y. Neural substrates underlying the effects of oxytocin: a quantitative meta-analysis of pharmaco-imaging studies. Soc Cogn Affect Neurosci. Oxford University Press; 2017;12:1565–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perez-Rodriguez M, Mahon K, Russo M, Ungar AK, Burdick KE. Oxytocin and social cognition in affective and psychotic disorders. Eur Neuropsychopharmacol. Elsevier B.V.; 2015;25:265–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shilling PD, Feifel D. Potential of Oxytocin in the Treatment of Schizophrenia. CNS Drugs. 2016;30:193–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **68.Keech B, Crowe S, Hocking DR. Intranasal oxytocin, social cognition and neurodevelopmental disorders: A meta-analysis. Psychoneuroendocrinology. Elsevier Ltd; 2018;87:9–19. [DOI] [PubMed] [Google Scholar]; A meta-analysis examining IN-OT effects on social cognition in neurodevelopmental disorders with known deficits in social functioning and abnormalities in the OT system (i.e., SCZ, ASD, PWS). The paper highlights the preliminary nature of OT-related improvement in social cognition for individuals with neurodevelopmental disorders and discusses challenges in the interpretation of IN-OT efficacy in these clinical conditions.
- 69.Domes G, Heinrichs M, Kumbier E, Grossmann A, Hauenstein K, Herpertz SC. Effects of intranasal oxytocin on the neural basis of face processing in autism spectrum disorder. Biol Psychiatry. Elsevier; 2013;74:164–71. [DOI] [PubMed] [Google Scholar]
- 70.Aoki Y, Yahata N, Watanabe T, Takano Y, Kawakubo Y, Kuwabara H, et al. Oxytocin improves behavioural and neural deficits in inferring others’ social emotions in autism. Brain. 2014;137:3073–86. [DOI] [PubMed] [Google Scholar]
- 71.Kruppa JA, Gossen A, Oberwelland Weiß E, Kohls G, Großheinrich N, Cholemkery H, et al. Neural modulation of social reinforcement learning by intranasal oxytocin in male adults with high-functioning autism spectrum disorder: a randomized trial. Neuropsychopharmacology. Nature Publishing Group; 2019;44:749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Watanabe T, Kuroda M, Kuwabara H, Aoki Y, Iwashiro N, Tatsunobu N, et al. Clinical and neural effects of six-week administration of oxytocin on core symptoms of autism. Brain. 2015;138:3400–12. [DOI] [PubMed] [Google Scholar]
- 73.Anagnostou E, Soorya L, Chaplin W, Bartz J, Halpern D, Wasserman S, et al. Intranasal oxytocin versus placebo in the treatment of adults with autism spectrum disorders: a randomized controlled trial. Mol Autism. Molecular Autism; 2012;3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bernaerts S, Boets B, Bosmans G, Steyaert J, Alaerts K. Behavioral effects of multiple-dose oxytocin treatment in autism: A randomized, placebo-controlled trial with long-term follow-up. Mol Autism. BioMed Central Ltd.; 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Savla GN, Vella L, Armstrong CC, Penn DL, Twamley EW. Deficits in Domains of Social Cognition in Schizophrenia: A Meta-Analysis of the Empirical Evidence. Schizophr Bull. 2012;39:979–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bürkner P-C, Williams DR, Simmons TC, Woolley JD. Intranasal Oxytocin May Improve High-Level Social Cognition in Schizophrenia, But Not Social Cognition or Neurocognition in General: A Multilevel Bayesian Meta-analysis. Schizophr Bull. 2017;43:1291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guastella AJ, Ward PB, Hickie IB, Shahrestani S, Hodge MAR, Scott EM, et al. A single dose of oxytocin nasal spray improves higher-order social cognition in schizophrenia. Schizophr Res. Elsevier B.V.; 2015;168:628–33. [DOI] [PubMed] [Google Scholar]
- 78.Bradley ER, Seitz A, Niles AN, Rankin KP, Mathalon DH, O’Donovan A, et al. Oxytocin increases eye gaze in schizophrenia. Schizophr Res. Elsevier B.V.; 2019;212:177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.De Coster L, Lin L, Mathalon DH, Woolley JD. Neural and behavioral effects of oxytocin administration during theory of mind in schizophrenia and controls: a randomized control trial. Neuropsychopharmacology. Nature Publishing Group; 2019;44:1925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee MR, Wehring HJ, McMahon RP, Liu F, Linthicum J, Buchanan RW, et al. The Effect of Intranasal Oxytocin on Measures of Social Cognition in Schizophrenia: A Negative Report. J Psychiatry Brain Sci. Hapres; 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jarskog LF, Pedersen CA, Johnson JL, Hamer RM, Rau SW, Elliott T, et al. A 12-week randomized controlled trial of twice-daily intranasal oxytocin for social cognitive deficits in people with schizophrenia. Schizophr Res. Elsevier B.V.; 2017;185:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Natelson Love MC, Ruff G, Geldmacher DS. Social Cognition in Older Adults: A Review of Neuropsychology, Neurobiology, and Functional Connectivity. Med Clin Rev. 2015;1:1–8. [Google Scholar]
- 83.Ebner NC, Fischer H. Emotion and aging: Evidence from brain and behavior. Front Psychol. 2014;5:996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scheibe S, Carstensen LL. Emotional aging: recent findings and future trends. Journals Gerontol Ser B Psychol Sci Soc Sci. Oxford University Press; 2010;65B:135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ebner NC, Kamin H, Diaz V, Cohen RA, MacDonald K. Hormones as “difference makers” in cognitive and socioemotional aging processes. Front Psychol. Frontiers; 2015;5:1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Plasencia G, Luedicke JM, Nazarloo HP, Carter CS, Ebner NC. Plasma oxytocin and vasopressin levels in young and older men and women: Functional relationships with attachment and cognition. Psychoneuroendocrinology. 2019;110:104419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Elabd C, Cousin W, Upadhyayula P, Chen RY, Chooljian MS, Li J, et al. Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nat Commun. Nature Publishing Group; 2014;5:4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Calzà L, Pozza M, Coraddu F, Farci G, Giardino L. Hormonal influences on brain ageing quality: Focus on corticotropin releasing hormone-, vasopressin- and oxytocin-immunoreactive neurones in the human brain. J Neural Transm. J Neural Transm (Vienna); 1997. p. 1095–100. [DOI] [PubMed] [Google Scholar]
- 89.Purba JS, Hofman MA, Swaab DF. Decreased number of oxytocin-immunoreactive neurons in the paraventricular nucleus of the hypothalamus in Parkinson’s disease. Neurology. 1994;44:84–84. [DOI] [PubMed] [Google Scholar]
- 90.Arsenijevic Y, Dreifuss JJ, Vallet P, Marguerat A, Tribollet E. Reduced binding of oxytocin in the rat brain during aging. Brain Res. 1995;698:275–9. [DOI] [PubMed] [Google Scholar]
- 91.Ivell R, Walther N. The role of sex steroids in the oxytocin hormone system. Mol Cell Endocrinol. Elsevier Ireland Ltd; 1999;151:95–101. [DOI] [PubMed] [Google Scholar]
- 92.Engelmann M, Landgraf R, Wotjak CT. The hypothalamic–neurohypophysial system regulates the hypothalamic–pituitary–adrenal axis under stress: An old concept revisited. Front Neuroendocrinol. Academic Press; 2004;25:132–49. [DOI] [PubMed] [Google Scholar]
- 93.Gaffey AE, Bergeman CS, Clark LA, Wirth MM. Aging and the HPA axis: Stress and resilience in older adults. Neurosci. Biobehav. Rev Elsevier Ltd; 2016. p. 928–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Russell JK, Jones CK, Newhouse PA. The Role of Estrogen in Brain and Cognitive Aging. Neurotherapeutics. Springer New York LLC; 2019. p. 649–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Quintana DS, Woolley JD. Intranasal Oxytocin Mechanisms Can Be Better Understood, but Its Effects on Social Cognition and Behavior Are Not to Be Sniffed At. Biol Psychiatry. Elsevier; USA; 2016;79:e49–50. [DOI] [PubMed] [Google Scholar]
- 96.Horta M, Ziaei M, Lin T, Porges EC, Fischer H, Feifel D, et al. Oxytocin alters patterns of brain activity and amygdalar connectivity by age during dynamic facial emotion identification. Neurobiol Aging. 2019;78:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grainger SA, Henry JD, Steinvik HR, Vanman EJ, Rendell PG, Labuschagne I. Intranasal oxytocin does not reduce age-related difficulties in social cognition. Horm Behav. Academic Press; 2018;99:25–34. [DOI] [PubMed] [Google Scholar]
- 98.Grainger SA, Henry JD, Steinvik HR, Vanman EJ. Intranasal oxytocin does not alter initial perceptions of facial trustworthiness in younger or older adults. J Psychopharmacol. 2019;33:250–4. [DOI] [PubMed] [Google Scholar]
- 99.Barraza JA, Grewal NS, Ropacki S, Perez P, Gonzalez A, Zak PJ. Effects of a 10-day oxytocin trial in older adults on health and well-being. Exp Clin Psychopharmacol. American Psychological Association; 2013;21:85–92. [DOI] [PubMed] [Google Scholar]
- 100.Maillet D, Rajah MN. Association between prefrontal activity and volume change in prefrontal and medial temporal lobes in aging and dementia: A review. Ageing Res. Rev Elsevier; 2013. p. 479–89. [DOI] [PubMed] [Google Scholar]
- 101.Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD. Differential aging of the medial temporal lobe: A study of a five-year change. Neurology. Lippincott Williams and Wilkins; 2004;62:433–8. [DOI] [PubMed] [Google Scholar]
- 102.Barnes J, Whitwell JL, Frost C, Josephs KA, Rossor M, Fox NC. Measurements of the amygdala and hippocampus in pathologically confirmed Alzheimer disease and frontotemporal lobar degeneration. Arch Neurol. American Medical Association; 2006;63:1434–9. [DOI] [PubMed] [Google Scholar]
- 103.Baez S, Pinasco C, Roca M, Ferrari J, Couto B, García-Cordero I, et al. Brain structural correlates of executive and social cognition profiles in behavioral variant frontotemporal dementia and elderly bipolar disorder. Neuropsychologia. Elsevier Ltd; 2019;126:159–69. [DOI] [PubMed] [Google Scholar]
- 104.Althammer F, Grinevich V. Diversity of oxytocin neurones: Beyond magno- and parvocellular cell types? J. Neuroendocrinol Blackwell Publishing Ltd; 2018. [DOI] [PubMed] [Google Scholar]
- 105.Busnelli M, Chini B. Molecular basis of oxytocin receptor signalling in the brain: what we know and what we need to know. Curr Top Behav Neurosci. Springer Verlag; 2018. p. 3–29. [DOI] [PubMed] [Google Scholar]
- 106.Quintana DS, Rokicki J, van der Meer D, Alnæs D, Kaufmann T, Córdova-Palomera A, et al. Oxytocin pathway gene networks in the human brain. Nat Commun. Nature Publishing Group; 2019;10:668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Boccia ML, Petrusz P, Suzuki K, Marson L, Pedersen CA. Immunohistochemical localization of oxytocin receptors in human brain. Neuroscience. Pergamon; 2013;253:155–64. [DOI] [PubMed] [Google Scholar]
- 108.Finger EC, MacKinley J, Blair M, Oliver LD, Jesso S, Tartaglia MC, et al. Oxytocin for frontotemporal dementia: A randomized dose-finding study of safety and tolerability. Neurology. 2015;84:174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.De Dreu CKW, Kret ME. Oxytocin Conditions Intergroup Relations Through Upregulated In-Group Empathy, Cooperation, Conformity, and Defense. Biol Psychiatry Elsevier; 2016;79:165–73. [DOI] [PubMed] [Google Scholar]
- 110.Sheng F, Liu Y, Zhou B, Zhou W, Han S. Oxytocin modulates the racial bias in neural responses to others’ suffering. Biol Psychol. Elsevier B.V.; 2013;92:380–6. [DOI] [PubMed] [Google Scholar]
- 111.Lane A, Mikolajczak M, Treinen E, Samson D, Corneille O, de Timary P, et al. Failed Replication of Oxytocin Effects on Trust: The Envelope Task Case. de Wit H, editor. PLoS One. 2015;10:e0137000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bänziger T, Mortillaro M, Scherer KR. Introducing the Geneva Multimodal expression corpus for experimental research on emotion perception. Emotion. 2012;12:1161–79. [DOI] [PubMed] [Google Scholar]
- 113.Piva M, Chang SWC. An integrated framework for the role of oxytocin in multistage social decision-making. Am J Primatol. 2018;e22735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Quintana DS, Westlye LT, Alnæs D, Kaufmann T, Mahmoud RA, Smerud KT, et al. Low-dose intranasal oxytocin delivered with Breath Powered device modulates pupil diameter and amygdala activity: a randomized controlled pupillometry and fMRI study. Neuropsychopharmacology. Nature Publishing Group; 2019;44:306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bethlehem RAI, Lombardo MV, Lai M-C, Auyeung B, Crockford SK, Deakin J, et al. Intranasal oxytocin enhances intrinsic corticostriatal functional connectivity in women. Transl Psychiatry. Nature Publishing Group; 2017;7:e1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bos PA, Spencer H, Montoya ER. Oxytocin reduces neural activation in response to infant faces in nulliparous young women. Soc Cogn Affect Neurosci. 2018;13:1099–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chu C, Hammock EAD, Joiner TE. Unextracted plasma oxytocin levels decrease following in-laboratory social exclusion in young adults with a suicide attempt history. J Psychiatr Res. Elsevier Ltd; 2020;121:173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Saxbe D, Khaled M, Horton KT, Mendez AJ. Maternal prenatal plasma oxytocin is positively associated with prenatal psychological symptoms, but method of immunoassay extraction may affect results. Biol Psychol. Elsevier B.V.; 2019;147:107718. [DOI] [PubMed] [Google Scholar]
- 119.Carter CS. Developmental consequences of oxytocin. Physiol Behav. Elsevier; 2003;79:383–97. [DOI] [PubMed] [Google Scholar]
- 120.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nat Rev Neurosci. Nature Publishing Group; 2011;12:524–38. [DOI] [PubMed] [Google Scholar]
- 121.Eckstein M, Bamert V, Stephens S, Wallen K, Young LJ, Ehlert U, et al. Oxytocin increases eye-gaze towards novel social and non-social stimuli. Soc Neurosci. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kemp AH, Guastella AJ. Oxytocin: Prosocial behavior, social salience, or approach-related behavior? Inflamm Alzheimer’s Dis. 2010;67:e33–e34. [DOI] [PubMed] [Google Scholar]
- 123.Kemp AH, Guastella AJ. The role of oxytocin in human affect: A novel hypothesis. Curr Dir Psychol Sci. 2011;20:222–31. [Google Scholar]
- 124.Olff M, Frijling JL, Kubzansky LD, Bradley B, Ellenbogen MA, Cardoso C, et al. The role of oxytocin in social bonding, stress regulation and mental health: An update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology. Pergamon; 2013;38:1883–94. [DOI] [PubMed] [Google Scholar]
- 125.Li SC. Biocultural Orchestration of Developmental Plasticity Across Levels: The Interplay of Biology and Culture in Shaping the Mind and Behavior Across the Life Span. Psychol. Bull 2003. p. 171–94. [DOI] [PubMed] [Google Scholar]
- 126.Hofer SM, Piccinin AM. Toward an Integrative Science of Life-Span Development and Aging. Journals Gerontol Ser B. 2010;65B:269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Roney JR. Theoretical frameworks for human behavioral endocrinology. Horm Behav. Academic Press; 2016;84:97–110. [DOI] [PubMed] [Google Scholar]
- 128.Schaie W, Baltes PB. On Sequential Strategies in Developmental Research. Hum Dev. Karger Publishers; 1975;18:384–90. [Google Scholar]
- 129.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. Public Library of Science; 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *130.Quintana DS. Revisiting non-significant effects of intranasal oxytocin using equivalence testing. Psychoneuroendocrinology. Elsevier Ltd; 2018;87:127–30. [DOI] [PubMed] [Google Scholar]; This paper reported the results of equivalence tests conducted on published and unpublished data from IN-OT studies, which indicated that various non-significant effects were due to data insensitivity over statistical equivalence. This paper highlights the importance of employing equivalence testing in IN-OT research to assess the absence of an effect for nonsignificant results.
- 131.Weisman O, Zagoory-Sharon O, Schneiderman I, Gordon I, Feldman R. Plasma oxytocin distributions in a large cohort of women and men and their gender-specific associations with anxiety. Psychoneuroendocrinology. 2013;38:694–701. [DOI] [PubMed] [Google Scholar]
- 132.Prakash VS, Mansukhani NA, Helenowski IB, Woodruff TK, Kibbe MR. Sex Bias in Interventional Clinical Trials. J Women’s Heal. Mary Ann Liebert Inc.; 2018;27:1342–8. [DOI] [PubMed] [Google Scholar]
- 133.Uhl K, Parekh A, Kweder S. Females in Clinical Studies: Where are We Going? Clin Pharmacol Ther. John Wiley & Sons, Ltd; 2007;81:600–2. [DOI] [PubMed] [Google Scholar]
- 134.Lussier D, Cruz-Almeida Y, Ebner NC. Musculoskeletal Pain and Brain Morphology: Oxytocin’s Potential as a Treatment for Chronic Pain in Aging. Front Aging Neurosci. Frontiers Media S.A.; 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xin F, Zhou F, Zhou X, Ma X, Geng Y, Zhao W, et al. Oxytocin Modulates the Intrinsic Dynamics Between Attention-Related Large-Scale Networks. Cereb Cortex. 2018; [DOI] [PubMed] [Google Scholar]
- 136.Brodmann K, Gruber O, Goya-Maldonado R. Intranasal Oxytocin Selectively Modulates Large-Scale Brain Networks in Humans. Brain Connect. Mary Ann Liebert Inc.; 2017;7:454–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *137.Bradley ER, Woolley JD. Oxytocin effects in schizophrenia: Reconciling mixed findings and moving forward. Neurosci Biobehav Rev. 2017;80:36–56. [DOI] [PMC free article] [PubMed] [Google Scholar]; A review of IN-OT effects in SCZ that demonstrated mixed findings for IN-OT treatment of SCZ symptoms and social-cognitive deficits. This paper suggested future research should investigate IN-OT effects on mentalizing, non-social cognition, olfaction, and facial expressivity in SCZ further.
- 138.Zik JB, Roberts DL. The many faces of oxytocin: Implications for psychiatry. Psychiatry Res. Elsevier Ireland Ltd; 2015. p. 31–7. [DOI] [PubMed] [Google Scholar]
- 139.MacLean EL, Wilson SR, Martin WL, Davis JM, Nazarloo HP, Carter CS. Challenges for measuring oxytocin: The blind men and the elephant? Psychoneuroendocrinology. Elsevier Ltd; 2019. p. 225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]


