Abstract
Bunyaviruses (Negarnaviricota: Bunyavirales) are a large and diverse group of viruses that include important human, veterinary, and plant pathogens. The rapid characterization of known and new emerging pathogens depends on the availability of comprehensive reference sequence databases that can be used to match unknowns, infer evolutionary relationships and pathogenic potential, and make response decisions in an evidence-based manner. In this study, we determined the coding-complete genome sequences of 99 bunyaviruses in the Centers for Disease Control and Prevention’s Arbovirus Reference Collection, focusing on orthonairoviruses (family Nairoviridae), orthobunyaviruses (Peribunyaviridae), and phleboviruses (Phenuiviridae) that either completely or partially lacked genome sequences. These viruses had been collected over 66 years from 27 countries from vertebrates and arthropods representing 37 genera. Many of the viruses had been characterized serologically and through experimental infection of animals but were isolated in the pre-sequencing era. We took advantage of our unusually large sample size to systematically evaluate genomic characteristics of these viruses, including reassortment, and co-infection. We corroborated our findings using several independent molecular and virologic approaches, including Sanger sequencing of 197 genome segments, and plaque isolation of viruses from putative co-infected virus stocks. This study contributes to the described genetic diversity of bunyaviruses and will enhance the capacity to characterize emerging human pathogenic bunyaviruses.
Author summary
Prior knowledge about families of pathogens can enhance efforts to prepare for and respond to emerging disease threats. The CDC’s Arbovirus Reference Collection (ARC) comprises a world reference repository of arthropod-borne viruses that are either pathogenic or are related to known pathogens. Many viruses in this collection were isolated before genome sequencing was readily available, resulting in an incomplete understanding of their potential relevance to human or animal health. In this study, we sequenced the genomes of 99 bunyaviruses in the ARC. We performed detailed phylogenetic analyses, described multiple well-supported instances of genome segment reassortment, and identified co-infection in several virus stocks. These sequences enhance the public database of known bunyaviruses which can be interrogated to identify emerging viruses during an outbreak, and contribute to a better understanding of bunyavirus evolution and pathogenic potential.
Introduction
New pathogens continue to emerge and threaten human, animal, and plant health. For humans alone, an average of two new disease-causing agents are reported each year [1–3]. These pathogens have primarily been viruses, have usually been associated with animal reservoirs, and many are vector-borne [2]. Bunyaviruses (Riboviria: Negarnaviricota: Bunyavirales) rank among the most serious infectious threats to humans (e.g. Crimean Congo hemorrhagic fever virus [4]), animals (e.g. Rift Valley fever virus [5]), and plants (e.g. tomato spotted wilt virus [6]) [7,8]. Bunyaviruses accounted for approximately 20% of the 188 human pathogenic viruses identified between 1901 and 2005 [3], and new pathogenic bunyaviruses continue to emerge [9–13]. Traditionally bunyaviruses have been grouped according to their serological cross-reactivity (i.e. they have been binned into serogroups), but molecular data and the discovery of many new viruses has driven the reorganization of the order. The order Bunyavirales now includes 12 families: Arenaviridae, Cruliviridae, Fimoviridae, Hantavirdae, Leishbuviridae, Mypoviridae, Nairoviridae, Peribunyaviridae, Phasmaviridae, Phenuiviridae,Tospoviridae, and Wupedeviridae [14,15].
Bunyaviruses have segmented, negative-sense or ambisense RNA genomes. Many currently characterized bunyavirus genomes are composed of three segments: a large (L) segment encoding a protein that functions as an RNA-directed RNA polymerase (RdRp); a medium (M) segment encoding glycoproteins (Gn and Gc); and a small (S) segment encoding a nucleoprotein (NP) and, in some clades, a non-structural protein, NSs. Some bunyavirus lineages have different numbers of genome segments. For instance, the majority of viruses in the Arenaviridae family are bisegmented, and some plant-infecting emaraviruses (family Fimoviridae) have 8 segments [16].
Like all viruses with segmented genomes, bunyaviruses are capable of reassortment, which appears to have occurred commonly during bunyavirus evolution [17]. Reassortment has happened between relatively distantly related bunyaviruses (intertypic reassortment) and between closely-related co-circulating strains (intratypic reassortment) [17,18], and can have epidemiological significance. For example, Ngari virus is a reassortant bunyavirus associated with outbreaks of hemorrhagic fever [19]. Iquitos and Itaya viruses are emerging reassortant viruses (Peribunyaviridae: Orthobunyavirus) that have both been associated with human illness in Peru [9,13]; an intra-lineage reassortant strain of Rift Valley fever virus (Phenuiviridae: Phlebovirus) was recently isolated from an ill traveler returning home to China [20]; and severe fever with thrombocytopenia syndrome virus (SFTSV) is a newly-recognized, tick-borne phenuivirus that causes severe human disease and for which reassortment has been recognized in strains detected in human patients [21]. Given the emergence potential of bunyaviruses and the contribution of reassortment to the generation of new virus strains, having the capability to recognize novel and reassortant viruses is paramount to public health preparedness.
The US Center for Disease Control and Prevention’s (CDC) Division of Vector-borne Diseases (DVBD), Arboviral Diseases Branch (ADB), maintains an Arbovirus Reference Collection (ARC). The ARC contains viruses that were isolated from arthropod vectors and vertebrate hosts, as well as from human clinical specimens. Isolates have been collected and deposited into the ARC over a 90-year period. As one of the largest collections of arboviruses in the world, the ARC is an invaluable resource for scientists and public health workers. The ARC includes 2,766 unique isolates, of which 835 are bunyaviruses. Some of these viruses have been genetically characterized, but many have not. Genetic characterization of the viruses in the ARC would expand the value and impact of this collection even further as a scientific and public health resource and infuse a significant amount of novel and important molecular data into the public domain for actionable use. Therefore, the objective of this project was to generate additional and novel genome sequence and phylogenetic data for a large group of bunyaviruses lacking genetic characterization to facilitate the rapid identification and characterization of emerging bunyaviruses.
Methods
Sample collection
Viruses represented a subset of bunyaviruses catalogued within the ARC [22] (Tables 1–3) that spanned most serogroups as well as ungrouped viruses. RNA was extracted from archived tissue culture supernatants or suckling mouse brain preparations using the QIAamp Viral RNA mini kit according to manufacturer’s instructions and eluted in 100μl AVE buffer (Qiagen). TURBO DNase was used to remove residual DNA (ThermoFisher), according to the manufacturer’s protocol. RNA concentrations were measured fluorometrically using the Qubit RNA HS Assay kit and a Qubit 3 fluorometer (Life Technologies).
Table 1. Sequenced genomes of 88 viruses belonging to Peribunyaviridae (Orthobunyavirus or Pacuvirus).
| Sequence Status * | Virus | Abbreviation | Strain (Passage**) | Serogroup | Collection Date | Collection Country*** | Isolation Source**** | Original Virus Description | GenBank Accession |
|---|---|---|---|---|---|---|---|---|---|
| 4 | Abras | ABRV | 75V1183 (P4V1) | Patois | 1974 | Ecuador | Culex mosquito | [46] | MK896654 –MK896656 |
| 1 | Acará | ACAV | BeAn 27639 (P2SM1) | Capim | 1961 | Brazil | Mouse | [47,48] | MK896651 –MK896653 |
| 2 | Aino | AINOV | JaNAr 28 (P9SM1) | Simbu | 1964 | Japan | Culex mosquito | [49] | MH484276 –MH484278 |
| 4 | Ananindeua | ANUV | BeAn 109303 (P36SM1) | Guamá | 1966 | Brazil | Opossum | [50] | MK896648 –MK896650 |
| 3 | Anopheles B | ANBV | Original | Anopheles B | 1940 | Colombia | Anopheles mosquito | [51] | MK896642 –MK896644 |
| 1 | Antequera | ANTV | AG80-226 (V2SM2V1) | Resistencia | 1980 | Argentina | Culex mosquito | [52,53] | MK896639 –MK896641 |
| 4 | Babahoyo | BABV | 75V2858 (P2SM2) | Patois | 1975 | Ecuador | Culex mosquito | [46] | MH484273 –MH484275 |
| 1 | Bakau | BAKV | MM-2325 (P8SM1) | Bakau | 1956 | Malaysia | Culex mosquito | [54] | MK896633 –MK896635 |
| 1 | Bangui | BGIV | DakHB 754 (P9SM1) | Ungrouped | 1970 | Central African Republic | Human | [55,56] | MK896630 –MK896632 |
| 1 | Barranqueras | BQSV | AG80-381 (V1SM3) | Resistencia | 1980 | Argentina | Culex mosquito | [52,53] | MK896627 –MK896629 |
| 3 | Batama | BMAV | DakAnB 1292 (P8SM2) | Tete | 1970 | Central African Republic | Bird | [57] | MK896624 –MK896626 |
| 1 | Belem | BLMV | BeAn 141106 (P11SM1) | Ungrouped | 1968 | Brazil | Bird | [58] | MK896621 –MK896623 |
| 1 | Benevides | BVSV = BENV | BeAr 153564 (P?SM2V1) | Capim | 1968 | Brazil | Mouse | MK896618 –MK896620 | |
| 1 | Benfica | BENV = BNFV | 71U344 (P?SM6) | Capim | 1971 | Peru | Hamster | [59] | MK896615 –MK896617 |
| 1 | Bertioga | BERV | 76V25643 (P?SM3) | Guamá | 1976 | Brazil | Culex mosquito | [60,61] | MK896612 –MK896614 |
| 4 | Birao | BIRV | DakArB 2198 (P?SM1) | Bunyamwera | 1969 | Central African Republic | Anopheles mosquito | [62] | MH484282 –MH484284 |
| 1 | Bobaya | BOBV | DakAnB 2208 (P9SM1) | Simbu | 1971 | Central African Republic | Bird | [56,57] | MW415980-MW415982 |
| 3 | Boracéia | BORV | SPAr 395 (P?SM2) | Anopheles B | 1962 | Brazil | Anopheles mosquito | [63] | MK896609—MK896611 |
| 2 | Boracéia | BORV | SPAr 4080 (P?) | Anopheles B | 1965 | Brazil | Anopheles mosquito | [63] | MK896606 –MK896608 |
| 4 | Bozo | BOZOV | DakArB 7343 (P?SM2) | Bunyamwera | 1975 | Central African Republic | Aedes mosquito | [64] | MH484285 –MH484287 |
| 2 | Bruconha | BRUV | 77V14676 (P?V1SM5) | C | 1976 | Brazil | Culex mosquito | [50] | MK896603 –MK896605 |
| 2 | Bunyamwera | BUNV | 46A-122 (V3) | Bunyamwera | 2006 | Kenya | Aedes mosquito | [65] | MH484288 –MH484290 |
| 1 | Bushbush | BSBV | TRVL 26668 (P?SM3) | Capim | 1959 | Trinidad & Tobago | Culex mosquito | [47,66] | MK896597 –MK896599 |
| 3 | Buttonwillow | BUTV | A 7956 (P?SM3H1SM1) | Simbu | 1961 | USA | Rabbit | [67,68] | MH484291 –MH484293 |
| 4 | Caimito | CAIV | VP 488A (P8V2SM1) | Ungrouped | 1971 | Panama | Sand fly | [69,70] | MK896592 –MK896594 |
| 1 | Cananéia | CNAV | SPAn 64962 (SM4V1) | Guamá | 1976 | Brazil | Mouse | [50] | MK896589 –MK896591 |
| 4 | Caraparú | CARV | BeAn 3994 (P?SM11) | C | 1956 | Brazil | Monkey | [71] | MK896586 –MK896588 |
| 2 | Enseada | ENSV | 78V213 (P?V1SM2) | Ungrouped | 1976 | Brazil | Culex mosquito | [50] | MK896583 –MK896585 |
| 4 | Fort Sherman | FSV | 86MSP18 (P2V1) | Bunyamwera | 1985 | Panama | Human | [72] | MH484294 –MH484296 |
| 2 | Gamboa | GAMV | 75V20086 (P?SM1V1) | Gamboa | 1975 | Ecuador | Aedeomyia squami-pennis mosquito | [73,74] | MK896574 –MK896576 |
| 2 | Guajará | GJAV | 18315 (P?SM4) | Capim | 1975 | Ecuador | Hamster | [46,60] | MK896571 –MK896573 |
| 1 | Guaratuba | GTBV | 76V25271 (P?SM3) | Guamá | 1976 | Brazil | Culex mosquito | [50] | MK896568 –MK896570 |
| 3 | Gumbo Limbo | GLV | FE3-71H (P4V1) | C | 1963 | USA | Culex mosquito | [75] | MK896565 –MK896567 |
| 4 | Itaquí | ITQV | BeAn 12797 (P?SM4V1SM2) | C | 1959 | Brazil | Mouse | [76,77] | MK896558 –MK896560 |
| 1 | Itimirim | ITIV | SPAn 47817 (SM6) | Guamá | 1976 | Brazil | Rat | [50] | MK896555 –MK896557 |
| 1 | Juan Díaz | JDV | MARU 8563 (P?SM5ppfV2SM1) | Capim | 1962 | Panama | Mouse | [73] | MK896552 –MK896554 |
| 3 | Kaikalur | KAIV | VRC 713423–2 (P7SM1) | Simbu | 1971 | India | Culex mosquito | [78] | MH484297 –MH484299 |
| 4 | Kairi | KRIV | TRVL 8900 (P13SM1) | Bunyamwera | 1955 | Trinidad & Tobago | Aedes mosquito | [79,80] | MH484300 –MH484302 |
| 1 | Ketapang | KETV | MM 2549 (P5SM2) | Bakau | 1956 | Malaysia | Culex mosquito | [54,60] | MK896546 –MK896548 |
| 1 | Las Maloyas | LMV | AG80-24 (P?V1SM4) | Anopheles A | 1980 | Argentina | Anopheles mosquito | [52,53] | MK896543 –MK896545 |
| 1 | Lednice | LEDV | 6118 (P3SM2) | Turlock | 1980 | Czechoslovakia | Culex mosquito | [81] | MK896540 –MK896542 |
| 2 | Lokern | LOKV | A 10391 (P?SM5) | Bunyamwera | NA | NA | NA | [82] | MH484303 –MH484305 |
| 2 | Lukuni | LUKV | ColAn 57389 (P?SM5) | Anopheles A | 1976 | Colombia | Vertebrate | [66,83] | MK896537 –MK896539 |
| 4 | Madrid | MADV | BT 4075 (P13SM2) | C | 1961 | Panama | Human | [84,85] | MK896531 –MK896533 |
| 2 | Main Drain | MDV | R4680 (SM3V1) | Bunyamwera | 1974 | USA | Anopheles mosquito | [86,87] | MH484309 –MH484311 |
| 2 | Main Drain | MDV | 72V2567 (V1SM1V2) | Bunyamwera | 1972 | USA | Aedes mosquito | [86,87] | MH484306 –MH484308 |
| 4 | Marituba | MTBV | BeAn 15 (SM3SH1V1SM2) | C | 1954 | Brazil | Monkey | [71,88] | MK896528 –MK896530 |
| 1 | Minatitlán | MNTV | M67U5 (P5SM1) | Minatitlán | 1967 | Mexico | Hamster | [46] | MK896525 –MK896527 |
| 4 | Mirim | MIRV | BeAn 7722 (SM1V1) | Guamá | 1957 | Brazil | Monkey | [89] | MK896522 –MK896524 |
| 1 | Moriche | MORV | TRVL 57896 (P?SM2V1) | Capim | 1964 | Trinidad & Tobago | Culex mosquito | [60,90] | MK896519 –MK896521 |
| 2, 3 | M’Poko | MPOV | BA 365 (P?AM1) | Turlock | 1966 | Central African Republic | Culex mosquito | [91] | MK896534 –MK896536 |
| 4 | Murutucú | MURV | BeAn 974 (P14SM2) | C | 1955 | Brazil | Monkey | [71] | MK896516 –MK896518 |
| 2 | Nepuyo | NEPV | HB7-451 (P?SM6) | C | 1967 | Honduras | Bat | [92,93] | MK896513 –MK896515 |
| 3 | Nola | NOLAV | DakAr B 2882 (P10SM2) | Bakau | 1970 | Central African Republic | Culex mosquito | [58] | MK896510 –MK896512 |
| 4 | Northway | NORV | 234 (P?SM1BHK(1)SM4) | Bunyamwera | 1971 | USA | Aedes mosquito | [94,95] | MH484312 –MH484314 |
| 1 | Okola | OKOV | YM 50 (P9SM1) | Tanga | 1964 | Cameroon | Eretmapodites mosquito | [58] | MK896507 –MK896509 |
| 4 | Oriboca | ORIV | BeAn 17 (P12V4ppf3V1SM1) | C | 1954 | Brazil | Monkey | [71,88] | MK896501 –MK896503 |
| 1 | Pacora | PCAV | J19 (P31SM1) | Ungrouped | 1958 | Panama | Culex mosquito | [58] | MK896498 –MK896500 |
| 1 | Pahayokee | PAHV | FE3-52F (P?BHK(1)SM1) | Patois | 1963 | USA | Culex mosquito | [96] | MK896495 –MK896497 |
| 2 | Patois | PATV | BT 4971 (SM7V1) | Patois | 1961 | Panama | Rat | [97,98] | MK896489 –MK896491 |
| 4 | Peaton | PEAV | CSIRO 110 (SM1) | Simbu | 1976 | Australia | Culicoides biting midge | [99] | MH484318 –MH484320 |
| 3 | Potosi | POTV | 89–3380 (V3SM2) | Bunyamwera | 2005 | USA | Aedes mosquito | [100] | MH484321 –MH484323 |
| 2 | Pueblo Viejo | PVV | E4-816 (V2) | Gamboa | 1974 | Ecuador | Aedeomyia squami-pennis mosquito | [74] | MK896484 –MK896486 |
| 1 | Resistencia | RTAV | AG80-504 (V1SM3V4SM3) | Resistencia | 1980 | Argentina | Culex mosquito | [52,53] | MK896478 –MK896480 |
| 3 | Restan | RESV | TRVL 51144 (SM2SH1SM2) | C | 1963 | Trinidad & Tobago | Culex mosquito | [101] | MK896475 –MK896477 |
| 1 | San Juan | SJV | 75V2374 (P?SM3) | Gamboa | 1975 | Ecuador | Aedeomyia squami-pennis mosquito | [74] | MK896472 –MK896474 |
| 1 | Santa Rosa | SARV | M2-1493 (SM5) | Bunyamwera | 1972 | Mexico | Aedes mosquito | [83] | MH484324 –MH484326 |
| 1 | Santarém | STMV | BeAn 238758 (P6SM1) | Ungrouped | 1973 | Brazil | Rat | [58] | MK896469 –MK896471 |
| 4 | Sedlec | SEDV | Av 172 (P4SM1BHK2) | Simbu | 1984 | Czechoslovakia | Bird | [102] | MH484327—MH484329 |
| 3 | Shokwe | SHOV | SAAr 4042 (P6SM2V1) | Bunyamwera | 1962 | South Africa | Aedes mosquito | [62,103,104] | MH484330 –MH484332 |
| 2 | Tacaiuma | TCMV | SpH 32580 (SM3) | Anopheles A | 1975 | Brazil | Human | [84] | MK896460 –MK896462 |
| 1 | Tanga | TANV | MP 1329 (P7SM1) | Tanga | 1962 | Tanzania | Anopheles mosquito | [105,106] | MK896457 –MK896459 |
| 2 | Tataguine | TATV | 79V1463 (SM2) | Tanga | 1979 | Gambia | Anopheles mosquito | [107] | MK896454 –MK896456 |
| 1 | Telok Forest | TFV | MalP 72–4 (P?SM2) | Bakau | 1972 | Malaysia | Monkey | [58] | MK896451 –MK896453 |
| 2 | Tensaw | TENV | A9-171B (P?SM5) | Bunyamwera | 1960 | USA | Anopheles mosquito | [108] | MH484333 –MH484335 |
| 1 | Termeil | TERV | BP 8090 (P3SM2) | Ungrouped | 1972 | Australia | Aedes mosquito | [109] | MK896448 –MK896450 |
| 2 | Thimiri | THIV | VRC 66414 (P15SM1) | Simbu | 1963 | India | Bird | [110] | MH484336 –MH484338 |
| 1 | Timboteua | TBTV | BeAn 116382 (SM2V1) | Guamá | 1967 | Brazil | Mouse | [111] | MK896445 –MK896447 |
| 4 | Tinaroo | TINV | CSIRO 153 (P?BHK4SM2) | Simbu | 1978 | Australia | Culicoides midge | [112] | MH484339 –MH484341 |
| 4 | Tlacotalpan | TLAV | 61D240 (P9V1) | Bunyamwera | 2005 | Mexico | Mansonia titillans mosquito | [113] | MH484342 –MH484344 |
| 2 | Turlock | TURV | USA 847–32 (P4SM2V1) | Turlock | 1954 | USA | Culex mosquito | [114,115] | MK896442-MK896444 |
| 2, 3 | Vinces | VINV | 24188 (P?SM4) | C | 1976 | Ecuador | Hamster | [46] | MK896439 –MK896441 |
| 2, 3 | Weldona | WELV | 77V5691 (P4V2) | Tete | 1976 | USA | midge (species unknown) | [116] | MK896433 –MK896435 |
| 1 | Wongal | WONV | MRM 168 (P6SM1) | Koongol | 1960 | Australia | Culex mosquito | [117,118] | MK896430 –MK896432 |
| 1 | Wyeomyia | WYOV | Original (P8SM1V1) | Wyeomia | 1940 | Colombia | Wyeomyia melanocephala mosquito | [51] | MH484345 –MH484347 |
| 3 | Yaba-7 | Y7V | Yaba 7 (P5SM1) | Simbu | 2005 | Nigeria | Mansonia africana mosquito | [119] | MH484348 –MH484350 |
| 4 | Zegla | ZEGV | BT 5012 (P?SM7) | Patois | 1961 | Panama | Rat | [84,98] | MK896421 –MK896423 |
* Sequence status as of October 3, 2019
1 = Completely new genome sequence
2 = New strain sequence information
3 = Addition of missing genome segments or coding complete segments
4 = Sequence information already existed. In almost all of these cases, sequences were deposited by other authors during the course of this study.
** Passage number as defined in ARC metadata
*** Currently-recognized country names
**** Based on ARC metadata compiled by the CDC
Table 3. Sequenced genomes of 5 viruses belonging to Nairoviridae (Orthonairovirus).
| Sequence status* | Virus | Abbreviation | Strain (Passage**) | Serogroup | Collection Date | Collection Country*** | Isolation Source**** | Original Virus Description | GenBank Accession |
|---|---|---|---|---|---|---|---|---|---|
| 4 | Estero Real | ERV | K 329 (P3SM1) | Ungrouped | 1980 | Cuba | Argasid tick | [126] | MK896577 –MK896579 |
| 2 | Hughes | HUGV | Dry Tortugas (P15SM2) | Hughes | 1962 | USA | Argasid tick | [127] | MK896561 –MK896563 |
| 4 | Sapphire II | SAPV | 75V8196 (P4SM1) | Hughes | 1975 | USA | Argasid tick | [128] | MK896466 –MK896468 |
| 1 | Wanowrie | WANV | Ig 700 (P7SM1) | Ungrouped | 1954 | India | Ixodid tick | [58,60] | MK896436 –MK896438 |
| 4 | Yogue | YOGV | DakAnD 5634 (P7SM1) | Yogue | 1968 | Senegal | Bat | [58] | MK896427 –MK896429 |
* Sequence status as of October 3, 2019
1 = Completely new genome sequence
2 = New strain sequence information
3 = Addition of missing genome segments or coding complete segments
4 = Sequence information already existed. In almost all of these cases, sequences were deposited by other authors during the course of this study.
** Passage number as defined in ARC metadata
*** Currently-recognized country names
**** Based on ARC metadata compiled by the CDC
Library preparation and sequencing
The KAPA RNA HyperPrep Kit was used to prepare sequencing libraries from total RNA according to the manufacturer’s protocol using half-scale reactions and the Kapa Dual-indexed Adapter Kit (Kapa Biosystems). Pooled libraries were length-selected for 375–500-bp fragments using a BluePippin 2% cassette (Sage Biosciences). If the length-selected concentration was less than 0.5 nM, additional PCR cycles were performed using the KAPA Library Amplification Kit, until the fluorescence reached that of the kit’s internal standard #1. Amplified libraries were cleaned using a 1:1.4 ratio of solid phase reversible immobilization (SPRI) beads (Kapa biosystems). Final length-selected and amplified libraries were diluted to 4 nM and the final concentration determined using the KAPA Library Quantification Kit. Paired-end 2x150bp sequencing was performed on an Illumina NextSeq, producing an average of 2.8 million read pairs per dataset.
Sequence analysis
Reads were demultiplexed and assessed for quality using FastQC [23]. Cutadapt was used to remove low quality and adapter-derived bases [24]. Read pairs with >96% pairwise nucleotide (nt) identity were collapsed using cd-hit-est to remove likely PCR duplicates [25,26]. Host sequences were filtered by mapping reads to the human (NCBI GCRh38) and/or the mouse (UCSC mm10) genomes using the Bowtie2 aligner with parameters,—local—sensitive—score-min C,60,0 using paired reads [27]. Host-filtered reads were assembled into contigs using SPAdes genome assembler [28]. BLASTn was used to taxonomically assign contigs by nucleotide similarity to the highest scoring sequence in the NCBI nt database with an expect (E) value less than 1e-8 [29,30]. Contigs not taxonomically assigned using BLASTn were assigned by protein-level similarity using DIAMOND aligner to query the NCBI protein database with an expect value of 1e-3 [31]. Viral contigs were validated by inspecting alignment of mapped reads to draft assemblies in Geneious v11.0.2 [32].
Draft bunyavirus genome assemblies were manually validated by aligning host-filtered reads to contigs using Bowtie2 as described above and alignments were independently validated by two people. Bases at the ends of genomes with less than 4x coverage of the same base were trimmed. After removing low quality data and duplicate and host reads, an average of 3.1% of read pairs remained. We performed de novo assembly to generate draft viral genome segment sequences, which were validated manually by remapping reads to draft assemblies and by Sanger sequencing. Viral reads accounted for an average of 88% of host filtered unique reads, which produced 748-fold mean coverage depth across virus genomes (S1 Fig). In all cases, sequences validated by Sanger sequencing matched NGS-generated sequences, confirming assemblies and ruling out sample mix-ups.
All genome sequences have been deposited into GenBank under accessions MH484273–MH484350, MK896421–MK896656, MK965544, and MW415980-MW415982. All sequences deposited in GenBank are coding-complete. Quality-filtered sequence reads have been deposited in the sequence read archive (SRA) under Bioproject ID PRJNA543521.
Validation of assemblies by sanger sequencing
Independent Sanger sequence confirmation from re-extracted RNA was obtained from at least one genome segment for 71 of 99 viruses (197 segments). This validation was performed to ensure that the sequencing data were derived from the intended virus stocks, especially when working with such a large sample set. For 54 viruses, sequences from all three genome segments were independently confirmed. Primers for each segment of each virus were designed from multiple sequence alignments of Illumina-generated sequences for viruses in each serogroup, using Geneious v11.0.2 [32]. Previously-published consensus primers were used when available, such as for viruses in the genus Orthonairovirus and in the genus Orthobunyavirus, phlebotomus fever serogroup [33], Bunyamwera serogroup, and Simbu serogroup [34] (S1 Table). Viral RNA was re-extracted from a separate vial of the same lot as the original isolate in a 96-well plate format using a Qiagen Biorobot 9604 (Qiagen, Valencia, CA, USA) according to manufacturer’s instructions. Nucleic acids were eluted in 100 μl AVE elution buffer supplied with the extraction kit, and stored at -20°C. Amplification of viral RNA was performed in both forward and reverse directions using the Qiagen OneStep RT-PCR kit (Qiagen, Valencia, CA, USA) according to manufacturer’s instructions and using the appropriate primer pair listed in S1 Table. Amplicons were purified using either the column-based QIAquick PCR Purification Kit (Qiagen) for individual samples or in a 96-well plate format using the Mag-Bind Viral DNA/RNA 96 Kit (Omega Bio-tek, Norcross, GA) on a KingFisher Flex System (ThermoFisher Scientific, Waltham, MA) according to the manufacturer’s instructions. Sanger sequencing services were provided by GeneWiz (South Plainfield, NJ, USA). Sequence files were imported into Geneious v11.0.2 for end-trimming and generation of consensus sequences and aligned to the Illumina-generated sequences in either Geneious v11.0.2 or MultiAlign (Corpet 1988) software.
Sequence alignments and phylogenetic analyses
To optimally align sequences, different strategies were used specific to the segment and bunyaviral genus. The open reading frame (ORF) encoding the RdRp protein on the L segment was used for all genera. The ORF of the M segment encoding the Gn/Gc glycoproteins was also used for all genera. The NSm ORF was removed from the alignment because outgroups contained a different genomic organization. Additionally, coding regions for the mucin and GP38 domains were removed from the orthonairovirus sequence alignment. S segments were aligned using the ORF encoding the nucleoprotein. Outgroups were used to root and provide polarity characteristics to the phylogenetic trees. Outgroups were chosen so as to not provide excessive gaps and disturbances in the alignments. Orthotospovirus sequences were used as the outgroups for orthobunyavirus and phlebovirus analyses. Orthohantavirus sequences were used as the outgroup for orthonairovirus analyses. All available reference sequences for each genus were pulled from GenBank’s RefSeq database and included in phylogenetic analyses. Alignments were performed on amino acid sequences using the Muscle algorithm and manually inspected afterwards using Seaview v4.1 [35,36]. After alignment, sequences were then converted back to their original nucleotide sequences for phylogenetic analysis. Poorly aligned, divergent positions characterized by excessive gaps in the alignment were removed using the Gblocks server under less strict conditions [37]. A coalescent phylogenetic analysis of the L, M, and S segments was conducted for orthonairoviruses, orthobunyaviruses, and phleboviruses: the L segment of pacuviruses was also executed. The analysis was conducted in MrBayes run once for 10 million steps, sampling every 5,000 steps and discarding the first 10% as burn-in [38,39]. The general time-reversible substitution model (GTR+I+Γ4) was used after determining this model to be optimal using ModelTest [40]. Convergence was assessed by examining the stationary ln-likelihood and effective sample size (ESS, >200) parameters using Tracer v1.4 (http://tree.bio.ed.ac.uk/software/tracer). All phylogenies were executed on the phylo.org server [41]. Output tree files were analyzed using FigTree v.1.3.1 (http://tree.bio.ed.ac.uk/software/figtree/).
Co-phylogenies were generated using these trees and a co-phylogeny visualization tool, available at https://github.com/stenglein-lab/TreeTangler, and were rooted using the outgroups described above. Prior to generation of co-phylogenies, nodes with support values lower than 0.95 were collapsed to polytomies using TreeGraph2 software [42].
To place the sequences in the context of all available related sequences, we downloaded all full-length L protein sequences annotated under the genera Orthobunyavirus (NCBI taxonomic ID [taxid] 11572) and Phlebovirus (taxid 11584), and the family Nairoviridae (taxid 1980415). We aligned these sequences using the MAFFT aligner, and, because of the large number of sequences, inferred maximum likelihood trees using FastTree software using model parameters -lg -gamma [43,44].
Co-infection analysis
To search for evidence of possible co-infections, we determined the ratio of the number of reads in each dataset that aligned to a given virus (virus self-mapping reads) to the number of reads that mapped to any bunyavirus protein sequence in the NCBI nr database, including bunyaviruses sequenced in this study. Self-mapping reads were quantified by aligning host-filtered reads to the corresponding assembled genomes using Bowtie2 as described above. Total bunyavirus reads were quantified by aligning host-filtered reads to a database composed of all the protein sequences for bunyavirus-annotated sequences in the NCBI database and from newly assembled genomes in this report, using DIAMOND as above. When the ratio of these two values was less than 0.95, we manually inspected contigs from the dataset to identify sequences of possible co-infecting viruses.
Candidate coinfections were validated using PCR and independent cell cultures. Specifically, PCR primers were designed that would discriminate between putative co-infecting viruses and the primary virus in each isolate. The originally sequenced stock virus from the ARC was also inoculated onto grivet (Chlorocebus aethiops) Vero cell (ATCC CCL-81) cultures for virus isolation, and supernatant was collected when cultures exhibited cytopathic effects (CPE). Approximately 1x106 cells were pelleted by centrifugation at 23 x g for 10 min at room temperature and then frozen at -80°C. RNA was extracted from cell pellets by adding 1 ml of Trizol (ThermoFisher) to pellets, pipetting to lyse, and incubating at room temperature for 5 minutes, following the Trizol protocol. cDNA was made using our standard reverse transcription protocol. Specifically, 500 ng of RNA or 5.5 μl of RNA was incubated at 65°C for 5 minutes and immediately placed on ice. Random 15-mer at 25 μM, 2 μl of 1X Superscript III buffer, 5 mM dithiothreitol, 1 mM each dNTPs (ThermoFisher), and 100 U Superscript III Reverse Transcriptase (Invitrogen) was added to this solution. Reaction mixtures were then incubated at 25°C for 5 minutes then at 42°C for 45 minutes, and finally at 70°C for 15 minutes with a 4°C hold. The resulting cDNA was then diluted 1:10 in water. Using segment-specific primers (S1 Table), qPCR was performed using LUNA Universal qPCR Master Mix (NEB M3003L) according to the manufacturer’s protocol.
In an attempt to separate and isolate the individual co-infecting viruses (see results), the original Abras isolate was plaqued on Vero cells. Ninety individual plaques were picked for RNA extraction and PCR-confirmation using segment-specific primers (S1 Table). Single virus stocks were cultured from plaques that were Sanger sequence-confirmed to have only one of the infecting genotypes present.
Results
Samples and sequencing
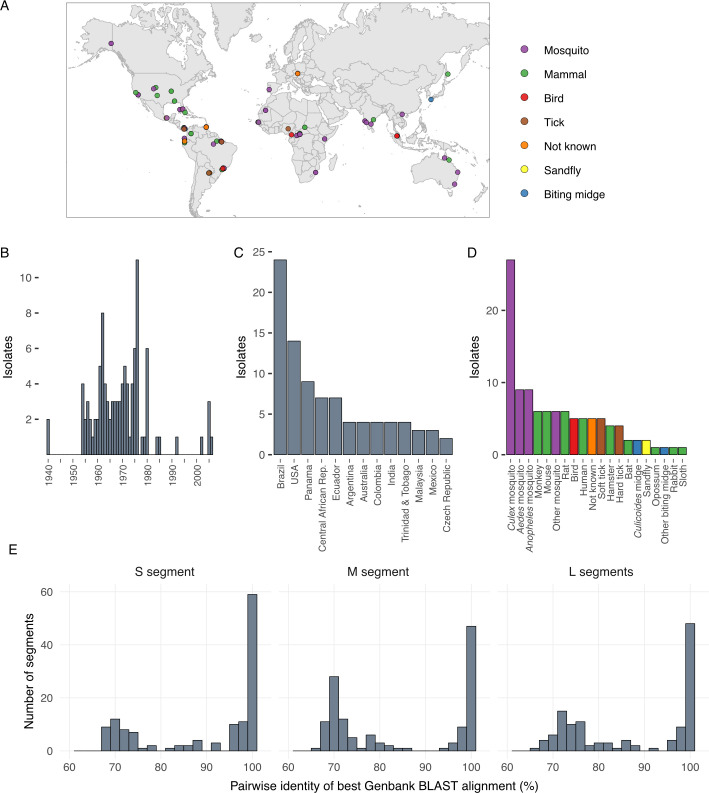
Coding-complete genomes were generated for 99 bunyaviruses [45]. We focused on viruses classified in the Peribunyaviridae (orthobunyaviruses), Nairoviridae (orthonairoviruses), and Phenuiviridae (banyangviruses and phleboviruses). These viruses constituted a globally and phylogenetically diverse set. The isolates were originally collected from vertebrate and arthropod hosts from 27 countries on six continents over the span of seven decades, from 1940 to 2005; Fig 1 and Tables 1–3). Most samples were collected during the 1950s, 60s, and 70s, and 67% of samples originated from North and South America (Fig 1B and 1C). Isolates originated from a variety of invertebrate and vertebrate hosts, though Aedes, Anopheles, and Culex mosquitoes were the predominant source of samples (Fig 1D). We focused on viruses for which there was limited existing sequence information at the initiation of this project (Fig 1E and Tables 1–3). Viruses had diverse passage histories. Some viruses were passaged through grivet (Chlorocebus aethiops) Vero or hamster (Mesocricetus auratus) BHK-21 cell cultures, others were passaged in suckling laboratory mice. Passage histories have been noted in Tables 1–3. More information on these viruses can be accessed through the Arbovirus Catalog (https://www.cdc.gov/arbocat) or the ARC website (https://www.cdc.gov/ncezid/dvbd/specimensub/arc/index.html), however taxonomy may vary between these sources and ICTV as taxonomic updates are instituted.
Fig 1. Characteristics of sequenced bunyavirus isolates.
Bunyavirus isolates were collected from around the world over the span of 7 decades from a variety of vertebrate and arthropod hosts. (A) Map showing original collection location of isolates, color-coded by host type, NK (not known); (B) histogram showing the number of isolates collected each year; (C) histogram showing the number of isolates from the indicated countries, for countries with 2 or more isolates; (D) histogram showing the number of isolates from the indicated host type. (E) histograms showing the pairwise percent nucleotide identity for the highest scoring BLASTn alignments to existing sequences in the NCBI nucleotide database for the sequenced S, M, and L segments.
Table 2. Sequenced genomes of 6 viruses belonging to Phenuiviridae (Phlebovirus and Uukuvirus).
| Sequence Status* | Virus | Abbreviation | Strain (Passage**) | Serogroup | Collection Date | Collection Country*** | Isolation Source**** | Original Virus Description | GenBank Accession |
|---|---|---|---|---|---|---|---|---|---|
| 4 | Bujaru | BUJV | BeAn 47693 (P10SM2) | Sandfly fever | 1962 | Brazil | Rat | [83] | MK896442 –MK896444 |
| 2 | Kaisodi | KASDV | G 14132 (P6SM1) | Sandfly fever | 1957 | India | Ixodid tick | [120,121] | MK896549 –MK896551 |
| 4 | Palma | PLMV | PoTi 4.92 (P?SM3) | Sandfly fever | 1992 | Portugal | Ixodid tick | [122] | MK896492 –MK896494 |
| 2 | Punta Toro | PTV | D-4021A (SM15) | Sandfly fever | 1966 | Panama | Human | [123] | MK896481 –MK896483 |
| 1 | Sunday Canyon | SCAV | RML 52301–11 (SM5V1SM2) | Sandfly fever | 1969 | USA | Argasid tick | [124] | MK896463 –MK896465 |
| 2 | Zaliv Terpeniya | ZTV | LEIV 21C (P7SM2) | Uukuniemi | 1969 | Russia | Ixodid tick | [125] | MK896424 –MK896426 |
* Sequence status as of October 3, 2019
1 = Completely new genome sequence
2 = New strain sequence information
3 = Addition of missing genome segments or coding complete segments
4 = Sequence information already existed. In almost all of these cases, sequences were deposited by other authors during the course of this study.
** Passage number as defined in ARC metadata
*** Currently-recognized country names
**** Based on ARC metadata compiled by the CDC
At the time of submission, 203 of the genome segment sequences we generated shared less than 97% pairwise nucleotide identity with existing sequences in the NCBI nucleotide database, as measured by blastn (Fig 1E). For 35 of the viruses, all 3 segments shared <85% nucleotide identity with existing sequences by blastn alignment. These 35 viruses represent previously unsequenced viruses (category 1 viruses in Tables 1–3 and Fig 1E). For 37 of the viruses, different strains of the same viruses had been previously sequenced or existing sequences were not complete (e.g., only one segment had been sequenced; categories 2 and 3 in Tables 1–3). For 26 of the viruses, coding complete genomes already existed (category 4 in Tables 1–3). In almost all of these 26 cases, sequences had been deposited by other groups during the course of our study.
Several viruses with nearly identical sequences had been given different names and, conversely, some viruses with the same name had quite different sequences. For instance, wongal virus had previously been classified with koongol virus in the species Koongol orthobunyavirus, but no sequence information for wongal virus was available [14]. Both viruses were isolated from Culex annulirostris mosquitoes several days apart from the same location in Australia [117]. We found that these viruses shared >99.7% pairwise nucleotide identity across all three segments. Thus, koongol and wongal viruses are the same virus. Similarly, all three segments of the Santa Rosa and Lokern (strain A 10391) viruses that we sequenced shared >98.5% identity.
In contrast, the Lukuni virus isolate that we sequenced (strain ColAn 57389) was unexpectedly different from the previously sequenced Lukuni virus (strain TRVL 10076)[129]. These two viruses shared <70% pairwise nucleotide identity across all three segments. Instead, the L and S segments of Lukuni virus strain ColAn 57389 were more similar to those of the Las Maloyas virus isolate that we sequenced (85% and 87% pairwise nucleotide identity). Likewise, the Guajará virus that we sequenced (strain 18315) and the previously sequenced Guajará virus (strain BeAn 10615) shared less than 78% pairwise nucleotide identity over all three segments [129]. These unexpected differences highlight the utility of sequencing additional strains of already-sequenced viruses.
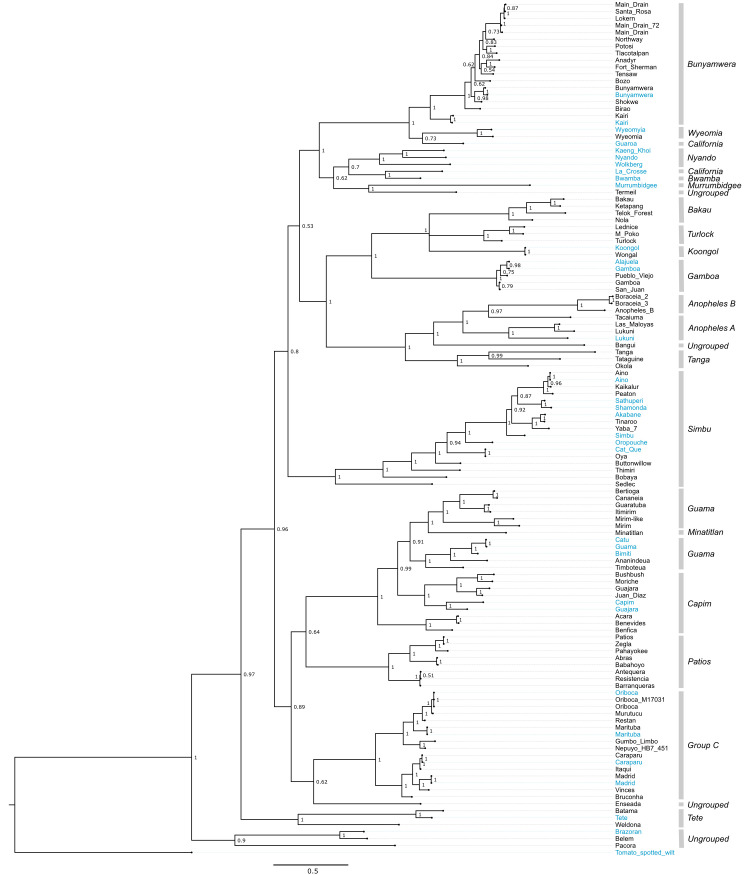
Phylogenetic analyses
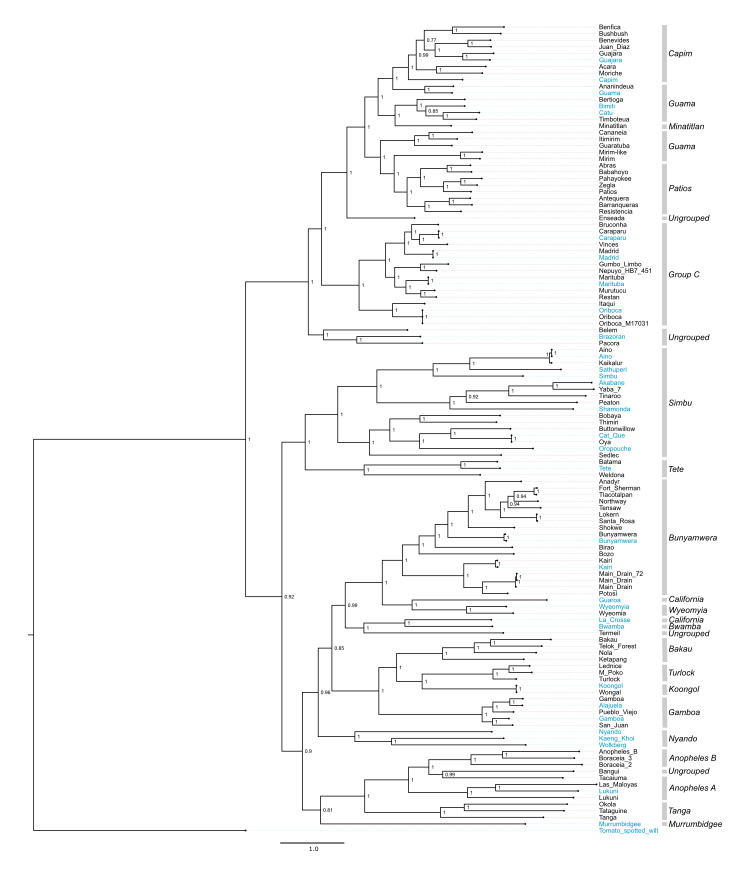
We performed a coalescent phylogenetic analysis of the L, M, and S segments (Figs 2–6). Trees included all of the viruses that we sequenced and all of the bunyavirus sequences from the genera in question in the NCBI RefSeq database as phylogenetic landmarks. We also created trees containing all available sequences annotated under the relevant taxa in the NCBI taxonomy database S2–S4 Figs). New orthobunyavirus genome sequences fell throughout the Orthobunyavirus genus (Figs 2–4 and S2–S4). The numbers of genome sequences sequenced in this study from the orthobunyavirus serogroups included: Anopheles A (3), Anopheles B (3), Bakau (4), Bunyamwera (16), Capim (7), Wyeomyia (1), Turlock (3), Koongol (1), Gamboa (3), Guama (7), Minatitlán (1), Group C (11), Patois (5), Simbu (9). Additionally, 5 viruses sequenced fell within the family Nairoviridae, 7 in the family Phenuiviridae and 2 in the genus Pacuvirus (Figs 5 and 6 and S5).
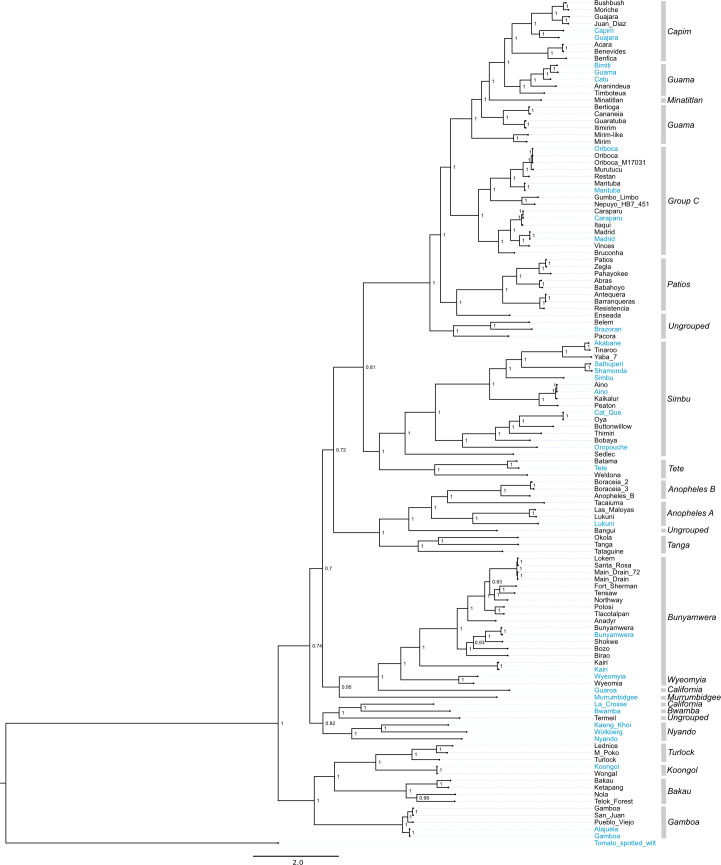
Fig 2. L segment phylogeny for viruses in Orthobunyavirus.
Bayesian phylogenetic tree using the ORF on the L segment. Numbers at nodes indicate posterior probability values. Reference sequences (sequences present in the NCBI RefSeq database) are included as phylogenetic landmarks and colored blue. Sequences from this study are colored black. Classical serogroup distinctions are indicated. Scale bars represent substitutions per site.
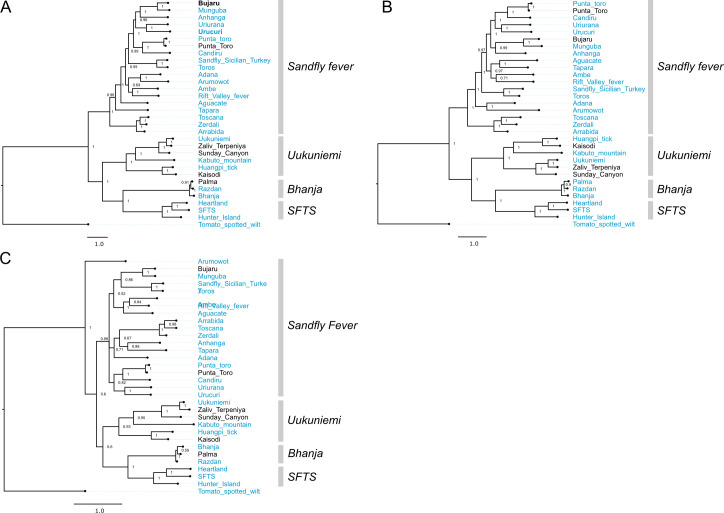
Fig 6. Phylogenetic analysis of viruses in Phenuiviridae.
Bayesian phylogenetic tree using (A) the ORF encoding the RdRp on the L segment, (B) the ORF encoding Gn/Gc on the M segment, and (C) the ORF encoding NP on the S segment. Numbers at nodes indicate posterior probability values. Reference sequences are included as phylogenetic landmarks and colored blue. Sequences generated in this study are colored black Classical serogroup distinctions are indicated. Scale bars represent substitutions per site.
Fig 4. S segment phylogeny for viruses in Orthobunyavirus.
Bayesian phylogenetic tree using the ORF encoding NP on the S segment. Numbers at nodes indicate posterior probability values. Reference sequences are colored blue and sequences generated as part of this study are colored black. Classical serogroup distinctions are indicated. Scale bars represent substitutions per site.
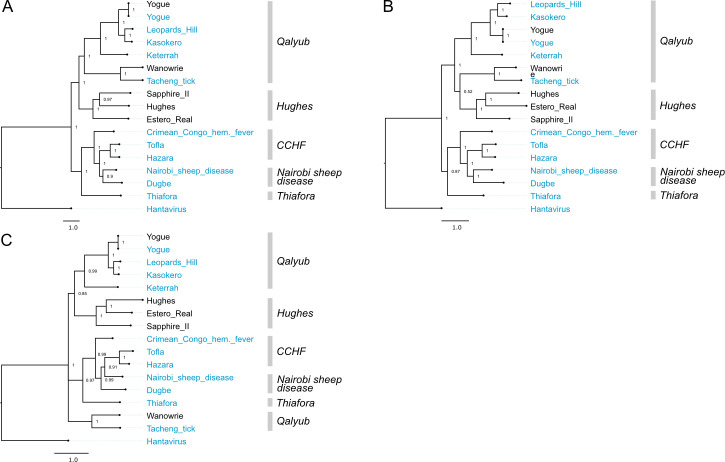
Fig 5. Phylogenetic analysis of viruses in Nairoviridae.
Bayesian phylogenetic tree using (A) the ORF encoding the RdRp on the L segment, (B) the ORF encoding Gn/NSm/Gc on the M segment, and (C) the ORF encoding NP on the S segment. Numbers at nodes indicate posterior probability values. Reference sequences are included as phylogenetic landmarks and colored blue. Sequences generated in this study are colored black. Classical serogroup distinctions are indicated. Scale bars represent substitutions per site.
Several virus genomes that were sequenced as part of this study had not been assigned to serogroups. We compared the phylogenetic placement of these viruses to that of viruses that had been categorized into established serogroups (Tables 1–3). Bangui virus was placed in L and S segment trees within an undesignated clade just prior to the divergence of the Anopheles A and B serogroup (Figs 2–4). The Belem, Pacora, and Brazoran viruses formed a monophyletic clade on all three orthobunyavirus trees (Figs 2–4), indicating a common ancestor between the three viruses. However, this clade was placed in different locations on the phylogenies for the different segments. On L and M segment trees, these three viruses branched basally to the Patois, Guama, Minatitlán, Group C, and Capim serogroups with strong posterior probability support (Figs 2 and 3). The S segment phylogeny showed this clade diverging from all other orthobunyaviruses with posterior probability support of 1.0 (Fig 4).
Fig 3. M segment phylogeny for viruses in Orthobunyavirus.
Bayesian phylogenetic tree using the ORF encoding Gn/Gc on the M segment. Numbers at nodes indicate posterior probability values. Reference sequences are colored blue and sequences generated in this project are colored black. Classical serogroup distinctions are indicated. Scale bars represent substitutions per site.
Enseada virus exhibited a similar discrepant placement in the 3 orthobunyavirus trees. The L segment of Enseada virus diverged off of the Patios serogroup (Fig 2). The M segment of Enseada virus diverged prior to the bifurcation of the Guama, Capim, Patois, and Minatitlan serogroups (Fig 3). Finally, the S segment of Enseada diverged just prior to the Group C serogroup (Fig 4). All of these placements were well-supported, with posterior support of 1.
Across all segments, Termeil virus branched basal to the California encephalitis and Bwamba serogroups. On the S segment phylogeny, Termeil virus grouped with Murrumbidgee virus (aka Trubanaman virus [130,131]), though posterior probability support was low (Fig 4).
The placement of two viruses in our trees diverged from their expected placements based on assigned serogroup classification. Tacaiuma virus has been assigned to the Anopheles A group. However, in the L, M, and S segment phylogenies, Tacaiuma virus instead clustered with high support with viruses in the Anopheles B serogroup (Figs 2–4). Guaroa virus has been assigned to the California serogroup, but did not cluster with La Crosse virus as this would suggest. Instead, across all segments, Guaroa virus branched basally to the Wyeomyia serogroup (Figs 2–4).
Caimito virus and Santarem virus grouped together with viruses in the genus Pacuvirus (S5 Fig). Our results coincide with the recent proposal to reclassify Caimito virus as belonging to the genus Pacuvirus (Peribunyaviridae) [132]. Our phylogenies consistently placed Santarem virus with Caimito virus. We propose Santarem be classified in the Pacuvirus genus along with Caimito virus.
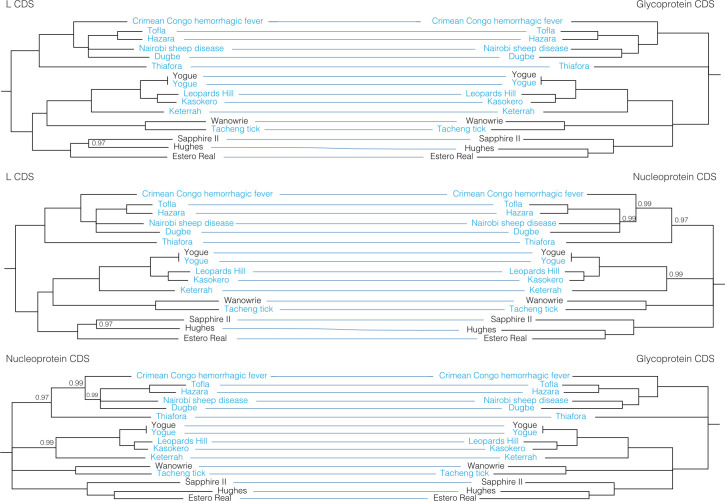
Among the nairoviruses, Wanowrie consistently fell within the Qalyub serogroup of the Orthonairovirus genus, most closely related to Tǎchéng tick virus, though there was insufficient support in the S segment tree to group these two viruses with the rest of those in the Qalyub serogroup (Fig 5). Estero Real virus was consistently placed within the Hughes serogroup for all three segments with strong posterior probability support (Fig 5).
In phenuivirus trees, Bujaru and Punta Toro viruses consistently grouped within the Sandfly fever serogroup, as expected (Fig 6). Also, expectedly, Zaliv Terpeniya and Sunday Canyon grouped within the Uukuniemi serogroup and Palma grouped with Bhanja serogroup across all segments (Fig 6). One deviation of note within the phenuivirus trees is the divergent placement of Arumowot virus S segment (Fig 6).
Reassortment
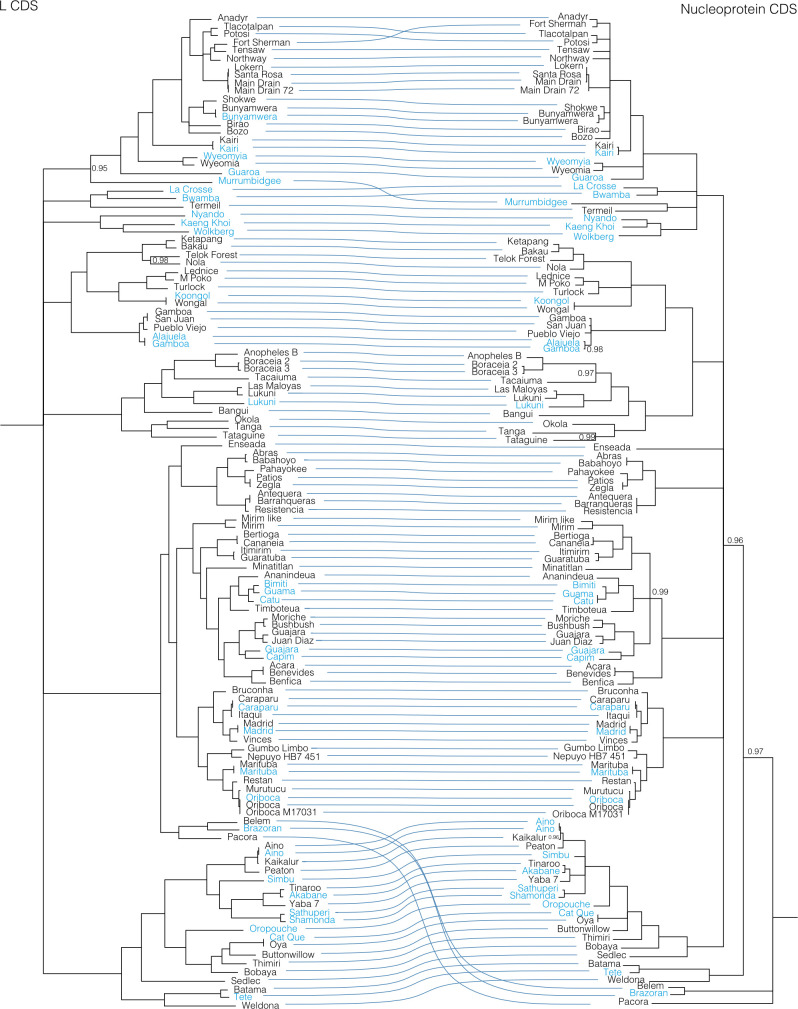
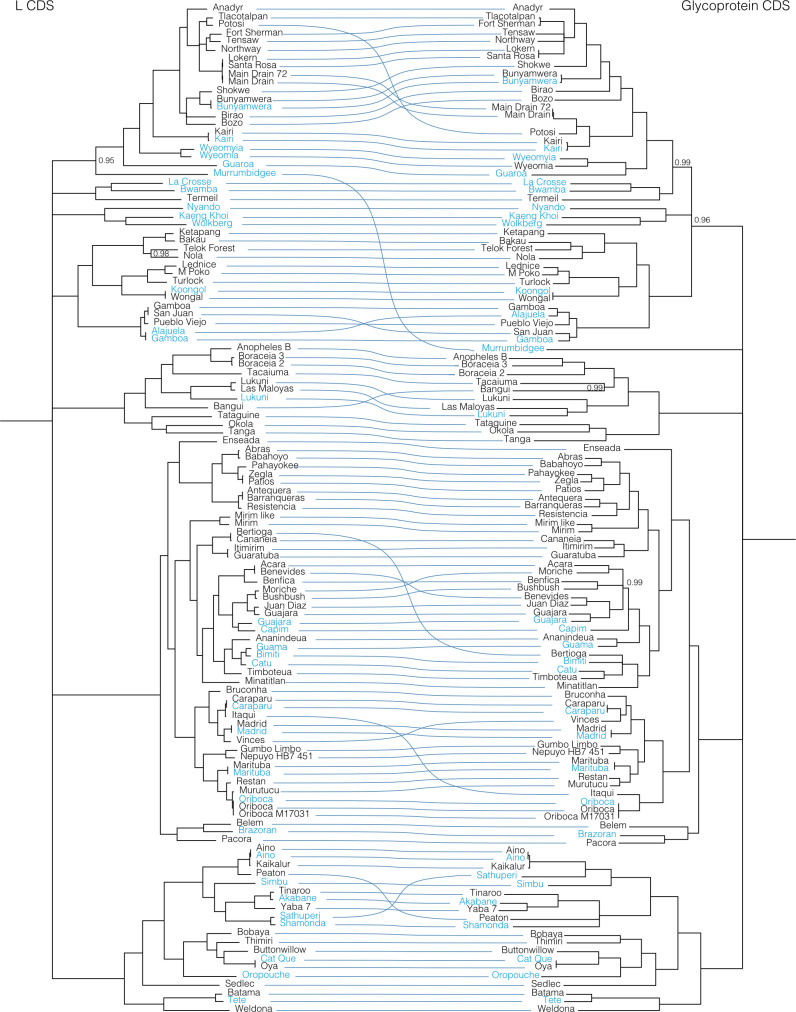
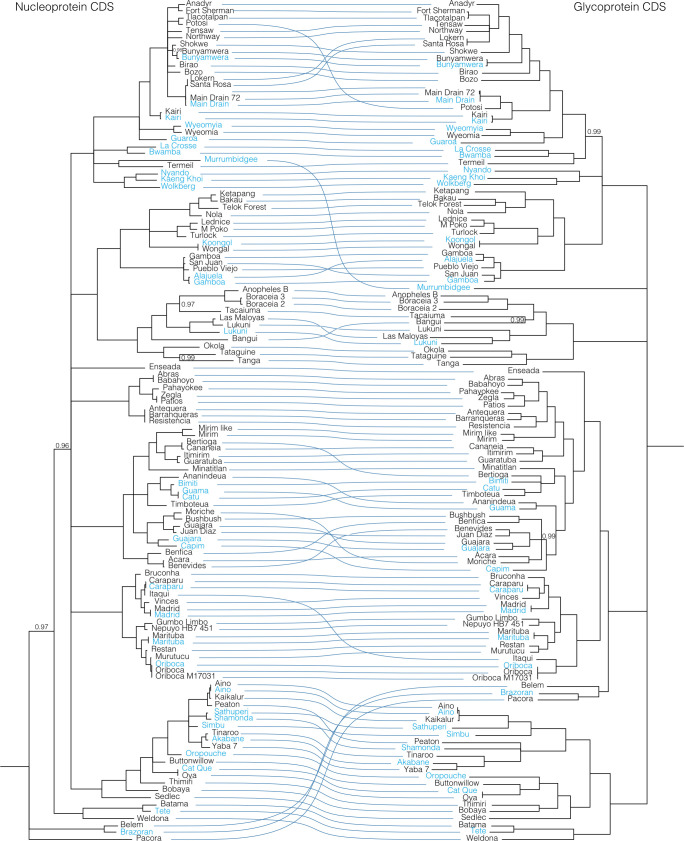
To further evaluate reassortment among the viruses we sequenced, we created co-phylogenies (tanglegrams) of L, M, and S segments (Figs 7–11). Prior to generation of these co-phylogenies, we collapsed interior nodes with support values < 0.95 so that poorly supported branching patterns would not produce false signals of reassortment. We also performed all possible pairwise global alignments of the coding regions of the segments to identify pairs of viruses with differential nucleotide identities between segments, for instance, pairs of viruses with closely related L and S segments but relatively divergent M segments (S2 Table). Numerous local topological incongruencies were apparent in the trees, but here we focus on the clearest examples of reassortment that were well supported by both phylogenetic discordance and large discrepancies in pairwise genetic distances.
Fig 7. Co-phylogenies of Orthobunyavirus L and S segment trees.
Branches with support values < 0.95 were converted to polytomies. All other branch support values were 1 unless indicated. Trees were rooted with outgroups as in Figs 2–4.
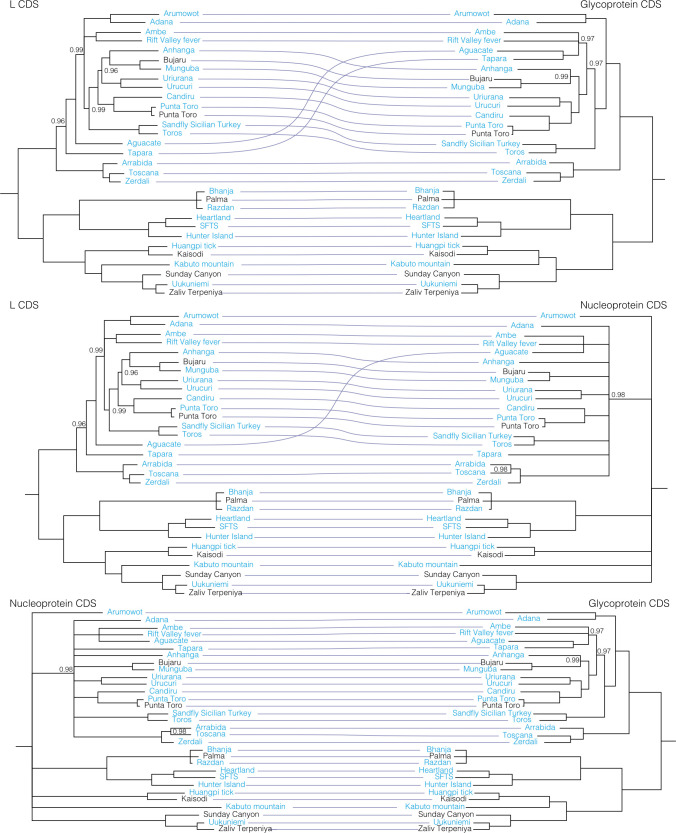
Fig 11. Co-phylogenies of Phenuiviridae L, M, and S segment trees.
Branches with support values < 0.95 were converted to polytomies. All other branch support values were 1 unless indicated. Trees were rooted with outgroups as in Fig 6.
Fig 8. Co-phylogenies of Orthobunyavirus L and M segment trees.
Branches with support values < 0.95 were converted to polytomies. All other branch support values were 1 unless indicated. Trees were rooted with outgroups as in Figs 2–4.
These analyses identified evidence of widespread reassortment among the orthobunyaviruses (Figs 7–9). In some cases, these corresponded to reassortment events that had been previously identified, but in other cases these were newly described. Most, but not all, well supported instances of reassortment involved viruses with similar L and S segments but relatively different M segments. This was evident in the relatively similar topologies of the L and S segment trees and the increased degree of crossing-over in the L-M and M-S tanglegrams. This corresponds to reassortant progeny that comprise the L and S segment of one co-infecting parent virus and the M segment of the second parent. In contrast, the differential placement of Brazoran, Belem and Pacora viruses on the L and M as opposed to the S segment phylogenies supports the hypothesis that this group of viruses resulted from an ancestral reassortment event involving the replacement of an S segment [12].
Fig 9. Co-phylogenies of Orthobunyavirus S and M segment trees.
Branches with support values < 0.95 were converted to polytomies. All other branch support values were 1 unless indicated. Trees were rooted with outgroups as in Figs 2–4.
Consistent with previous reports, there was evidence of extensive reassortment involving all 3 segments in the Bunyamwera serogroup [19,133,134]. The Fort Sherman and Anadyr virus isolates sequenced in this study have S segments with 90% pairwise nucleotide identity but relatively different L and M segments that shared <75% pairwise nucleotide identity. This is one of the few examples we identified of viruses with closely related S segments but relatively different L and M segments. The S and L segments of Potosi virus were most closely related to those of Tlacotalpan virus, but the M segment was more related to Kairi and Main Drain viruses (Figs 2–4 and 7–9 and S2 Table). Both Main Drain isolates that we sequenced had S and L segments that were >95% identical to those of Santa Rosa and Lokern viruses. But the Main Drain M segments clustered instead with those of Potosi and Kairi viruses. Shokwe and Bunyamwera viruses L and S segments grouped together, but their M segments did not form a monophyletic cluster.
In the Bakau serogroup, Ketapang and Bakau viruses had similar L and S segments (81 and 88% identical) but relatively different M segments (66% identical). The M segment of Bakau virus was instead more closely related to that of Telok Forest virus, with which it shared 70% pairwise nucleotide identity.
The two Boracéia virus strains that we sequenced (SPAr 395 and SPAr 4080) were isolated from Anopheles cruzii mosquitoes in São Paulo, Brazil in 1962 and 1965, respectively (Table 1). These isolates had closely related L and S segments with >91% pairwise nucleotide identity but M segments that shared only 63% identity (Fig 7 and S2 Table). The Bangui virus L and S segments branched basally to the viruses in the Anopheles A and B groups in the L and S segment trees, but in the M segment tree, Bangu virus fell on a well-supported branch with Tacaiuma virus nested within these groups. Patois and Zegla viruses had nearly identical L and S segments with 97% and 99.7% pairwise nucleotide identity, but their M segments shared only 73% identity.
In the Simbu group viruses, Aino, Kaikalur and Peaton viruses formed a well-supported cluster on L and S segment trees, but the M segment of Peaton virus was at the end of a long, separately placed branch. The Tinaroo and Akabane virus L and S segments were 87 and 95% identical, but the M segments shared only 65% pairwise nucleotide identity. A similar pattern was evident for Athuperi and Shamonda viruses.
Antequera, Barrenqueras, and Resistencia viruses exhibited patterns of sequence relatedness indicative of multiple past reassortment events. The S segments of these three viruses were all ≈99% identical. The L segments of Antequera and Barrenqueras were also nearly identical (99%), but these were both only ≈90% identical to the L segment of Resistencia virus. The M segments of the three viruses all shared <71% pairwise nucleotide identity.
In the Guama and Capim group viruses, there were several examples of apparent M segment reassortment. Bertioga and Cananeia viruses had highly related L and S segments but M segments that were only 67% identical. The same pattern was evident for Itimirim and Guaratuba viruses, for Moriche and Bushbush viruses, for Acara and Benevides viruses, for Guajara and Juan Diaz viruses, and for the trio of Bimiti, Guama and Catu viruses.
Reassortment has also clearly shaped the evolution of the Group C orthobunyaviruses, as has been previously noted [17,135,136]. Caraparu and Itaqui viruses had nearly identical L and S segments but quite different M segments. The M segment of Itaqui virus was instead much more similar to that of Oriboca virus. The L and S segments of Vinces virus clustered with those of Madrid virus, but the Vinces virus M segment was more closely related to that of Caraparu virus. Restan, Murutucu, and Oriboca virus formed a well-supported cluster on L and S segment trees, but on the M segment tree, Restan amd Murutucu viruses remained clustered together while the Oriboca virus M segment was on a branch basal to the other Group C viruses with Itaqui virus.
There was little evidence for reassortment among the orthonairoviruses in our analysis. The only instance of possible reassortment was for Hughes virus. Despite similar pairwise nucleotide identities between the three segments of Hughes, Sapphire II, and Estero Real viruses, these three viruses had different branching patterns on the three segment trees, albeit with long branch lengths and support values <1 (Fig 10 and S2 Table). In phlebovirus tanglegrams, Aguacate and Tapara viruses showed evidence of reassortment, but there was no strong evidence for reassortment among the phleboviruses sequenced in this study (Fig 11).
Fig 10. Co-phylogenies of Nairoviridae L, M, and S segment trees.
Branches with support values < 0.95 were converted to polytomies. All other branch support values were 1 unless indicated. Trees were rooted with outgroups as in Fig 5.
Co-infection
We identified evidence of co-infection (i.e. RNA from more than one virus) in four virus stocks: Abras virus, Guaratuba virus, Hughes virus, and one of the two Main Drain virus isolates. The initial indication that there might be co-infection in these stocks was the assembly of more than the expected three bunyavirus contigs from the corresponding datasets, and was followed up with additional computational and molecular tests to validate possible co-infections.
In the Abras virus dataset, six coding-complete sequences assembled, including 2 L, 2 M, and 2 S segments. Three of the segments matched the previously sequenced Abras virus isolate 75V1183 (the same isolate that we sequenced) with 99–100% nucleotide identity [137]. The other three segments belonged to a previously unsequenced bunyavirus in the Guama serogroup most closely related to Mirim virus, with which it shared 77–81% pairwise nucleotide identity. Abras virus was present at a much higher abundance: 33,462 reads mapped to the three Abras virus segments, whereas only 2,091 reads mapped to the Mirim-like segments. Using RT-PCR with segment-specific primers (S1 Table), and RNA re-extracted from another vial of the isolate, we successfully amplified all six segments to confirm the coinfection. Additionally, we attempted to plaque-purify both viruses from the original isolate by Vero cell plaque assay. However, among the 90 viral plaques screened, none purely represented the co-infecting Guama serogroup virus, and passage of the co-infecting virus on Vero cells in an attempt to isolate it repeatedly failed. Additional preparations of Abras virus in the ARC that had been passaged in suckling mouse brain did not contain this co-infecting virus, leading us to suspect that the contaminant was introduced during original preparation of this particular virus stock that was sequenced during this study. Notably, Aguilar et al. (2018) did not report a co-infecting virus after also sequencing Abras isolate 75V1183 [137].
The Guaratuba virus dataset also produced 6 coding complete or partial bunyavirus segment sequences. Coding-complete sequences were assembled for all three segments of Guaratuba virus, as inferred based on their phylogenetic placement with other Guama serogroup viruses. An additional L, M, and S segment were assembled, but only the S segment was coding-complete. The additional partial L, M, and S sequences were ≈98% identical at the nucleotide level to strain 76V-25880 of Enseada virus [50,138]. The Guaratuba virus sequences had ≈7x more mapping reads (3,164) than the coinfecting Enseada-like virus (457). The Enseada virus-like sequences from the Guaratuba datasets were only 85–93% identical to the Enseada virus isolate strain 78V-213 genome that we sequenced for this project, indicating that these sequences were not attributable to cross-contamination during sample processing and library preparation. We used RT-qPCR to confirm the presence of all six segments in the original RNA from which the sequencing library was made. An independent RNA extract from Vero cells infected with Guaratuba virus from a second vial from the same lot was also positive for all 6 segments by RT-qPCR.
The Hughes virus dataset produced 4 bunyavirus contigs corresponding to a complete L, M, and S segment, and a partial M segment of about 1.2 kb. This partial M segment was nearly identical to a previously published Hughes virus M segment sequence (99.4% identical to strain DT-1, accession KP792739.1) The full length M sequence was only 81% identical to Hughes virus strain DT-1 and 72% identical to Hughes virus strain G2126 (accession KU925471). The partial M sequence had a coverage level of 2.4% relative to the full-length M sequence.
For Main Drain virus strain 72V2567, we assembled coding complete sequences for the L, M, and S segments with 452,386 mapping reads. In addition, 90 reads mapped to Cache Valley virus strain 6V633. We were able to corroborate the detection of the Cache Valley virus-like sequences using RT-qPCR with discriminating primers (S1 Table). The L, M, and S partial sequences were 98.5%, 95.9%, and 99% identical to Cache Valley virus respectively (MH166879.1, MH166878.1, MH166877.1).
Discussion
Bunyaviruses comprise one of the largest viral orders, and some bunyaviruses pose substantial threats to humans, animals, and plants (3). We generated complete genome sequences for 99 bunyaviruses belonging to the families Nairoviridae, Peribunyaviridae, and Phenuiviridae, and analyzed them phylogenetically. Our results were consistent with recently published phylogenies on the Patois serogroup viruses [137] and a diversity of orthobunyaviruses in the Anopheles A, Capim, Guamá, koongol, Mapputta, Tete, and Turlock serogroups [129], but difficult to compare entirely given the inclusion of different viruses in each published analysis. Given the large number of viruses sequenced in this study, we elected to limit additional sequences in our phylogenies to new sequences plus those in NCBI RefSeq.
The phylogenies recapitulated assigned serogroups relatively well, however there were several points of inconsistency between the two. It is not that surprising that the determined phylogenetic relationships did not completely recapitulate established serogroups. Serology is based on similarities/dissimilarities in the surface proteins of the virion particle (encoded exclusively by M segments), whereas phylogenetic analysis can assess the relationships between any genes of any viral genome segment. Although one might expect the M segment to recapitulate the classical serogroup distinctions, this was not the case (Figs 3, 5B, and 6B). The L segment is the most conserved segment among all three families and most closely recapitulates the classical serogroup distinctions (Figs 2, 5A, and 6A). However, we found that several serogroups were not monophyletic in L segment phylogenies. For example, the Guama serogroup consisted of polyphyletic clades on all segments (Figs 2–4). Capim and Patois serogroups were also polyphyletic in the S segment tree (Figs 4, 5C, and 6C). Additionally, we were unable to resolve several ancestral branches which resulted in several polytomies, particularly in orthobunyavirus trees (Fig 4).
Many human pathogenic viruses in the genus Orthobunyavirus are found within the California, Bunyamwera, Simbu, and Group C serogroups. To these serogroups we have collectively added 33 coding-complete genome sequences. Although the pathogenic potential remains to be determined for many of the viruses sequenced in this study, viruses falling within serogroups for which there were previously no genomic data available will facilitate the identification and prioritization of novel potentially pathogenic strains for further study.
Reassortment
These new sequences also contribute to a richer database with which to resolve the origins of reassortant viruses. Briese et al. [17] proposed that many, if not all, currently recognized bunyaviruses are the product of recent or ancient reassortants between known and/or possibly extinct viruses. High-level analysis for evidence of reassortment supported this hypothesis, and the conclusion that reassortment is a major factor driving bunyavirus genetic variability particularly in the Bunyamwera, Capim, and Group C serogroups (Figs 7–9). Reassortment among bunyaviruses has the potential to result in the emergence of novel human pathogens, as has been the case with Iquitos virus [9], Itaya virus [13], and Ngari virus [139].
L and S segment trees were largely concordant, and reassortment was, in almost all cases, evident as pairs or groups of viruses that had similar L and S segments but different M segments. Previous studies have reported that reassortant bunyaviruses tend to combine the S and L segments from one parent with the M segment from the other parent, making this a seemingly common phenomenon [19,139–141], although this is not always the case [17]. Linkage disequilibrium analysis of LACV isolates from field-collected mosquitoes showed evidence of frequent reassortment among strains in the field, and among all three genome segments [130]. Among the viruses we analyzed, there were several examples of pairs of viruses that had nearly identical S segments but relatively different L and M segments, suggesting recent reassortment events that may have involved the joining of the S segment of one parental virus with the L and M segment of the second parent. The reassortment that has happened frequently throughout bunyavirus evolution has introduced phylogenetic scrambling that makes it difficult to tease apart exact relationships. Sequencing and analysis of larger numbers of bunyaviruses will continue to shed light on the reassortment that has driven and continues to shape bunyavirus evolution.
Co-infection of viral stocks
We also uncovered evidence of co-infections present in four virus stocks. It is possible that multiple viruses could be co-isolates from pools of mosquitoes containing multiple viruses. Overlapping ranges of the co-infecting viruses could provide support for this hypothesis. For instance, we detected Cache Valley virus reads in the Main Drain strain 72V2567 isolate. This Main Drain virus stock was derived from Aedes vexans mosquitoes collected in New Mexico, USA, in 1972, and Cache Valley virus is broadly distributed in Northern and Central America [142]. Similarly, there was evidence of an Enseada virus co-infection in the Guaratuba virus isolate that we sequenced. Guaratuba and Enseada virus have both been isolated from pools of Culex mosquitoes collected in 1976 in Brazil [50,138].
Ultimately, however, the true origins of these apparent co-infections are not knowable from our data alone. It is possible that cross-contamination may have occurred during isolation or passage. The failure to identify the co-infecting Mirim-like virus in an independent stock of Abras virus supports this alternative in that case. We can exclude cross-contamination during RNA extraction, library preparation, and sequencing because the co-infecting viruses were not identical to any of the other viruses we sequenced, and we confirmed the presence of co-infecting RNAs in separate vials of a stock for the Abras, Hughes, and Guaratuba virus isolates. Testing of the original samples and resequencing earlier passages of a virus could resolve ambiguity about the origin of co-infections, but it is likely that the source material for most of these isolates is no longer available. Continuing surveillance and direct sequencing of virus genomes in field samples without virus passage in cell culture will provide additional insight into the extent to which individual vectors or vector populations harbor bunyavirus co-infections, and the extent to which this impacts reassortment potential.
Taxonomic implications
The recent expansion in sequenced bunyaviral genomes–and of RNA virus genomes in general–has led to a restructuring of bunyavirus taxonomy [14,15,143–146]. Some of the viruses sequenced in this study will undoubtedly lead to the establishment of new genera and species, particularly within the Orthobunyavirus genus. Our data highlight some unresolved issues with bunyavirus taxonomy that are attributable to pervasive reassortment. For instance, the question of how should pairs of viruses that are nearly identical in 2 of the 3 genome segments but highly divergent in the 3rd, like Bertioga and Cananéia viruses, or Santa Rosa and Main Drain viruses, be classified?
Conclusions
This study contributed 35 totally new bunyavirus genome sequences to the public domain. These sequences further enrich the reference data available for the identification of emerging bunyaviruses, facilitate the resolution of phylogenetic relationships among known and newly-described viruses, and provide additional context towards the identification of reassortant strains. In addition, the generation of such a large dataset permitted expanded analyses of co-infection and reassortment. Each of these analyses provided foundational data which will support future investigations on the genetic diversity, reassortment, and virus-vector-host interactions in this important group of emerging human pathogens.
Supporting information
Coverage represents mean coverage for non-overlapping 10 nucleotide windows.
(PDF)
All complete L protein sequences annotated under the Orthobunyavirus genus in the NCBI Taxonomy database were downloaded and used to infer a maximum likelihood phylogeny. Sequences that we generated in the course of this study are indicated in red. Tree is midpoint rooted. Scale bar indicates substitutions per site.
(PDF)
All complete L protein sequences annotated under the Phlebovirus genus in the NCBI Taxonomy database were downloaded and used to infer a maximum likelihood phylogeny. Sequences that we generated in the course of this study are indicated in red. Tree is midpoint rooted. Scale bar indicates substitutions per site.
(PDF)
All complete L protein sequences annotated under the Nairoviridae family in the NCBI Taxonomy database were downloaded and used to infer a maximum likelihood phylogeny. Sequences that we generated in the course of this study are indicated in red. Tree is midpoint rooted. Scale bar indicates substitutions per site.
(PDF)
The virus sequences we generated in this study are shown in black.
(PDF)
(XLSX)
Virus names with _reference in them derive from RefSeq sequences.
(XLSX)
Acknowledgments
We thank Dr. Amy Lambert for project support and technical guidance.
Data Availability
All sequencing datasets have been deposited into GenBank under accessions MH484273–MH484350 and MK896421–MK896656 and MK965544. Quality-filtered sequence reads have been deposited in the sequence read archive (SRA) under Bioproject ID PRJNA543521.
Funding Statement
This research was funded by the Colorado State University Office of the Vice President for Research (RCK, MDS), the CSU Infectious Disease Research Center (RCK). KB, HRH, and BR are employees of the US Centers for Disease Control and Prevention Division of Vector-Borne Diseases and contributed to study design, data analysis, and manuscript preparation as part of their normal duties. Computational resources were supported by NIH/NCATS Colorado CTSA Grant Number UL1 TR002535 (MDS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bebber DP, Marriott FHC, Gaston KJ, Harris SA, Scotland RW. Predicting unknown species numbers using discovery curves. Proc Biol Sci. 2007;274: 1651–1658. 10.1098/rspb.2007.0464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woolhouse M, Guant E. Ecological origins of novel human pathogens. Critical Reviews in Microbiology. 2007;33: 1–12. 10.1080/10408410601172164 [DOI] [PubMed] [Google Scholar]
- 3.Woolhouse MEJ, Howey R, Gaunt E, Reilly L, Chase-Topping M, Savill N. Temporal trends in the discovery of human viruses. Proc Biol Sci. 2008;275: 2111–2115. 10.1098/rspb.2008.0294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wahid B, Altaf S, Naeem N, Ilyas N, Idrees M. Scoping Review of Crimean-Congo Hemorrhagic Fever (CCHF) Literature and Implications of Future Research. J Coll Physicians Surg Pak. 2019;29: 563–573. 10.29271/jcpsp.2019.06.563 [DOI] [PubMed] [Google Scholar]
- 5.Wanyoike F, Mtimet N, Bett B. Willingness to pay for a Rift valley fever (RVF) vaccine among Kenyan cattle producers. Prev Vet Med. 2019;171: 104763. 10.1016/j.prevetmed.2019.104763 [DOI] [PubMed] [Google Scholar]
- 6.Roselló S, Díez MJ, Nuez F. Viral diseases causing the greatest economic losses to the tomato crop. I. The Tomato spotted wilt virus—a review. Scientia Horticulturae. 1996;67: 117–150. 10.1016/S0304-4238(96)00946-6 [DOI] [Google Scholar]
- 7.Elliott RM, Schmaljohn CS. Bunyaviridae. 6th ed. In: Knipe D M, Howley P M, editors. Fields Virology. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2013. pp. 1244–1282. [Google Scholar]
- 8.Maes P, Adkins S, Alkhovsky SV, Avšič-Županc T, Ballinger MJ, Bente DA, et al. Taxonomy of the order Bunyavirales: second update 2018. Arch Virol. 2019;164: 927–941. 10.1007/s00705-018-04127-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aguilar PV, Barrett AD, Saeed MF, Watts DM, Russell K, Guevara C, et al. Iquitos Virus: A Novel Reassortant Orthobunyavirus Associated with Human Illness in Peru. PLoS Negl Trop Dis. 2011;5. 10.1371/journal.pntd.0001315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu X-J, Liang M-F, Zhang S-Y, Liu Y, Li J-D, Sun Y-L, et al. Fever with Thrombocytopenia Associated with a Novel Bunyavirus in China. N Engl J Med. 2011;364: 1523–1532. 10.1056/NEJMoa1010095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann B, Scheuch M, Höper D, Jungblut R, Holsteg M, Schirrmeier H, et al. Novel Orthobunyavirus in Cattle, Europe, 2011. Emerg Infect Dis. 2012;18: 469–472. 10.3201/eid1803.111905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanciotti RS, Kosoy OI, Bosco-Lauth AM, Pohl J, Stuchlik O, Reed M, et al. Isolation of a novel orthobunyavirus (Brazoran virus) with a 1.7kb S segment that encodes a unique nucleocapsid protein possessing two putative functional domains. Virology. 2013;444: 55–63. 10.1016/j.virol.2013.05.031 [DOI] [PubMed] [Google Scholar]
- 13.Hontz RD, Guevara C, Halsey ES, Silvas J, Santiago FW, Widen SG, et al. Itaya virus, a Novel Orthobunyavirus Associated with Human Febrile Illness, Peru. Emerg Infect Dis. 2015;21: 781–788. 10.3201/eid2105.141368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abudurexiti A, Adkins S, Alioto D, Alkhovsky SV, Avšič-Županc T, Ballinger MJ, et al. Taxonomy of the order Bunyavirales: update 2019. Arch Virol. 2019;164: 1949–1965. 10.1007/s00705-019-04253-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes HR, Adkins S, Alkhovskiy S, Beer M, Blair C, Calisher CH, et al. ICTV Virus Taxonomy Profile: Peribunyaviridae. J Gen Virol. 2020;101: 1–2. 10.1099/jgv.0.001365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tatineni S, McMechan AJ, Wosula EN, Wegulo SN, Graybosch RA, French R, et al. An eriophyid mite-transmitted plant virus contains eight genomic RNA segments with unusual heterogeneity in the nucleocapsid protein. J Virol. 2014;88: 11834–11845. 10.1128/JVI.01901-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Briese T, Calisher CH, Higgs S. Viruses of the family Bunyaviridae: Are all available isolates reassortants? Virology. 2013;446: 207–216. 10.1016/j.virol.2013.07.030 [DOI] [PubMed] [Google Scholar]
- 18.Reese SM, Blitvich BJ, Blair CD, Geske D, Beaty BJ, Black WC. Potential for La Crosse virus segment reassortment in nature. Virology Journal. 2008;5: 164. 10.1186/1743-422X-5-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerrard SR, Li L, Barrett AD, Nichol ST. Ngari virus is a Bunyamwera virus reassortant that can be associated with large outbreaks of hemorrhagic fever in Africa. J Virol. 2004;78: 8922–8926. 10.1128/JVI.78.16.8922-8926.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Sun Y, Shi W, Tan S, Pan Y, Cui S, et al. The first imported case of Rift Valley fever in China reveals a genetic reassortment of different viral lineages. Emerg Microbes Infect. 2017;6: e4–e4. 10.1038/emi.2016.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding N-Z, Luo Z-F, Niu D-D, Ji W, Kang X-H, Cai S-S, et al. Identification of two severe fever with thrombocytopenia syndrome virus strains originating from reassortment. Virus Res. 2013;178: 543–546. 10.1016/j.virusres.2013.09.017 [DOI] [PubMed] [Google Scholar]
- 22.Arbovirus Catalog—CDC Division of Vector-Borne Diseases (DVBD). [cited 9 Apr 2019]. Available: https://wwwn.cdc.gov/arbocat/
- 23.Babraham Bioinformatics—FastQC A Quality Control tool for High Throughput Sequence Data. Available: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/
- 24.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal. 2011;17: 10–12. 10.14806/ej.17.1.200 [DOI] [Google Scholar]
- 25.Li W, Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006;22: 1658–1659. 10.1093/bioinformatics/btl158 [DOI] [PubMed] [Google Scholar]
- 26.Li W, Jaroszewski L, Godzik A. Clustering of highly homologous sequences to reduce the size of large protein databases. Bioinformatics. 2001;17: 282–283. 10.1093/bioinformatics/17.3.282 [DOI] [PubMed] [Google Scholar]
- 27.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9: 357–359. 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19: 455–477. 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215: 403–410. 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- 30.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, et al. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10: 421. 10.1186/1471-2105-10-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buchfink B, Xie C, Huson DH. Fast and sensitive protein alignment using DIAMOND. Nat Methods. 2015;12: 59–60. 10.1038/nmeth.3176 [DOI] [PubMed] [Google Scholar]
- 32.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28: 1647–1649. 10.1093/bioinformatics/bts199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lambert AJ, Lanciotti RS. Consensus amplification and novel multiplex sequencing method for S segment species identification of 47 viruses of the Orthobunyavirus, Phlebovirus, and Nairovirus genera of the family Bunyaviridae. J Clin Microbiol. 2009;47: 2398–2404. 10.1128/JCM.00182-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kono Y, Yusnita Y, Mohd Ali AR, Maizan M, Sharifah SH, Fauzia O, et al. Characterization and identification of Oya virus, a Simbu serogroup virus of the genus Bunyavirus, isolated from a pig suspected of Nipah virus infection. Arch Virol. 2002;147: 1623–1630. 10.1007/s00705-002-0838-y [DOI] [PubMed] [Google Scholar]
- 35.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32: 1792–1797. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galtier N, Gouy M, Gautier C. SEAVIEW and PHYLO_WIN: two graphic tools for sequence alignment and molecular phylogeny. Comput Appl Biosci. 1996;12: 543–548. 10.1093/bioinformatics/12.6.543 [DOI] [PubMed] [Google Scholar]
- 37.Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol. 2000;17: 540–552. 10.1093/oxfordjournals.molbev.a026334 [DOI] [PubMed] [Google Scholar]
- 38.Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17: 754–755. 10.1093/bioinformatics/17.8.754 [DOI] [PubMed] [Google Scholar]
- 39.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19: 1572–1574. 10.1093/bioinformatics/btg180 [DOI] [PubMed] [Google Scholar]
- 40.Posada D, Crandall KA. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14: 817–818. 10.1093/bioinformatics/14.9.817 [DOI] [PubMed] [Google Scholar]
- 41.Miller MA, Pfeiffer W, Schwartz T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. 2010 Gateway Computing Environments Workshop (GCE). New Orleans, LA, USA: IEEE; 2010. pp. 1–8. 10.1109/GCE.2010.5676129 [DOI]
- 42.Stöver BC, Müller KF. TreeGraph 2: Combining and visualizing evidence from different phylogenetic analyses. BMC Bioinformatics. 2010;11: 7. 10.1186/1471-2105-11-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katoh K, Standley DM. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol Biol Evol. 2013;30: 772–780. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Price MN, Dehal PS, Arkin AP. FastTree 2—approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5: e9490. 10.1371/journal.pone.0009490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ladner JT, Beitzel B, Chain PSG, Davenport MG, Donaldson EF, Frieman M, et al. Standards for sequencing viral genomes in the era of high-throughput sequencing. MBio. 2014;5: e01360–01314. 10.1128/mBio.01360-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calisher CH, Gutierrez VE, Bruce Francy D, Aracely Alava A, Muth DJ, Lazuick JS. Identification of hitherto unrecognized arboviruses from Ecuador: Members of serogroups B, C, Bunyamwera, Patois, and Minatitlan. Am J Trip Med Hyg. 1983;32: 877–885. [DOI] [PubMed] [Google Scholar]
- 47.Simpósio sobre a Biota Amazônica Belém, Lent H. Atas do simpósio sobre a biota amazônica Belém, Pará, Brasil, Junho 6–11, 1966. Rio de Janeiro Conselho Nacional de Pesquisas (CNPQ); 1967. Available: https://www.biodiversitylibrary.org/item/194233 [Google Scholar]
- 48.Taylor RM, American Committee on Arthropod-borne Viruses. Subcommittee on Information Exchange; National Institute of Allergy and Infectious Diseases (U.S.). Catalogue of arthropod-borne viruses of the world; a collection of data on registered arthropod-borne animal viruses. Washington, Public Health Service; for sale by the Supt. of Docs., U.S. Govt. Print. Off.; 1967. [Google Scholar]
- 49.Takahashi K, Oya A, Okazda T, Matsuo R, Kuma M. Aino virus, a new a new member of simbu group of arbovirus from mosquitoes in Japan. Jpn J Med Sci Biol. 1968;21: 95–101. 10.7883/yoken1952.21.95 [DOI] [PubMed] [Google Scholar]
- 50.Calisher CH, Coimbra TL, Lopez O de S, Muth DJ, Sacchetta L de A, Francy DB, et al. Identification of new Guama and Group C serogroup bunyaviruses and an ungrouped virus from Southern Brazil. Am J Trop Med Hyg. 1983;32: 424–431. 10.4269/ajtmh.1983.32.424 [DOI] [PubMed] [Google Scholar]
- 51.Roca-Garcia M. The Isolation of Three Neurotropic Viruses from Forest Mosquitoes in Eastern Colombia. The Journal of Infectious Diseases. 1944;75: 160–169. [Google Scholar]
- 52.Calisher CH, Monath TP, Mitchell CJ, Sabattini MS, Cropp CB, Kerschner J, et al. Arbovirus investigations in Argentina, 1977–1980. III. Identification and characterization of viruses isolated, including new subtypes of western and Venezuelan equine encephalitis viruses and four new bunyaviruses (Las Maloyas, Resistencia, Barranqueras, and Antequera). Am J Trop Med Hyg. 1985;34: 956–965. [PubMed] [Google Scholar]
- 53.Mitchell CJ, Monath TP, Sabattini MS, Cropp CB, Daffner JF, Calisher CH, et al. Arbovirus investigations in Argentina, 1977–1980. II. Arthropod collections and virus isolations from Argentine mosquitoes. Am J Trop Med Hyg. 1985;34: 945–955. [PubMed] [Google Scholar]
- 54.Karabatsos N, Buckley SM. Susceptibility of the baby-hamster kidney-cell line (BHK-21) to infection with arboviruses. Am J Trop Med Hyg. 1967;16: 99–105. 10.4269/ajtmh.1967.16.99 [DOI] [PubMed] [Google Scholar]
- 55.Digoutte JP, Robin Y, Cagnard VJ. [Bangui virus (HB 70–754), a new virus isolated from a case of acute exanthemata]. Ann Microbiol (Paris). 1973;124: 147–153. [PubMed] [Google Scholar]
- 56.El Mekki AA, Nieuwenhuysen P, van der Groen G, Pattyn SR. Characterization of some ungrouped viruses. Trans R Soc Trop Med Hyg. 1981;75: 799–806. 10.1016/0035-9203(81)90416-8 [DOI] [PubMed] [Google Scholar]
- 57.Digoutte JP. Rapport Annuel de l’Institut Pasteur de Bangui. 1970. 5478269 [Google Scholar]
- 58.Zeller HG, Karabatsos N, Calisher CH, Digoutte JP, Cropp CB, Murphy FA, et al. Electron microscopic and antigenic studies of uncharacterized viruses. II. Evidence suggesting the placement of viruses in the family Bunyaviridae. Arch Virol. 1989;108: 211–227. 10.1007/BF01310935 [DOI] [PubMed] [Google Scholar]
- 59.Calisher CH, Shope RE. Bunyaviridae: The Bunyaviruses. Laboratory Diagnosis of Infectious Diseases Principles and Practice. New York, NY: Springer New York; 1988. pp. 626–646. 10.1007/978-1-4612-3900-0_32 [DOI] [Google Scholar]
- 60.Stim TB. Arbovirus Plaquing in Two Simian Kidney Cell Lines. Journal of General Virology. 1969;5: 329–338. 10.1099/0022-1317-5-3-329 [DOI] [Google Scholar]
- 61.DE Souza Lopes O, DE Abreu Sacchetta L, Fonseca IE, Lacerda JP. Bertioga (Guama group) and Anhembi (Bunyamwera group), two new arboviruses isolated in São Paulo, Brazil. Am J Trop Med Hyg. 1975;24: 131–134. 10.4269/ajtmh.1975.24.131 [DOI] [PubMed] [Google Scholar]
- 62.Hunt AR, Calisher CH. Relationships of bunyamwera group viruses by neutralization. Am J Trop Med Hyg. 1979;28: 740–749. [PubMed] [Google Scholar]
- 63.Lopes O de S, Forattini OP, Fonseca IE, Lacerda JP, Sacchetta LA, Rabello EX. Boracéia virus. A new virus related to anopheles B virus. Proc Soc Exp Biol Med. 1966;123: 502–504. 10.3181/00379727-123-31526 [DOI] [PubMed] [Google Scholar]
- 64.Saluzzo J-F, Germain M, Huard M, Robin Y, Gonzalez J-P, Herve J-P, et al. Le virus bozo (ArB 7343): Un nouvel arbovirus du groupe bunyamwera isolé en république centrafricaine; sa transmission expérimentale par Aedes aegypti. Annales de l’Institut Pasteur / Virologie. 1983;134: 221–232. 10.1016/S0769-2617(83)80061-6 [DOI] [Google Scholar]
- 65.Smithburn KC, Haddow AJ, Mahaffy AF. A Neurotropic Virus Isolated from Aedes Mosquitoes Caught in the Semliki Forest. The American Journal of Tropical Medicine and Hygiene. 1946;s1-26: 189–208. 10.4269/ajtmh.1946.s1-26.189 [DOI] [PubMed] [Google Scholar]
- 66.Spence L, Anderson CR, Aitken TH, Downs WG. Bushbush, Ieri and Lukuni viruses, three unrelated new agents isolated from Trinidadian forest mosquitoes. Proc Soc Exp Biol Med. 1967;125: 45–50. 10.3181/00379727-125-32009 [DOI] [PubMed] [Google Scholar]
- 67.Reeves WC, Scrivani RP, Hardy JL, Roberts DR, Nelson RL. Buttonwillow virus, a new Arbovirus isolated from mammals and Culicoides midges in Kern County, California. Am J Trop Med Hyg. 1970;19: 544–551. 10.4269/ajtmh.1970.19.544 [DOI] [PubMed] [Google Scholar]
- 68.Hardy JL, Scrivani RP, Lyness RN, Nelson RL, Roberts DR. Ecologic studies of Buttonwillow virus in Kern County, California, 1961–1968. Am J Trop Med Hyg. 1970;19: 552–563. 10.4269/ajtmh.1970.19.552 [DOI] [PubMed] [Google Scholar]
- 69.Tesh RB, Chaniotis BN, Peralta PH, Johnson KM. Ecology of Viruses Isolated from Panamanian Phlebotomine Sandflies. The American Journal of Tropical Medicine and Hygiene. 1974;23: 258–269. 10.4269/ajtmh.1974.23.258 [DOI] [PubMed] [Google Scholar]
- 70.Tesh RB, Boshell J, Young DG, Morales A, Corredor A, Modi GB, et al. Biology of Arboledas virus, a new phlebotomus fever serogroup virus (Bunyaviridae: Phlebovirus) isolated from sand flies in Colombia. Am J Trop Med Hyg. 1986;35: 1310–1316. 10.4269/ajtmh.1986.35.1310 [DOI] [PubMed] [Google Scholar]
- 71.Henderson JR, Taylor RM. Propagation of certain arthropod-borne viruses in avian and primate cell cultures. J Immunol. 1960;84: 590–598. [PubMed] [Google Scholar]
- 72.Mangiafico JA, Sanchez JL, Figueiredo LT, LeDuc JW, Peters CJ. Isolation of a newly recognized Bunyamwera serogroup virus from a febrile human in Panama. Am J Trop Med Hyg. 1988;39: 593–596. 10.4269/ajtmh.1988.39.593 [DOI] [PubMed] [Google Scholar]
- 73.Catalogue of arthropod-borne viruses of the world. The Subcommittee on Information Exchange. The American Committee on Arthropod-borne Viruses. Am J Trop Med Hyg. 1970;19: Suppl:1149–50. 10.4269/ajtmh.1970.19.1082 [DOI] [PubMed] [Google Scholar]
- 74.Calisher CH, Lazuick JS, Justines G, Francy DB, Monath TP, V EG, et al. Viruses Isolated from Aedeomyia Squamipennis Mosquitoes Collected in Panama, Ecuador, and Argentina: Establishment of the Gamboa Serogroup. The American Journal of Tropical Medicine and Hygiene. 1981;30: 219–223. 10.4269/ajtmh.1981.30.219 [DOI] [PubMed] [Google Scholar]
- 75.Henderson BE, Calisher CH, Coleman PH, Fields BN, Work TH. Gumbo Limbo, a new group C arbovirus from the Florida everglades. Am J Epidemiol. 1969;89: 227–231. 10.1093/oxfordjournals.aje.a120933 [DOI] [PubMed] [Google Scholar]
- 76.Shope RE, Causey CE, Causey OR. Itaqui Virus, a New Member of Arthropod-Borne Group C*. The American Journal of Tropical Medicine and Hygiene. 1961;10: 264–265. 10.4269/ajtmh.1961.10.264 [DOI] [Google Scholar]
- 77.Buckley SM, Shope RE. Comparative Assay of Arthropod-Borne Group C Virus Antibodies by Tissue Culture Neutralization and Hemagglutination-Inhibition Tests. The American Journal of Tropical Medicine and Hygiene. 1961;10: 53–61. 10.4269/ajtmh.1961.10.53 [DOI] [Google Scholar]
- 78.Rodrigues FM, Singh PB, Dandawate CN, Soman RS, Bhatt PN. Kaikalur virus—a new arthropod-borne virus belonging to the Simbu group isolated in India from Culex tritaeniorhynchus (Giles). Indian J Med Res. 1977;66: 719–725. [PubMed] [Google Scholar]
- 79.Anderson CR, Aitken TH, Spence LP, Downs WG. Kairi virus, a new virus from Trinidadian forest mosquitoes. Am J Trop Med Hyg. 1960;9: 70–72. 10.4269/ajtmh.1960.9.70 [DOI] [PubMed] [Google Scholar]
- 80.Casals J, Whitman L. A New Antigenic Group of Arthropod-Borne Viruses. The American Journal of Tropical Medicine and Hygiene. 1960;9: 73–77. 10.4269/ajtmh.1960.9.73 [DOI] [PubMed] [Google Scholar]
- 81.Málková D. Yaba 1 virus in Czechoslovakia. I. Some physical and chemical properties of yaba 1 (Lednice 110) virus. Acta Virol. 1972;16: 264–266. [PubMed] [Google Scholar]
- 82.Main OM, Hardy JL, Reeves WC. Growth of Arboviruses and Other Viruses in a Continuous Line of Culex Tarsalis Cells. J Med Entomol. 1977;14: 107–112. 10.1093/jmedent/14.1.107 [DOI] [PubMed] [Google Scholar]
- 83.Simpósio sobre a Biota Amazônica, Simpósio sobre a Biota Amazônica, Woodall JP. Atas do simpósio sobre a biota amazônica Belém, Pará, Brasil, Junho 6–11, 1966. Rio de Janeiro: Conselho Nacional de Pesquisas (CNPQ); 1967. 10.5962/bhl.title.110138 [DOI] [Google Scholar]
- 84.Causey OR, Causey CE, Maroja OM, Macedo DG. The isolation of arthropod-borne viruses, including members of two hitherto undescribed serological groups, in the Amazon region of Brazil. Am J Trop Med Hyg. 1961;10: 227–249. 10.4269/ajtmh.1961.10.227 [DOI] [PubMed] [Google Scholar]
- 85.Derodaniche E, Paesdeandrade A, Galindo P. Isolation of Two Antigenically Distinct Arthropod-Borne Viruses of Group C in Panama. Am J Trop Med Hyg. 1964;13: 839–843. [PubMed] [Google Scholar]
- 86.Porterfield JS, Casals J, Chumakov MP, Gaidamovich SY, Hannoun C, Holmes IH, et al. Bunyaviruses and Bunyaviridae. Intervirology. 1975;6: 13–24. 10.1159/000149449 [DOI] [PubMed] [Google Scholar]
- 87.Mellor PS, Boorman J, Loke R. The multiplication of Main Drain Virus in two species of Culicoides (Diptera, Ceratopogonidae). Archiv f Virusforschung. 1974;46: 105–110. 10.1007/BF01240210 [DOI] [PubMed] [Google Scholar]
- 88.Sunaga H, Taylor RM, Henderson JR. Comparative Sensitivity of Viruses to Treatment with Diethyl Ether and Sodium Desoxycholate. The American Journal of Tropical Medicine and Hygiene. 1960;9: 419–424. 10.4269/ajtmh.1960.9.419 [DOI] [PubMed] [Google Scholar]
- 89.Buckley SM. Applicability of the Hela (Gey) Strain of Human Malignant Epithelial Cells to the Propagation of Arboviruses. Proc Soc Exp Biol Med. 1964;116: 354–358. 10.3181/00379727-116-29246 [DOI] [PubMed] [Google Scholar]
- 90.Jonkers AH, Spence L, Downs WG, Aitken THG, Tikasingh ES. Arbovirus Studies in Bush Bush Forest, Trinidad, W. I., September 1959–December 1964. The American Journal of Tropical Medicine and Hygiene. 1968;17: 276–284. 10.4269/ajtmh.1968.17.276 [DOI] [PubMed] [Google Scholar]
- 91.Henderson BE. East African Virus Research Institute Report. 1969;No. 18:31–33. [Google Scholar]
- 92.Spence L, Anderson CR, Aitken TH, Downs WG. Nepuyo virus, a new group C agent isolated in Trinidad and Brazil. I. Isolation and properties of the Trinidadian strain. Am J Trop Med Hyg. 1966;15: 71–74. 10.4269/ajtmh.1966.15.71 [DOI] [PubMed] [Google Scholar]
- 93.Shope RE, Whitman L. Nepuyo virus, a new group C agent isolated in Trinidad and Brazil. II. Serological studies. Am J Trop Med Hyg. 1966;15: 772–774. 10.4269/ajtmh.1966.15.772 [DOI] [PubMed] [Google Scholar]
- 94.Calisher CH, Lindsey HS, Ritter DG, Sommerman KM. Northway virus: a new Bunyamwera group arbovirus from Alaska. Can J Microbiol. 1974;20: 219–223. 10.1139/m74-034 [DOI] [PubMed] [Google Scholar]
- 95.Akashi H, Kaku Y, Kong X, Pang H. Antigenic and genetic comparisons of Japanese and Australian Simbu serogroup viruses: evidence for the recovery of natural virus reassortants. Virus Res. 1997;50: 205–213. 10.1016/s0168-1702(97)00071-3 [DOI] [PubMed] [Google Scholar]
- 96.Fields BN, Henderson BE, Coleman PH, Work TH. Pahayokee and Shark River, two new arboviruses related to Patois and Zegla from the Florida everglades. Am J Epidemiol. 1969;89: 222–226. 10.1093/oxfordjournals.aje.a120932 [DOI] [PubMed] [Google Scholar]
- 97.Srihongse S, Galindo P, Grayson MA. Isolation of group C arboviruses in Panama including two new members, Patois and Zegla. Am J Trop Med Hyg. 1966;15: 379–384. 10.4269/ajtmh.1966.15.379 [DOI] [PubMed] [Google Scholar]
- 98.Galindo P, Srihongse S, Rodaniche ED, Grayson MA. An Ecological Survey for Arboviruses in Almirante, Panama, 1959–1962*. The American Journal of Tropical Medicine and Hygiene. 1966;15: 385–400. 10.4269/ajtmh.1966.15.385 [DOI] [PubMed] [Google Scholar]
- 99.St George TD, Standfast HA, Cybinski DH, Filippich C, Carley JG. Peaton virus: a new Simbu group arbovirus isolated from cattle and Culicoides brevitarsis in Australia. Aust J Biol Sci. 1980;33: 235–243. 10.1071/bi9800235 [DOI] [PubMed] [Google Scholar]
- 100.Francy DB, Karabatsos N, Wesson DM, Moore CG, Lazuick JS, Niebylski ML, et al. A new arbovirus from Aedes albopictus, an Asian mosquito established in the United States. Science. 1990;250: 1738–1740. 10.1126/science.2270489 [DOI] [PubMed] [Google Scholar]
- 101.Jonkers AH, Metselaar D, de Andrade AH, Tikasingh ES. Restan virus, a new group C arbovirus from Trinidad and Surinam. Am J Trop Med Hyg. 1967;16: 74–78. 10.4269/ajtmh.1967.16.74 [DOI] [PubMed] [Google Scholar]
- 102.Hubálek Z, Juricová Z, Halouzka J, Butenko AM, Kondrasina NG, Guscina EA, et al. Isolation and characterization of Sedlec virus, a new bunyavirus from birds. Acta Virol. 1990;34: 339–345. [PubMed] [Google Scholar]
- 103.Bishop DHL, Shope RE. Comprehensive Virology. N.Y.: Plenum Press; 1979. pp. 1–156. [Google Scholar]
- 104.Cornet M, Robin Y, Chateau R, Heme G, Adam C, Valade M, et al. Isolation of arboviruses from mosquitoes in eastern Senegal Notes on epidemiology of aedes—borne viruses especially yellow fever virus. Cahiers ORSTOM Serie entomologie medicale et parasitologie. 1979;17: 149–163. [Google Scholar]
- 105.Woodall JP, Williams MC. Tanga virus: a hitherto undescribed virus from Anopheles mosquitoes from Tanzania. East African Medical Journal. 1967;44: 83–6. [PubMed] [Google Scholar]
- 106.Ardoin PM, Simpson DI. Artificial infection of Aedes (Stegomyia) aegypti (Linnaeus) with five African arboviruses. J Med Entomol. 1967;4: 189–191. 10.1093/jmedent/4.2.189 [DOI] [PubMed] [Google Scholar]
- 107.Brès P, Williams M, Chambon L. Isolement au Sénégal d’un nouveau prototype d’arbovirus, la souche “Tataguine” (IPD/A 252). Ann Inst Pasteur. 1966;11: 585–591. [Google Scholar]
- 108.Murphy FA, Harrison AK, Tzianabos T. Electron microscopic observations of mouse brain infected with Bunyamwera group arboviruses. J Virol. 1968;2: 1315–1325. 10.1128/JVI.2.11.1315-1325.1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marshall ID, Woodroofe GM, Gard GP. Arboviruses of Coastal South-Eastern Australia. Australian Journal of Experimental Biology and Medical Science. 1980;58: 91–102. 10.1038/icb.1980.9 [DOI] [PubMed] [Google Scholar]
- 110.Carey DE, Reuben R, George S, Shope RE, Myers RM. Kammavanpettai, Kannamangalam, Sembalam and Thimiri viruses: four unrelated new agents isolated from birds in India. Indian J Med Res. 1971;59: 1708–1711. [PubMed] [Google Scholar]
- 111.Carvalho VL, Nunes MRT, Medeiros DBA, da Silva SP, Lima CPS, Inada DT, et al. New Virus Genome Sequences of the Guama Serogroup (Genus Orthobunyavirus, Family Bunyaviridae), Isolated in the Brazilian Amazon Region. Genome Announc. 2017;5. 10.1128/genomeA.01750-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.St George TD, Cybinski DH, Filippich C, Carley JG. The isolation of three Simbu group viruses new to Australia. Aust J Exp Biol Med Sci. 1979;57: 581–582. 10.1038/icb.1979.60 [DOI] [PubMed] [Google Scholar]
- 113.Scherer WF, Campillo-Sainz C, Dickerman RW, Diaz-Najera A, Madalengoitia J. Isolation of Tlacotalpan virus, a new Bunyamwera-group virus from Mexican mosquitoes. Am J Trop Med Hyg. 1967;16: 79–91. 10.4269/ajtmh.1967.16.79 [DOI] [PubMed] [Google Scholar]
- 114.Lennette EH, Ota MI, Fujimoto FY, Wiener A, Loomis EC. Turlock virus: a presumably new arthropod-borne virus; isolation and identification. Am J Trop Med Hyg. 1957;6: 1024–1035. 10.4269/ajtmh.1957.6.1024 [DOI] [PubMed] [Google Scholar]
- 115.Lennette EH, Ota MI, Hoffman MN. Turlock virus: a description of some of its properties. Am J Trop Med Hyg. 1957;6: 1036–1046. 10.4269/ajtmh.1957.6.1036 [DOI] [PubMed] [Google Scholar]
- 116.Calisher CH, McLean RG, Zeller HG, Francy DB, Karabatsos N, Bowen RA. Isolation of Tete serogroup bunyaviruses from Ceratopogonidae collected in Colorado. Am J Trop Med Hyg. 1990;43: 314–318. 10.4269/ajtmh.1990.43.314 [DOI] [PubMed] [Google Scholar]
- 117.Doherty RL, Carley JG, Mackerras MJ, Marks EN. Studies of Arthropod-Borne Virus Infections in Queensland. Australian Journal of Experimental Biology and Medical Science. 1963;41: 17–39. 10.1038/icb.1963.2 [DOI] [PubMed] [Google Scholar]
- 118.Casals J. New developments in the classification of arthropod-borne viruses. Anais de Microbiolgia. 1963; 13–34. [Google Scholar]
- 119.Causey OR, Kemp GE, Causey CE, Lee VH. Isolations of Simbu-group viruses in Ibadan, Nigeria 1964–69, including the new types Sango, Shamonda, Sabo and Shuni. Ann Trop Med Parasitol. 1972;66: 357–362. 10.1080/00034983.1972.11686835 [DOI] [PubMed] [Google Scholar]
- 120.Pavri KM, Casals J. Kaisodi virus, a new agent isolated from Haemaphysalis spinigera in Mysore state, South India. Am J Trop Med Hyg. 1966;15: 961–963. 10.4269/ajtmh.1966.15.961 [DOI] [PubMed] [Google Scholar]
- 121.Bhatt PN, Kulkarni KG, Boshell J, Rajagopalan PK, Patil AP, Goverdhan MK, et al. Kaisodi virus, a new agent isolated from Haemaphysalis spinigera in Mysore State, South India. I. Isolation of strains. Am J Trop Med Hyg. 1966;15: 958–960. 10.4269/ajtmh.1966.15.958 [DOI] [PubMed] [Google Scholar]
- 122.Filipe AR, Alves MJ, Karabatsos N, Matos APA de, Núncio MS, Bacellar F. Palma Virus, a New Bunyaviridae Isolated from Ticks in Portugal. Intervirology. 1994;37: 348–351. 10.1159/000150399 [DOI] [PubMed] [Google Scholar]
- 123.Murphy FA, Harrison AK, Whitfield SG. Bunyaviridae: Morphologic and Morphogenetic Similarities of Bunyamwera Serologic Supergroup Viruses and Several Other Arthropod-Borne Viruses. Intervirology. 1973; 297–316. 10.1159/000148858 [DOI] [PubMed] [Google Scholar]
- 124.Yunker CE, Clifford CM, Thomas LA, Keirans JE, Casals J, George JE, et al. Sunday Canyon virus, a new ungrouped agent from the tick Argas (A.) cooleyi in Texas. Acta Virol. 1977;21: 36–44. [PubMed] [Google Scholar]
- 125.Lvov DK, Timopheeva AA, Gromashevski VL, Gostinshchikova GV, Veselovskaya OV, Chervonski VI, et al. “Zaliv Terpeniya” virus, a new Uukuniemi group arbovirus isolated from Ixodes (Ceratixodes) putus Pick.-Camb. 1878 on Tyuleniy Island (Sakhalin region) and Commodore Islands (Kamchatsk region). Arch Gesamte Virusforsch. 1973;41: 165–169. 10.1007/BF01252761 [DOI] [PubMed] [Google Scholar]
- 126.Málková D, Holubová J, Cerný V, Daniel M, Fernández A, de la Cruz J, et al. Estero real virus: a new virus isolated from argasid ticks Ornithodoros tadaridae in Cuba. Acta Virol. 1985;29: 247–250. [PubMed] [Google Scholar]
- 127.Philip CB. Hughes Virus, A New Arboviral Agent from Marine Bird Ticks. J Parasitol. 1965;51: 252. [PubMed] [Google Scholar]
- 128.Yunker CE, Clifford CM, Thomas LA, Cory J, George JE. Isolation of viruses from swallowticks, Argas cooleyi, in the southwestern United States. Acta Virol. 1972;16: 415–421. [PubMed] [Google Scholar]
- 129.Shchetinin A, Lvov D, Deriabin P, Botikov A, Gitelman A, Kuhn J, et al. Genetic and Phylogenetic Characterization of Tataguine and Witwatersrand Viruses and Other Orthobunyaviruses of the Anopheles A, Capim, Guamá, Koongol, Mapputta, Tete, and Turlock Serogroups. Viruses. 2015;7: 5987–6008. 10.3390/v7112918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Huang B, Firth C, Watterson D, Allcock R, Colmant AMG, Hobson-Peters J, et al. Genetic Characterization of Archived Bunyaviruses and their Potential for Emergence in Australia. Emerg Infect Dis. 2016;22: 833–840. 10.3201/eid2205.151566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gauci PJ, McAllister J, Mitchell IR, Boyle DB, Bulach DM, Weir RP, et al. Genomic characterisation of three Mapputta group viruses, a serogroup of Australian and Papua New Guinean bunyaviruses associated with human disease. PLoS ONE. 2015;10: e0116561. 10.1371/journal.pone.0116561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hughes H, Lambert A. ICTV Taxonomy Proposal. Move one species from the phenuivirid genus Phlebovirus to the peribunyavirid genus Pacuvirus, and create one novel pacuvirus species. 2019022M. 2019. [Google Scholar]
- 133.Groseth A, Vine V, Weisend C, Guevara C, Watts D, Russell B, et al. Maguari Virus Associated with Human Disease. Emerg Infect Dis. 2017;23: 1325–1331. 10.3201/eid2308.161254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tangudu CS, Charles J, Blitvich BJ. Evidence that Lokern virus (family Peribunyaviridae) is a reassortant that acquired its small and large genome segments from Main Drain virus and its medium genome segment from an undiscovered virus. Virol J. 2018;15. 10.1186/s12985-018-1031-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nunes MRT, Travassos da Rosa APA, Weaver SC, Tesh RB, Vasconcelos PFC. Molecular epidemiology of group C viruses (Bunyaviridae, Orthobunyavirus) isolated in the Americas. J Virol. 2005;79: 10561–10570. 10.1128/JVI.79.16.10561-10570.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Castillo Oré RM, Caceda RE, Huaman AA, Williams M, Hang J, Juarez DE, et al. Molecular and antigenic characterization of group C orthobunyaviruses isolated in Peru. PLoS ONE. 2018;13: e0200576. 10.1371/journal.pone.0200576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Aguilar PV. Genetic Characterization of the Patois Serogroup (Genus Orthobunyavirus; Family Peribunyaviridae) and Evidence That Estero Real Virus is a Member of the Genus Orthonairovirus. Am J Trip Med Hyg. 2018;99(2): 451–457. 10.4269/ajtmh.18-0201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.de Souza WM, Acrani GO, Romeiro MF, Reis O, Tolardo AL, da Silva SP, et al. Molecular characterization of Capim and Enseada orthobunyaviruses. Infection, Genetics and Evolution. 2016;40: 47–53. 10.1016/j.meegid.2016.02.024 [DOI] [PubMed] [Google Scholar]
- 139.Briese T, Bird B, Kapoor V, Nichol ST, Lipkin WI. Batai and Ngari viruses: M segment reassortment and association with severe febrile disease outbreaks in East Africa. J Virol. 2006;80: 5627–5630. 10.1128/JVI.02448-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yanase T, Aizawa M, Kato T, Yamakawa M, Shirafuji H, Tsuda T. Genetic characterization of Aino and Peaton virus field isolates reveals a genetic reassortment between these viruses in nature. Virus Res. 2010;153: 1–7. 10.1016/j.virusres.2010.06.020 [DOI] [PubMed] [Google Scholar]
- 141.Yanase T, Kato T, Aizawa M, Shuto Y, Shirafuji H, Yamakawa M, et al. Genetic reassortment between Sathuperi and Shamonda viruses of the genus Orthobunyavirus in nature: implications for their genetic relationship to Schmallenberg virus. Arch Virol. 2012;157: 1611–1616. 10.1007/s00705-012-1341-8 [DOI] [PubMed] [Google Scholar]
- 142.Calisher CH, Francy DB, Smith GC, Muth DJ, Lazuick JS, Karabatsos N, et al. Distribution of Bunyamwera serogroup viruses in North America, 1956–1984. Am J Trop Med Hyg. 1986;35: 429–443. 10.4269/ajtmh.1986.35.429 [DOI] [PubMed] [Google Scholar]
- 143.Adams MJ, Lefkowitz EJ, King AMQ, Harrach B, Harrison RL, Knowles NJ, et al. Changes to taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2017). Archives of Virology. 2017;162: 2505–2538. 10.1007/s00705-017-3358-5 [DOI] [PubMed] [Google Scholar]
- 144.Marklewitz M, Palacios G, Ebihara H, Kuhn J, Junglen S. Create six new genera, create eight-five new species, rename/move ten species and abolish two species in the family Phenuiviridae, order Bunyavirales. ICTV [International Committee for Taxonomy of Viruses]. 2019;Proposal (Taxoprop) No. 2019.026M. [Google Scholar]
- 145.Maes P, Adkins S, Alkhovsky SV, Avšič-Županc T, Ballinger MJ, Bente DA, et al. Taxonomy of the order Bunyavirales: second update 2018. Arch Virol. 2019;164: 927–941. 10.1007/s00705-018-04127-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Blitvich BJ, Beaty BJ, Blair CD, Brault AC, Dobler G, Drebot MA, et al. Bunyavirus Taxonomy: Limitations and Misconceptions Associated with the Current ICTV Criteria Used for Species Demarcation. Am J Trop Med Hyg. 2018;99: 11–16. 10.4269/ajtmh.18-0038 [DOI] [PMC free article] [PubMed] [Google Scholar]