Abstract
BACKGROUND:
Factors associated with red blood cell (RBC), plasma, and platelet transfusions in hospitalized neonates and children across the United States have not been well characterized.
METHODS:
Data from the Kids’ Inpatient Database (KID) 2016 were analyzed. KID is a random sample of 10% of all uncomplicated in-hospital births and 80% of remaining pediatric discharges from approximately 4200 US hospitals. Sampling weights were applied to generate nationally representative estimates. Primary outcome was one or more RBC transfusion procedures; plasma and platelet transfusions were assessed as secondary outcomes. Analysis was stratified by age: neonates (NEO; ≤28 d), and nonneonates (PED; >28 d and <18 y). Multivariable logistic regression was used to estimate adjusted odds ratios (aORs) and 95% confidence intervals (95% CIs).
RESULTS:
Among 5,604,984 total hospitalizations, overall prevalence of transfusions was 1.07% (95% CI, 0.94%−1.22%) for RBCs, 0.17% (95% CIs, 0.15%−0.21%) for plasma and 0.35% (95% CI, 0.30%−0.40%) for platelet transfusions. RBC transfusions occurred among 0.43% NEO admissions and 2.63% PED admissions. For NEO admissions, RBC transfusion was positively associated with nonwhite race, longer length of hospitalization, highest risk of mortality (aOR, 86.58; 95% CI, 64.77–115.73) and urban teaching hospital location. In addition to the above factors, among PED admissions, RBC transfusion was positively associated with older age, female sex (aOR, 1.10; 95% CI, 1.07–1.13), and elective admission status (aOR, 1.62; 95% CI, 1.46–1.80). Factors associated with plasma and platelet transfusions were largely similar to those associated with RBC transfusion, except older age groups had lower odds of plasma transfusion among PED admissions.
CONCLUSIONS:
While there is substantial variability in the proportion of neonates and nonneonatal children transfused nationally, there are several similar, yet unique, nonlaboratory predictors of transfusion identified in these age groups.
Transfusions of red blood cells (RBCs), platelets, and plasma are critical therapies for pediatric and neonatal populations.1,2 Among the pediatric patient population, the indications for transfusions and associated practices vary tremendously based on a host of factors, notably age and disease severity.3,4
Although nationally representative studies have described the transfusion practices and associated factors for transfusion in the US adult population,5,6 no nationally representative studies have exclusively evaluated the epidemiology and correlates of transfusions in the pediatric in-patient population.7Further, survey data from the AABB (formerly the American Association of Blood Banks) and US Centers for Disease Control and Prevention offer insight into blood component collections, distributions, and transfusions in the United States,8–11 yet none of the surveys —to date—have focused specifically on the pediatric or neonatal populations. A recent nationally representative study highlighted a decline in RBC transfusions among hospitalized patients across all age ranges, with the exception of pediatric patient admissions.12
This study uses a large, nationally representative pediatric inpatient database to characterize the demographic and hospital-level (i.e., nonlaboratory) correlates of RBC, plasma, and platelet transfusions in hospitalized pediatric and neonatal patients across the United States.
METHODS
Data source
Kids’ Inpatient Database (KID) designed as an initiative of the Healthcare Cost and Utilization Projectʼs (HCUP) is the largest publicly available all-payer pediatric inpatient care database in the United States13 and has been used previously to define the epidemiology of general and freestanding childrenʼs hospitals in the United States.14 Both freestanding childrenʼs hospitals, defined as hospitals that exclusively admit children, and general hospitals, those that admit children as well as adults, are included in KID database. The 2016 KID, sponsored by the Agency for Healthcare Research and Quality, includes data from 4200 community hospitals across 47 participating states, ultimately encompassing a national sample of pediatric discharges (i.e., patient age ≤20 y at admission). The American Hospital Association defines a community hospital as a short-term, nonfederal, general and specialty hospital, excluding rehabilitation hospitals. A community hospital per this definition could thus imply a teaching or nonteaching/nonacademic hospital.
KID randomly samples 10% of all uncomplicated in-hospital births and 80% of all other pediatric discharges after sorting by hospital and diagnosis-related group (DRG). Observations were self-weighted and calculated by strata, which were defined by census division, bed size, location, teaching status, and ownership, and freestanding childrenʼs hospital status. Per-stratum discharge weights were created separately for newborn and nonnewborn pediatric discharges, in proportion to the total number of newborn and nonnewborn pediatric discharges in the American Hospital Association universe. Strata containing fewer than two hospitals, 30 uncomplicated births, 30 complicated births, and/or 30 nonbirth pediatric discharges were combined with a stratum containing hospitals with similar characteristics.
Each inpatient discharge record includes patient-level characteristics including demographics (age, sex, and race), elective versus nonelective admission type, patient outcomes (length of stay, number of diagnoses, number of procedures, and in-patient mortality); primary insurance/payer type, up to 15 International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) diagnostic codes, and up to 15 ICD-10-CM procedure codes. The hospital-level characteristics included the hospital location classified as being urban/rural and hospital teaching status, number of beds, and freestanding childrenʼs hospital status versus not.
Since the KID is a deidentified publicly available data set, the Johns Hopkins Medical Institutions Institutional Review Board exempted this study from review. This study was conducted in accordance with the HCUP data use agreement guidelines.
Study outcomes
The primary outcome was the percentage of hospitalizations with one or more allogenic RBC transfusion procedures, determined as having at least one of the following ICD-10 procedure codes: 30233N1, 30233P1, 30243N1, 30243P1, 30253N1, 30263N1, or 30623P1. Secondary outcomes were the percentage of hospitalizations with one or more plasma transfusions (ICD-10 procedure codes 30233R1, 30243R1, 30253R1, and 30253R1) and the percentage of hospitalizations with one or more platelet transfusions (ICD-10 procedure codes 30233J1, 30233K1, 30233L1, 30233M1, 30243J1, 30243K1, 30243L1, and 30243M1). All outcomes were measured as a binary variable: having received at least one transfusion during the course of the hospitalization versus none. Data on the number of units/volume transfused were not available in the database.
Statistical analyses
This analysis was restricted to patients aged 18 years or less at admission. As the unit of analysis is a hospital discharge, an individual patient may be included multiple times in the database. To derive national estimates, discharge weights provided by HCUP were used. All reported estimates are weighted unless specified otherwise. Taylor series linearization was used to estimate standard errors. All data analyses were performed using “svy” commands in Stata/MP, version 15.2 (Statacorp).
Neonatal (NEO; age ≤28 d) and nonneonatal childhood (PED; age >28 days and <18 years) admissions were examined separately. Characteristics of the study population examined were further stratified by RBC transfusion status using descriptive statistics. The percentage of hospitalizations with one or more RBC transfusion procedure, one or more plasma transfusion, and one or more platelet transfusion were separately examined by individual-level and hospital-level factors. Estimates with low cell counts (n < 10) were not reported in accordance with HCUPʼs privacy policies. The NEO admissions included both complicated and uncomplicated newborn births as well as neonatal admissions. In a sensitivity analysis, we looked at RBC transfusion patterns specifically among the high-risk neonatal births. We identified this subgroup by a composite variable using the HCUP KID identifier variable for complicated births (I10_UNCBRTH) and in hospital birth (I10_HOSPBRTH) as well as restricted the analysis to hospitalizations with a length of stay of more than 3 days. Another sensitivity analysis was conducted by freestanding childrens hospital status.
For each outcome, adjusted odds ratios (aORs) and 95% confidence intervals (95% CIs) were estimated using multivariable logistic regression. Multivariable models included all covariates determined either to be clinically relevant a priori and/or significant in univariate models (two-sided p value < 0.05). The multivariable models for NEO admissions included sex, race, length of hospital stay (days), all patient refined diagnosis-related group (APR-DRG) mortality risk subclass, primary payer, hospital location, and teaching status. The multivariable models for PED admissions included age group, sex, race, elective admission status, length of hospital stay (days), APR-DRG mortality risk subclass, primary payer, hospital location, and teaching status. Highly collinear factors were excluded from multivariable models (e.g., APR-DRG risk severity subclass being collinear with APR-DRG mortality risk subclass). Elective admission status was not included as a covariate in the neonatal models since elective admissions were rare in neonates (only 0.72% admissions were reported as elective in NEO admissions as compared to 19.6% elective PED admissions). An available case approach was used to handle missing data (i.e., discharges that were missing any examined factors were excluded from the multivariable models) (Table S1, available as supporting information in the online version of this paper).
RESULTS
This study analyzed data from 2,634,337 unweighted hospitalizations recorded in 2016, representative of 5,604,984 weighted pediatric hospitalizations in 2016 in the United States. NEO (age ≤28 d) admissions included births and were the most common of all ages (70.83%). Table 1 shows characteristics of the study population among NEO and PED admissions. Among PED admissions, 80.10% were non-elective. The all-cause mortality rate among NEOs was 0.36%, and 0.39% among PEDs. The median length of hospital stay for NEO and PED admissions were 2 days (interquartile range [IQR], 2–3 days) and 2 days (IQR, 1–4 days), respectively.
TABLE 1.
Study population characteristics of inpatient hospitalizations in the 2016 Kids’ Inpatient Database (KID)
| Neonates |
Nonneonates |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall (N = 3,970,065) |
RBC transfusion (n = 16,947) |
No RBC transfusion (n = 3,953,118) |
Overall (N = 1,634,918) |
RBC transfusion (n = 43,062) |
No RBC transfusion (n = 1,591,856) |
|||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Sex | ||||||||||||
| Male | 2,040,741 | (51.40) | 9,321 | (55.00) | 2,031,419 | (51.39%) | 826,742 | (50.57) | 21,723 | (50.45) | 805,019 | (50.57) |
| Female | 1,927,012 | (48.54) | 7,624 | (44.99) | 1,919,388 | (48.55%) | 807,771 | (49.41) | 21,301 | (49.47) | 786,469 | (49.41) |
| Age group | ||||||||||||
| Infant (29 d to <1 y) | … | … | … | … | … | … | 282,637 | (17.29) | 6,993 | (16.24) | 275,644 | (17.32) |
| Toddler (1–2 y) | … | … | … | … | … | … | 135,836 | (8.31) | 2,949 | (6.85) | 132,887 | (8.35) |
| Early childhood age (2–5 y) | … | … | … | … | … | … | 307,073 | (18.78) | 9,490 | (22.04) | 297,583 | (18.69) |
| Middle childhood age (6–11 y) | … | … | … | … | … | … | 317,854 | (19.44) | 9,380 | (21.78) | 308,474 | (19.38) |
| Adolescent (12–18 y) | … | … | … | … | … | … | 591,517 | (36.18) | 14,250 | (33.09) | 577,268 | (36.26) |
| Race/Ethnicity | ||||||||||||
| White | 1,843,370 | (46.43) | 5,506 | (32.49) | 1,837,864 | (46.49) | 727,671 | (44.51) | 15,174 | (35.24) | 712,497 | (44.76) |
| African American | 516,989 | (13.02) | 4,153 | (24.50) | 512,836 | (12.97) | 273,951 | (16.76) | 9,086 | (21.10) | 264,865 | (16.64) |
| Hispanic | 718,686 | (18.10) | 3,308 | (19.52) | 715,378 | (18.10) | 345,138 | (21.11) | 9,264 | (21.51) | 335,874 | (21.10) |
| Asian or Pacific Islander | 217,086 | (5.47) | 737 | (4.35) | 216,350 | (5.47) | 48,350 | (2.96) | 1,832 | (4.25) | 46,518 | (2.92) |
| Other | 264,387 | (6.66) | 1,203 | (7.10) | 263,184 | (6.66) | 96,801 | (5.92) | 2,587 | (6.01) | 94,214 | (5.92) |
| Admission type | ||||||||||||
| Nonelective | … | … | … | … | … | … | 1,309,608 | (80.10) | 30,653 | (71.18) | 1,278,954 | (80.34) |
| Elective | … | … | … | … | … | … | 320,137 | (19.58) | 12,186 | (28.30) | 307,951 | (19.35) |
| Length of stay (days)* | 2 (2–3) | 51 (18–85) | 2 (2–3) | 2 (1–4) | 6 (3–13) | 2 (1–4) | ||||||
| APR-DRG risk mortality subclass | ||||||||||||
| 1 | 3,822,562 | (96.28) | 2,973 | (17.54) | 3,819,590 | (96.62) | 1,320,023 | (80.74) | 18,037 | (41.89) | 1,301,986 | (81.79) |
| 2 | 80,237 | (2.02) | 3,814 | (22.51) | 76,423 | (1.93) | 220,394 | (13.48) | 13,561 | (31.49) | 206,832 | (12.99) |
| 3 | 38,930 | (0.98) | 6,702 | (39.55) | 32,228 | (0.82) | 69,557 | (4.25) | 7,757 | (18.01) | 61,800 | (3.88) |
| 4 | 21,479 | (0.54) | 3,152 | (18.60) | 18,327 | (0.46) | 23,857 | (1.46) | 3,670 | (8.59) | 20,157 | (1.27) |
| APR-DRG risk severity subclass | ||||||||||||
| 1 | 2,854,232 | (71.89) | 391 | (2.31) | 2,853,841 | (72.19) | 655,030 | (40.07) | 5,464 | (12.69) | 649,567 | (40.81) |
| 2 | 780,846 | (19.67) | 1,783 | (10.52) | 779,063 | (19.71) | 632,366 | (38.68) | 12,660 | (29.40) | 619,706 | (38.93) |
| 3 | 278,212 | (7.01) | 5,549 | (32.74) | 272,663 | (6.90) | 269,062 | (16.46) | 14,333 | (33.29) | 254,729 | (16.00) |
| 4 | 49,918 | (1.26) | 8,918 | (52.62) | 41,000 | (1.04) | 77,373 | (4.73) | 10,598 | (24.61) | 66,775 | (4.19) |
| Primary payer | ||||||||||||
| Medicaid | 1,823,748 | (45.94) | 9,800 | (57.83) | 1,813,947 | (45.89) | 893,602 | (54.66) | 22,021 | (51.14) | 871,581 | (54.75) |
| Medicare | 14,176 | (0.36) | 36 | (0.21) | 14,140 | (0.36) | 5,928 | (0.36) | 172 | (0.40) | 5,756 | (0.36) |
| Private | 1,830,825 | (46.12) | 6,031 | (35.59) | 1,824,794 | (46.16) | 628,881 | (38.47) | 16,723 | (38.83) | 612,159 | (38.46) |
| Self | 180,890 | (4.56) | 283 | (1.67) | 180,608 | (4.57) | 38,157 | (2.33) | 1,120 | (2.6) | 37,037 | (2.33) |
| No charge/other | 115,779 | (2.92) | 788 | (4.65) | 114,991 | (2.91) | 65,605 | (4.01) | 2,988 | (6.94) | 62,617 | (3.93) |
| In-hospital mortality | ||||||||||||
| No | 3,952,490 | (99.56) | 14,812 | (87.40) | 3,937,678 | (99.61) | 1,627,188 | (99.53) | 41,818 | (97.11) | 1,585,371 | (99.59) |
| Yes | 14,406 | (0.36) | 2,120 | (12.51) | 12,286 | (0.31) | 6,415 | (0.39) | 1,243 | (2.89) | 5,173 | (0.32) |
| Hospital control | ||||||||||||
| Government, nonfederal | 491,838 | (12.39) | 2,355 | (13.90) | 489,483 | (12.38) | 200,435 | (12.26) | 4,829 | (11.21) | 195,606 | (12.29) |
| Private, nonprofit | 2,910,780 | (73.32) | 13,029 | (76.88) | 2,897,751 | (73.30) | 1,264,881 | (77.37) | 35,797 | (83.13) | 1,229,084 | (77.21) |
| Private, investor-owned | 567,447 | (14.29) | 1,563 | (9.22) | 565,884 | (14.31) | 169,602 | (10.37) | 2,435 | (5.66) | 167,166 | (10.50) |
| Hospital location/teaching | ||||||||||||
| Urban teaching | 2,628,001 | (66.20) | 15,184 | (89.60) | 2,612,817 | (66.10) | 1,387,542 | (84.87) | 41,013 | (95.24) | 1,346,529 | (84.59) |
| Urban nonteaching | 959,609 | (24.17) | 1,631 | (9.62) | 957,979 | (24.23) | 175,944 | (10.76) | 1,640 | (3.81) | 174,304 | (10.95) |
| Rural | 382,455 | (9.63) | 132 | (0.78) | 382,323 | (9.67) | 71,433 | (4.37) | 409 | (0.95) | 71,024 | (4.46) |
All data are weighted using survey weights provided by the Healthcare Cost and Utilization Project (HCUP). Red blood cell transfusion (RBC) defined as at least one RBC transfusion procedure, identified using International Classification of Diseases, 10th Revision codes: 30233 N1, 30233P1, 30243 N1, 30243P1, 30253 N1, 30263 N1, and/or 30623P1. Data may not sum to 100% due to missingness.
Data are median and corresponding interquartile range (IQR).
APR-DRG = all patient refined diagnosis-related group.
Among all pediatric (PED and NEO) admissions, overall 1.07% (95% CI, 0.94%−1.22%) received one or more RBC transfusion, 0.17% (95% CI, 0.15%−0.21%) received one or more plasma transfusion, and 0.35% (95% CI, 0.30%−0.40%) received one or more platelet transfusion (Fig. 1). Among NEO admissions alone, 0.43% received an RBC transfusion, 0.10% received a plasma transfusion, and 0.14% received a platelet transfusion (Tables 2, 3, and 4). Among PED admissions, 2.63% received an RBC transfusion, 0.35% received a plasma transfusion, and 0.85% received a platelet transfusion (Tables 2, 3, and 4). On a sensitivity analysis assessing the subgroup of neonates stratified as complicated births whose length of stay was more than 3 days only (as defined above in methods), the percentage of hospitilizations with RBCs transfused increased from 0.43% in all neonatal births to 2.71% of hospitilizations involving complicated neonatal births (Table 5). Likewise, the percentage of patients receiving platelet transfusions increased from 0.14% to 0.78%, and plasma from 0.10% to 0.44%.
Fig 1.
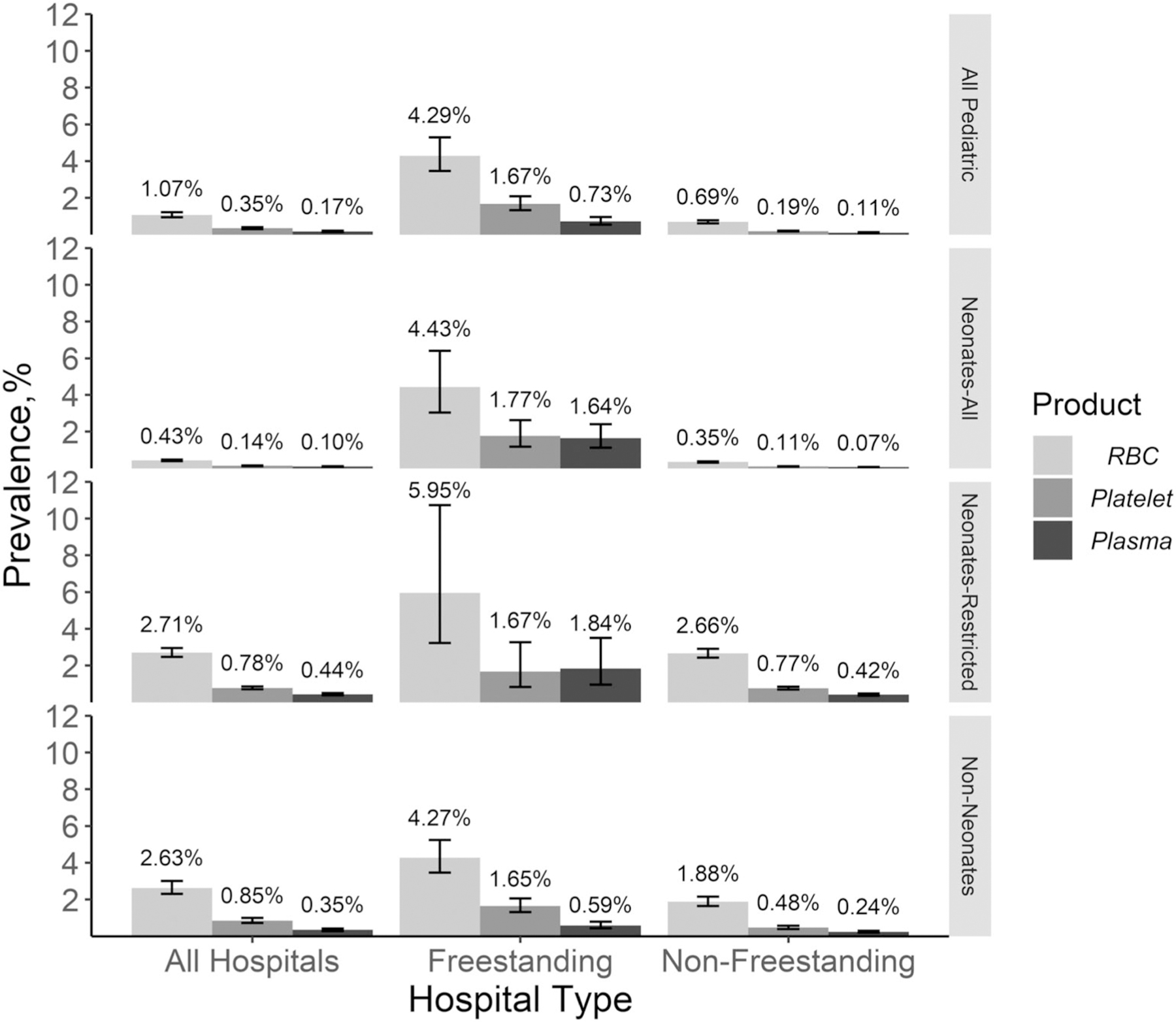
Prevalence of one or more red blood cell (RBC), platelet, and plasma transfusion during inpatient hospitalizations for all pediatric age groups and hospital types and stratified by all neonates (NEO), neonates restricted to complicated hospital births whose length of stay was greater than 3 days, and non-neonates (PED) and freestanding and nonfreestanding childrenʼs hospitals in the 2016 Kidʼs Inpatient Database (KID).
TABLE 2.
Factors associated with one or more RBC transfusions during inpatient hospitalizations in the 2016 Kids’ Inpatient Database (KID) neonate
| Neonates |
Nonneonates |
|||||||
|---|---|---|---|---|---|---|---|---|
| No. transfused (n = 16,947) | % transfused (0.43) | OR (95% CI) | aOR (95% CI) | No. transfused (n = 43,062) | % transfused (2.63) | OR (95% CI) | aOR (95% CI) | |
| Sex | ||||||||
| Male | 9,321 | 0.46 | Reference | Reference | 21,723 | 2.63 | Reference | Reference |
| Female | 7,624 | 0.40 | 0.87 (0.83–0.90)* | 1.00 (0.95–1.04) | 21,301 | 2.64 | 1.00 (0.98–1.03) | 1.10 (1.07–1.13)* |
| Age group | ||||||||
| Infant (29 d to <1 y) | … | … | … | … | 6,993 | 2.47 | Reference | Reference |
| Toddler (1–2 y) | … | … | … | … | 2,949 | 2.17 | 0.87 (0.78–0.98)† | 1.00 (0.89–1.11) |
| Early childhood age (2–5 y) | … | … | … | … | 9,490 | 3.09 | 1.26 (1.17–1.36)* | 1.38 (1.27–1.49)* |
| Middle childhood age (6–11 y) | … | … | … | … | 9,380 | 2.95 | 1.20 (1.10–1.30)* | 1.35 (1.23–1.49)* |
| Adolescent (12–18 y) | … | … | … | … | 14,250 | 2.41 | 0.97 (0.89–1.07) | 1.25 (1.14–1.38)* |
| Race/Ethnicity | ||||||||
| White | 5,506 | 0.30 | Reference | Reference | 15,174 | 2.09 | Reference | Reference |
| African American | 4,153 | 0.80 | 2.70 (2.42–3.01)* | 1.67 (1.46–1.91)* | 9,086 | 3.32 | 1.61 (1.43–1.81)* | 1.81 (1.61–2.04)* |
| Hispanic | 3,308 | 0.46 | 1.54 (1.34–1.78)* | 1.42 (1.22–1.64)* | 9,264 | 2.68 | 1.30 (1.12–1.49)* | 1.38 (1.20–1.58)* |
| Asian or Pacific Islander | 737 | 0.34 | 1.14 (0.93–1.39) | 1.36 (1.16–1.58)* | 1,832 | 3.79 | 1.85 (1.53–2.23)* | 1.66 (1.40–1.99)* |
| Other | 1,203 | 0.46 | 1.53 (1.30–1.79)* | 1.26 (1.07–1.49)† | 2,587 | 2.67 | 1.29 (1.07–1.56)† | 1.29 (1.08–1.55)† |
| Admission type | ||||||||
| Nonelective | … | … | … | … | 30,653 | 2.34 | Reference | Reference |
| Elective | … | … | … | … | 12,186 | 3.81 | 1.65 (1.48–1.84)* | 1.62 (1.46–1.80)* |
| Length of stay (d)§ | … | 51 (18–85) | 1.05 (1.05–1.05)* | 1.02 (1.02–1.02)* | … | 6 (3–13) | 1.03 (1.02–1.03)* | 1.01 (1.01–1.02)* |
| APR-DRG risk mortality subclass | ||||||||
| 1 | 2,973 | 0.08 | Reference | Reference | 18,037 | 1.37 | Reference | Reference |
| 2 | 3,814 | 4.75 | 64.13 (59.60–69.00)* | 38.29 (34.96–41.93)* | 13,561 | 6.15 | 4.73 (4.40–5.09) | 4.15 (3.85–4.48)* |
| 3 | 6,702 | 17.21 | 267.19 (241.77–295.28)* | 95.36 (83.10–109.42)* | 7,757 | 11.15 | 9.06 (8.21–10.00)* | 7.08 (6.44–7.80)* |
| 4 | 3,152 | 14.68 | 221.02 (166.36–293.66)* | 86.58 (64.77–115.73)* | 3,700 | 15.51 | 13.25 (11.73–14.97)* | 9.41 (8.23–10.75)* |
| APR-DRG risk severity subclass‖ | ||||||||
| 1 | 391 | 0.01 | Reference | … | 5,464 | 0.83 | Reference | … |
| 2 | 1,783 | 0.23 | 16.69 (14.26–19.53)* | … | 12,660 | 2.00 | 2.43 (2.28–2.59)* | … |
| 3 | 5,549 | 1.99 | 148.41 (128.17–171.85)* | … | 14,333 | 5.33 | 6.69 (6.09–7.34)* | … |
| 4 | 8,918 | 17.86 | 1586.10 (1307.26–1924.42)* | … | 10,598 | 13.70 | 18.87 (16.64–21.40)* | … |
| Primary payer¶ | ||||||||
| Medicaid | 9,800 | 0.54 | Reference | Reference | 22,021 | 2.46 | Reference | Reference |
| Medicare | 36 | 0.25 | 0.47 (0.27–0.81)† | 0.99 (0.53–1.86) | 172 | 2.90 | 1.18 (0.79–1.77) | 0.88 (0.65–1.21) |
| Private | 6,031 | 0.33 | 0.61 (0.56–0.66)* | 0.99 (0.90–1.09) | 16,723 | 2.66 | 1.08 (0.99–1.18) | 1.20 (1.09–1.32)* |
| Self | 283 | 0.16 | 0.29 (0.23–0.36)* | 0.65 (0.52–0.81)* | 1,120 | 2.94 | 1.20 (0.92–1.55) | 1.27 (1.02–1.58)‡ |
| No charge/Other | 788 | 0.68 | 1.27 (0.90–1.79) | 1.21 (0.91–1.62) | 2,988 | 4.55 | 1.89 (1.31–2.73)* | 1.72 (1.29–2.28)* |
| In-hospital mortality‖ | ||||||||
| No | 14,812 | 0.37 | Reference | … | 41,818 | 2.57 | Reference | … |
| Yes | 2,120 | 14.72 | 45.88 (42.00–50.11)* | … | 1,243 | 19.37 | 9.11 (7.89–10.52)* | … |
| Hospital control‖ | ||||||||
| Government, nonfederal | 2,355 | 0.48 | Reference | … | 4,829 | 2.41 | Reference | … |
| Private, nonprofit | 13,029 | 0.45 | 0.93 (0.70–1.24) | … | 35,797 | 2.83 | 1.18 (0.84–1.66) | … |
| Private, investor-owned | 1,563 | 0.28 | 0.57 (0.39–0.85)† | … | 2,435 | 1.44 | 0.59 (0.31–1.12) | … |
| Hospital location/teaching¶ | ||||||||
| Urban teaching | 15,184 | 0.58 | Reference | Reference | 41,061 | 2.96 | Reference | Reference |
| Urban nonteaching | 1,631 | 0.17 | 0.29 (0.23–0.38)* | 0.72 (0.55–0.94)‡ | 1,640 | 0.93 | 0.31 (0.19–0.50)* | 0.47 (0.28–0.78)† |
| Rural | 132 | 0.03 | 0.06 (0.03–0.12)* | 0.22 (0.12–0.43)* | 409 | 0.57 | 0.19 (0.15–0.23)* | 0.34 (0.28–0.42)* |
All data are weighted using survey weights provided by The Healthcare Cost and Utilization Project (HCUP). RBC transfusion defined as at least one RBC transfusion procedure, identified using International Classification of Diseases, 10th Revision codes: 30233 N1, 30233P1, 30243 N1, 30243P1, 30253 N1, 30263 N1, and/or 30623P1. Data may not sum to 100% due to missingness.
Bold type refers to any p <0.05.
p < 0.001.
p < 0.010.
p < 0.05.
Data are median and corresponding interquartile range (IQR) for admissions with one or more RBC transfusion.
Excluded from multivariable model due to high collinearity.
Medicare primary payer status and rural hospital data estimates have a coefficient of variation above the threshold of 30%.
APR-DRG = all patient refined diagnosis-related group.
TABLE 3.
Factors associated with one or more plasma transfusions during inpatient hospitalizations in the 2016 Kids’ Inpatient Database (KID) neonate
| Neonates |
Nonneonates |
|||||||
|---|---|---|---|---|---|---|---|---|
| No. transfused (n = 4007) | % transfused (0.10) | OR (95% CI) | aOR (95% CI) | No. transfused (n = 5757) | % transfused (0.35) | OR (95% CI) | aOR (95% CI) | |
| Sex | ||||||||
| Male | 2391 | 0.12 | Reference | Reference | 3154 | 0.38 | Reference | Reference |
| Female | 1613 | 0.08 | 0.71 (0.66–0.77)* | 0.87 (0.80–0.95)† | 2602 | 0.32 | 0.84 (0.79–0.90)* | 0.95 (0.88–1.02) |
| Age group | ||||||||
| Infant (29 d to <1 y) | … | … | … | … | 1663 | 0.59 | Reference | Reference |
| Toddler (1–2 y) | … | … | … | … | 387 | 0.28 | 0.48 (0.39–0.59)* | 0.57 (0.46–0.71)* |
| Early childhood age (2–5 y) | … | … | … | … | 1079 | 0.35 | 0.60 (0.53–0.67)* | 0.64 (0.56–0.72)* |
| Middle childhood age (6–11 y) | … | … | … | … | 918 | 0.29 | 0.49 (0.42–0.57)* | 0.54 (0.46–0.65)* |
| Adolescent (12–18 y) | … | … | … | … | 1710 | 0.29 | 0.49 (0.41–0.59)* | 0.68 (0.55–0.83)* |
| Race/ethnicity | ||||||||
| White | 1354 | 0.07 | Reference | Reference | 2100 | 0.29 | Reference | Reference |
| African American | 731 | 0.14 | 1.93 (1.65–2.25)* | 1.00 (0.79–1.28) | 913 | 0.33 | 1.16 (0.97–1.37) | 1.32 (1.12–1.56)† |
| Hispanic | 850 | 0.12 | 1.61 (1.29–2.01)* | 1.45 (1.18–1.80)* | 1383 | 0.40 | 1.39 (1.14–1.70)† | 1.51 (1.26–1.80)* |
| Asian or Pacific Islander | 189 | 0.09 | 1.19 (0.95–1.49) | 1.44 (1.20–1.75)* | 288 | 0.60 | 2.07 (1.60–2.68)* | 1.78 (1.45–2.19)* |
| Other | 291 | 0.11 | 1.50 (1.20–1.86)* | 1.20 (0.98–1.47) | 369 | 0.38 | 1.32 (1.05–1.66)† | 1.32 (1.03–1.68)‡ |
| Admission type | ||||||||
| Non-elective | … | … | … | … | 3366 | 0.26 | Reference | Reference |
| Elective | … | … | … | … | 2322 | 0.73 | 2.84 (2.41–3.34)* | 3.42 (2.89–4.07)* |
| Length of stay (days)§ | … | 20 (8–50) | 1.03 (1.03–1.03)* | 1.00 (1.00–1.00)‡ | - | 8 (5–19) | 1.02 (1.02–1.02)* | 1.00 (1.00–1.01)* |
| APR-DRG risk mortality subclass | ||||||||
| 1 | 192 | 0.01 | Reference | Reference | 363 | 0.06 | Reference | Reference |
| 2 | 504 | 0.63 | 125.65 (101.90–154.95)* | 107.96 (86.46–134.81)* | 642 | 010 | 5.51 (4.58–6.35)* | 4.62 (3.77–5.64)* |
| 3 | 1606 | 4.12 | 855.01 (687.99–1062.56)* | 672.99 (528.73–856.61)* | 1825 | 0.68 | 27.58 (22.19–34.29)* | 22.14 (17.84–27.50)* |
| 4 | 1656 | 7.71 | 1660.64 (1196.45–2304.92)* | 1359.42 (950.92–1943.42)* | 2927 | 3.78 | 72.74 (57.08–92.70)* | 66.85 (52.29–85.46)* |
| APR-DRG risk severity subclass‖ | ||||||||
| 1 | 55 | 0.00 | Reference | … | 1261 | 0.10 | Reference | … |
| 2 | 181 | 0.02 | 12.15 (8.15–18.10)* | … | 1156 | 0.52 | 1.83 (1.51–2.23)* | … |
| 3 | 992 | 0.36 | 187.11 (133.80–261.66)* | … | 1788 | 2.57 | 12.31 (9.19–16.48)* | … |
| 4 | 2730 | 5.67 | 3023.94 (2101.60–4351.08)* | … | 1552 | 6.51 | 70.86 (50.43–99.57)* | … |
| Primary payer¶ | ||||||||
| Medicaid | 2114 | 0.12 | Reference | Reference | 2811 | 0.31 | Reference | Reference |
| Medicare | … | … | … | … | 24 | 0.40 | 1.27 (0.61–2.67) | 0.99 (0.47–2.09) |
| Private | 1512 | 0.08 | 0.71 (0.62–0.82)* | 1.12 (0.96–1.30) | 2230 | 0.35 | 1.13 (0.98–1.29) | 1.22 (1.05–1.42)‡ |
| Self | 103 | 0.06 | 0.49 (0.36–0.66)* | 0.79 (0.56–1.10) | 126 | 0.33 | 1.05 (0.80–1.39) | 1.23 (0.92–1.63) |
| No charge/other | 269 | 0.23 | 2.01 (1.24–3.25)† | 1.83 (1.28–2.61)† | 558 | 0.85 | 2.72 (1.57–4.71)* | 2.10 (1.39–3.17)† |
| In-hospital mortality‖ | ||||||||
| No | 2928 | 0.07 | Reference | - | 5052 | 0.31 | Reference | … |
| Yes | 1077 | 7.48 | 109.03 (96.48–123.21)* | - | 705 | 10.98 | 39.62 (33.31–47.12)* | … |
| Hospital control‖ | ||||||||
| Government, nonfederal | 445 | 0.09 | Reference | … | 627 | 0.31 | Reference | … |
| Private, nonprofit | 3130 | 0.11 | 1.19 (0.81–1.74) | … | 4846 | 0.38 | 1.23 (0.79–1.90) | … |
| Private, investor-owned | 432 | 0.08 | 0.84 (0.47–1.51) | … | 284 | 0.17 | 0.53 (0.19–1.51) | … |
| Hospital location/teaching¶ | ||||||||
| Urban teaching | 3649 | 0.14 | Reference | Reference | 5598 | 0.40 | Reference | Reference |
| Urban nonteaching | 345 | 0.04 | 0.26 (0.17–0.40)* | 0.69 (0.41–1.14) | 143 | 0.08 | 0.20 (0.12–0.34)* | 0.39 (0.22–0.70)† |
| Rural | … | … | … | … | 16 | 0.02 | 0.05 (0.03–0.10)* | 0.15 (0.08–0.30)* |
All data are weighted using survey weights provided by The Healthcare Cost and Utilization Project (HCUP). Plasma transfusion defined as at least one plasma transfusion procedure, identified using International Classification of Diseases, 10th Revision codes: 30233R1, 30243R1, 30253R1, and/or 30263R1. Data may not sum to 100% due to missingness. Bold type refers to any p <0.05.
p < 0.001.
p < 0.010.
p < 0.05.
Data are median and corresponding interquartile range (IQR) for admissions with at least one plasma transfusion.
Excluded from multivariable model due to high collinearity.
Neonates: Medicare primary payer status and rural hospital data are not reported due to low sample size (<10) in accordance with HCUPʼs privacy policy and reporting guidelines. Nonneonates: Medicare primary payer status, no charge/other primary payer status, urban nonteaching and rural hospital data estimates have a coefficient of variation above the threshold of 30%.
APR-DRG = all patient refined diagnosis-related group.
TABLE 4.
Factors associated with one or more platelet transfusion during inpatient hospitalizations in the 2016 Kids’ Inpatient Database (KID) neonate
| Neonates |
Nonneonates |
|||||||
|---|---|---|---|---|---|---|---|---|
| No. transfused (n = 5541) | % transfused (0.14) | OR (95% CI) | aOR (95% CI) | No. transfused (n = 13,843) | % transfused (0.85) | OR (95% CI) | aOR (95% CI) | |
| Sex | ||||||||
| Male | 3,215 | 0.16 | Reference | Reference | 7,498 | 0.91 | Reference | Reference |
| Female | 2,321 | 0.12 | 0.76 (0.72–0.81)* | 0.91 (0.85–0.97)† | 6,330 | 0.78 | 0.86 (0.82–0.91)‡ | 0.97 (0.92–1.03) |
| Age group | ||||||||
| Infant (29 d to <1 y) | … | … | … | … | 1,844 | 0.65 | Reference | Reference |
| Toddler (1–2 y) | … | … | … | … | 925 | 0.68 | 1.04 (0.89–1.22) | 1.28 (1.09–1.51)† |
| Early childhood age (2–5 y) | … | … | … | … | 3,716 | 1.21 | 1.86 (1.61–2.16)* | 2.10 (1.78–2.47)* |
| Middle childhood age (6–11 y) | … | … | … | … | 3,513 | 1.11 | 1.70 (1.47–1.96)* | 1.98 (1.68–2.34)* |
| Adolescent (12–18 y) | … | … | … | … | 3,844 | 0.65 | 1.00 (0.86–1.16) | 1.40 (1.18–1.66)* |
| Race/Ethnicity | ||||||||
| White | 1,905 | 0.10 | Reference | Reference | 5,615 | 0.77 | Reference | Reference |
| African American | 1,207 | 0.23 | 2.26 (1.98–2.58)* | 1.25 (1.05–1.49)‡ | 1,602 | 0.58 | 0.76 (0.66–0.89)† | 0.88 (0.75–1.03) |
| Hispanic | 1,068 | 0.15 | 1.44 (1.22–1.70)* | 1.28 (1.10–1.49)† | 3,390 | 0.98 | 1.28 (1.05–1.55)‡ | 1.40 (1.17–1.67)* |
| Asian or Pacific Islander | 231 | 0.11 | 1.03 (0.80–1.33) | 1.21 (0.99–1.47) | 673 | 1.38 | 1.82 (1.40–2.36)* | 1.52 (1.22–1.90)* |
| Other | 393 | 0.15 | 1.44 (1.16–1.78)* | 1.15 (0.94–1.39) | 889 | 0.92 | 1.19 (0.92–1.54) | 1.19 (0.93–1.52) |
| Admission type | ||||||||
| Nonelective | … | … | … | … | 9,312 | 0.71 | Reference | Reference |
| Elective | 4,463 | 1.39 | 1.97 (1.66–2.34)* | 1.74 (1.46–2.07)* | ||||
| Length of stay (days)§ | … | 27 (11–68) | 1.03 (1.03–1.03)* | 1.01 (1.00–1.01)‡ | 9 (4–22) | 1.02 (1.02–1.03)* | 1.01 (1.01–1.02)* | |
| APR-DRG risk mortality subclass- | ||||||||
| 1 | 630 | 0.02 | Reference | Reference | 3,209 | 0.24 | Reference | Reference |
| 2 | 907 | 1.13 | 69.39 (61.36–78.46)* | 57.91 (50.68–66.18)* | 5,701 | 2.59 | 10.90 (9.96–11.93)* | 8.92 (7.98–9.96)* |
| 3 | 2,326 | 5.97 | 385.71 (338.23–439.85)* | 261.43 (224.55–304.37)* | 3,287 | 4.73 | 20.36 (18.43–22.48)* | 15.42 (13.82–17.21)* |
| 4 | 1,591 | 7.41 | 485.73 (368.89–639.57)* | 341.89 (252.65–462.65)* | 1,646 | 6.90 | 30.42 (26.38–35.08)* | 21.16 (17.88–25.04)* |
| APR-DRG risk severity subclass‖ | ||||||||
| 1 | 199 | 0.01 | Reference | … | 644 | 0.10 | Reference | … |
| 2 | 629 | 0.08 | 11.57 (9.54–14.04)* | … | 3,074 | 0.49 | 4.97 (4.20–5.87)* | … |
| 3 | 1,536 | 0.55 | 79.71 (65.34–97.23)* | … | 5,553 | 2.06 | 21.43 (18.08–25.39)* | … |
| 4 | 3,089 | 6.19 | 946.92 (754.75–1188.03)* | … | 4,573 | 5.91 | 63.88 (53.36–76.46)* | … |
| Primary payer | ||||||||
| Medicaid | 3,083 | 0.17 | Reference | Reference | 6232 | 0.70 | Reference | Reference |
| Medicare | … | … | … | … | 31 | 0.52 | 0.74 (0.39–1.43) | 0.54 (0.31–0.96)‡ |
| Private | 2,081 | 0.11 | 0.67 (0.61–0.74)* | 1.06 (0.95–1.19) | 6,031 | 0.96 | 1.38 (1.24–1.53)* | 1.41 (1.26–1.57)* |
| Self | 100 | 0.06 | 0.33 (0.24–0.44)* | 0.61 (0.45–0.84)† | 400 | 1.05 | 1.51 (0.90–2.53) | 1.50 (0.90–2.49) |
| No charge/other | 269 | 0.23 | 1.37 (0.94–2.00) | 1.24 (0.93–1.65) | 1,135 | 1.73 | 2.51 (1.59–3.95)* | 1.93 (1.41–2.64)* |
| In-hospital mortality‖ | ||||||||
| No | 4,438 | 0.11 | Reference | … | 13,200 | 0.81 | Reference | … |
| Yes | 1,097 | 7.62 | 73.36 (65.58–82.07)* | … | 642 | 10.01 | 13.60 (11.62–15.93)* | … |
| Hospital control‖ | ||||||||
| Government, nonfederal | 595 | 0.12 | Reference | … | 1,141 | 0.57 | Reference | … |
| Private, nonprofit | 4,482 | 0.15 | 1.27 (0.92–1.75) | … | 12,002 | 0.95 | 1.67 (1.09–2.56)‡ | … |
| Private, investor-owned | 463 | 0.08 | 0.67 (0.42–1.10) | … | 699 | 0.41 | 0.72 (0.29–1.79)‡ | … |
| Hospital location/teaching | ||||||||
| Urban teaching | 5,057 | 0.19 | Reference | Reference | 13,508 | 0.97 | Reference | Reference |
| Urban nonteaching | 460 | 0.05 | 0.25 (0.19–0.33)* | 0.64 (0.46–0.88)† | 319 | 0.18 | 0.18 (0.10–0.35)* | 0.35 (0.18–0.68)† |
| Rural | 23 | 0.01 | 0.03 (0.01–0.07)* | 0.12 (0.04–0.33)* | 16 | 0.02 | 0.02 (0.01–0.05)* | 0.05 (0.02–0.11)* |
All data are weighted using survey weights provided by The Healthcare Cost and Utilization Project (HCUP). Platelet transfusion defined as at least one platelet transfusion procedure, identified using International Classification of Diseases, 10th Revision codes: 30233 J1, 30233 K1, 30233 L1, 30233 M1, 30243 J1, 30243 K1, 30243 L1, and/or 30243 M1. Data may not sum to 100% due to missingness. Bold type refers to any p <0.05.
p < 0.001.
p < 0.010.
p < 0.05.
Data are median and corresponding interquartile range (IQR) for admissions with one or more platelet transfusions.
Excluded from multivariable model due to high collinearity.
Neonates: Medicare data are not reported due to low sample size (<10) in accordance with HCUPʼs privacy policy and reporting guidelines. No charge/other primary payer status and rural hospital estimates have a coefficient of variation above the threshold of 30%. Nonneonates: Urban nonteaching and rural hospital estimates have a coefficient of variation above the threshold of 30.
TABLE 5.
Sensitivity analysis showing factors associated with one or more RBC transfusions during inpatient hospitalizations in the 2016 Kids’ Inpatient Database (KID) for neonates (age ≤28 d) complicated births where the length of hospitalization stay was more than 3 days
| No. transfused (n = 10,387) | % transfused (2.71) | OR (95% CI) | p value | aOR (95% CI) | p value | |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 5,531 | 2.63 | Reference | … | Reference | … |
| Female | 4,854 | 2.80 | 1.07 (1.02–1.12) | 0.005 | 1.05 (1.00–1.11) | 0.062 |
| Race/Ethnicity | ||||||
| White | 3,288 | 1.94 | Reference | - | Reference | - |
| African American | 2,946 | 4.54 | 2.40 (2.15–2.68) | <0.001 | 1.88 (1.64–2.16) | <0.001 |
| Hispanic | 2,077 | 3.25 | 1.70 (1.47–1.96) | <0.001 | 1.51 (1.30–1.79) | <0.001 |
| Asian or Pacific Islander | 512 | 2.62 | 1.36 (1.09–1.69) | 0.006 | 1.44 (1.21–1.70) | <0.001 |
| Other | 723 | 2.62 | 1.26 (1.12–1.64) | 0.001 | 1.22 (1.00–1.49) | 0.058 |
| Length of stay (d)* | 1.04 (1.03–1.04) | <0.001 | 1.02 (1.02–1.02) | <0.001 | ||
| APR-DRG risk mortality subclass | ||||||
| 1 | 2,000 | 0.64 | Reference | … | Reference | … |
| 2 | 2,688 | 6.36 | 10.62 (9.76–11.55) | <0.001 | 6.89 (6.24–7.60) | <0.001 |
| 3 | 4,088 | 20.74 | 40.91 (36.68–45.61) | <0.001 | 16.00 (13.85–18.47) | <0.001 |
| 4 | 1,413 | 25.28 | 52.88 (45.25–61.80) | <0.001 | 18.06 (14.76–22.09) | <0.001 |
| APR-DRG risk severity subclass† | ||||||
| 1 | 160 | 0.14 | Reference | … | … | … |
| 2 | 1,216 | 0.89 | 6.31 (5.19–7.67) | <0.001 | … | … |
| 3 | 3,748 | 3.35 | 24.40 (20.15–29.55) | <0.001 | … | … |
| 4 | 5,065 | 24.65 | 230.50 (188.14–282.39) | <0.001 | … | … |
| Primary payer‡ | ||||||
| Medicaid | 27 | 2.47 | Reference | … | Reference | … |
| Medicare | 6,060 | 3.03 | 0.81 (0.47–1.40) | 0.447 | 1.27 (0.62–2.59) | 0.516 |
| Private | 3,766 | 2.37 | 0.78 (0.71–0.85) | <0.001 | 1.05 (0.95–1.17) | 0.320 |
| Self | 144 | 1.26 | 0.41 (0.32–0.53) | <0.001 | 0.76 (0.57–1.01) | 0.061 |
| No charge/other | 383 | 3.15 | 1.04 (0.80–1.35) | 0.775 | 0.91 (0.67–1.23) | 0.523 |
| In-hospital mortality† | ||||||
| No | 9,493 | 2.49 | Reference | … | … | … |
| Yes | 885 | 32.48 | 18.80 (16.41–21.55) | <0.001 | … | … |
| Hospital control† | ||||||
| Government, nonfederal | 1,740 | 3.42 | Reference | … | … | … |
| Private, nonprofit | 7,544 | 2.69 | 0.78 (0.60–1.01) | 0.062 | … | … |
| Private, investor-owned | 1,103 | 2.11 | 0.61 (0.42–0.88) | 0.009 | … | … |
| Hospital location/teaching‡ | ||||||
| Urban teaching | 9,054 | 3.07 | Reference | … | Reference | … |
| Urban nonteaching | 1,265 | 1.73 | 0.55 (0.44–0.70) | <0.001 | 0.99 (0.76–1.29) | 0.921 |
| Rural | 68 | 0.43 | 0.14 (0.06–0.29) | <0.001 | 0.41 (0.21–0.79) | 0.008 |
All data are weighted using survey weights provided by The Healthcare Cost and Utilization Project (HCUP). RBC transfusion defined as at least one RBC transfusion procedure, identified using International Classification of Diseases, 10th Revision codes: 30233 N1, 30233P1, 30243 N1, 30243P1, 30253 N1, 30263 N1, and/or 30623P1. Bold type refers to any p <0.05.
Data may not sum to 100% due to missingness.
Data are median and corresponding interquartile range (IQR) for admissions with one or more RBC transfusion.
Excluded from multivariable model due to high collinearity.
Medicare primary payer status and rural hospital data estimates have a coefficient of variation above the threshold of 30%.
Among NEO admissions, RBC transfusion was more common among nonwhite races compared to white race (African Americans vs. whites (aOR, 1.67; 95% CI, 1.46–1.91), Hispanics versus whites (aOR,1.42; 95% CI, 1.22–1.64), and Asian or Pacific Islanders versus whites (aOR, 1.36; 95% CI, 1.16–1.58) (Table 2). Besides these racial differences, RBC transfusion was more common among higher APR-DRG risk mortality subclasses (aOR, 86.58; 95% CI, 64.77–115.73) for APR-DRG risk mortality Subclass 4 versus Subclass 1 and those admitted to urban teaching hospitals. Median hospital length of stay was higher among those who received a blood transfusion as compared to those who did not for both NEO (51 days, IQR, 18–85 vs. 2 days, IQR, 2–3) and PED (6 days, IQR, 3–13 vs. 2 days, IQR 1–4; p < 0.001; Table 2). Urban teaching hospitals had the highest prevalence of RBC transfusions (2.96%) among PED, as compared to 0.93% in urban nonteaching hospitals and 0.57% in rural hospitals. The aOR of RBC among NEO use in urban nonteaching hospitals as compared to urban teaching hospitals was 0.72 (95% CI, 0.55–0.94) and rural hospitals as compared to urban teaching was 0.22 (95% CI, 0.12–0.43).
Among the PED admissions, many of the associations were similar to those observed in NEO admissions (Table 2). Specifically, the racial association with RBC transfusion showed similar trends with higher prevalence of a transfusion in African Americans versus whites (aOR, 1.81; 95% CI, 1.61–2.04); Hispanics versus whites (aOR, 1.38; 95% CI, 1.20–1.58) and Asian or Pacific Islanders versus whites (aOR, 1.66; 95% CI, 1.40–1.99). In addition, elective admissions were associated with a higher odds of RBC transfusion (aOR, 1.62; 95% CI, 1.46–1.80). Among PED age groups, compared to infants as reference age, admissions among early childhood ages (aOR, 1.38; 95% CI, 1.27–1.49), middle childhood ages (adjOR, 1.35; 95% CI, 1.23–1.49), and adolescents (aOR, 1.25; 95% CI, 1.14–1.38) had higher odds of RBC transfusion. Unlike NEO admissions, female sex was associated with a higher odds of RBC transfusion among PED admissions (aOR, 1.10; 95% CI, 1.07–1.13). PED admissions that had Medicaid as the primary insurance payer had the lowest prevalence of RBC transfusions (2.46%); those admissions that were paid by another mechanism including other insurance groups, self-pay, and no-charge patients had significantly higher odds of RBC transfusion (Table 2).
For both NEO and PED admissions, the factors associated with plasma transfusions were similar to those associated with RBC transfusion (Table 3). However, among NEO, female sex was associated with lower odds of plasma transfusion (aOR, 0.87; 95% CI, 0.80–0.95). In contrast to RBC transfusions, all older age groups were associated with lower odds of plasma transfusion as compared to infants, with admissions among toddlers having the lowest prevalence of plasma transfusions (aOR, 0.57; 95% CI, 0.46–0.71).
Again, the factors associated with platelet transfusions for neonates and children were similar to those associated with RBC transfusions for both NEO and PED admissions (Table 4). Unlike RBC transfusions, female sex was independently associated with lower odds of platelet transfusions (aOR, 0.91; 95% CI, 0.85–0.97). For PED admissions, increasing age, increasing APR-DRG risk mortality subclass, longer length of stay, elective admission versus nonelective, and hospitalization at an urban teaching hospital versus urban nonteaching and rural hospitals were all associated with a higher odds of platelet transfusion. However, female sex (aOR, 0.97; 95% CI, 0.92–1.03) was not associated with platelet transfusion.
In a subgroup analyses restricted to hospitalizations at freestanding childrenʼs hospitals, the overall prevalence of RBC transfusion was 4.29% (95% CI, 3.46%−5.30%) and 0.73% for plasma (95% CI, 0.55%−0.96%) and 1.67% for platelet transfusions (95% CI, 1.33%−2.09%) (Fig. 1) The largest proportion of hospitalizations at freestanding childrenʼs hospitals was among adolescents (25.26%) and those paid primarily with Medicaid (51.78%). None of the freestanding childrenʼs hospitals were under nonfederal government control or were located in rural locations. There were no rural teaching hospitals. The overall prevalence of RBC transfusion in nonfreestanding childrenʼs hospitals was 0.69% (95% CI, 0.61%−0.78%), 0.11% for plasma transfusions (95% CI, 0.09%−0.13%), and 0.19% for platelet transfusions (95% CI, 0.17%−0.22%) (Fig. 1).
Table S2, available as supporting information in the online version of this paper, shows the study population characteristics of inpatient hospitalizations at freestanding childrenʼs hospitals in the 2016 KID. Among NEO admissions to freestanding childrenʼs hospitals, African American race, length of stay, and increasing APR-DRG risk mortality subclass were associated with increased odds of RBC transfusion (Table S3, available as supporting information in the online version of this paper). Among PED admissions analyzed by different age groups, toddlers had higher odds of RBC transfusion as compared with infants (aOR, 1.25; 95% CI, 1.11–1.40; Table S4, available as supporting information in the online version of this paper). African American, Hispanic, and Asian or Pacific Islander race/ethnicity as compared to white race; elective admission; increased length of stay; increased APR-DRG risk mortality subclass; and hospitalizations paid primarily by self-pay or no charge/other payment as compared to hospitalizations with Medicaid as primary payer were also associated with higher odds of RBC transfusion. Hospitalizations at urban teaching hospitals were not independently associated with higher odds of RBC transfusion at freestanding childrenʼs hospitals (aOR, 0.28; 95% CI, 0.08–1.01). In nonfreestanding childrenʼs hospital, among all ages, being nonwhite, APR-DRG risk mortality status, length of stay, were associated with higher odds of receiving an RBC, while being at a rural hospital was associated with lower odds of receiving an RBC. Among PEDs, elective admission was also significantly associated with higher odds of receiving an RBC.
DISCUSSION
Multiple clinical practice guidelines for RBC, plasma, and platelet transfusion practices in adults have been published over the past decade.15–20 While lagging behind adult recommendations, there are several recently published pediatric transfusion guidelines and consensus recommendations including those from the British Society of Hematology, the Australian Society of Blood Transfusion, and the Transfusion and Anemia Expertise.21–23 In addition, transfusion guidelines have been developed for perioperative patient blood management and injured children.24,25 However, most of these recommendations have focused on laboratory parameters and thresholds as determinants of transfusion decisions. However, despite these recommendations, there is a wide variability in transfusion practices.26 This suggests that there likely are other nonlaboratory determinants of transfusion decisions in neonates and older children. Understanding the epidemiology of current transfusion practices and the various patient- and hospital-level correlates of transfusion decisions is foundational to understanding the immense heterogeneity in pediatric and neonatal transfusion practices and aim to standardize these critical interventions.3
This analysis of a nationally representative, pediatric-specific, inpatient database, represents over 5 million neonatal and nonneonatal hospitalizations, including approximately 500,000 hospitalizations in freestanding childrenʼs hospitals. The findings offer insight into the epidemiology of in-hospital pediatric and neonatal transfusion practices, including various patient and hospital-level correlates of RBC, plasma, and platelet transfusions in hospitalized neonatal and pediatric patients.
NEO RBC transfusions were associated with nonwhite race (higher prevalence of transfusions in African-Americans, Hispanics, and Asian and Pacific Islanders as compared to whites), longer length of stay, higher APR-DRG risk mortality subclass, and admission to an urban teaching hospital (rather than an urban nonteaching or rural hospital). Among PED admissions, RBC transfusions were associated with female sex, older childhood and adolescent age groups, elective admission type, length of stay, higher APR-DRG risk mortality subclass, and hospitalization at an urban teaching hospital. Pertinent to insurance status, primary payment by Medicaid was associated with lower rates of blood transfusion than private insurance, self-pay, or other payment types. While insurance status has been shown to be an important socioeconomic predictor of access to health care, it must be noted that this analysis does not account for the secondary insurance payer mix, as it was not available for 201627; thus, this study may not accurately capture the effect of the insurance status of transfusion decisions.13
While pediatric transfusion medicine is not just about transfusing “little adults” or administering “little volumes,” many of the factors associated with RBC transfusions (e.g., female sex, minority race/ethic status, severity of illness, increased length of stay, etc.) are similar to those of adults.6 Unlike adults, however, RBC transfusion in children and neonates were more common in urban teaching hospitals. Multiple prior small or single-center studies in children have highlighted significant variation in clinical transfusion practices and hemoglobin transfusion thresholds.26
Hemostatic blood products, such as plasma and platelets, are frequently transfused to children more commonly as prophylactic transfusions to prevent bleeding as well as less commonly as therapeutic interventions to control bleeding.28,29 The factors associated with plasma transfusions and platelet transfusions were similar to those in both NEOs and PEDs. Notably, however, African American race was not associated with platelet transfusions among NEOs; and older age groups were associated with lower odds of plasma transfusion among PED admissions as compared to infants.
This study also highlights the prevalence of any blood transfusion during a hospitalization in freestanding childrenʼs hospitals; while the overall RBC transfusion prevalence across all hospitalizations in children was approximately 1.1%, RBC transfusions at freestanding childrenʼs hospitals alone were approximately fourfold higher than hospitalizations in other hospital types combined. While freestanding childrenʼs hospitalizations had about 10% of all the burden of total number of PED and NEO hospitalizations nationally, they accounted for about 40% of all hospitalizations with transfusions and the overall utilization of RBC transfusions. This likely reflects the greater acuity of cases that are treated in freestanding childrenʼs hospitals including those in pediatric and neonatal intensive care units and Level 1 trauma centers, as well as more complex surgical procedures being performed at these centers. Similar findings have been reported previously by Slonim et al.30 using data from pediatric patients who were hospitalized at 35 academic childrenʼs hospitals that are members of the Pediatric Health Information System. Other than AABB data by Sapiano et al.11 reporting the number of children receiving plasma and platelet concentrates over a single year, few studies have examined the epidemiology of plasma and platelet transfusions in pediatric patients as a whole. Many subgroups have been described. For example, 3.4% of critically ill children receive a plasma transfusion, and 3.3% receive a platelet transfusion during their admission.28,29
Several studies have reported data on demographic and epidemiologic characterization of transfusion recipients. These have largely been derived from population-based studies or lookback investigations in adults. Few studies to date have evaluated children.22,31 The Recipient Epidemiology and Donor Evaluation Study–III program, which evaluated RBC, platelet, and plasma transfusions in 12 hospitals focusing on the laboratory parameters, sociodemographic data, and transfusion reactions, included a small number of pediatric and neonatal transfusions.7,32 The National Institutes of Health recognizes this large gap in research and recently funded the Recipient Epidemiology and Donor Evaluation Study–IV-Pediatrics to improve transfusion safety in infants and children. This paper should begin to assist in the understanding of transfusions and their occurence in neonates and children at the national level and offer insights into differences in transfusion practice in freestanding childrenʼs hospitals versus other hospitals.
This study has several limitations. Given the cross-sectional nature of this analysis, all reported associations should be descriptively interpreted. The unit of analysis is a single hospitalization and not a specific patient; therefore, a single patient may be represented in multiple observations. The likelihood of a patient being readmitted cannot be predicted. Thus, it was not possible to determine if any transfusions recurred in the same patient. Due to missing data, estimates for race and its prediction on blood component transfusions may be biased. Additionally, given the available case approach, multivariable models may be biased due to missing data. Laboratory and medical record data including the indication for transfusion and the number of units transfused were not available. ICD-10-CM coding was introduced widely in the fourth quarter of 2015, meaning its use was relatively new at the time of these data and the codes used in this analysis have not yet been validated against ICD-9-CM codes or blood bank transfusion records. Finally, this analysis was limited to inpatient transfusions, which may not be broadly generalizable to all pediatric patients, particularly those in an outpatient setting.
Nationally representative data evaluating factors associated with transfusions in the neonatal and pediatric inpatient population are limited, but this study shows substantial heterogeneity in neonatal and transfusion practices. While the decision to transfuse is thought to be based on temporally proximal laboratory data, this study suggests that there are also key nonlaboratory correlates of transfusion in children and neonates.
Supplementary Material
Acknowledgments
This work was supported in part by the National Institutes of Health (R01AI120938 and R01AI128779 to A.A.R.T., T32AI102623 to E.U.P., and K23HL151826 to E.M.B.).
ABBREVIATIONS:
- aOR
adjusted odds ratio
- APR-DRG
all patient refined diagnosis-related group
- DRG
diagnosis-related group
- HCUP
Healthcare Cost and Utilization Project
- ICD-10-CM
International Classification of Diseases, 10th Revision, Clinical Modification
- KID
Kids’ Inpatient Database
- NEO
neonate
- PED
nonneonate
Footnotes
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
Appendix S1. Supporting Information.
REFERENCES
- 1.Jacquot C, Mo YD, Luban NLC. New approaches and trials in pediatric transfusion medicine. Hematol Oncol Clin North Am 2019;33:507–20. [DOI] [PubMed] [Google Scholar]
- 2.Cure P, Bembea M, Chou S, et al. 2016 proceedings of the National Heart, Lung, and Blood Instituteʼs scientific priorities in pediatric transfusion medicine. Transfusion 2017;57:1568–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Josephson CD, Luban NL. Pediatric and neonatal transfusion medicine: a roadmap for research. Transfus Med Rev 2016;30: 157–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nellis ME, Goel R, Karam O. Transfusion management in pediatric oncology patients. Hematol Oncol Clin North Am 2019;33: 903–13. [DOI] [PubMed] [Google Scholar]
- 5.Roubinian NH, Murphy EL, Swain BE, et al. Predicting red blood cell transfusion in hospitalized patients: role of hemoglobin level, comorbidities, and illness severity. BMC Health Serv Res 2014;14:213–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goel R, Patel EU, White JL, et al. Factors associated with red blood cell, platelet, and plasma transfusions among inpatient hospitalizations: a nationally representative study in the United States. Transfusion 2019;59:500–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karafin MS, Bruhn R, Westlake M, et al. Demographic and epidemiologic characterization of transfusion recipients from four US regions: evidence from the REDS-III recipient database. Transfusion 2017;57:2903–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitaker B, Rajbhandary S, Kleinman S, et al. Trends in United States blood collection and transfusion: results from the 2013 AABB Blood Collection, Utilization, and Patient Blood Management Survey. Transfusion 2016;56:2173–83. [DOI] [PubMed] [Google Scholar]
- 9.Chung KW, Basavaraju SV, Mu Y, et al. Declining blood collection and utilization in the United States. Transfusion 2016;56: 2184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellingson KD, Sapiano MRP, Haass KA, et al. Continued decline in blood collection and transfusion in the United States-2015. Transfusion 2017;57(Suppl 2):1588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sapiano MRP, Savinkina AA, Ellingson KD, et al. Supplemental findings from the National Blood Collection and Utilization Surveys, 2013 and 2015. Transfusion 2017;57(Suppl 2):1599–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goel R, Chappidi MR, Patel EU, et al. Trends in red blood cell, plasma, and platelet transfusions in the United States, 1993–2014. JAMA 2018;319:825–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Healthcare Cost and Utilization Project (HCUP). Overview of the kidsʼ inpatient database (KID). 2009. [cited 2019 Nov 15]. Available from https://www.hcup-us.ahrq.gov/kidoverview.jsp.
- 14.Leyenaar JK, Ralston SL, Shieh MS, et al. Epidemiology of pediatric hospitalizations at general hospitals and freestanding childrenʼs hospitals in the United States. J Hosp Med 2016;11:743–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qaseem A, Humphrey LL, Fitterman N, et al. Treatment of anemia in patients with heart disease: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2013;159: 770–9. [DOI] [PubMed] [Google Scholar]
- 16.Roback JD, Caldwell S, Carson J, et al. Evidence-based practice guidelines for plasma transfusion. Transfusion 2010;50:1227–39. [DOI] [PubMed] [Google Scholar]
- 17.Carson JL, Guyatt GH, Heddle NM, et al. Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA 2016;316:2025–35. [DOI] [PubMed] [Google Scholar]
- 18.Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2015;162:205–13. [DOI] [PubMed] [Google Scholar]
- 19.Retter A, Wyncoll D, Pearse R, et al. Guidelines on the management of anaemia and red cell transfusion in adult critically ill patients. Br J Haematol 2013;160:445–64. [DOI] [PubMed] [Google Scholar]
- 20.Estcourt LJ, Birchall J, Allard S, et al. Guidelines for the use of platelet transfusions. Br J Haematol 2017;176:365–94. [DOI] [PubMed] [Google Scholar]
- 21.New HV, Berryman J, Bolton-Maggs PH, et al. Guidelines on transfusion for fetuses, neonates and older children. Br J Haematol 2016;175:784–828. [DOI] [PubMed] [Google Scholar]
- 22.Gauvin F, Champagne MA, Robillard P, et al. Long-term survival rate of pediatric patients after blood transfusion. Transfusion 2008;48:801–8. [DOI] [PubMed] [Google Scholar]
- 23.Valentine SL, Bembea MM, Muszynski JA, et al. Consensus recommendations for RBC transfusion practice in critically ill children from the pediatric critical care transfusion and anemia expertise initiative. Pediatr Crit Care Med 2018;19:884–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trappey AF 3rd, Thompson KM, Kuppermann N, et al. Development of transfusion guidelines for injured children using a modified delphi consensus process. J Trauma Acute Care Surg 2019;87:935–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faraoni D, Meier J, New HV, et al. Patient blood management for neonates and children undergoing cardiac surgery: 2019 NATA guidelines. J Cardiothorac Vasc Anesth 2019;33: 3249–63. [DOI] [PubMed] [Google Scholar]
- 26.Klaus SA, Frank SM, Salazar JH, et al. Hemoglobin thresholds for transfusion in pediatric patients at a large academic health center. Transfusion 2015;55:2890–7. [DOI] [PubMed] [Google Scholar]
- 27.Healthcare Cost and Utilization Project (HCUP). KID description of data elements. 2019. https://www.hcup-us.ahrq.gov/db/nation/kid/kiddde.jsp. Accessed Sept 1, 2019.
- 28.Nellis ME, Karam O, Mauer E, et al. Platelet transfusion practices in critically ill children. Crit Care Med 2018;46:1309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karam O, Demaret P, Shefler A, et al. Indications and effects of plasma transfusions in critically ill children. Am J Respir Crit Care Med 2015;191:1395–402. [DOI] [PubMed] [Google Scholar]
- 30.Slonim AD, Joseph JG, Turenne WM, et al. Blood transfusions in children: a multi-institutional analysis of practices and complications. Transfusion 2008;48:73–80. [DOI] [PubMed] [Google Scholar]
- 31.Vamvakas EC. Long-term survival rate of pediatric patients after blood transfusion. Transfusion 2008;48:2478–80. [DOI] [PubMed] [Google Scholar]
- 32.Triulzi D, Gottschall J, Murphy E, et al. A multicenter study of plasma use in the United States. Transfusion 2015;55:1313–9 quiz 1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


