Abstract
In searching for Parkinson’s disease (PD) pharmacotherapies we began studying FTY720, a food and drug administration (FDA) approved drug. We also created derivatives, FTY720-C2 and FTY720-Mitoxy, and began assessing them. Here we treated dopaminergic MN9D cells with FTY720s then measured microRNA (miRNA) levels by PCR arrays. We discovered that all three FTY720s increased miR376b-3p, while FTY720-C2 also increased miR-128–3p, miR-146b-5p, miR-7a-5p, and miR-9–5p, and FTY720-Mitoxy also increased miR-30d-5p. Investigations revealed that some miRNAs downregulate alpha-synuclein, while others reduce apoptosis, suggesting that FTY720s may act to reduce synucleinopathy and dopaminergic neuron loss in PD and related disorders.
Keywords: Neuroprotection, Dopaminergic MN9D cells, miRNA upregulation
GRAPHICAL ABSTRACT
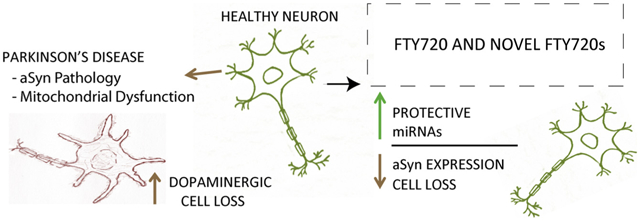
Parkinson’s disease (PD) is a neurodegenerative disorder with neither a cure nor an approved treatment that will slow or stop disease progression. PD motor symptoms arise after extensive loss of substantia nigra pars compacta dopaminergic neurons. In searching for novel PD therapeutics, our laboratory identified FTY720 (Gilenya, Fingolimod), an FDA-approved multiple sclerosis drug with neuroprotective effects [1]. We also synthesized FTY720-derivatives, FTY720-C2 and FTY720-Mitoxy, and have shown that all three FTY720s: protect MN9D dopaminergic cells from TNF-α-mediated apoptosis, increase cellular metabolism, and increase neuroprotective brain derived neurotrophic factor (BDNF) at both the mRNA and protein level [2]. In preclinical mouse models, FTY720 reduces enteric nervous system Lewy-body-like aSyn aggregation, improves behavior, and diminishes dopaminergic neurotoxicity [1,3,4]. Importantly, all three FTY720s target the brain. We have also shown that FTY720-C2 and FTY720-Mitoxy are not phosphorylated and thus will not cause immunosuppression [5,6]. In addition, BDNF protein levels are increased in A53T mutant transgenic mice treated with FTY720, which is associated with a decrease in miR206–3p expression [1], a micro RNA (miRNA) that downregulates BDNF levels [1].
Endogenous miRNAs are 17–24 base-pair (bp) single-stranded non-coding RNAs. Regulation of gene expression by miRNAs has been reviewed [7] and shows that miRNAs are transcribed from the genome as primary miRNA (pri-miRNA), which has a hairpin structure where a mature miRNA is implanted. In the nucleus, pri-miRNA is cleaved by Drosha, an RNase III that generates precursor miRNA (pre-miRNA). Pre-miRNA is then exported to the cytosol where it is cleaved by Dicer, another RNase III that releases a ~22 base pair miRNA duplex, after which one of the duplex strands is the mature miRNA. Mature miRNA is then assembled into the miRNA Induced Silencing Complex (RISC) to target a complementary mRNA. Since miRNAs target mRNA transcripts to stimulate mRNA degradation or to repress translation of mRNA into protein, it can be said that an inverse relationship exists between mature miRNAs and the protein product of targeted mRNAs. In this study, we measured the effects of FTY720s on mature neuronal miRNA expression as detailed below.
MN9D dopaminergic cells of low-passage, were grown on TPP® plates (LPS, Inc., Rochester, NY, USA) in Dulbecco’s modified Eagle’s medium (DMEM; D5648; Sigma-Aldrich Co, St Louis, MO, USA) supplemented with 10% fetal bovine serum (HyClone, Logan, UT, USA) at 37 °C in 5% CO2. As determined in previous time-dependent and dose-dependent-response studies [2], we used the optimal low dose to treat MN9D cells, 160 nM FTY720, FTY720-C2, FTY720-Mitoxy or vehicle (ethanol) alone for 24 h. Our prior studies also confirmed that FTY720s used at this dose do not stimulate cell proliferation but do enhance metabolism [2]. Afterward, cells were harvested for miRNA extraction using the miRNeasy kit (Qiagen, Cat# 217004, Germantown, MD, USA) as per the manufacturer with the added on-column treatment of an RNase-free DNase kit (Qiagen, Cat# 79254). RNA concentration and quality were confirmed using Nanodrop 2000 (Thermo Scientific, Waltham, MA, USA) and a bleach gel [8], respectively. Once we confirmed RNA quality, reverse transcription was performed using miScript II RT kit (Qiagen, Cat# 218160) and the cDNA product was then used as the starting material for quantitative PCR (qPCR) using the commercially available miRNA PCR Array plate (Qiagen, Cat# MIMM-107Z) and the miScript SYBR Green PCR kit (Qiagen, Cat# 218075). The array plates were run using the Mastercycler Eppendorf Gradient S thermocycler (Eppendorf, Hauppauge, New York, USA). Data were analyzed with Qiagen web-based tools [9], using the following PCR controls SNORD68, SNORD72, SNORD95, SNORD96A, and RUN6–6P for normalization. An analysis of putative mRNA targets for miRNAs whose expression was significantly increased by FTY720s was made using online miRDB [10] and TargetScan [11] databases.
We analyzed the expression levels of 84 neuronal miRNAs in MN9D neuronal cells in response to FTY720s using qPCR. Data from three independent experiments confirmed that miR376b-3p levels were increased after treating cells with FTY720, FTY720-C2, or FTY720-Mitoxy for 24 h (Fig. 1). The fold changes in miR376b-3p were increased by 52, 42, and 53% above the vehicle group respectively. Regarding FTY720-C2, this FTY720-derivative significantly increased the levels of miR-128–3p, miR-146b-5p, miR-7a-5p, and miR-9–5p by 27, 33, 38, and 51% above vehicle respectively (Table 1). Furthermore, FTY720-Mitoxy also significantly increased miR-30d-5p by 14% (Table 1).
Fig. 1.
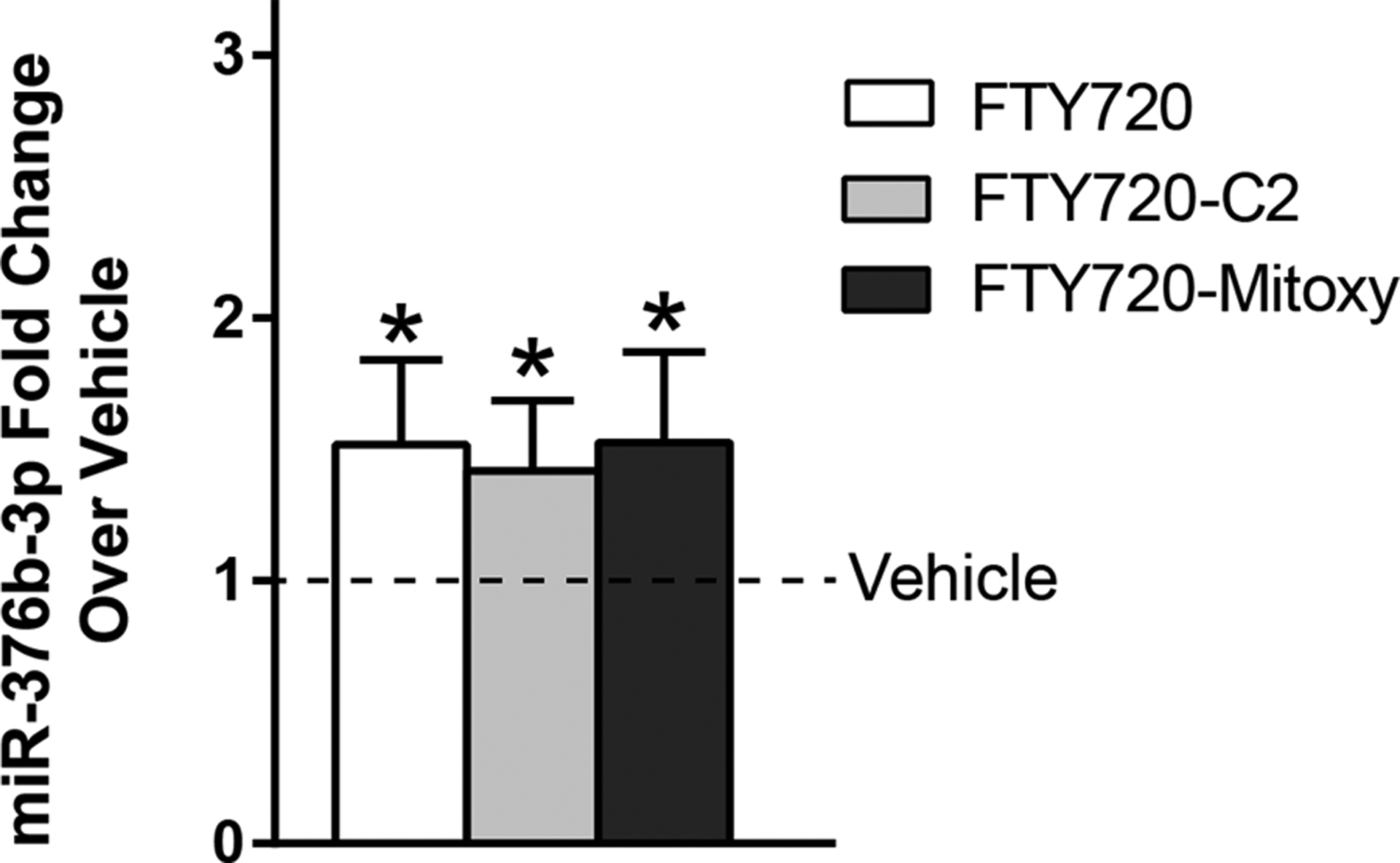
Effect of FTY720s on miR-376b-3p levels. Levels of miR-376b-3p are elevated in MN9D cells treated 24 h at 160 nM of all three FTY720s: FTY720, FTY720-C2, and FTY720-Mitoxy as compared to vehicle treated control cells. Data represent the mean fold change of 3 independent experiments ± 95% confidence intervals. Student’s t-tests, *p < 0.05.
Table 1.
Effects of FTY720s on additional neuronal miRNAs.
| Micro RNA | FTY720-C2 | FTY720-Mitoxy | FTY720 |
|---|---|---|---|
| miR-128–3p | 1.27 (1.06–1.47) * | 1.10 (0.92–1.27)ns | 1.08 (0.86–1.30)ns |
| miR-146b-5p | 1.31 (0.87–1.75) * | 1.07 (0.88–1.26)ns | 1.02 (0.87–1.16)ns |
| miR-30d-5p | 1.13 (1.00–1.26)ns | 1.14 (1.03–1.26) * | 1.04 (0.91–1.18)ns |
| miR-7a-5p | 1.38 (1.12–1.63) * | 1.05 (0.75–1.36)ns | 1.19 (0.79–1.58)ns |
| miR-9–5p | 1.51 (1.05–1.96) * | 1.26 (0.83–1.69)ns | 1.10 (0.70–1.49)ns |
Data represent the mean fold change of 3 independent experiments ± 95% confidence intervals. Student’s t-tests,
p < 0.05 are highlighted,
not significant.
In many diseases including PD, mitochondrial dysfunction plays a role in neurodegeneration. Identifying treatments that can improve mitochondrial function may thus identify beneficial new therapeutics. As mentioned above, miR-376b-3p was up-regulated by all three FTY720s and it is known that miR-376b-3p targets a protein called mitochondrial fission factor (MFF) in a manner to suppress its ability to induce mitochondria fission [12]. In response to stress, MFF recruits dynamin-related protein 1 (Drp1) then promotes excessive mitochondrial fission, which can induce apoptosis. Inhibition of Drp1 has been shown to be protective of dopaminergic neurons [13]. Our results suggest that all three FTY720s have the ability to suppress MFF/Drp1-dependent mitochondrial fission and should thus help protect cells from apoptotic death.
It was exciting to see that FTY720-C2 significantly increased the levels of four additional miRNAs, miR-128–3p, miR-146b-5p, miR-7a-5p, and miR-9–5p (Table 1). Recently, it was demonstrated that miR-128, acting through the neurotrophic receptor tyrosine kinase 3 (NTRK3), upregulates the protein B-cell lymphoma 2 (Bcl-2), an anti-apoptotic protein that reduces cell death [14]. Regarding miR-146b-5p, it directly attenuates interleukin (IL)-1b-induced IL-5 release in human astrocytes, an important mechanism in inflammation that can lead to apoptosis [15], suggesting that miR-146b-5p may help reduce neuroinflammation and neuronal cell death. Among the most important findings for FTY720-C2 with regard to PD is the significant increase in miR-7a-5p, which targets the PD-associated SNCA gene. Though mice express miR-7a and 7b, humans express only miR-7, and all three miR-7s target the same 8-mer sequence of SNCA. Binding of miR-7 to SNCA is neuroprotective as it serves to reduce alpha-synuclein (aSyn) protein levels, which then reduces the risk of aSyn PD pathology (synucleinopathy) [16]. Using in silico analyses we found that miR-7a-5p targets caspase 9, a pro-apoptotic protein, further suggesting that miR-7a-5p is an anti-apoptotic miRNA. Regarding the miR-9 family, it has received considerable attention based on its role in the regulation of neurogenesis [17]. Although the miR-9–5p literature remains somewhat limited, its demonstrated role in neurogenesis is promising. Finally, we discovered that FTY720-Mitoxy significantly increased miR-30d-5p, which prevents cerebral injury by inhibiting autophagy-mediated microglial effects that modulate the inflammatory response [18]. Our study thus shows important discoveries regarding protective miRNA increases that may help preserve dopaminergic function while reducing aSyn pathology in response to FTY720s, which may provide beneficial therapeutics for PD and related disorders. In future cell and mouse studies, we plan to assess if the identified miRNAs indeed regulate the proposed targets in a manner to enhance neuronal viability related to PD.
Acknowledgments
The authors are grateful to Texas Tech University Health Sciences Center El Paso Graduate School of Biomedical Sciences for support (NTG and ISU), Fogarty International Center - U.S./Costa Rica Neuropsychiatric Genetics Research Training Program (NCOD-5D43TW008333; ISU; RGP, Michael Ecamilla), Hoy Family Research, Perez Family Research, Lizanell and Colbert Coldwell Foundation, Ms. Anna Mae Doyle Gift Funds, the Multiple System Atrophy (MSA) Coalition, and the El Paso Community Foundation to (RGP). This work is dedicated to D. Byer and M. J. Fox, and in loving memory of L. “Rusty” Lanelli, S. M. Hoy, and J. Cordy.
Footnotes
Conflict of interest
The corresponding author has filed a patent, “Compositions and Methods for the Treatment of Parkinson’s Disease”, US 20150290145, CA 2888634, which does not alter adherence to Neuroscience Letters policies.
References
- [1].Vidal-Martinez G, Vargas-Medrano J, Gil-Tommee C, et al. , FTY720/fingolimod reduces synucleinopathy and improves gut motility in A53T mice: contributions of pro-brain-derived neurotrophic factor (Pro-BDNF) and mature BDNF, J. Biol. Chem 291 (39) (2016) 20811–20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Vargas-Medrano J, Krishnamachari S, Villanueva E, et al. , Novel FTY720-based compounds stimulate neurotrophin expression and phosphatase activity in dopaminergic cells, ACS Med. Chem. Lett 5 (7) (2014) 782–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ren M, Han M, Wei X, et al. , FTY720 attenuates 6-OHDA-associated dopaminergic degeneration in cellular and mouse parkinsonian models, Neurochem. Res 42 (2) (2017) 686–696. [DOI] [PubMed] [Google Scholar]
- [4].Zhao P, Yang X, Yang L, et al. , Neuroprotective effects of fingolimod in mouse models of Parkinson’s disease, FASEB J. 31 (1) (2017) 172–179. [DOI] [PubMed] [Google Scholar]
- [5].Enoru JO, Yang B, Krishnamachari S, et al. , Preclinical metabolism pharmacokinetics and in vivo analysis of new blood-brain-barrier penetrant fingolimod analogues: FTY720-C2 and FTY720-Mitoxy, PLoS One 11 (9) (2016) e0162162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Segura-Ulate I, Belcher TK, Vidal-Martinez G, Vargas-Medrano J, Perez RG, FTY720-derivatives do not induce FTY720-like lymphopenia, J. Pharmacol. Sci 133(3) (2017) 187–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kosik KS, The neuronal microRNA system, Nat. Rev. Neurosci 7 (12) (2006) 911–920. [DOI] [PubMed] [Google Scholar]
- [8].Aranda PS, LaJoie DM, Jorcyk CL, Bleach gel: a simple agarose gel for analyzing RNA quality, Electrophoresis 33 (2) (2012) 366–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Qiagen Data Analysis Center. https://www.qiagen.com/us/shop/genes-and-pathways/data-analysis-center-overview-page/. (Accessed 9 August 2018).
- [10].Wong N, Wang X, miRDB: an online resource for microRNA target prediction and functional annotations, Nucleic Acids Res. 43 (D1) (2015) D146–D152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Agarwal V, Bell GW, Nam J-W, Bartel DP, Predicting effective microRNA target sites in mammalian mRNAs, eLife 4 (2015) e05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sun YL, Li SH, Yang L, Wang Y, miR-376b-3p attenuates mitochondrial fission and cardiac hypertrophy by targeting mitochondrial fission factor, Clin. Exp. Pharmacol. Physiol 45 (8) (2018) 779–787. [DOI] [PubMed] [Google Scholar]
- [13].Filichia E, Hoffer B, Qi X, Luo Y, Inhibition of Drp1 mitochondrial translocation provides neural protection in dopaminergic system in a Parkinson’s disease model induced by MPTP, Sci. Rep 6 (2016) 32656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Guidi M, Muiños-Gimeno M, Kagerbauer B, Martí E, Estivill X, Espinosa-Parrilla Y, Overexpression of miR-128 specifically inhibits the truncated isoform of NTRK3 and upregulates BCL2 in SH-SY5Y neuroblastoma cells, BMC Mol. Biol 11(1) (2010) 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Moore C, Rao V, Fuh S-C, et al. , Implicating microRNAs as regulators of microglia and astrocyte responses in human CNS inflammatory disease (P5.018), Neurology 82 (10 Suppl) (2014). [Google Scholar]
- [16].Mouradian MM, MicroRNAs in Parkinson’s disease, Neurobiol. Dis 46 (2) (2012) 279–284. [DOI] [PubMed] [Google Scholar]
- [17].Coolen M, Katz S, Bally-Cuif L, miR-9: a versatile regulator of neurogenesis, Front. Cell. Neurosci 7 (220) (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jiang M, Wang H, Jin M, et al. , Exosomes from miR-30d-5p-ADSCs reverse acute ischemic stroke-induced autophagy-mediated brain injury by promoting M2 microglial/macrophage polarization, Cell. Physiol. Biochem 47 (2) (2018) 864–878. [DOI] [PubMed] [Google Scholar]


