Abstract
Background:
Depression has been associated with low-grade elevation of plasma cytokines (e.g. interleukin-6, IL-6; tumor necrosis factor alpha, TNFα) in both cross-sectional and longitudinal studies in adults. Preclinical and clinical studies also suggest that IL-6 and TNFα elevation are associated with anhedonia. However, few studies have examined longitudinal relationships between cytokines and depression/anhedonia in clinically depressed samples, particularly adolescents.
Methods:
Thirty-six adolescents with a depressive disorder receiving standard-of-care community treatment were assessed at a baseline and a follow-up timepoint. Self-report and clinical measures of depression and anhedonia, along with plasma IL-6 and TNFα levels, were obtained at both timepoints. Baseline cytokine measures were examined in association with baseline and follow-up clinical measures. On an exploratory basis, change in clinical measures over time was examined in relation to change in cytokine levels over time.
Results:
Higher baseline TNFα levels predicted higher follow-up depression severity after approximately four months (controlling for baseline depression). Higher baseline TNFα levels also associated positively with baseline anhedonia and predicted higher anhedonia at follow-up (controlling for baseline anhedonia). No association was found between change in clinical measures and change in cytokine levels over time.
Conclusions:
Among adolescents receiving standard-of-care community treatment for depression, higher levels of TNFα predicted greater depressive symptoms at 4-month follow-up, suggesting this cytokine may be used to help identify patients in need of more intensive treatment. Elevated TNFα levels were also associated with concurrent and future anhedonia symptoms, suggesting a specific mechanism in which TNFα affects depression trajectories. Future studies should examine the relationships between cytokine levels and depression/anhedonia symptoms at multiple timepoints in larger cohorts of depressed adolescents.
Keywords: Cytokine, Adolescent, Anhedonia, Depression, TNF alpha, Longitudinal
1. Introduction
Adolescent depression is often characterized by low mood, anhedonia, sleep disturbance, or low energy, and, with an 11% prevalence in the United States, is relatively common in pediatric populations (Avenevoli et al., 2015). Adolescent depression is linked to negative psychosocial outcomes related to social interactions, family functioning, and academic performance (Jaycox et al., 2009), and, given its wide spectrum of negative effects, has been designated a major public health concern (Perou et al., 2013). Adolescent depression is also a major risk factor for suicide, which is the second leading cause of death in this age group, highlighting the importance for effective treatments for depression (Shain, 2016). However, despite improvements in both psychosocial and psychotropic treatments, pediatric depression continues to be associated with a long duration of illness (e.g. 6 + months), high rates of recurrence (20%–60%) (Birmaher and Brent, 2007), and depression in adulthood (Lewinsohn et al., 1999), with lack of objective biological markers that can predict individual-level differences in improvements in depressive symptoms. Objective biological markers would help better identify which patients may need greater targeted interventions. Furthermore, objective biological markers could also elucidate the molecular pathophysiology of depression, advancing pharmacological interventions and treatments for depression.
In recent years, activation of the innate immune system and related biological markers of inflammation have been associated with depression and implicated as a potential pathophysiological factor in the development or prolongation of depressive disorders (Raison et al., 2006; Petralia et al., 2020). Specifically, proinflammatory cytokines, small signaling proteins that coordinate the innate inflammatory response, have been shown to modulate activation of the hypothalamicpituitary axis and access the central nervous system to affect depression-relevant neural circuits and neurotransmitter functioning (Miller et al., 2009). Such effects (among others) have been linked to development of depressive symptomology (Jeon and Kim, 2016). Several meta-analyses in adults have robustly demonstrated cross-sectional elevations of proinflammatory cytokines in depressed individuals as compared to healthy controls, most consistently the cytokines interleukin-6 (IL-6) and tumor necrosis factor alpha (TNFα) (Osimo et al., 2020; Dowlati et al., 2010; Enache et al., 2019). Some, but not all, studies show positive cross-sectional correlations between depression symptom severity and proinflammatory cytokine levels (Raison et al., 2006). Pediatric studies similarly suggest that TNFα and other inflammatory markers (Tabatabaeizadeh et al., 2018) are elevated in adolescents with depressive disorders as compared to healthy controls, although meta-analyses are restricted by small sample sizes and a limited number of studies (D’Acunto et al., 2019; Kim et al., 2014).
However, cross sectional studies may not capture potential associations of IL-6 and TNFα on prospective depressive symptomology, which could differ from cross-sectional cytokine-depression relationships and might be useful for clinical decision-making and/or for generating novel mechanistic insights regarding the temporal precedence of inflammatory factors and subsequent symptom trajectories. Longitudinal studies examining IL-6/TNFα and depressive/anhedonic symptoms could help parse this relationship. Adult studies have examined longitudinal associations of cytokine changes and depression severity after treatment with antidepressants or psychotherapy (Lopresti et al., 2014; Strawbridge et al., 2015). These studies suggest that higher levels of pro-inflammatory markers (particularly TNFα) at baseline predict poor depression treatment response. Some large adult community studies have found that higher inflammatory markers (e.g. IL-6) predict risk for future depression (Valkanova et al., 2013) and that depression predicts future higher levels of inflammatory markers (e.g. C-Reactive Protein (CRP)) (Huang et al., 2019). However, these results are not consistent, even among large studies with similar study designs (Kennis et al., 2019; Glaus et al., 2018; Lamers et al., 2019). Similarly, community pediatric studies find positive bidirectional longitudinal relationships between depressive symptoms and peripheral pro-inflammatory markers (TNFα, IL-6, CRP) (Miller and Cole, 2012; Moriarity et al., 2019; Khandaker et al., 2014), but in three smaller studies of clinically depressed adolescents, no longitudinal associations between depression and cytokines were found (Pérez-Sánchez et al., 2018; Lee et al., 2020a; Becerril-Villanueva et al., 2019).
Mixed findings in longitudinal studies examining cytokines and their association with depression may partly be due to the heterogeneity of depression itself (Fried and Nesse, 2015; Lamers et al., 2018). For example, a robust preclinical and clinical literature suggests that proinflammatory cytokines may have a greater influence on anhedonia as compared to global depression (Felger and Treadway, 2017). Anhedonia is often described as the reduced ability or motivation to experience pleasure and is a core clinical feature of depression. Two cytokines - IL-6 and TNFα - are closely associated with anhedonia in both rodent models (van Heesch et al., 2013) and in studies of human patients who receive treatment with IL-6-inducing immunostimulants such as INFα (Felger and Treadway, 2017; Ito et al., 1996). Preclinical and clinical studies also suggest that IL-6 or TNFα may selectively disrupt striatal activity (Treadway et al., 2017) or striatal dopaminergic activity (McCoy et al., 2006), effects which are then theorized to lead to anhedonic symptoms (Eisenberger et al., 2010). Importantly, anhedonia is associated with both depression and suicidal ideation/behavior (Ducasse et al., 2018). Thus, understanding longitudinal relationships of cytokines with both depression and anhedonia is important.
An understanding of factors that contribute to symptoms of depression in the pediatric population is crucial because both depression (Thapar et al., 2012) and reward pathway dysfunction (Forbes and Dahl, 2012) emerge in adolescence. Given the intrinsic sensitivity of this developmental period, treatment interventions and/or greater understanding (which might guide future interventions) could both reduce negative symptomology burden and improve the trajectory of future psychological functioning. Accordingly, longitudinal studies are needed to examine cytokine-depression relationships in depressed adolescents, but few such studies have been conducted, and no prior studies to our knowledge have examined longitudinal associations of cytokines and anhedonia in adolescents with depressive disorders.
Given the importance of understanding both cross-sectional and longitudinal associations between cytokines and depression/anhedonia in adolescents, we conducted an initial examination of the cross-sectional and longitudinal associations of IL-6 and TNFα with depression severity and anhedonia severity, in a sample of 36 adolescents with depression who received outpatient naturalistic depression treatment (e.g. standard-of-care community psychotherapy with possible psychotropic medication treatment). We hypothesized that (1) baseline IL-6/TNFα would be positively associated with baseline depression severity and symptoms of anhedonia and that (2) baseline IL-6/TNFα would be positively associated with more symptoms of depression and anhedonia over a 4-month follow-up. On an exploratory basis, change in clinical measures over time was examined in relation to change in cytokine levels over time.
2. Materials and methods
2.1. Participants
Participants included 36 adolescents aged 12–18 years with a DSMIV unipolar depressive disorder (e.g. Major Depressive Disorder) who were enrolled in community psychotherapeutic treatment. Participants were recruited between 2018 and 2019 through flyers, clinical referral from outpatient clinics, or a local research registry. Informed consent was obtained from all participants who were 18 years old. For participants younger than 18 years old, informed consent was obtained from parents, while informed assent was obtained from adolescents.
Exclusion criteria included presence of any acute viral illness or bacterial illness in the week preceding or following the blood draw (e.g. cold, flu, or gastroenteritis symptoms) based on self-report and medical chart review, acute or chronic medical illness potentially affecting the hypothalamic–pituitaryadrenal (HPA) axis or systemic immune system (e.g. ulcerative colitis, Cushing’s syndrome), recent use of medications affecting the immune system (e.g. NSAIDs, methotrexate, antibiotics/antiviral medications, corticosteroids), current pregnancy, or psychiatric diagnoses of anorexia nervosa, bipolar spectrum disorder, or primary psychotic disorder. Participants were excluded if meeting exclusion criterion based on individual interview, medical chart review, and parent interview (if applicable). The Institutional Review Board (IRB) of the University of Pittsburgh approved this study and patients were recruited in accordance with the IRB guidelines for Human Subjects Protection.
At baseline and throughout the follow-up period, and as a condition of study eligibility, all participants were receiving naturalistic standard-of-care community treatment, which included psychotherapeutic treatment (e.g. cognitive behavior therapy or dialectical behavior therapy). At baseline, most adolescents (67%) were receiving concurrent antidepressant treatment, and most adolescents (83%) received antidepressant treatment at some timepoint in the study (see Supplement Table S1 for list of antidepressant treatments).
2.2. Assessment
Participants completed two assessments – one assessment at baseline and one assessment at follow-up, which took place after approximately 4 months (range 3 – 7.5 months), scheduled flexibly based on the participant’s and laboratory’s availability. On each occasion, assessment included a clinical interview, self-report assessments, parent interview/report (if applicable), along with a venous blood draw (which was done within one week of the interviews/assessments).
2.3. Behavioral and medical assessment
Depressive disorder diagnosis was based on clinical interview and medical chart review conducted by a board-certified child and adolescent psychiatrist. Both parent and participants were interviewed if younger than 18, while the parent interview was optional for participants who were 18 years old.
Depression severity was determined using the clinician-rated Children’s Depression Rating Scale-Revised (CDRS-R), which measures recent depressive symptoms (e.g. past month), with higher scores indicating greater depression severity (potential score range 17 to 113) (Mayes et al., 2010). Depression severity was also assessed with adolescent-report Mood and Feelings Questionnaire-Child (MFQ-C), to assess adolescent depressive symptoms in the past two weeks, with higher scores indicating greater depression severity (potential score range 0 to 66) (Burleson Daviss et al., 2006). Anhedonia was measured using the self-report Snaith-Hamilton Pleasure Scale (SHAPS), which has robust validity in adolescent populations, with higher scores indicating greater anhedonia severity in the past few days (potential score range 0 to 14) (Leventhal et al., 2015). Medical assessment included measurement of body mass index (BMI), blood pressure, and heart rate.
2.4. Plasma cytokine measurement and analysis
Plasma was collected from a 20 mL blood draw that was conducted between 9:45am and 1:45 pm at both study visits. All samples were centrifuged for 15 min, and then immediately stored at −80 °C (or transported via dry ice prior to storage at −80 °C). Plasma levels of IL-6 and TNFα were measured with Simple Plex assays using the antibodybased Ella™ system (ProteinSimple, Biotechne) per the manufacturer’s instructions. This system employs a solid-phase sandwich immunoassay run in triplicate. Levels of IL-6 and TNFα assessed using ELLA correlate highly with those assessed by ELISA (p’s > 0.97) (Aldo et al., 2016). Lower limits of detection using this platform are 0.26 pg/mL for IL-6 and 0.03 pg/mL for TNFα (2nd generation). The average intra-assay CV was 6.56% for IL-6 and 2.15% for TNFα. The manufacturer-reported inter-assay CV ranges between 9.6 and 10.2% for IL-6 and 8.5–9% for TNFα. All plasma samples had detectable cytokine levels. Of note, time of blood draw or blood transport time (i.e. time between blood draw and placement of sample in freezer/dry ice) was not associated with cytokine levels when combining these measures across timepoints (p’s > 0.1) and sensitivity analysis including these as covariates did not alter any significant results in our primary analysis. However, baseline blood transport time (mean = 30.3 min) was positively associated with baseline IL-6 values (r = 0.39, p = 0.031), and thus we also present results from an exploratory sensitivity analyses including baseline blood transport time as a covariate for analyses involving IL-6.
Commonly reported normative values of IL-6 range between 0.5 and 1.75 pg/mL in healthy adolescents and between 0.4 and 2 pg/mL in depressed adolescents, while TNFα commonly ranges between 4.9 and 6.7 pg/mL in healthy adolescents and between 4 and 19 pg/mL in depressed adolescents (Lee et al., 2020a; Gabbay et al., 2009; Amitai et al., 2016; González-Gil et al., 2018; Wennberg et al., 2002). While our values fell uniformly within these previously published ranges, absolute ranges taken from other studies may have significantly limited applicability given inter-assay and inter-plate variability in cytokine levels (particularly in smaller samples of depressed adolescents) and effects of confounders (e.g. BMI and sex) on cytokine levels.
2.5. Statistical analysis
Prior to any analysis, cytokine values were Winsorized and log-transformed to reduce the effect of outliers. Overall, less than 12% of all data points at each timepoint were adjusted by Winsorizing. To determine association of baseline IL-6 and TNFα with baseline/follow-up clinical measures, linear regression was used with depression severity or anhedonia as the dependent variable and cytokines as an independent variable, and statistically adjusting in all analyses for biological sex and body mass index (BMI), given their previously reported robust relationship with peripheral cytokine levels (O’Connor et al., 2009). In analyses examining follow-up clinical measures, baseline clinical measures were also included as a covariate to adjust for baseline effects. In exploratory analyses, to determine association of cytokine level change and clinical measure change, linear regression was used with change (delta) in clinical measures as an independent variable and change in cytokines as a dependent variable, controlling for sex and BMI.
Sensitivity analyses were performed using linear regression models to examine the influence on significant findings of additional covariates including age, race, smoking status, socioeconomic status, SSRI use, or any antidepressant use, in analyses both individually including covariates and including all covariates (see Supplemental Table S2). The patterns and effect sizes of findings did not change appreciably in these analyses and none of these covariates were significant predictors of dependent measures in these analyses. Significant results were unchanged when analyzing data after removal of significant univariate outliers as defined by Grubb’s test. Significant results were also unchanged in sensitivity analyses examining data without Winsorizing of data points. Lastly, given our small sample size, nonparametric bootstrapping with replacement with 1000 replicates was done for all significant linear regression models, without appreciable change in results (see Supplemental Table S3).
Partial regression plots were used to optimize graphical visualization of the nature of the relationship between relevant independent and dependent variables, by accounting for the effects of covariates in regression models through use of residual values. Time series plots were used to qualitatively illustrate change in symptoms and cytokine measures (see Supplemental Fig. S1). For descriptive purposes and interested readers, correlational statistics (see Supplemental Table S4) and scatterplots (see Supplemental Fig. S2) between cytokine values and clinical measures are provided in the Supplement. All statistical analyses were performed with the statistical software R version 3.5.2, with usage of packages boot, DescTools, ggplot2, outliers, lme4, MASS, and lmerTest.
3. Results
3.1. Demographic and clinical characteristics
Participants were on average 16.1 ± 1.9 years of age, 75% female, and 77.8% Caucasian. The average duration between baseline and follow-up assessments was 131.3 ± 38 days (~19 weeks). The average baseline CDRS-R score was 55.9 ± 14.3 and MFQ-C score was 37.2 ± 12.1, corresponding to mild to moderate depression severity based on prior reported norms and pediatric antidepressant trials (Mayes et al., 2010; Burleson Daviss et al., 2006; March et al., 2007; Plener et al., 2012). Between baseline and follow-up timepoints, significant decreases were found for CDRS-R (55.9 to 44.9, p < 0.001), MFQ-C (37.2 to 27.8, p < 0.001) and SHAPS scores (4.9 to 3.4, p = 0.001) in the context of patients receiving naturalistic community treatment (see Supplemental Table S5).
3.2. Cytokine characteristics
Average baseline and follow-up IL-6 plasma levels respectively were 1.47 ± 0.8 pg/mL and 1.36 ± 0.7 pg/mL. Average baseline and follow-up TNFα plasma levels were 7.22 ± 1.2 pg/mL and 7.47 ± 1.3 pg/mL. Participants’ cytokine levels did not differ between baseline and follow-up assessments (see Supplemental Table S6).
To assess surface validity of IL-6 assay results, we examined correlation of IL-6 with BMI at baseline (r = 0.62, p < 0.001, df = 34) and at follow-up (r = 0.74, p < 0.001, df = 34), given that BMI has been strongly associated with IL-6 in larger samples (O’Connor et al., 2009). Similar surface validity tests with BMI were not done for TNFα, given that peripheral TNFα levels are not consistently associated with BMI (Haack et al., 1999) and not as strongly tied to adipocyte activity (Grunfeld and Palladino, 1990). To assess reliability of cytokine assay results, we also examined within-individual correlation between timepoints of cytokine values for IL-6 (r = 0.49, p = 0.003, df = 34) and TNFα (r = 0.7, p < 0.001, df = 34).
3.3. Baseline cytokine associations with baseline clinical measures
After controlling for sex and BMI, baseline TNFα was positively associated with higher baseline SHAPS scores (B = 11.28, p = 0.012, n = 36), (see Fig. 1). However, baseline TNFα was not associated with any other baseline clinical measure including the CDRS-R (p = 0.16) or MFQ-C (p = 0.09). Baseline IL-6 was not associated with any baseline clinical measure including the SHAPS, CDRS-R, or MFQ-C (p’s > 0.2). However, on an exploratory sensitivity analysis including baseline blood transport time as a covariate, baseline IL-6 was positively associated with baseline SHAPS scores (B = 4.21, p = 0.049, n = 30).
Fig. 1.
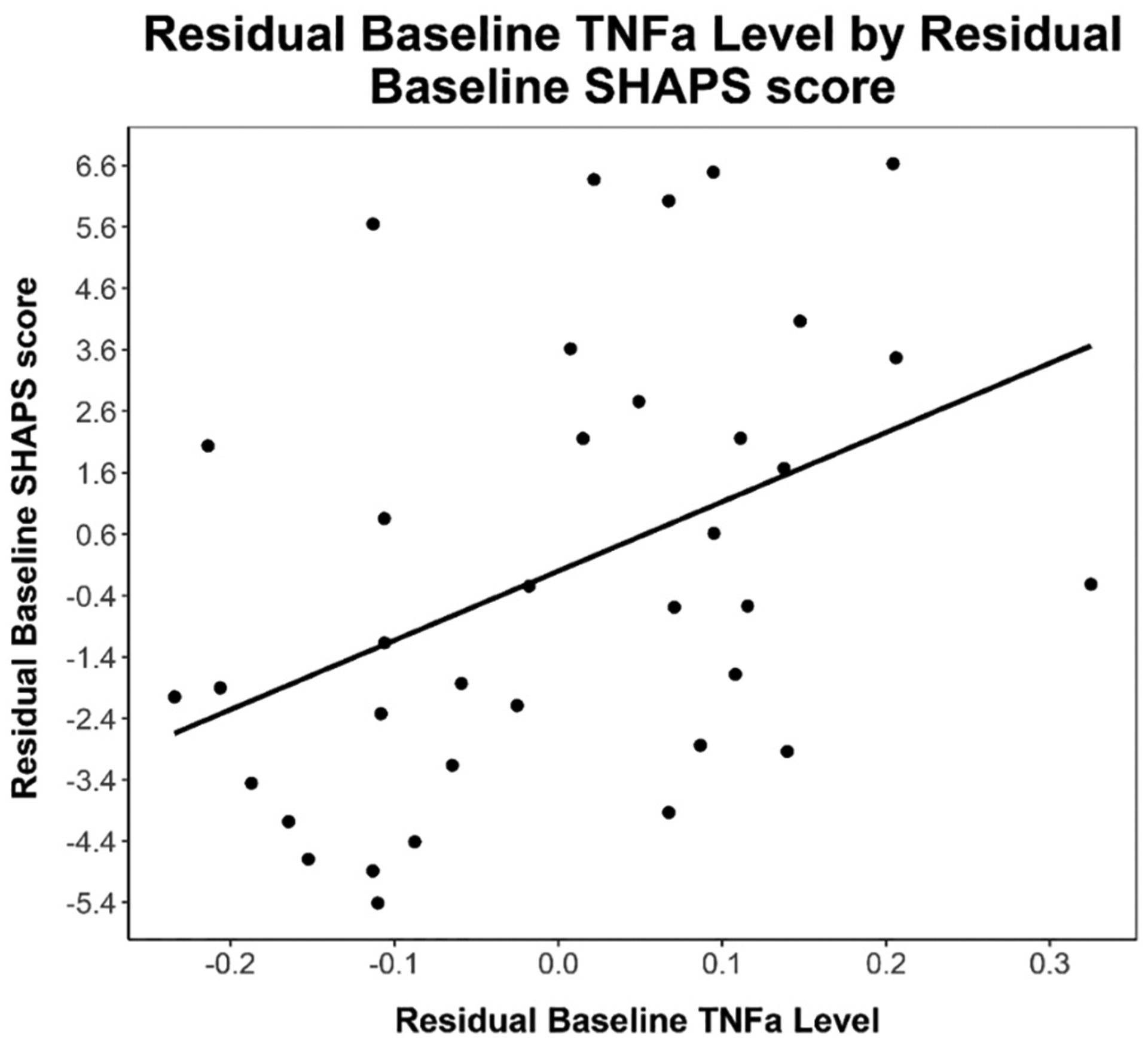
Note. Partial regression plot of residual baseline TNFα levels by residual baseline SHAPS anhedonia scores. Partial regression plot is provided to visualize relationship between independent and dependent variable while statistically adjusting for BMI and biological sex. Line of best fit is provided for descriptive purposes (Equation: Baseline SHAPS Residual = 11.3 * Baseline TNFα Residual).
3.4. Baseline cytokine associations with follow-up clinical measures and exploratory analyses
Baseline TNFα was positively associated with follow-up CDRS-R (B = 33.62, p = 0.029, n = 36) and SHAPS anhedonia scores (B = 6.8, p = 0.043, n = 36) in models that adjusted for baseline CDRS-R and SHAPS scores respectively (see Figs. 2 & 3). Baseline TNFα was not associated with follow-up MFQ-C scores (p = 0.3). Baseline IL-6 was not associated with follow-up CDRS-R, MFQ-C, or SHAPS scores (p’s > 0.36).
Fig. 2.
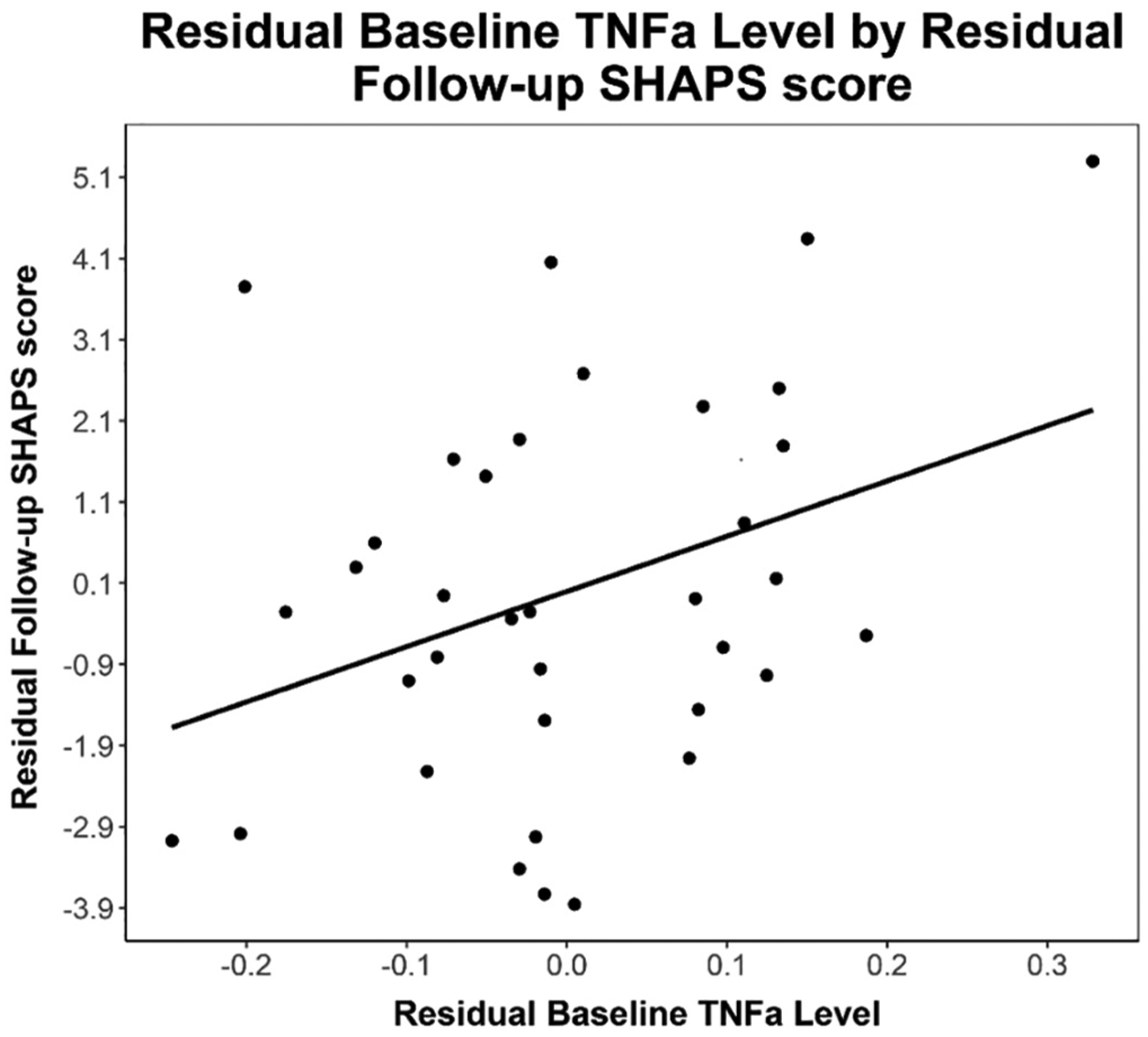
Note. Partial regression plot of residual baseline TNFα levels by residual follow-up SHAPS anhedonia scores. Partial regression plot is provided to visualize relationship between independent and dependent variable while statistically adjusting for BMI, biological sex, and baseline SHAPS anhedonia scores. Line of best fit provided for descriptive purposes (Equation: Follow-Up SHAPS Residual = 6.8 * Baseline TNFα Residual).
Fig. 3.
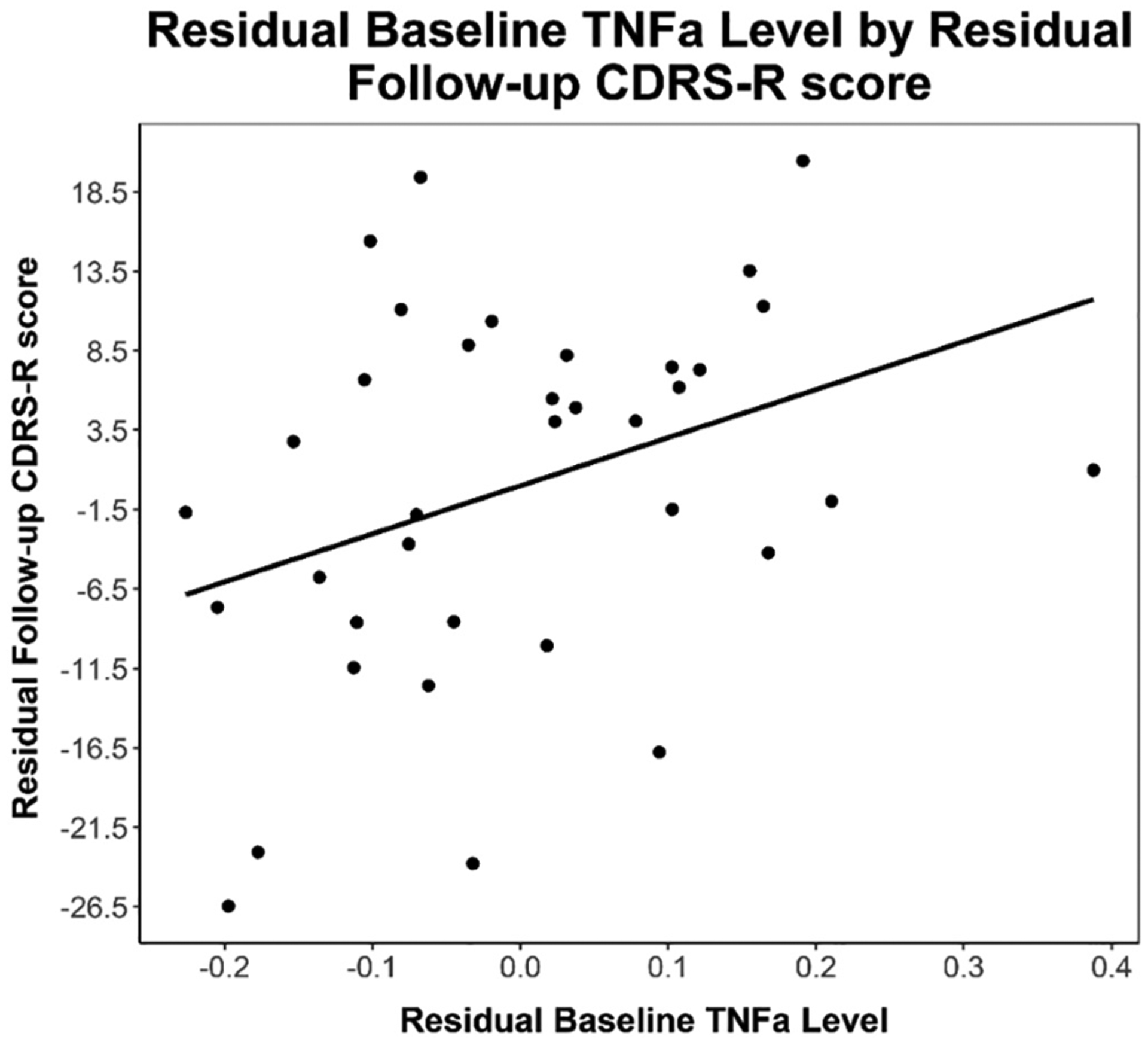
Note. Partial regression plot of residual baseline TNF-alpha levels by residual follow-up CDRS-R depression severity scores. Partial regression plot is provided to visualize relationship between independent and dependent variable while statistically adjusting for BMI, biological sex, and baseline CDRS-R depression scores. Line of best fit provided for descriptive purposes. (Equation: Follow-up CDRS-R Residual = 34 * Baseline TNFα Residual).
In exploratory analyses, no cytokine level changes over time were associated with changes in clinical measure scores (CDRS-R, MFQ-C, or SHAPS) over time (p’s > 0.15). Similarly, no follow-up cytokine levels were associated with follow-up clinical measures (p’s > 0.11). See Supplemental Fig. S1 for graphical time series visualization of change in cytokine/symptom measures.
4. Discussion
In this study of depressed adolescents, we found that elevated baseline TNFα predicted greater depression severity (as measured by the CDRS-R) following an average of 19 weeks of community standard-of-care treatment. Elevated baseline TNFα also predicted greater anhedonia (as measured by the SHAPS) both cross-sectionally (at baseline) and longitudinally (at follow-up). To our knowledge, this is the first report of elevated TNFα being associated with prospective depressive symptoms or anhedonia in depressed adolescents, and one of few studies examining longitudinal cytokine-symptom relationships in depressed adolescents. Together, these cross-sectional and longitudinal findings suggest that in the context of adolescent depression, there may be a more immediate and acute link between TNFα and anhedonia, which over time (and in the context of standard treatment) appears to culminate in a less favorable clinical trajectory, including both sustained anhedonia and more severe overall depression.
In terms of findings between depressive symptoms and cytokines, our findings are consistent with community-based studies of adults and adolescents (Kautz et al., 2019; Miller and Cole, 2012; Moriarity et al., 2019; Khandaker et al., 2014), where raised inflammatory markers (such as IL-6, TNFα, and CRP) have been associated with subsequent development of depressive symptoms (Valkanova et al., 2013), and studies of clinically depressed adults, where raised inflammatory markers are linked to poor treatment response (Strawbridge et al., 2015; Rengasamy et al., 2018). Of the few studies examining clinically depressed adolescents (Pérez-Sánchez et al., 2018; Lee et al., 2020a; Becerril-Villanueva et al., 2019), none have linked individual differences in cytokine levels with prospective improvement in depression after treatment. However, one study of adolescents with MDD (Lee et al., 2020a) found increases in plasma IL-2, IFNγ and IL-10 after twelve weeks of antidepressant treatment, while a study of a cohort of adolescents with mixed depressive/anxiety disorders found that pretreatment cytokine levels (specifically TNFα, IL-6 and IL-1β) were associated with non-response (defined by the Clinical Global Impressions scale) to eight weeks of open-label treatment with fluoxetine (Amitai et al., 2016). In contrast to our study, prior studies involved mandatory psychotropic medication treatment, did not describe if psychotherapeutic treatment was provided, did not always exclude for acute viral/bacterial illness or infection at time of blood draw (Lee et al., 2020a), were of shorter duration than our study, or did not report on effects of sex and BMI on cytokine levels (sex and BMI have been robustly associated with cytokine levels in numerous studies) (O’Connor et al., 2009). These factors limit comparisons of those studies to our own but suggest that additional rigorous studies are needed to specifically examine if baseline cytokine levels predict changes in prospective depressive symptoms. Our findings extend the current knowledge base by providing novel evidence of longitudinal effects of TNFα levels on depressive symptoms in depressed adolescents.
From a broad pathophysiological perspective, dysfunction in neurotransmitter processes, dysregulation in neural circuits, HPA axis perturbations, kynurenine pathway alterations, changes in the gutbrain axis, or nitrosoxidative system effects may contribute to depressive symptoms in adolescents (Raison et al., 2006; Miller et al., 2009, 2013; Jeon and Kim, 2016). Low grade TNFα elevation may affect these processes, resulting in behavioral changes related to depression (Raison et al., 2006). Furthermore, associations of elevated TNFα have been associated with reduced serotonergic or glutaminergic signaling which may limit effects of treatment, such as antidepressant treatment (Haroon et al., 2018). However, longitudinal studies are required to identify potential causal cytokine-driven mechanisms contributing to adolescent depression/anhedonia to determine the specific processes that may influence this relationship.
In terms of anhedonic symptoms, we found that elevated baseline plasma TNFα was positively associated with both baseline and follow-up anhedonia severity. Prior studies in adults similarly have shown that anhedonia is cross-sectionally associated with raised inflammatory markers (primarily IL-6/CRP but also TNFα) in both samples of both depressed patients and non-depressed patients, even while adjusting for depression severity (Majd et al., 2020; Felger et al., 2016). Similarly, longitudinal studies have shown that TNFα predicts development of anhedonia in adults at high clinical risk for psychosis (Goldsmith et al., 2019) and suggested that IL-6 in childhood may predict anhedonia in adulthood (Chu et al., 2019). Consistent with our results, one recent study found transdiagnostic associations of several pro-inflammatory cytokines with greater anhedonia severity in adolescents, although IL-6 or TNFα were not examined (Freed et al., 2019). Our findings are particularly novel given that no prior adult or pediatric studies to our knowledge have found longitudinal anhedonia-cytokine relationships in patients with depressive disorders. Importantly, the associations with baseline TNFα and follow-up anhedonia severity persisted after addition of baseline anhedonia as a covariate, suggesting a unique between-timepoint association of TNFα and anhedonia.
Taken together, these results related to anhedonia suggest that TNFα has both immediate effects on anhedonia and sustained downstream effects that limit improvements in anhedonia in the context of treatment for depression. Theoretically, elevations in TNFα may activate physiological processes leading to anhedonia that are not targeted or successfully remediated by psychotherapy or psychotropic treatment. Such effects may explain why certain patients have less improvements in depression or are treatment-refractory. These effects of TNFα on anhedonia may be mediated by increased downstream cytokine activation and enhanced immune cell mobilization, particularly in the central nervous system (Stanton et al., 2019). More specifically, TNFα or its downstream mediators may affect neurotransmitters such as dopamine (particularly in striatal regions) or glutamate, neuron cell structure, corticostriatal reward neurocircuitry (Felger et al., 2016), and/or the kynurenine pathway (Stanton et al., 2019; Haroon et al., 2020). These effects may ultimately lead to greater symptoms of anhedonia.
Surprisingly, we did not find any associations between IL-6 and clinical measures, or between change in clinical measures and change in any cytokine levels in our primary analyses. On exploratory sensitivity analyses, when adding baseline blood transport time as a covariate, greater baseline IL-6 was associated with greater symptoms of anhedonia. This IL-6 and anhedonia relationship may be present in adolescents, consistent with the broader cytokine and anhedonia literature, and given the preliminary nature of our analysis, requires examination in future studies. In terms of our null findings, our small sample size may have limited our ability to detect such relationships, and thus future studies with larger sample sizes may detect such potential relationships if existent. The null findings related to change measures may also be present because significant reductions for both depression and anhedonia scores were observed between baseline and follow-up timepoints, while there was no significant difference between IL-6 and TNFα scores between timepoints. Alternatively, IL-6 has not been robustly associated with depression or anhedonia in adolescents (particularly as compared to adults) (D’Acunto et al., 2019), and may not have associations with depression/anhedonia in adolescent populations. Additionally, we did not find any associations between the self-reported depression severity (MFQ-C) and cytokine levels. However, in adolescents, clinician administered measures (such as the CDRS-R) may have greater validity compared to self-report measures (Shain et al., 1990), given that adolescents may underreport symptoms or be more influenced by their affective state, exemplified by the use of clinicianreported measures as primary outcome measures in pediatric antidepressant trials (March et al., 2004).
Our findings should be taken in the context of certain limitations. As described previously, some null findings might be secondary to our study being underpowered with a small sample size. Second, we were unable to exhaustively control for all potential confounders and could not have done such an analysis reliably in our small sample with an observational design. Additionally, some patients were taking psychotropic medication at the time of the blood draw. However, we examined antidepressant and SSRI medication use in sensitivity analyses and these did not appreciably change our results (see Supplemental Table S2), consistent with multiple studies finding no effect of antidepressants on cytokine levels in adolescents (Pérez-Sánchez et al., 2018; Amitai et al., 2019). We were unable to control for individual treatment effects given that patients were receiving naturalistic standard-of-care treatment (e.g. psychotherapy +/− medications), but such treatment is the most common treatment for depressive disorders (Olfson et al., 2016), enhancing the generalizability of our findings to real-world clinical conditions. Similarly, given we did not have an active treatment with comparator placebo treatment, our finding preclude determination of whether these results are secondary to specific effects of treatment or general improvement in depressive symptomology. Future research should consider treatment-specific moderation effects on cytokine-symptom associations. Although one study limitation is that we did not perform for multiple testing correction, doing so would have adversely affected statistical power. Our statistical analysis techniques reflect the preliminary nature of our results, consistent with other studies examining psychoneuroimmunological interactions in adolescents (Lee et al., 2020a). Lastly, our use of the SHAPS as a measure of anhedonia does preclude specificity in identifying types of anhedonia that these cytokines may affect, e.g. anticipatory or consummatory anhedonia.
If our results are replicated in larger samples, TNFα could potentially be used as a risk biomarker identifying depressed adolescents who may continue to have elevated symptoms of depression or anhedonia after treatment. This knowledge could be used to identify individuals needing more intensive treatment or aid in development of targeted treatments of adolescents with elevated inflammatory markers. For instance, in adults, elevated inflammatory markers are associated with better antidepressant response to non-serotonergic treatments and anti-inflammatory treatments (Arteaga-Henríquez et al., 2019), such as the anti-TNFα agent infliximab (Raison et al., 2013). Although less research has been done on targeted treatments for anhedonia, a recent RCT of adults with bipolar depression found that infliximab reduced anhedonic symptoms, and that such reductions were moderated by baseline TNFα levels, suggesting that TNFα may be a specific marker for certain treatments to reduce anhedonic depressive symptoms (Lee et al., 2020b). Similarly, one treatment study found that elevations in the inflammatory marker S100B were associated with smaller reduction in anhedonia symptoms in depressed adults receiving monotherapy SSRI treatment (Jha et al., 2019). Understanding the individual-level factors that contribute to treatment efficacy is particularly important in depressed adolescents who are in a vulnerable developmental period. More effective treatments during this time period may improve longerterm trajectories of psychosocial dysfunction, physical health problems, and risk of suicide (Thapar et al., 2012; Pettit et al., 2009).
In summary, our study suggests that in adolescents with depressive disorders, elevated baseline TNFα may be an indicator of poor response to standard-of-care treatment for depression, which might have important future treatment implications. Furthermore, baseline TNFα was positively associated with both baseline and subsequent anhedonia, suggesting this specific symptom of depression was more tightly coupled with TNFα at multiple timepoints and may represent an earlier link along the longitudinal pathway from inflammatory processes to poor overall depression prognosis. Such results add to the extant literature identifying a potential pathophysiologic role of TNFα in the development of anhedonia, and additionally extend these findings to adolescent populations. Future pediatric depression studies should examine larger sample sizes and multiple timepoints to more clearly delineate the relationship between cytokines, depression severity and anhedonia severity.
Supplementary Material
Acknowledgments
This research was supported by funding from the American Academy of Child and Adolescent Psychiatry (AACAP) Pilot Research Award for Junior Faculty and Child and Adolescent Psychiatry Fellows. Support was provided by anonymous donors to the Children’s Hospital of Pittsburgh Foundation. Funding from the Ruth L. Kirschstein National Research Service Award Institutional Research Training Grants sponsored by the National Institutes of Health (NIH T32 MH018951: Brent) also supported this research. Participant recruitment in the study was supported by grant funding from National Institutes of Health through grant UL1TR001857. We acknowledge Dr. Nadine Melhem for her greatly valued assistance in reviewing this manuscript. We thank Aruna Saminathan for her assistance with methodological aspects of this manuscript.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbi.2020.09.004.
References
- Aldo P, et al. , 2016. Simple Plex™: a novel multi-analyte, automated microfluidic immunoassay platform for the detection of human and mouse cytokines and chemokines. Am. J. Reprod. Immunol 75 (6), 678–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai M, et al. , 2016. The relationship between plasma cytokine levels and response to selective serotonin reuptake inhibitor treatment in children and adolescents with depression and/or anxiety disorders. J. Child Adolesc. Psychopharmacol 26 (8), 727–732. [DOI] [PubMed] [Google Scholar]
- Amitai M, et al. , 2019. Increased circulatory IL-6 during 8-week fluoxetine treatment is a risk factor for suicidal behaviors in youth. Brain Behav. Immun [DOI] [PubMed] [Google Scholar]
- Arteaga-Henríquez G, et al. , 2019. Low-grade inflammation as a predictor of antidepressant and anti-inflammatory therapy response in MDD patients: a systematic review of the literature in combination with an analysis of experimental data collected in the EU-Moodinflame consortium. Front. Psychiatry 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenevoli S, et al. , 2015. Major depression in the National Comorbidity Survey-Adolescent Supplement: prevalence, correlates, and treatment. J. Am. Acad. Child Adolescent Psychiatry 54 (1), 37–44 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerril-Villanueva E, et al. , 2019. Alterations in the levels of growth factors in adolescents with major depressive disorder: a longitudinal study during the treatment with fluoxetine. Mediators Inflamm. 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Brent D, A.W.G.o.Q. Issues, 2007. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J. Am. Acad. Child Adolescent Psychiatry 46 (11), 1503–1526. [DOI] [PubMed] [Google Scholar]
- Burleson Daviss W, et al. , 2006. Criterion validity of the Mood and Feelings Questionnaire for depressive episodes in clinic and non-clinic subjects. J. Child Psychol. Psychiatry 47 (9), 927–934. [DOI] [PubMed] [Google Scholar]
- Chu AL, et al. , 2019. Longitudinal association between inflammatory markers and specific symptoms of depression in a prospective birth cohort. Brain Behav. Immun 76, 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Acunto G, et al. , 2019. Inflammatory cytokines in children and adolescents with depressive disorders: a systematic review and meta-analysis. J. Child Adolesc. Psychopharmacol [DOI] [PubMed] [Google Scholar]
- Dowlati Y, et al. , 2010. A meta-analysis of cytokines in major depression. Biol. Psychiatry 67 (5), 446–457. [DOI] [PubMed] [Google Scholar]
- Ducasse D, et al. , 2018. Anhedonia is associated with suicidal ideation independently of depression: a meta-analysis. Depression Anxiety 35 (5), 382–392. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, et al. , 2010. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol. Psychiatry 68 (8), 748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enache D, Pariante C, Mondelli V, 2019. Markers of central inflammation in major depressive disorder: a systematic review and meta-analysis of studies examining cerebrospinal fluid, positron emission tomography and post-mortem brain tissue. Brain Behav. Immun [DOI] [PubMed] [Google Scholar]
- Felger JC, et al. , 2016. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol. Psychiatry 21 (10), 1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Treadway MT, 2017. Inflammation effects on motivation and motor activity: role of dopamine. Neuropsychopharmacology 42 (1), 216–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE, 2012. Research review: altered reward function in adolescent depression: what, when and how? J. Child Psychol. Psychiatry 53 (1), 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed RD, et al. , 2019. Anhedonia as a clinical correlate of inflammation in adolescents across psychiatric conditions. World J. Biol. Psychiatry 20 (9), 712–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried EI, Nesse RM, 2015. Depression is not a consistent syndrome: an investigation of unique symptom patterns in the STAR* D study. J. Affect. Disord 172, 96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, et al. , 2009. Immune system dysregulation in adolescent major depressive disorder. J. Affect. Disord 115 (1), 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaus J, et al. , 2018. Mood disorders and circulating levels of inflammatory markers in a longitudinal population-based study. Psychol. Med 48 (6), 961–973. [DOI] [PubMed] [Google Scholar]
- Goldsmith DR, et al. , 2019. Association of baseline inflammatory markers and the development of negative symptoms in individuals at clinical high risk for psychosis. Brain Behav. Immun 76, 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Gil E, et al. , 2018. Inflammation in metabolically healthy and metabolically abnormal adolescents: the HELENA study. Nutr., Metab. Cardiovasc. Dis 28 (1), 77–83. [DOI] [PubMed] [Google Scholar]
- Grunfeld C, Palladino M, 1990. Tumor necrosis factor: immunologic, antitumor, metabolic, and cardiovascular activities. Adv. Intern. Med 35, 45–72. [PubMed] [Google Scholar]
- Haack M, et al. , 1999. Plasma levels of cytokines and soluble cytokine receptors in psychiatric patients upon hospital admission: effects of confounding factors and diagnosis. J. Psychiatr. Res 33 (5), 407–418. [DOI] [PubMed] [Google Scholar]
- Haroon E, et al. , 2018. Antidepressant treatment resistance is associated with increased inflammatory markers in patients with major depressive disorder. Psychoneuroendocrinology 95, 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, et al. , 2020. Associations among peripheral and central kynurenine pathway metabolites and inflammation in depression. Neuropsychopharmacology 45 (6), 998–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, et al. , 2019. Longitudinal association of inflammation with depressive symptoms: a 7-year cross-lagged twin difference study. Brain Behav. Immun 75, 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito N, et al. , 1996. Induction of interleukin-6 by interferon alfa and its abrogation by a serine protease inhibitor in patients with chronic hepatitis C. Hepatology 23 (4), 669–675. [DOI] [PubMed] [Google Scholar]
- Jaycox LH, et al. , 2009. Impact of teen depression on academic, social, and physical functioning. Pediatrics 124 (4), e596–e605. [DOI] [PubMed] [Google Scholar]
- Jeon SW, Kim YK, 2016. Neuroinflammation and cytokine abnormality in major depression: cause or consequence in that illness? World J. Psychiatry 6 (3), 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, et al. , 2019. Higher S100B levels predict persistently elevated anhedonia with escitalopram monotherapy versus antidepressant combinations: findings from COMED trial. Pharmaceuticals 12 (4), 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautz MM, et al. , 2019. Longitudinal changes of inflammatory biomarkers moderate the relationship between recent stressful life events and prospective symptoms of depression in a diverse sample of urban adolescents. Brain Behav. Immun [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennis M, et al. , 2019. Prospective biomarkers of major depressive disorder: a systematic review and meta-analysis. Mol. Psychiatry 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker GM, et al. , 2014. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry 71 (10), 1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-W, et al. , 2014. Inflammatory markers and the pathogenesis of pediatric depression and suicide: a systematic review of the literature. J. Clin. Psychiatry 75 (11), 1242–1253. [DOI] [PubMed] [Google Scholar]
- Lamers F, et al. , 2019. Longitudinal association between depression and inflammatory markers: results from the Netherlands study of depression and anxiety. Biol. Psychiatry [DOI] [PubMed] [Google Scholar]
- Lamers F, Milaneschi Y, Penninx BW, 2018. Depression subtypes and inflammation: atypical rather than melancholic depression is linked with immunometabolic dysregulations. In: Inflammation and Immunity in Depression. Elsevier, pp. 455–471. [Google Scholar]
- Lee H, et al. , 2020a. Prospective study on cytokine levels in medication-naïve adolescents with first-episode major depressive disorder. J. Affect. Disord 266, 57–62. [DOI] [PubMed] [Google Scholar]
- Lee Y, et al. , 2020b. Efficacy of Adjunctive Infliximab vs. Placebo in the Treatment of Anhedonia in Bipolar I/II Depression. Brain Behav. Immun [DOI] [PubMed] [Google Scholar]
- Leventhal AM, et al. , 2015. Measuring anhedonia in adolescents: a psychometric analysis. J. Pers. Assess 97 (5), 506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn PM, et al. , 1999. Natural course of adolescent major depressive disorder: I. Continuity into young adulthood. J. Am. Acad. Child Adolesc. Psychiatry 38 (1), 56–63. [DOI] [PubMed] [Google Scholar]
- Lopresti AL, et al. , 2014. A review of peripheral biomarkers in major depression: the potential of inflammatory and oxidative stress biomarkers. Prog. Neuropsychopharmacol. Biol. Psychiatry 48, 102–111. [DOI] [PubMed] [Google Scholar]
- Majd M, Saunders EF, Engeland CG, 2020. Inflammation and the dimensions of depression: a review. Front. Neuroendocrinol 56, 100800. [DOI] [PubMed] [Google Scholar]
- March J, et al. , 2004. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents with Depression Study (TADS) randomized controlled trial. JAMA 292 (7), 807–820. [DOI] [PubMed] [Google Scholar]
- March JS, et al. , 2007. The Treatment for Adolescents with Depression Study (TADS): long-term effectiveness and safety outcomes. Arch. Gen. Psychiatry 64 (10), 1132–1144. [DOI] [PubMed] [Google Scholar]
- Mayes TL, et al. , 2010. Psychometric properties of the Children’s Depression Rating Scale-Revised in adolescents. J. Child Adolesc. Psychopharmacol 20 (6), 513–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy MK, et al. , 2006. Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson’s disease. J. Neurosci 26 (37), 9365–9375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, et al. , 2013. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety 30 (4), 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cole SW, 2012. Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biol. Psychiatry 72 (1), 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL, 2009. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 65 (9), 732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarity DP, et al. , 2019. Inflammatory proteins predict change in depressive symptoms in male and female adolescents. Clin. Psychol. Sci 7 (4), 754–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor M-F, et al. , 2009. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav. Immun 23 (7), 887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson M, Blanco C, Marcus SC, 2016. Treatment of adult depression in the United States. JAMA Int. Med 176 (10), 1482–1491. [DOI] [PubMed] [Google Scholar]
- Osimo EF, et al. , 2020. Inflammatory markers in depression: a meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain Behav. Immun [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Sánchez G, et al. , 2018. Inflammatory profiles in depressed adolescents treated with fluoxetine: an 8-week follow-up open study. Mediators Inflamm. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perou R, Bitsko RH, Blumberg SJ, Pastor P, Ghandour RM, Gfroerer JC, Huang LN, 2013. Mental health surveillance among children—United States, 2005–2011. MMWR Suppl. 62 (2), 1–35. [PubMed] [Google Scholar]
- Petralia MC, et al. , 2020. The cytokine network in the pathogenesis of major depressive disorder. Close to translation? Autoimmun. Rev, 102504. [DOI] [PubMed] [Google Scholar]
- Pettit J, et al. , 2009. The long-term course of depression: development of an empirical index and identification of early adult outcomes. Psychol. Med 39 (3), 403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plener PL, et al. , 2012. Convergence of children’s depression rating scale-revised scores and clinical diagnosis in rating adolescent depressive symptomatology. Mental Illness 4 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, et al. , 2013. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry 70 (1), 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH, 2006. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 27 (1), 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH, 2006. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengasamy M, et al. , 2018. Associations of plasma interleukin-6 with plasma and cerebrospinal fluid monoamine biosynthetic pathway metabolites in treatment-resistant depression. Neurol., Psychiatry Brain Res 30, 39–46. [Google Scholar]
- Shain B, 2016. Suicide and suicide attempts in adolescents. Pediatrics, e20161420. [DOI] [PubMed] [Google Scholar]
- Shain BN, Naylor M, Alessi N, 1990. Comparison of self-rated and clinician-rated measures of depression in adolescents. Am. J. Psychiatry [DOI] [PubMed] [Google Scholar]
- Stanton CH, et al. , 2019. From stress to anhedonia: molecular processes through functional circuits. Trends Neurosci. 42 (1), 23–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawbridge R, et al. , 2015. Inflammation and clinical response to treatment in depression: a meta-analysis. Eur. Neuropsychopharmacol 25 (10), 1532–1543. [DOI] [PubMed] [Google Scholar]
- Tabatabaeizadeh S-A, et al. , 2018. There is an association between serum high-sensitivity C-reactive protein (hs-CRP) concentrations and depression score in adolescent girls. Psychoneuroendocrinology 88, 102–104. [DOI] [PubMed] [Google Scholar]
- Thapar A, et al. , 2012. Depression in adolescence. Lancet 379 (9820), 1056–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, et al. , 2017. Association between interleukin-6 and striatal prediction-error signals following acute stress in healthy female participants. Biol. Psychiatry 82 (8), 570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkanova V, Ebmeier KP, Allan CL, 2013. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord. 150 (3), 736–744. [DOI] [PubMed] [Google Scholar]
- van Heesch F, et al. , 2013. Systemic tumor necrosis factor-alpha decreases brain stimulation reward and increases metabolites of serotonin and dopamine in the nucleus accumbens of mice. Behav. Brain Res 253, 191–195. [DOI] [PubMed] [Google Scholar]
- Wennberg P, et al. , 2002. TNF-a gene polymorphism and plasma TNF-a levels are related to lumbar spine bone area in healthy female Caucasian adolescents. Eur. J. Endocrinol 146, 629–634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


