Abstract
Previous research suggests the presence of subtle semantic decline in early stages of Alzheimer’s disease. This study investigated associations between amyloid burden, a biomarker for Alzheimer’s disease, and tasks of semantic impairment in older individuals without dementia. A systematic search in MEDLINE, PsycINFO, and Embase yielded 3691 peer-reviewed articles excluding duplicates. After screening, 41 studies with overall 7495 participants were included in the meta-analysis and quality assessment. The overall weighted effect size of the association between larger amyloid burden and larger semantic impairment was 0.10 (95% CI [−0.03; 0.22], p = 0.128) for picture naming, 0.19 (95% CI [0.11; 0.27], p < 0.001) for semantic fluency, 0.15 (95% CI [−0.15; 0.45], p = 0.326) for vocabulary, and 0.10 (95% CI [−0.14; 0.35], p = 0.405; 2 studies) for WAIS Information. Risk of bias was highest regarding comparability, as effect sizes were often not calculated on covariate-adjusted statistics. The relevance of the indicated amyloid-related decline in semantic fluency for research and clinical applications is likely negligible due to the effect’s small magnitude. Future research should develop more sensitive metrics of semantic fluency to optimize its use for early detection of Alzheimer’s disease-related cognitive impairment.
Keywords: Dementia, Preclinical, Prodromal, Non-demented, Neuropathology, Neuropsychology, Category fluency, Animal fluency, CSF, PET
1. Introduction
Alzheimer’s disease evolves from early pathophysiological changes in amyloid and tau proteins in the brain to manifest clinical dementia with overt cognitive and functional impairment across an estimated time span of 20 years or more (Bateman et al., 2012; Jansen et al., 2015; Villemagne et al., 2013). This process puts Alzheimer’s disease on a continuum of slow development, including a long preclinical phase in which the disease process evolves but no clinical diagnosis has been established yet. This preclinical phase is crucial for clinical trials aimed at intervention and for timely diagnosis for patients and caregivers.
The preclinical phase of Alzheimer’s disease is typically identified using biomarkers associated with the pathophysiological changes of the disease (Dubois et al., 2014). In the temporal evolution of Alzheimer’s disease biomarkers, amyloid-beta has been proposed to be among the first observable ones (Jack and Holtzman, 2013). Amyloid-beta can be detected using different methods, primarily by using histopathology, cerebrospinal fluid (CSF) assays, positron emission tomography (PET), or blood plasma assays. While Alzheimer’s disease biomarkers are often used in research, clinical application of these biomarkers including amyloid is less common due to considerations regarding expenses, invasiveness, and time to administer or evaluate (Zhang, 2019). Importantly, not everyone with elevated levels of brain amyloid develops dementia, and not everyone with clinically diagnosed Alzheimer’s disease has substantial amyloid burden (Brookmeyer and Abdalla, 2018; Glymour et al., 2018; Ossenkoppele et al., 2015).
Despite these discrepancies in the relationship between amyloid burden and development of dementia, the presence of amyloid in older adults without dementia has been consistently related to faster cognitive decline over time (e.g., Baker et al., 2017; Papp et al., 2016) and higher risk of incident Alzheimer’s disease (e.g., Chouraki et al., 2015; Koyama et al., 2012). In contrast to these associations over time with cognitive decline and future clinical endpoints, the cross-sectional relationship of amyloid burden with cognition in older adults without dementia is less clear across studies (Roostaei et al., 2017).
Tasks of episodic memory and semantic fluency (i.e., naming as many exemplars of a certain category as possible in a limited time) are the most reliable neuropsychological markers of progression to Alzheimer’s disease (Drago et al., 2011). Episodic memory is the most characteristic cognitive function impaired in early Alzheimer’s disease (Bäckman et al., 2001; Hodges, 1998). Additionally, a large body of research has demonstrated the role of subtle semantic decline in early stages of the disease or in individuals at high risk for Alzheimer’s disease (e.g., Chertkow et al., 2008; Papp et al., 2016; Vonk et al., 2019a). While a decline in episodic memory is not only present in Alzheimer’s disease but to a lesser extent also in normal aging, several aspects of semantic cognition stay relatively intact with normal aging (Horn and Cattell, 1966, 1967; Park et al., 2002; Salthouse, 2010; Vonk et al., 2020). Therefore, semantic cognition could play an important diagnostic and prognostic role in preclinical Alzheimer’s disease (Venneri et al., 2018).
A growing body of literature shows the presence of semantic memory impairment in preclinical AD and mild cognitive impairment (MCI) (Clark et al., 2009; Joubert et al., 2010; Murphy et al., 2006; Rao et al., 2015; Venneri et al., 2016), particularly in relation to amyloid burden (e.g., Papp et al., 2016, 2017; Snitz et al., 2013; Wirth et al., 2013). The pathogenic mechanisms that lead to the clinical manifestation of AD are still debated, including two recognized hypotheses: the amyloid cascade hypothesis (Hardy and Allsop, 1991) and the tau hyperphosphorylation hypothesis (Wischik et al., 1988). In the amyloid cascade hypothesis, in a complex pathway of events, amyloidosis leads to cognitive decline via synaptic dysfunctioning (amyloid plaques), and in the tau hyperphosphorylation hypothesis, in a complex pathway of events, extensive phosphorylation of tau leads to cognitive decline via microtubule dysfunctioning (neurofibrillary tangles) (Fan et al., 2019). The theoretical framework at the basis of the current study follows the amyloid cascade hypothesis. In both hypotheses, the dysfunctioning at the cell-level leads to neuronal loss in a network of brain regions, starting in the medial temporal lobe and subsequently spreading via the lateral temporal and parietal lobes to the frontal lobe (Dickerson et al., 2009). The brain regions affected early in the AD process, particularly the medial temporal lobe and temporal-parietal region, play a key role in semantic processing (Binder et al., 2009; Didic et al., 2011; Vonk et al., 2019b).
To date, two meta-analyses have investigated relationships between amyloid burden and cognition across multiple cognitive domains, including semantic memory, in older adults without dementia (Baker et al., 2017; Hedden et al., 2013). Both studies analyzed semantic cognition as a domain and included studies in their analyses that used a composite or factor score comprised of multiple tasks. However, there is a lot of variety in tests that are included in these domain scores, and some included tests may not reflect semantic processing. For example, Baker et al. (2017) classified letter fluency (i.e., naming as many exemplars starting with a certain letter as possible in a limited time) to represent semantic memory, while this task is generally thought to reflect executive functioning (Shao et al., 2014; Vonk et al., 2019b). Other semantic tasks, such as picture naming, typically suffer from a ceiling effect in healthy older adults (Moreno-Martínez and Laws, 2007). It is therefore important to consider these tasks separately in their relationship to amyloid burden to investigate subtle amyloid-related semantic impairment. This systematic review and meta-analysis investigated the presence and magnitude of associations between amyloid burden and semantic impairment across different types of semantic tasks in older individuals without dementia. We hypothesized that the increased power of a meta-analysis would reveal a small-to-moderate association between amyloid burden and performance on some but not all semantic tasks; we particularly expected an effect for the semantic fluency task, following previous findings on performance on this task among older individuals without dementia who were at higher risk for dementia (Papp et al., 2016; Vonk et al., 2019a).
2. Methods
The protocol for this systematic review and meta-analysis was registered in PROSPERO and is available in Supplementary Text 1. This systematic review and meta-analysis are reported following the PRISMA guidelines (Moher et al., 2009).
2.1. Search and selection
This systematic review and meta-analysis aimed to include all observational studies that reported on associations between amyloid burden and semantic cognition—measured within one year from each other—in older adults without dementia. We performed an electronic search on May 23, 2020 in MEDLINE (via the PubMed interface), PsycINFO, and Embase for peer-reviewed articles with no date or language restrictions. Unpublished materials, conference abstracts, and grey literature were not included.
The search string was developed for PubMed by adapting the search strings reported in Hedden et al. (2013) and Baker et al. (2017) in consultation with a librarian (PW, acknowledgments). The adjusted search string was tested for inclusion of the articles using semantic tasks in the meta-analyses by Hedden et al. (2013) and Baker et al. (2017), and subsequently translated to Embase and PsychINFO (Supplementary Text 2). Duplicates were removed using EndNote reference management software, and screening was performed using the Rayyan app (Ouzzani et al., 2016).
All studies that were identified in the search were first independently screened on title and abstract for inclusion based on eligibility criteria by two reviewers (JV and ET). The reviewers were blinded to each other’s decisions and resulting disagreements were resolved by discussion between the reviewers. Next, potentially suitable full texts as well as all studies using semantic tasks in the meta-analyses by Hedden et al. (2013) and Baker et al. (2017) were extracted and screened for eligibility. The reference lists from the selected studies were screened for additional articles (i.e., snowballing) and Scopus was used to screen articles that have subsequently cited the selected studies (i.e., reverse snowballing). The study selection processes were recorded using a PRISMA flow diagram (Fig. 1).
Fig. 1.
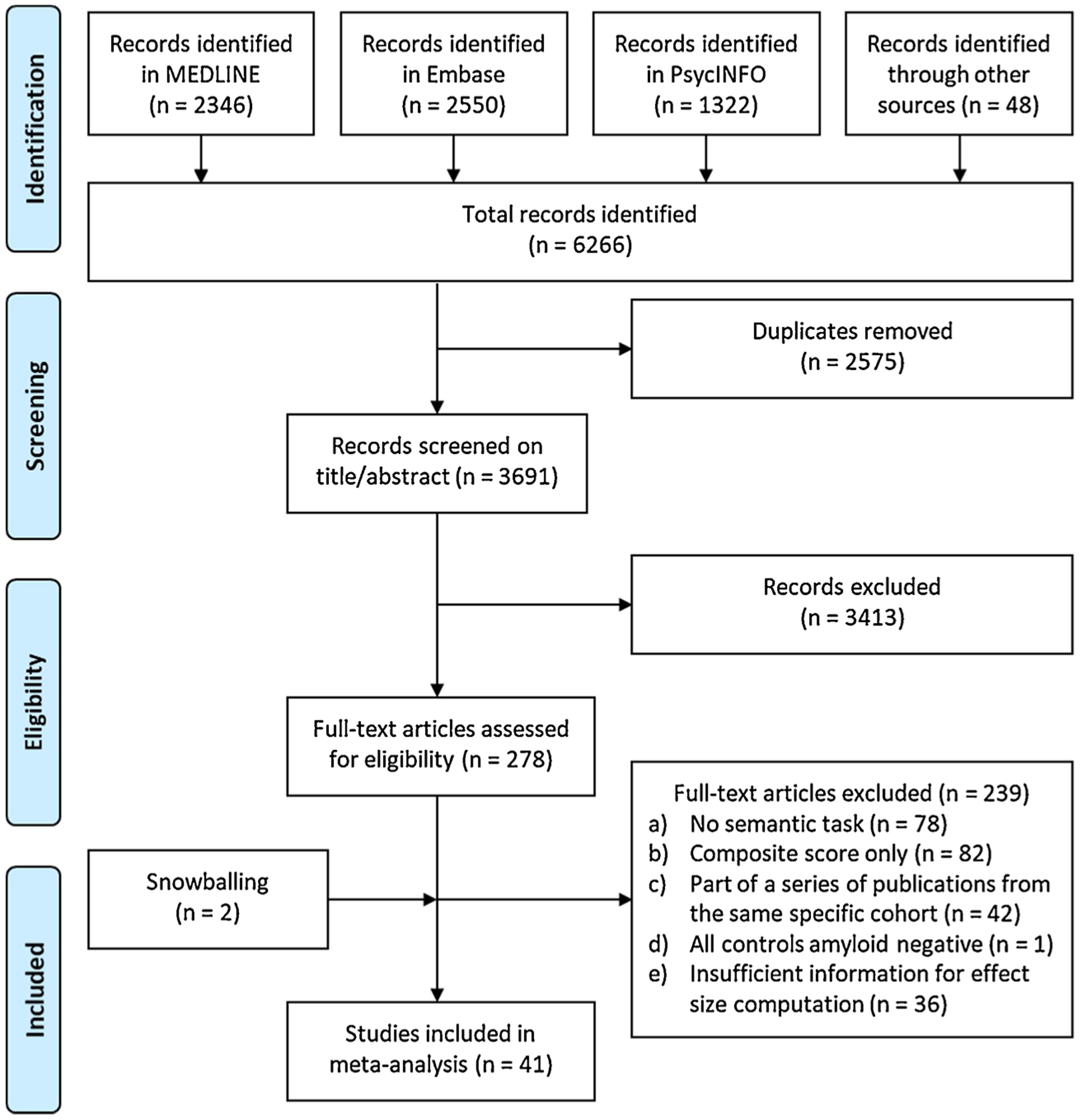
Flowchart study selection.
2.2. Eligibility criteria
A study was included if the study 1) reported associations between amyloid burden and semantic cognition, 2) measured amyloid and tasks of semantic cognition within one year from each other, 3) reported results for one or more separate semantic tasks, 4) reported results for nondemented older adults with an average age of >50 years, and 5) provided sufficient information in the publication for effect size computation.
Studies were excluded if they were limited to adults with an average age <50 years, individuals with all the same amyloid status (i.e., all amyloid positive or all amyloid negative), individuals with a diagnosis of MCI, and individuals with a diagnosis of neurodegenerative disease. Moreover, studies were excluded if they did not report results for individual semantic tasks but only reported results for a semantic, language, or executive function composite or domain score, if they did not reported amyloid status for the cognitively normal group, and if studies were published in another language than Dutch, English, German, or Farsi. If multiple studies reported results on the same task(s) from the same ongoing observational study, we included only the largest sample to represent the data for that semantic task(s) from that cohort to avoid introducing bias due to duplicate data.
2.3. Classification of determinant and outcome
The determinant of interest was amyloid burden, defined as either a continuous variable on a scale from no or low brain amyloid levels to high brain amyloid levels, or categorically as presence or absence of brain amyloid-positivity based on a cut-off value. Amyloid levels were determined using PET ligands, CSF or blood plasma assays, or histopathological evaluation.
The outcome of interest was performance on a semantic cognition task, including the following tasks: a) Boston Naming Test, Action Naming Test and other picture naming tasks or object naming tasks, b) semantic fluency, also called category fluency or animal fluency, and part of the Isaac Set Test, c) category verification task, d) synonym judgment task, e) WAIS Information, f) WAIS Similarities, g) WAIS Vocabulary or other vocabulary tasks, h) Pyramids and Palm Trees Test, Camel and Cactus Test, and other picture association tasks or word association tasks, i) word–picture matching. The outcome of interest was continuous, as the semantic tasks under consideration all had continuous outcome scores. We only considered performance on individual semantic tasks, not semantic domain or composite scores that included multiple different semantic tasks. We analyzed outcomes of semantic cognition performance in cross-sectional studies and scores at baseline in longitudinal studies, but not the rate of change from baseline to the last available follow-up in longitudinal studies.
2.4. Data extraction and risk of bias assessment
Information about study design and methodology, participant demographics and characteristics, amyloid method (PET, CSF, blood plasma, or histopathology) and metric (continuous or categorical), semantic cognition performance, and the associations between amyloid burden and semantic cognition tasks were extracted from the included studies and reviewed by two authors (JV and ET). If both unadjusted and adjusted effects were provided, preference was given to adjusted effects. If multiple methods were used, preference was given to methods in the following order: 1) histopathology, 2) CSF, 3) PET, and 4) blood plasma. If multiple categories of semantic fluency were reported separately, preference was given to semantic fluency of the category animals; not all cohorts use multiple categories, but typically all administer at least animals. Animals are the customary category because other categories are structurally biased by demographic factors, e.g., gender (Capitani et al., 1999; Marra et al., 2007).
Risk of bias in included studies was assessed at outcome-level using a modified version of the Newcastle-Ottawa Quality Assessment Scale Cohort Studies (Peterson et al., 2011). This modified scale using a star-based rating system consists of seven items to critically appraise a study on the quality of participant selection (maximum 3 stars), comparability of cohorts on the basis of the design or analysis (maximum 4 stars), and the quality of outcome assessment (maximum 2 stars) (Supplementary Text 3). Disagreements between judgments over the risk of bias in particular studies were resolved by discussion between the reviewers (JV and ET). If a sufficient number of studies (>10) was available for a semantic task, publication bias was assessed using funnel plots and by computing Egger’s t statistics.
2.5. Statistical analysis
Findings from the included studies were aggregated in overview tables and figures, and meta-analytically analyzed. All outcomes were transformed into effect sizes by using the studies’ reported statistics, e. g., mean and standard deviation or standard error, or results from analyses including t-tests, analysis of variance, correlations, regressions, and linear mixed-effects models. If available, values from analyses adjusted for age, sex, education, or potentially other variables were used. All effects were translated into standardized mean difference (Cohen’s d; for dichotomous measurements: mean difference/pooled standard deviations, for continuous measurements: standardized regression coefficients). If needed, the sign of effect sizes was changed so that positive effect sizes reflected greater amyloid burden associated with greater semantic impairment.
To obtain the pooled estimate for each semantic task, random-effects models with inverse variance weighting were used if a sufficient number of studies (5+) was identified. If only 2–4 studies were identified for a certain semantic task, we were bound to use a fixed-effects model with inverse variance weighting for methodological reasons, although results may be too optimistic when using a fixed-effects model. The models used a DerSimonian-Laird estimator for τ2. Differences in the association between amyloid burden and semantic cognition across the different semantic tasks were tested with subgroup analyses between tasks. We performed pre-specified subgroup analyses for amyloid assessment method (PET/CSF/blood/histopathology), use of a continuous versus categorical scale of amyloid burden, if a study did or did not control for demographic covariates (e.g., age, sex, education, or other), and if a study included only individuals with subjective cognitive complaints. We additionally performed posthoc subgroup analyses between participant samples with a mean age below versus above 70 years. A p-value below 0.05 was considered as a statistically significant result except for the Q-test (used in subgroup analyses), for which the threshold of statistical significance is typically set at p = .10 (Pereira et al., 2010).
Heterogeneity of the results was assessed using visual inspection of overlap in confidence intervals in the forest plot, Cochran’s Q test, and I-squared statistic. The amount of heterogeneity was interpreted according to the recommendations by the Cochrane Handbook (Higgins et al., 2019), i.e., 0–40% might not be important, 30–60% moderate heterogeneity, 50–90% substantial heterogeneity, and 75–100% considerable heterogeneity. The amount and impact of between-study variance was calculated using tau-square. The analyses and generation of figures (i.e., forest plots) were performed in R Version 3.6.0 (R Core Team, 2018).
3. Results
3.1. Search results and study characteristics
The search returned a total of 3691 studies after exclusion of duplicates. As detailed in the flow diagram in Fig. 1, screening yielded 41 studies for inclusion in the meta-analysis. The key characteristics of the included studies are presented in Table 1. The selected studies included a total of 7495 participants with a mean age ranging from 56.9–94.1, mean education (if reported in years) ranging from 9.3–17.3 years, and the proportion of female participants ranging from 39% to 74%. Of the 41 included studies, 21 (51.2%) reported on tasks of picture naming (3070 participants), 34 (82.9%) reported on tasks of semantic fluency (6753 participants), 2 (4.9%) reported on vocabulary (186 participants), 2 (4.9%) reported on WAIS Information (285 participants), and 1 (2.4%) on word-picture matching (118 participants). Amyloid-beta was analyzed as a continuous determinant in 9 (22.0%) studies. The method of amyloid assessment (for the majority of individuals in a study if multiple methods were used) was histopathology in 5 (12.2%) studies, CSF in 19 (46.3%) studies, PET in 15 (36.6%) studies, and blood plasma in 2 (4.9%) studies. In 29 (70.7%) studies, the sample size included 50 or more cognitively normal individuals. The provided information to calculate effect sizes was controlled for age, education, sex/gender, or other variables in 7 (17.1%) of the studies.
Table 1.
Individual study characteristics.
| N | Cohort/sample origin | Language of administration | Age | Education (yr, or alternative) | Sex/gender | |
|---|---|---|---|---|---|---|
| Study | m (SD, range)1 | m (SD, range)1 | woman | |||
| Aizenstein et al. (2008) | 38 | Pittsburgh community | English | 74.4 (6.1) | 15.1 (2.8) | 63% |
| Amariglio et al. (2012) | 131 | Harvard Aging Brain Study (HABS) | English | 73.5 (6) | 16.1 (2.9) | 53% |
| Bennett et al. (2006) | 134 | Religious Orders Study (ROS), Memory and Aging Project (MAP) | English | 83.3 (6.4) | 17.3 (4.3) | 55% |
| Bos et al. (2018) | 907 | Barcelona St. Pau, EDAR, Gipuzkoa Alzheimer Project, Gothenburg MCI study, IDIBAPS, IMAP+, Leuven, EMIF preclinical-AD study, ADNI | Spanish, Swedish, Dutch, German, Greek, Danish | 68.0 (9.1) | 14.7 (3.7) | 53% |
| Clark et al. (2018) | 314 | Wisconsin Alzheimer’s Disease Research Center (ADRC) | English | 58.8 (37–85) | 16.3 (2.5) (8–25) | 69% |
| Doraiswamy et al. (2014) | 67 | AV45-A11 | English | 70.0 (11.3) | 15.3 (2.2) | 60% |
| Dubois et al. (2018) | 318 | INSIGHT-preAD Study | French | 76.0 (3.5) (70–85) | 68% ≥ HS | 63% |
| Edelman et al. (2017) | 44 | Pittsburgh community | English | 76.4 (5.6) | ≥ 12 years | 73% |
| Edmonds et al. (2015) | 570 | Alzheimer’s Disease Neuroimaging Initiative (ADNI) | English | 73.1 (7.1) | 16.4 (2.6) | 46% |
| Funaki et al. (2019) | 42 | Keio University Hospital Memory Clinic | Japanese | 74.4 (4.7) | 15.1 (2.1) | 48% |
| Harrington et al. (2017) | 335 | Australian Imaging, Biomarker, and Lifestyle Study (AIBL) | English | 68.3 (5.7) (60–85) | Not specified | 58% |
| Hassenstab et al. (2016) | 264 | Washington University Knight ADRC | English | 72.1 (5.4) (65–86) | 15.5 (2.8) | 59% |
| Hilal et al. (2017) | 1201 | Rotterdam Study | Dutch | 61.8 (7.2) | 12.1 (4.0) | 53% |
| Hulette et al. (1998) | 11 | University of Miami ADRC | English | 81.6 (6.0) | 16.6 (2.8) | 50% |
| Jicha et al. (2012) | 126 | UK-ADC | English | 83.7 (7.8) | 16.0 (2.4)2 | 48% |
| Kawas et al. (2013) | 13 | The 90+ Study | English | 94.1 (median) (90–99) | 54% > HS | 69% |
| Kim et al. (2016) | 13 | Yeungnam University Hospital in Daegu, Korea | Korean | 72.0 (4.0) | 9.5 (5.5) | 54% |
| Lee et al. (2020) | 97 | Seoul, Korea | Korean | 70.9 (7.5) | 13.8 (3.6) | 60% |
| Li et al. (2007) | 72 | Community University of Washington | English | 67.9 (9.0) | 15.7 (3.0) | 54% |
| Li et al. (2014) | 315 | ADRC+ | English | 57.4 (18.1) | 16.1 (2.6) | 54% |
| Lim et al. (2013) | 178 | AIBL | English | 71.6 (7.5) | Not specified | 50% |
| Loewenstein et al. (2016) | 23 | University of Miami School of Medicine, Mount Sinai Medical Center, University of Florida | English | 74.6 (8.1) | 15.7 (2.9) | 74% |
| Mathis et al. (2013) | 152 | Ginkgo Evaluation of Memory | English | 85.5 (2.9) | 14.8 (2.6) | 42% |
| Mielke et al. (2016) | 464 | Mayo Clinic Study of Aging | English | 62.7 (5.4) (50–69) | 15.3 (2.3) | 48% |
| Morris et al. (1996) | 21 | Washington University Faculty of Medicine | English | 84.5 (6.6) | 14.8 (3.3) | 48% |
| Oh et al. (2011) | 52 | Community from Berkley | English | 74.1 (6.0) | 17.2 (1.9) | 65% |
| Oh et al. (2012) | 189 | Community from Berkley | English | 74.2 (6.0) | 17.2 (2.0) | 65% |
| Papp et al. (2017) | 279 | HABS | English | 73.4 (6.0) | 15.9 (3.0) | 59% |
| Payoux et al. (2015) | 235 | Multidomain Alzheimer Preventive Trial (MAPT) | French | 74.7 (4.3) | 30% >12 yr | 60% |
| Perrotin et al. (2012) | 48 | Berkley Aging Cohort Study | English | 73.5 (5.9) | 17.3 (1.9) | 65% |
| Price et al. (2009) | 97 | National Alzheimer Coordinating Center (NACC)-supported Neuropsychological Database Initiative | English | 84.3 (8.6) | 15.4 (2.9) | 57% |
| Rami et al. (2011) | 17 | Alzheimer’s Disease & Other Cognitive Disorders Unit of the Hospital Clinic in Barcelona | Spanish | 68.7 (6.8) | 9.3 (4.0) | N/A |
| Rosenberg et al. (2013) | 15 | John’s Hopkins ADRC, Joint Memory Clinics Baltimore, Memory Enhancement Center Eatontown, Community Health Center North East | English | 74.5 (10.4) | 17.3 (2.5) | 39% |
| Sala-Llonch et al. (2017) | 89 | Oslo | Norwegian | 73.1 (6.0) (65–90) | Not specified | 48% |
| Snitz et al. (2020) | 118 | Monongahela-Youghiogheny Healthy Aging Team-Neuroimaging, Heart Strategies Concentrating on Risk Evaluation parent study | English | 76.3 (5.7) (65–91) | 74% > HS | 58% |
| Soldan et al. (2016) | 222 | BIOCARD | English | 56.9 (10.1) (22–85) | 17.2 (2.3) (12–20) | 60% |
| Sperling et al. (2013) | 78 | AV45-A05 study/24 different sites | English | 69.4 (11.1) | 15.2 (2.3) | 56% |
| Um et al. (2017) | 50 | Catholic Geriatric Neuroimaging Database | Korean | 67.5 (4.7) | 9.6 (2.2) | 64% |
| Vannini et al. (2013) | 41 | Brigham and Women’s Hospital and Massachusetts General Hospital | English | 73.7 (0.5) | 15.6 (0.5) | 68% |
| Verberk et al. (2020) | 241 | SCIENCe and Amsterdam Dementia Cohort | Dutch | 61.0 (9.0) | 5.0 (1.0)3 | 40% |
| Verfaillie et al. (2019) | 63 | SCIENCe | Dutch | 63.5 (8.2) | 5.6 (1.3)3 | 43% |
Note.
if available;
due to a typo in original paper the AB- group was reported to have an average of 60 years of education, thus we omitted this group in calculating mean years of education;
Dutch educational scale (Verhage, 1965);
m = mean, SD = standard deviation; cat = categorical, cont = continuous; PET = positron emission tomography, CSF = cerebrospinal fluid; yr = years, HS = high school.
3.2. Risk of bias within and across studies
Quality assessment for risk of bias within the included studies is presented in Table 2. Studies scored between 3 and 9 stars, with 17 studies (41.5%) obtaining 5 or more stars. On selection criteria, studies lost stars because their sample was not representative of the average older adult without dementia in the community (16 studies, 39.0%) or because the categorization of amyloid was not based on established or published cut-offs (16 studies, 39.0%). On comparability, 38 studies (92.7%) lost stars because their effect sizes could not be calculated on information that was adjusted for age, sex/gender, education, and other covariates. All studies scored the maximum number of stars on the assessment of outcome.
Table 2.
Newcastle-Ottawa scale for assessment of quality of included studies.
| Selection | Comparability | Outcome | Overall | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | 1. Representative | 2. Selection | 3. Exposure | Age | Sex/gender | Education | Other factors | 1. Outcome | 2. Same method | (max. 9) |
| Aizenstein et al. (2008) | ✶ | ✶ | – | – | – | – | – | ✶ | ✶ | 4 |
| Amariglio et al. (2012) | – | ✶ | – | – | – | – | – | ✶ | ✶ | 3 |
| Bennett et al. (2006) | – | ✶ | ✶ | – | – | – | – | ✶ | ✶ | 4 |
| Bos et al. (2018) | ✶ | ✶ | – | – | – | – | – | ✶ | ✶ | 4 |
| Clark et al. (2018) | ✶ | ✶ | – | – | – | – | – | ✶ | ✶ | 4 |
| Doraiswamy et al. (2014) | ✶ | ✶ | – | – | – | – | – | ✶ | ✶ | 4 |
| Dubois et al. (2018) | – | ✶ | ✶ | – | – | – | – | ✶ | ✶ | 4 |
| Edelman et al. (2017) | ✶ | ✶ | – | – | – | – | – | ✶ | ✶ | 4 |
| Edmonds et al. (2015) | ✶ | ✶ | ✶ | – | – | – | – | ✶ | ✶ | 5 |
| Funaki et al. (2019) | – | ✶ | – | – | – | – | – | ✶ | ✶ | 3 |
| Harrington et al. (2017) | – | ✶ | ✶ | ✶ | – | – | ✶ | ✶ | ✶ | 6 |
| Hassenstab et al. (2016) | – | ✶ | ✶ | – | – | – | – | ✶ | ✶ | 4 |
| Hilal et al. (2017) | ✶ | ✶ | ✶ | ✶ | ✶ | ✶ | ✶ | ✶ | ✶ | 9 |
| Hulette et al. (1998) | ✶ | ✶ | ✶ | – | – | – | – | ✶ | ✶ | 5 |
| Jicha et al. (2012) | ✶ | ✶ | ✶ | – | – | – | – | ✶ | ✶ | 5 |
| Kawas et al. (2013) | ✶ | ✶ | – | – | – | – | – | ✶ | ✶ | 4 |
| Kim et al. (2016) | – | ✶ | ✶ | – | – | – | – | ✶ | ✶ | 4 |
| Lee et al. (2020) | ✶ | ✶ | ✶ | ✶ | ✶ | ✶ | – | ✶ | ✶ | 8 |
| Li et al. (2007) | – | ✶ | ✶ | ✶ | ✶ | ✶ | ✶ | ✶ | ✶ | 8 |
| Li et al. (2014) | – | ✶ | – | – | – | – | – | ✶ | ✶ | 3 |
| Lim et al. (2013) | ✶ | ✶ | – | – | – | – | – | ✶ | ✶ | 4 |
| Loewenstein et al. (2016) | ✶ | ✶ | ✶ | – | – | – | – | ✶ | ✶ | 5 |
| Mathis et al. (2013) | ✶ | ✶ | – | – | – | – | – | ✶ | ✶ | 4 |
| Mielke et al. (2016) | – | ✶ | ✶ | ✶ | ✶ | ✶ | – | ✶ | ✶ | 7 |
| Morris et al. (1996) | ✶ | ✶ | ✶ | – | – | – | – | ✶ | ✶ | 5 |
| Oh et al. (2011) | ✶ | ✶ | – | – | – | – | – | ✶ | ✶ | 4 |
| Oh et al. (2012) | ✶ | ✶ | – | – | – | – | – | ✶ | ✶ | 4 |
| Papp et al. (2017) | ✶ | ✶ | ✶ | – | – | – | – | ✶ | ✶ | 5 |
| Payoux et al. (2015) | ✶ | ✶ | – | – | – | – | – | ✶ | ✶ | 4 |
| Perrotin et al. (2012) | ✶ | ✶ | ✶ | – | – | – | – | ✶ | ✶ | 5 |
| Price et al. (2009) | – | ✶ | ✶ | – | – | – | – | ✶ | ✶ | 4 |
| Rami et al. (2011) | – | ✶ | ✶ | – | – | – | – | ✶ | ✶ | 4 |
| Rosenberg et al. (2013) | ✶ | ✶ | ✶ | – | – | – | – | ✶ | ✶ | 5 |
| Sala-Llonch et al. (2017) | – | ✶ | ✶ | – | – | – | – | ✶ | ✶ | 4 |
| Snitz et al. (2020) | ✶ | ✶ | ✶ | ✶ | ✶ | ✶ | ✶ | ✶ | ✶ | 9 |
| Soldan et al. (2016) | – | ✶ | – | – | – | – | – | ✶ | ✶ | 3 |
| Sperling et al. (2013) | ✶ | ✶ | – | – | – | – | – | ✶ | ✶ | 4 |
| Um et al. (2017) | ✶ | ✶ | ✶ | – | – | – | – | ✶ | ✶ | 5 |
| Vannini et al. (2013) | ✶ | ✶ | ✶ | – | – | – | – | ✶ | ✶ | 5 |
| Verberk et al. (2020) | – | ✶ | ✶ | ✶ | ✶ | ✶ | – | ✶ | ✶ | 7 |
| Verfaillie et al. (2019) | – | ✶ | ✶ | – | – | – | – | ✶ | ✶ | 4 |
Note. Each star represents if individual criterion within the subsection was fulfilled.
Funnel plots for picture naming and semantic fluency to assess risk of bias across the included studies were not fully symmetric (Fig. 2). However, the Egger’s t statistic for asymmetry was non-significant for both picture naming (b for bias = 0.88, SE = 0. 59; t(19) = 1.509, p = 0.148) and semantic fluency (b for bias = 0.70, SE = 0.37; t(32) = 1.901, p = 0.066). For tasks of vocabulary, WAIS information, and word-picture matching not enough studies reported effects to assess publication bias for these tasks.
Fig. 2.
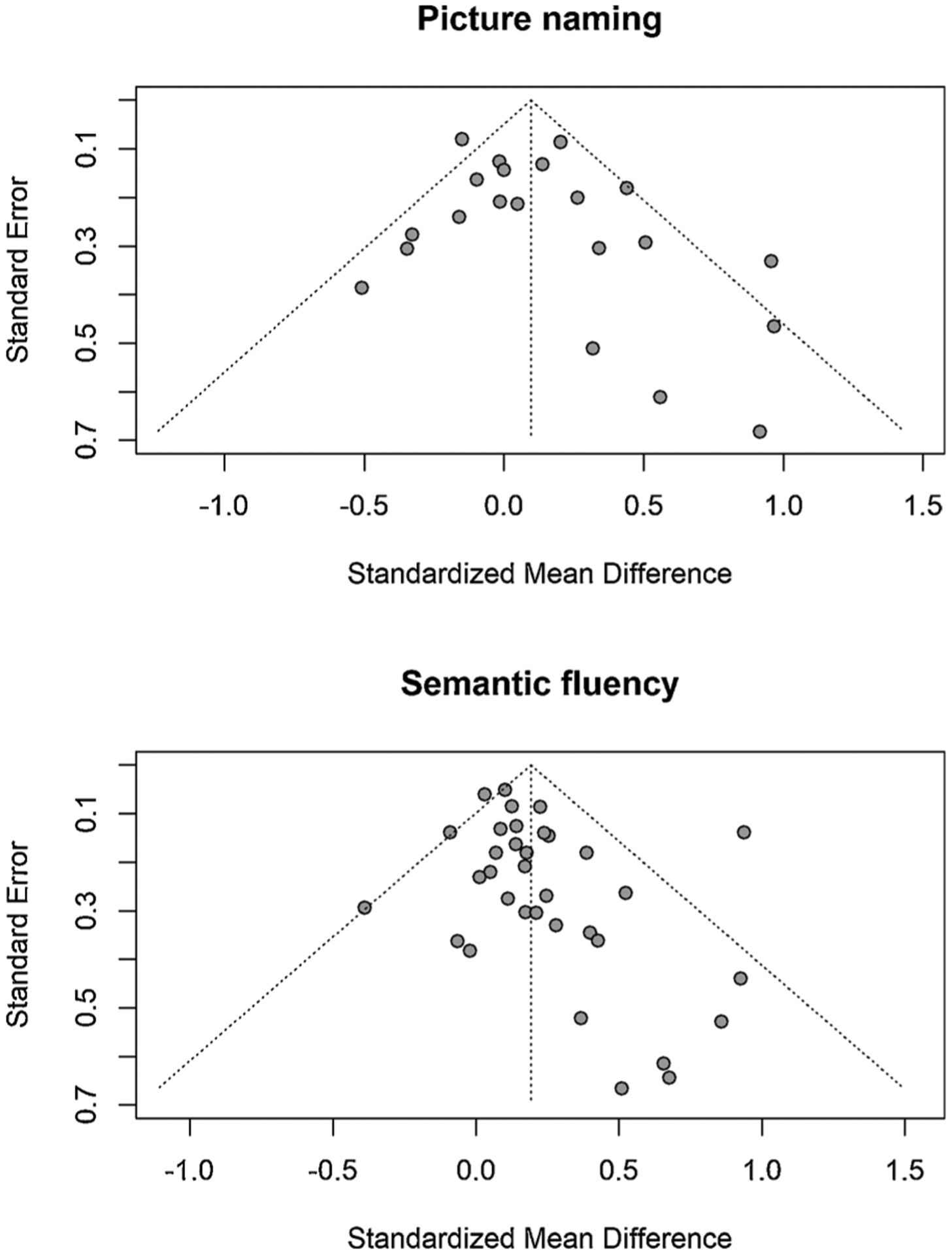
Funnel plots to assess publication bias.
3.3. Meta-analysis
Individual study effect sizes (Cohen’s d) for the four different semantic tasks that could be included in the meta-analysis, i.e., picture naming, semantic fluency, vocabulary, and WAIS Information are presented in Table 3. Figs. 3–6 show the forest plots per semantic task including effect sizes per study with 95% confidence intervals (CI) as well as the pooled results. The overall weighted effect size of the association between larger amyloid burden and larger semantic impairment was 0.10 (95% CI [−0.03; 0.22], p = 0.128) for picture naming, 0.19 (95% CI [0.11; 0.27], p < 0.001) for semantic fluency, 0.15 (95% CI [−0.15; 0.45], p = 0.326) for vocabulary, and 0.10 (95% CI [−0.14; 0.35], p = 0.405; 2 studies) for WAIS Information. These effect sizes are considered to be in the small-sized range, given that an effect size of d = 0.20 is considered small, d = 0.50 medium and d = 0.80 large (Cohen, 1988). Moderate heterogeneity in the pooled estimate of effect size was detected for picture naming (Q = 40.65, p = 0.004, I2 = 50.8%) and semantic fluency (Q = 59.00, p = 0.036, I2 = 44.1%), but not for vocabulary (Q = 1.07, p = 0.301, I2 = 6.7%) or WAIS Information (Q = 0.46, p = 0.499, I2 = 0.0%). For word-picture matching, which could not be meta-analyzed as only one study reported this task, the relationship with amyloid was −0.12 [−0.26; 0.02] (Snitz et al., 2020).
Table 3.
Individual study’s analytic specifications and effect sizes with standard error.
| Author | Total N | AB− n | AB + n | Amyloid scale | Amyloid method | SCD only | Covariate-controlled | Picture naming | Semantic fluency | Vocabulary | WAIS Information | Word-picture matching |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aizenstein et al. (2008) | 38 | 29 | 9 | categorical | PET | no | no | −0.51 (0.39) | −0.02 (0.38) | – | – | – |
| Amariglio et al. (2012) | 131 | 97 | 34 | categorical | PET | no | no | 0.26 (0.2) | – | – | – | – |
| Bennett et al. (2006) | 134 | 84 | 50 | categorical | histology | no | no | 0.44 (0.18) | 0.39 (0.18) | 0.05 (0.18) | – | – |
| Bos et al. (2018) | 907 | 526 | 190 | categorical | CSF or PET | no | no | – | 0.12 (0.08) | – | – | – |
| Clark et al. (2018) | 314 | 240 | 104 | categorical | CSF | no | no | – | 0.94 (0.14) | – | – | – |
| Doraiswamy et al. (2014) | 67 | 57 | 10 | categorical | PET | no | no | – | 0.4 (0.34) | – | – | – |
| Dubois et al. (2018) | 318 | 230 | 88 | categorical | PET | yes | no | −0.02 (0.13) | 0.14 (0.13) | – | – | – |
| Edelman et al. (2017) | 44 | 23 | 21 | categorical | PET | no | no | 0.34 (0.3) | 0.17 (0.3) | – | – | – |
| Edmonds et al. (2015) | 570 | 142 | 428 | categorical | CSF | no | no | 0.2 (0.09) | 0.22 (0.09) | – | – | – |
| Funaki et al. (2019) | 42 | 32 | 10 | categorical | PET | no | no | – | −0.07 (0.36) | – | – | – |
| Harrington et al. (2017) | 335 | 277 | 58 | categorical | PET | no | yes | – | 0.25 (0.14) | – | – | – |
| Hassenstab et al. (2016) | 264 | 177 | 87 | categorical | CSF | no | no | 0.14 (0.13) | 0.09 (0.13) | – | 0.13 (0.13) | – |
| Hilal et al. (2017) | 1201 | – | – | continuous | plasma | no | yes | – | 0.28 (0.33) | – | – | – |
| Hulette et al. (1998) | 11 | 7 | 4 | categorical | histology | no | no | – | 0.68 (0.64) | – | – | – |
| Jicha et al. (2012) | 126 | 71 | 54 | categorical | histology | no | no | – | 0.18 (0.18) | – | – | – |
| Kawas et al. (2013) | 13 | 9 | 4 | Categorical | PET | no | no | 0.56 (0.61) | 0.66 (0.61) | – | – | – |
| Kim et al. (2016) | 13 | 10 | 3 | continuous | PET | yes1 | no | 0.92 (0.68) | 0.51 (0.67) | – | – | – |
| Lee et al. (2020) | 97 | – | – | continuous | plasma | no | yes | 0.05 (0.21) | – | – | – | – |
| Li et al. (2007) | 72 | 51 | 21 | categorical | CSF | no | no | – | 0.52 (0.26) | – | – | – |
| Li et al. (2014) | 315 | – | – | continuous | CSF | no | yes | – | 0.1 (0.05) | – | – | – |
| Lim et al. (2013) | 178 | 123 | 55 | categorical | PET | yes | no | −0.1 (0.16) | – | – | – | – |
| Loewenstein et al. (2016) | 23 | – | – | continuous | PET | no | no | – | 0.93 (0.44) | – | – | – |
| Mathis et al. (2013) | 152 | 74 | 78 | categorical | PET | no | no | – | 0.14 (0.16) | – | – | – |
| Mielke et al. (2016) | 464 | 383 | 81 | categorical | PET | no | yes | −0.16 (0.24) | 0.05 (0.22) | – | – | – |
| Morris et al. (1996) | 21 | 12 | 9 | categorical | histology | no | no | 0.97 (0.47) | – | – | −0.18 (0.44) | – |
| Oh et al. (2011) | 52 | 33 | 19 | categorical | PET | no | no | 0.51 (0.29) | – | 0.41 (0.29) | – | – |
| Oh et al. (2012) | 189 | 34 | 18 | categorical | PET | no | no | – | −0.39 (0.29) | – | – | – |
| Papp et al. (2017) | 279 | 209 | 70 | categorical | PET | no | no | – | −0.09 (0.14) | – | – | – |
| Payoux et al. (2015) | 235 | 158 | 77 | categorical | PET | no | no | – | 0.24 (0.14) | – | – | – |
| Perrotin et al. (2012) | 48 | 27 | 11 | categorical | PET | no | no | – | 0.43 (0.36) | – | – | – |
| Price et al. (2009) | 97 | 59 | 38 | categorical | histology | no | no | −0.01 (0.21) | 0.17 (0.21) | – | – | – |
| Rami et al. (2011) | 17 | 11 | 6 | categorical | CSF | yes | no | 0.32 (0.51) | 0.86 (0.53) | – | – | – |
| Rosenberg et al. (2013) | 15 | – | – | continuous | PET | no | no | – | 0.37 (0.52) | – | – | – |
| Sala-Llonch et al. (2017) | 89 | 62 | 27 | categorical | CSF | no | no | – | 0.01 (0.23) | – | – | – |
| Snitz et al. (2020) | 118 | – | – | continuous | PET | no | yes | – | 0.07 (0.18) | – | – | −0.12 (.07) |
| Soldan et al. (2016) | 222 | 148 | 74 | categorical | CSF | no | no | 0 (0.14) | – | – | – | – |
| Sperling et al. (2013) | 78 | 60 | 18 | categorical | PET | no | no | – | 0.25 (0.27) | – | – | – |
| Um et al. (2017) | 50 | 34 | 16 | categorical | PET | no | no | −0.35 (0.31) | 0.21 (0.3) | – | – | – |
| Vannini et al. (2013) | 41 | 22 | 19 | categorical | PET | no | no | 0.96 (0.33) | – | – | – | – |
| Verberk et al. (2020) | 241 | – | – | continuous | CSF | yes | yes | −0.15 (0.08) | 0.03 (0.06) | – | – | – |
| Verfaillie et al. (2019) | 63 | 44 | 19 | categorical | PET or CSF | yes | no | −0.33 (0.28) | 0.11 (0.27) | – | – | – |
Note. SCD = subjective cognitive decline; PET = positron emission tomography, CSF = cerebrospinal fluid;
All subjects had a history of major depressive disorder in addition to SCD.
Fig. 3.
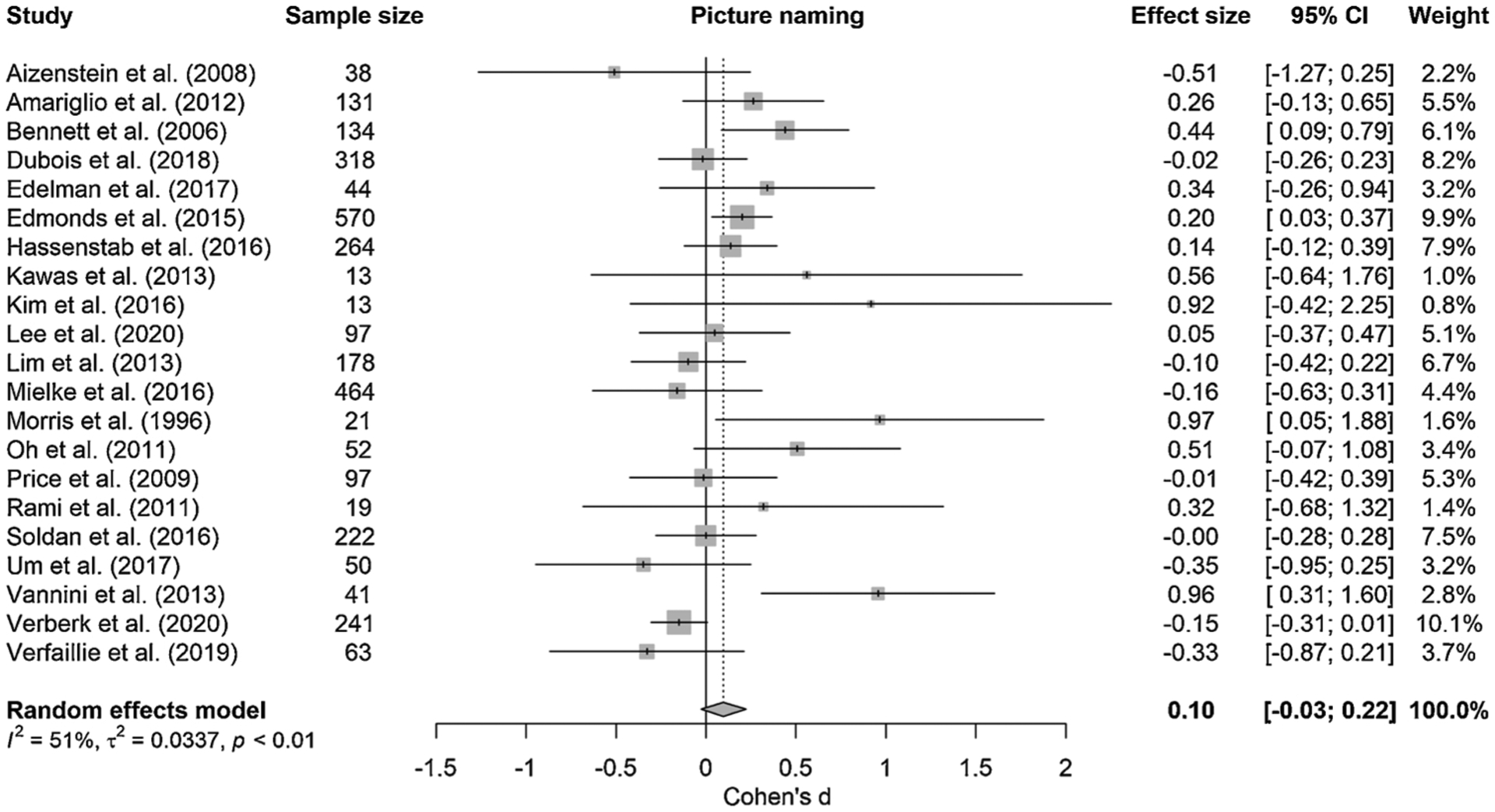
Forest plot of meta-analysis of the relationship between amyloid burden and picture naming; positive values represent greater impairment in performance in the presence of higher amyloid burden, dotted line represents no effect; size of the squares represents study weight.
Fig. 6.
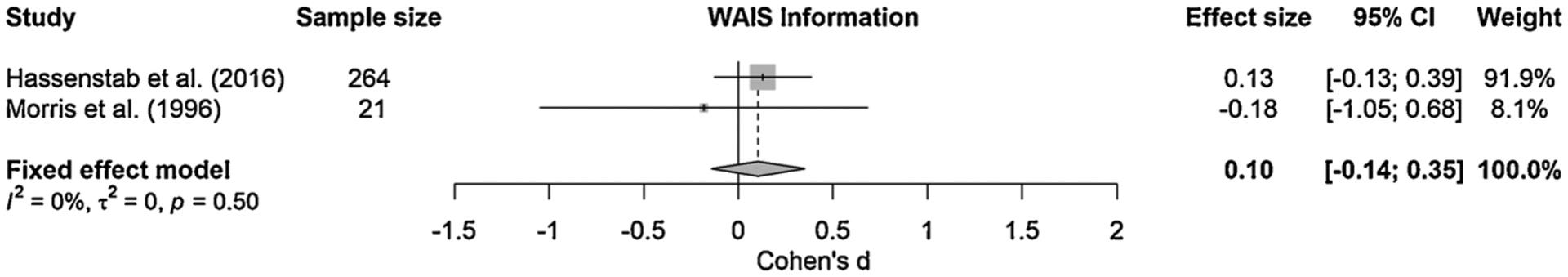
Forest plot of meta-analysis of the relationship between amyloid burden and WAIS Information; positive values represent greater impairment in performance in the presence of higher amyloid burden, dotted line represents no effect; size of the squares represents study weight.
To investigate whether the association between amyloid burden and semantic cognition differed across tasks, we tested the difference between pairs of tasks with a subgroup analysis. We found no difference in pooled estimates across any pairs of tests: picture naming and semantic fluency (Q = 1.58, p = 0.208), picture naming and vocabulary (Q = 0.11, p = 0.736), picture naming and WAIS Information (= < 0.01, p = 0.956), semantic fluency and vocabulary (Q = 0.05, p = 0.821), semantic fluency and WAIS Information (Q = .44, p = 0.507), and vocabulary and WAIS Information (Q = 0.06, p = 0.805).
3.4. Subgroup analyses
We performed subgroup analyses for tasks of picture naming and semantic fluency, as the number of studies available for vocabulary (n = 2) and WAIS Information (n = 2) did not allow for stratified analyses.
For picture naming, the association with amyloid was stronger among studies with individuals who were not selected on having subjective complaints compared to studies that included only individuals with subjective complaints (15 vs. 6 studies; Q = 8.72, df = 1, p = 0.003), when the cohort’s mean age was above 70 years old compared to a mean cohort age below 70 years (14 vs. 7 studies; Q = 11.02, df = 1, p = 0.001), and when a study’s analysis did not control for any covariates compared to those that controlled for at least one covariate (18 vs. 3 studies; Q = 7.49, df = 1, p = 0.006). We observed no subgroup differences when stratifying the studies based on amyloid assessment method (Q = 2.12, df = 3, p = 0.547) or when an analysis used a categorical as opposed to a continuous value of amyloid (4 vs. 17 studies; Q = 2.09, df = 1, p = 0.148). Forest plots of these subgroup analyses, including more detailed results, are available in Supplementary Figs. 1–5.
For semantic fluency, the association with amyloid was stronger among studies with individuals who were not selected on having subjective complaints compared to studies that included only individuals with subjective complaints (29 vs. 5 studies; Q = 4.12, df = 1, p = 0.042), controlled versus uncontrolled for covariates (6 vs. 28 studies; Q = 4.87, df = 1, p = 0.027), and when an analysis used a categorical as opposed to a continuous value of amyloid burden (25 vs. 9 studies; Q = 4.07, df = 1, p = 0.044). We found no differences between subgroups when stratifying the studies based on amyloid assessment method (Q = 3.89, df = 3, p = 0.273), or if a cohort’s mean age was below versus above 70 years (12 vs. 22 studies; Q = 1.26, df = 1, p = 0.262). Forest plots of these subgroup analyses, including more detailed results, are available in Supplementary Figs. 6–10.
4. Discussion
This systematic review and meta-analysis summarized the evidence on the cross-sectional association between amyloid burden and semantic cognition in older adults without dementia. By pooling effect sizes of this relationship across multiple studies for separate tasks of semantic cognition, we increased the power and precision of the estimated effect size compared to individual studies. We found that higher amyloid burden was associated with more impairment in tasks of semantic fluency, but not picture naming, vocabulary, and WAIS Information. The magnitude of the effect of amyloid burden on semantic fluency was, following established conventions (Cohen, 1988), small. We detected moderate statistical heterogeneity signaling a certain inconsistency of effects across studies. Subgroup analyses showed that subjective cognitive impairment and covariate adjustment modified the effect of amyloid burden on both picture naming and semantic fluency, while age only modified the effect for picture naming and categorical/continuous amyloid scale only modified the effect for semantic fluency. Amyloid method (i.e., histopathology, CSF, PET, blood) did not modify the effect for either task. We did not find evidence for publication bias. Risk of bias within studies was highest with regard to comparability, as the majority of effect sizes could not be calculated on covariate-adjusted statistics.
The presence of a cross-sectional relationship between amyloid burden and semantic fluency differs from what is reported across both individual studies as well as meta-analyses. In the individual studies included in this meta-analysis, few studies reported a cross-sectional relationship between amyloid burden and semantic fluency. The lack of a relationship could be observed in the CIs of individual studies’ effect sizes, which nearly all contained the value zero, even though the vast majority of the studies reported a positive effect estimate between amyloid burden and semantic fluency impairment. This discrepancy between the pooled estimate versus individual study effects can be explained by the high variance in individual studies, which is reduced when the effect sizes are pooled across all studies due to increased power by enlarging the sample size.
The discrepancy at the meta-analytic level between this study and the pooled estimates by Hedden et al. (2013) and Baker et al. (2017) is likely not due to insufficient power to detect effects, since all three meta-analyses included at least 14 studies to calculate a pooled effect size for semantic cognition. Instead, the discrepancy is most probably due to the use of semantic domain scores in the analyses by Hedden et al. (2013) and Baker et al. (2017). Hedden et al. (2013) argued for the use of domain scores under the assumption that individual tests of a cognitive domain are similar in their representation of that domain. They noted, however, that this assumption is relatively difficult to test due to the wide variability in tests for certain domains. Because of the increase in the number of studies on the relationship between amyloid burden and cognition in recent years, we could test this assumption by investigating the relationship between amyloid burden and semantic cognition separately across different tasks. Our results demonstrated that not all semantic tasks are equally strongly related to amyloid burden. Thus, the assumption by Hedden et al. (2013) does not hold for the semantic domain, and combining these tasks in a domain score would dilute the presence of task-specific effects.
Differences in the magnitude of effect sizes across picture naming, semantic fluency, vocabulary, and WAIS Information may be caused for various reasons. Semantic cognition has multiple components, including semantic control, semantic memory efficiency, and semantic representation (Jefferies, 2013; Vonk et al., 2020; Whitney et al., 2011). Different semantic tasks may tap into these components with different weights, which may make the tasks differentially sensitive to early cognitive symptoms in the course of Alzheimer’s disease. Tasks of semantic cognition may also vary in their sensitivity to detect impairment in cognitively normal older adults due to limitations in their metrics. For example, picture naming typically suffers from a ceiling effect in cognitively normal individuals (Moreno-Martínez and Laws, 2007), since a task like the Boston Naming Test—the most popular picture naming task across studies—was developed for individuals with cognitive impairment due to aphasia (Kaplan et al., 1983).
While semantic fluency has no maximum score and no floor effect in older individuals without dementia, the traditional metric of total number of words generated may be too coarse to detect subtle semantic decline cross-sectionally at such an early stage of Alzheimer’s disease in individual studies. For example, the traditional metric of semantic fluency was not sensitive enough to distinguish amyloid-positive from amyloid-negative individuals at baseline but explained unique variance in amyloid-related decline over time (Papp et al., 2017). Moreover, the traditional metric of semantic fluency (total number of items) has been shown to fail at distinguishing non-demented APOE e4 carriers (i.e., genetic risk for Alzheimer’s disease) from non-carriers, while an alternative item-level metric was able to distinguish these groups (Vonk et al., 2019a). Since the current meta-analysis provided evidence for the presence of semantic impairment in the early preclinical phase, future research should focus on developing more sensitive metrics of semantic fluency or other sensitive semantic measures to be able to detect a larger effect in a smaller sample (Venneri et al., 2018, 2019; Vonk et al., 2019a).
Another explanation for the relatively weak association between amyloid burden and semantic cognition in this study, as well as with other cognitive domains in previous meta-analyses (Baker et al., 2017; Hedden et al., 2013), may be that the relationship between amyloid and cognition is mediated by tau pathology, following the tau hyperphosphorylation hypothesis. Increased amyloid may be a signal for increased tau pathology and associated neurodegeneration in semantic networks later in the pathway (e.g., Han et al., 2012; Hanseeuw et al., 2019). A recent study by Weigand et al. (2020) among 301 older adults without dementia from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) contrasted cognitive performance across groups with and without amyloid and/or tau pathology. They showed that in the absence of tau pathology, no differences were found between amyloid-positive versus amyloid-negative individuals in memory, language, or executive functioning, while the largest effect sizes for all three domains were found when contrasting tau-positive versus tau-negative individuals in the amyloid-positive subgroup (Weigand et al., 2020). Another study showed that tau pathology, but not antecedent amyloid accumulation, correlated with cognition in individuals who were cognitively normal or had early symptoms of Alzheimer’s disease (Tosun et al., 2017). A literature review by Nelson et al. (2012) reported that at autopsy, the extent of cognitive impairment correlated more strongly with tau pathology than amyloid pathology. Thus, the specific impact of early Alzheimer’s disease on aspects of episodic memory and semantic cognition may be more strongly associated with tau pathology than amyloid pathology. Future research should explore differences in the strength of association among the three types of biomarkers of Alzheimer’s disease (i.e., amyloid, tau, and neurodegeneration) with episodic memory and semantic impairment for timely identification of individuals at high risk for clinical dementia.
Subgroup analyses contrasted various clinical and methodological factors of variability to explore sources of the moderate heterogeneity observed across effect estimates for both picture naming and semantic fluency. The relationship between amyloid and both tasks was stronger in studies that did not select only individuals with subjective impairment compared to studies that did. The etiology of subjective complaints can be highly heterogeneous (e.g., due to Alzheimer’s disease, other forms of dementia, depression, personality characteristics), particularly in a population that is different than one recruited in a memory clinic. More studies are needed to investigate the underlying cause of this difference across subgroups in relation to subjective cognitive impairment. The use of amyloid as a continuous versus categorical measurement revealed subgroup differences in semantic fluency, as the effect was weaker when amyloid was used as a continuous metric compared to a categorical metric. While not significant for picture naming, a similar pattern was observed in effect sizes between these subgroups. The use of amyloid as a continuous metric assumes a linear relationship with semantic impairment. Previous studies have shown that amyloid accumulation follows a sigmoid curve (Jack et al., 2013), with relatively slow accumulation at subthreshold biomarker levels followed by a relatively linear increase post-threshold until the accumulation rate levels off again at very high amyloid burden. Thus, using amyloid burden as a continuous metric may result in a weaker association between early amyloid burden and early semantic impairment due to this initial non-linear development of amyloid burden at subthreshold levels.
Several limitations of this research should be acknowledged, including various sources of bias. To avoid statistical bias by including the same individuals more than once, we had to exclude studies that reported results from the same cohort. We decided a priori to include the study with the largest sample size from a cohort. We should thus acknowledge that there are 42 more studies available that we could not include, but that have also investigated the association between amyloid burden and semantic impairment. We additionally had to exclude 36 studies that did not report sufficient information to compute an effect size of the association between amyloid burden and semantic cognition in cognitively normal older adults. We do not expect that exclusion of these studies substantially affected the pooled estimate of effect sizes, since we were able to include a substantial number of studies and we did not detect publication bias. However, additional inclusion of these studies—if sufficient information would have been reported—could have potentially reduced the uncertainty around the estimate, providing a more precise 95% CI. Future studies should adopt the standard practice of reporting effect sizes and confidence intervals with statistical estimates (Andrade, 2019), in addition to an extensive description of participant characteristics across all variables involved, including means and standard deviations across subsets of participants. Lastly, some of the subgroup analyses included relatively small groups of studies, which may have impacted the reliability of those results. Strengths of our study include the large number of included studies yielded by our thorough systematic search and the implementation of a quality assessment to outline the risk of bias within studies, which was not reported in either meta-analysis by Hedden et al. (2013) or Baker et al. (2017).
Assessment of cognitive abilities through neuropsychological testing is relatively easy, low-cost, and non-invasive compared to biomarker assessment—particularly in the context of primary care—and correlates with pathophysiological changes throughout the Alzheimer’s disease continuum (Baker et al., 2017; Hedden et al., 2013; Zhang, 2019). The results of this study confirmed the role of semantic impairment in early stages of Alzheimer’s disease. However, the relevance of the indicated amyloid-related decline in semantic fluency for research and clinical applications is likely negligible due to the effect’s small magnitude. Development of more sensitive semantic cognition markers of Alzheimer’s disease, in combination with biomarkers, could improve identification of high-risk individuals for early diagnosis and participation in clinical trials, and timely detection of Alzheimer’s disease-related symptoms in primary care settings.
Supplementary Material
Fig. 4.
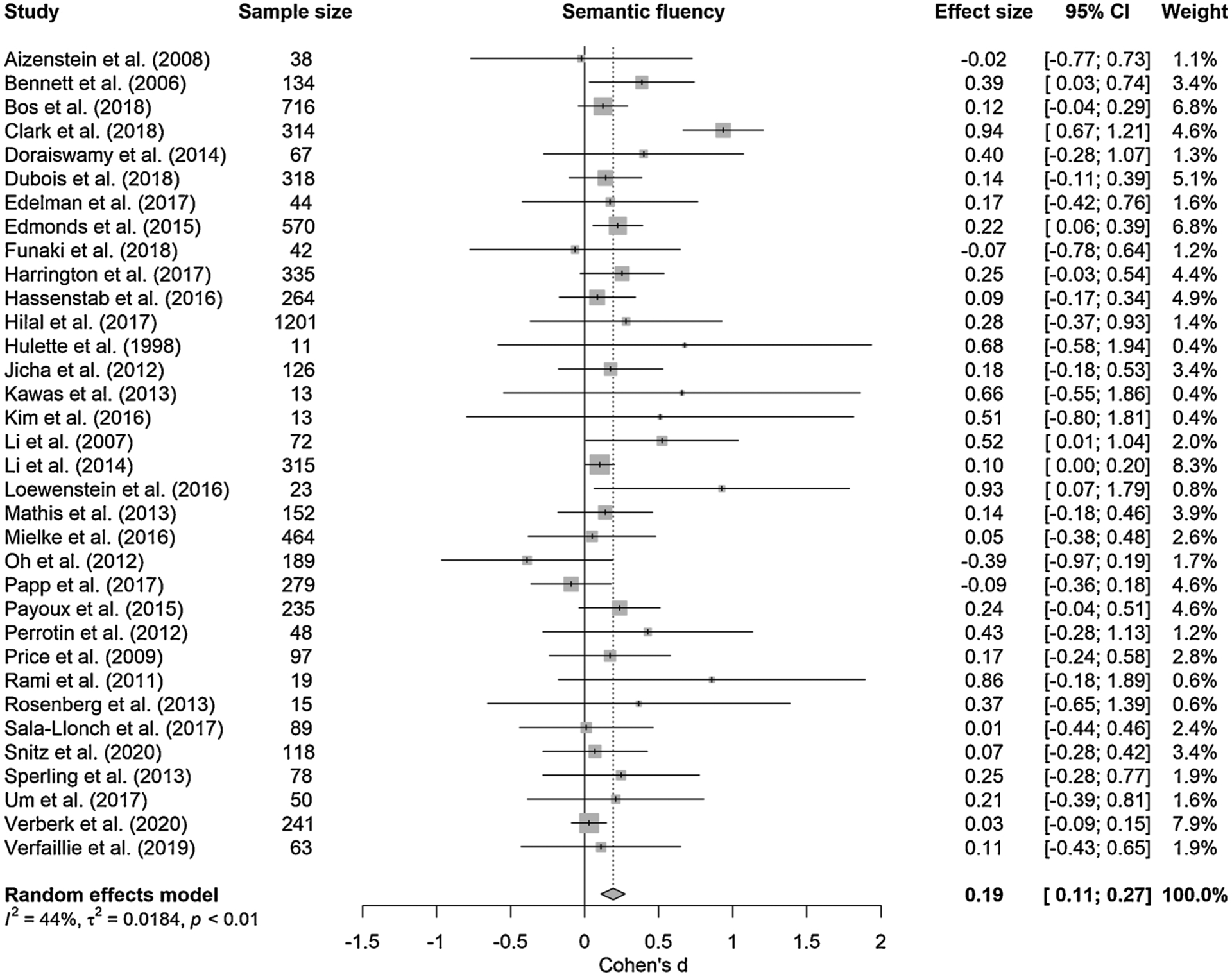
Forest plot of meta-analysis of the relationship between amyloid burden and semantic fluency; positive values represent greater impairment in performance in the presence of higher amyloid burden, dotted line represents no effect; size of the squares represents study weight.
Fig. 5.
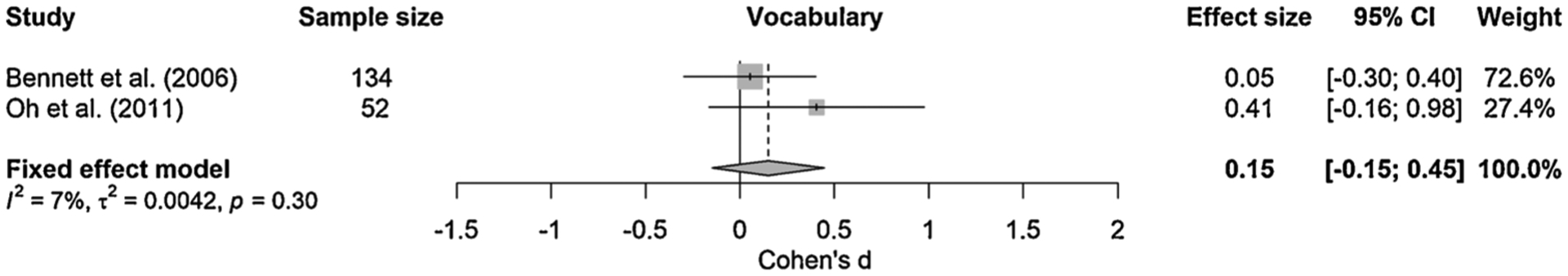
Forest plot of meta-analysis of the relationship between amyloid burden and vocabulary; positive values represent greater impairment in performance in the presence of higher amyloid burden, dotted line represents no effect; size of the squares represents study weight.
Acknowledgments
This work was supported by an Alzheimer Nederland Fellowship to J. M.J. Vonk (WE.15-2018-05), National Institute on Aging (NIA) K99/R00 award to J.M.J. Vonk (K99AG066934), and ZonMw NWO Veni grant to J.M.J. Vonk (project number 09150161810017). This study was conducted in the context of the Netherlands Consortium of Dementia Cohorts (NCDC); NCDC receives funding in the context of Deltaplan Dementie from ZonMw (project number 73305095005) and Alzheimer Nederland. We thank Paulien Wiersma, Librarian Medical Sciences at University Utrecht, for her help in preparing the systematic search strategy. We also thank Dr. Lotte Gerritsen for sharing her R code to perform the meta-analysis.
Footnotes
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.mad.2020.111386.
References
- Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA, Snitz BE, Houck PR, 2008. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch. Neurol 65, 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amariglio RE, Becker JA, Carmasin J, Wadsworth LP, Lorius N, Sullivan C, Maye JE, Gidicsin C, Pepin LC, Sperling RA, Johnson KA, Rentz DM, 2012. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia 50, 2880–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade C, 2019. The P value and statistical significance: misunderstandings, explanations, challenges, and alternatives. Indian J. Psychol. Med 41, 210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckman L, Small BJ, Fratiglioni L, 2001. Stability of the preclinical episodic memory deficit in Alzheimer’s disease. Brain 124, 96–102. [DOI] [PubMed] [Google Scholar]
- Baker JE, Lim YY, Pietrzak RH, Hassenstab J, Snyder PJ, Masters CL, Maruff P, 2017. Cognitive impairment and decline in cognitively normal older adults with high amyloid-beta: a meta-analysis. Alzheimers Dement. (Amst) 6, 108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, 2012. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N. Engl. J. Med 367, 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D, Schneider J, Arvanitakis Z, Kelly J, Aggarwal N, Shah R, Wilson R, 2006. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 66, 1837–1844. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL, 2009. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb. Cortex 19, 2767–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos I, Vos SJ, Jansen WJ, Vandenberghe R, Gabel S, Estanga A, Ecay-Torres M, Tomassen J, den Braber A, Lleó A, 2018. Amyloid-β, Tau, and cognition in cognitively normal older individuals: examining the necessity to adjust for biomarker status in normative data. Front. Aging Neurosci 10, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookmeyer R, Abdalla N, 2018. Estimation of lifetime risks of Alzheimer’s disease dementia using biomarkers for preclinical disease. Alzheimer’s Dementia 14, 981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitani E, Laiacona M, Barbarotto R, 1999. Gender affects word retrieval of certain categories in semantic fluency tasks. Cortex 35, 273–278. [DOI] [PubMed] [Google Scholar]
- Chertkow H, Whatmough C, Saumier D, Duong A, 2008. Cognitive neuroscience studies of semantic memory in Alzheimer’s disease. Prog. Brain Res 169, 393–407. [DOI] [PubMed] [Google Scholar]
- Chouraki V, Beiser A, Younkin L, Preis SR, Weinstein G, Hansson O, Skoog I, Lambert J-C, Au R, Launer L, 2015. Plasma amyloid-β and risk of Alzheimer’s disease in the Framingham Heart Study. Alzheimer’s Dementia 11 (249–257), e241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LJ, Gatz M, Zheng L, Chen YL, McCleary C, Mack WJ, 2009. Longitudinal verbal fluency in normal aging, preclinical, and prevalent Alzheimer’s disease. Am. J. Alzheimers Dis. Other Demen 24, 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LR, Berman SE, Norton D, Koscik RL, Jonaitis E, Blennow K, Bendlin BB, Asthana S, Johnson SC, Zetterberg H, Carlsson CM, 2018. Age-Accelerated cognitive decline in asymptomatic adults with csf β-Amyloid. Neurology 90, e1306–e1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, 1988. Statistical Power Analysis for the Behavioral Sciences. Lawrence Erlbaum Associates, Inc, Hillsdale, N.J. [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Grodstein F, Wright CI, Blacker D, Rosas HD, Sperling RA, Atri A, Growdon JH, Hyman BT, Morris JC, Fischl B, Buckner RL, 2009. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cerebral Cortex 19, 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didic M, Barbeau EJ, Felician O, Tramoni E, Guedj E, Poncet M, Ceccaldi M, 2011. Which memory system is impaired first in Alzheimer’s disease? J. Alzheimer Dis 27, 11–22. [DOI] [PubMed] [Google Scholar]
- Doraiswamy PM, Sperling RA, Johnson K, Reiman EM, Wong TZ, Sabbagh MN, Sadowsky CH, Fleisher AS, Carpenter A, Joshi AD, Lu M, Grundman M, Mintun MA, Skovronsky DM, Pontecorvo MJ, Duara R, Sabbagh M, Ahern GL, Holub RF, Farmer MV, Safirstein BE, Alva G, Longmire CF, Jewell G, Johnson KA, Korn R, Wendt JK, Wong D, Coleman RE, Devous M, Jennings D, Weiner MW, Murphy CA, Kovnat KD, Williamson JD, 2014. Florbetapir F 18 amyloid PET and 36-month cognitive decline:a prospective multicenter study. Mol. Psychiatry 19, 1044–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drago V, Babiloni C, Bartres-Faz D, Caroli A, Bosch B, Hensch T, Didic M, Klafki HW, Pievani M, Jovicich J, Venturi L, Spitzer P, Vecchio F, Schoenknecht P, Wiltfang J, Redolfi A, Forloni G, Blin O, Irving E, Davis C, Hardemark HG, Frisoni GB, 2011. Disease tracking markers for Alzheimer’s disease at the prodromal (MCI) stage. J. Alzheimers Dis 26 (Suppl 3), 159–199. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, DeKosky ST, Gauthier S, Selkoe D, Bateman R, 2014. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 13, 614–629. [DOI] [PubMed] [Google Scholar]
- Dubois B, Epelbaum S, Nyasse F, Bakardjian H, Gagliardi G, Uspenskaya O, Houot M, Lista S, Cacciamani F, Potier MC, Bertrand A, Lamari F, Benali H, Mangin JF, Colliot O, Genthon R, Habert MO, Hampel H, Audrain C, Auffret A, Baldacci F, Benakki I, Bertin H, Boukadida L, Cavedo E, Chiesa P, Dauphinot L, Dos Santos A, Dubois M, Durrleman S, Fontaine G, Genin A, Glasman P, Jungalee N, Kas A, Kilani M, La Corte V, Lehericy S, Letondor C, Levy M, Lowrey M, Ly J, Makiese O, Metzinger C, Michon A, Mochel F, Poisson C, Ratovohery S, Revillon M, Rojkova K, Roy P, Santos-Andrade K, Schindler R, Seux L, Simon V, Sole M, Tandetnik C, Teichmann M, Thiebaut de Shotten M, Younsi N, 2018. Cognitive and neuroimaging features and brain β-amyloidosis in individuals at risk of Alzheimer’s disease (INSIGHT-preAD): a longitudinal observational study. Lancet Neurol. 17, 335–346. [DOI] [PubMed] [Google Scholar]
- Edelman K, Tudorascu D, Agudelo C, Snitz B, Karim H, Cohen A, Mathis C, Price J, Weissfeld L, Klunk W, Aizenstein H, 2017. Amyloid-beta deposition is associated with increased medial temporal lobe activation during memory encoding in the cognitively normal elderly. Am. J. Geriatr. Psychiatry 25, 551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds EC, Delano-Wood L, Galasko DR, Salmon DP, Bondi MW, 2015. Subtle cognitive decline and biomarker staging in preclinical alzheimer’s disease. J. Alzheimer Dis 47, 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Mao C, Xu Y, Shi C, Hu X, Zhang S, Yang Z, Hu Z, Sun H, Fan Y, 2019. New insights into the pathogenesis of alzheimer’s disease. Front. Neurol 10, 1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funaki K, Nakajima S, Noda Y, Wake T, Ito D, Yamagata B, Yoshizaki T, Kameyama M, Nakahara T, Murakami K, Jinzaki M, Mimura M, Tabuchi H, 2019. Can we predict amyloid deposition by objective cognition and regional cerebral blood flow in patients with subjective cognitive decline? Psychogeriatrics 19, 325–332. [DOI] [PubMed] [Google Scholar]
- Glymour MM, Brickman AM, Kivimaki M, Mayeda ER, Cĥene G, Dufouil C, Manly JJ, 2018. Will biomarker-based diagnosis of Alzheimer’s disease maximize scientific progress? Evaluating proposed diagnostic criteria. Eur. J. Epidemiol 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SD, Gruhl J, Beckett L, Dodge HH, Stricker NH, Farias S, Mungas D, Initiative, As.D.N., 2012. Beta amyloid, tau, neuroimaging, and cognition: sequence modeling of biomarkers for Alzheimer’s disease. Brain Imaging Behav. 6, 610–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanseeuw BJ, Betensky RA, Jacobs HI, Schultz AP, Sepulcre J, Becker JA, Cosio DMO, Farrell M, Quiroz YT, Mormino EC, 2019. Association of amyloid and tau with cognition in preclinical Alzheimer disease: a longitudinal study. JAMA Neurol. 76, 915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Allsop D, 1991. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol. Sci 12, 383–388. [DOI] [PubMed] [Google Scholar]
- Harrington KD, Lim YY, Ames D, Hassenstab J, Laws SM, Martins RN, Rainey-Smith S, Robertson J, Rowe CC, Salvado O, 2017. Amyloid β–associated cognitive decline in the absence of clinical disease progression and systemic illness. Alzheimer’s Dementia 8, 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassenstab J, Chasse R, Grabow P, Benzinger TLS, Fagan AM, Xiong C, Jasielec M, Grant E, Morris JC, 2016. Certified normal: alzheimer’s disease biomarkers and normative estimates of cognitive functioning. Neurobiol. Aging 43, 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Oh H, Younger AP, Patel TA, 2013. Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology 80, 1341–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, 2019. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilal S, Akoudad S, Van Duijn CM, Niessen WJ, Verbeek MM, Vanderstichele H, Stoops E, Arfan Ikram M, Vernooij MW, 2017. Plasma Amyloid-β levels, cerebral small vessel disease, and cognition: the rotterdam study. J. Alzheimer Dis 60, 977–987. [DOI] [PubMed] [Google Scholar]
- Hodges J, 1998. The amnestic prodrome of Alzheimer’s disease. Brain 121, 1601. [DOI] [PubMed] [Google Scholar]
- Horn JL, Cattell RB, 1966. Age differences in primary mental ability factors. J. Gerontol 21, 210–220. [DOI] [PubMed] [Google Scholar]
- Horn JL, Cattell RB, 1967. Age differences in fluid and crystallized intelligence. Acta Psychol. (Amst) 26, 107–129. [DOI] [PubMed] [Google Scholar]
- Hulette CM, Welsh-Bohmer KA, Murray MG, Saunders AM, Mash DC, McIntyre LM, 1998. Neuropathological and neurolasychological changes in’ normal’ aging: evidence for preclinical Alzheimer Disease in cognitively normal individuals. J. Neuropathol. Exp. Neurol 57, 1168–1174. [DOI] [PubMed] [Google Scholar]
- Jack CR, Holtzman DM, 2013. Biomarker modeling of Alzheimer’s disease. Neuron 80, 1347–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Wiste HJ, Lesnick TG, Weigand SD, Knopman DS, Vemuri P, Pankratz VS, Senjem ML, Gunter JL, Mielke MM, 2013. Brain β-amyloid load approaches a plateau. Neurology 80, 890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FR, Visser PJ, Aalten P, Aarsland D, Alcolea D, 2015. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA 313, 1924–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies E, 2013. The neural basis of semantic cognition: converging evidence from neuropsychology, neuroimaging and TMS. Cortex 49, 611–625. [DOI] [PubMed] [Google Scholar]
- Jicha GA, Abner EL, Schmitt FA, Kryscio RJ, Riley KP, Cooper GE, Stiles N, Mendiondo MS, Smith CD, Van Eldik LJ, Nelson PT, 2012. Preclinical AD Workgroup staging: pathological correlates and potential challenges. Neurobiol. Aging 33, 622.e621–622.e616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert S, Brambati SM, Ansado J, Barbeau EJ, Felician O, Didic M, Lacombe J, Goldstein R, Chayer C, Kergoat M-J, 2010. The cognitive and neural expression of semantic memory impairment in mild cognitive impairment and early Alzheimer’s disease. Neuropsychologia 48, 978–988. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S, 1983. Boston Naming Test. Lea & Febiger, Philadelphia, PA. [Google Scholar]
- Kawas CH, Greenia DE, Bullain SS, Clark CM, Pontecorvo MJ, Joshi AD, Corrada MM, 2013. Amyloid imaging and cognitive decline in nondemented oldest-old: the 90+ Study. Alzheimer’s Dementia 9, 199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H-G, Kong E-J, Cheon E-J, Kim H-W, Koo B-H, 2016. Association between cerebral amyloid deposition and clinical factors including cognitive function in geriatric depression: pilot study using amyloid positron emission tomography. Clin. Psychopharmacol. Neurosci 14, 378–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama A, Okereke OI, Yang T, Blacker D, Selkoe DJ, Grodstein F, 2012. Plasma amyloid-β as a predictor of dementia and cognitive decline: a systematic review and meta-analysis. Arch. Neurol 69, 824–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JJ, Choi Y, Chung S, Yoon DH, Choi SH, Kang SM, Seo D, Park KI, 2020. Association of plasma oligomerized beta amyloid with neurocognitive battery using Korean version of consortium to establish a registry for Alzheimer’s disease in health screening population. Diagnostics 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Sokal I, Quinn JF, Leverenz JB, Brodey M, Schellenberg GD, Kaye JA, Raskind MA, Zhang J, Peskind ER, Montine TJ, 2007. CSF tau/Abeta42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurology 69, 631–639. [DOI] [PubMed] [Google Scholar]
- Li G, Millard SP, Peskind ER, Zhang J, Yu CE, Leverenz JB, Mayer C, Shofer JS, Raskind MA, Quinn JF, Galasko DR, Montine TJ, 2014. Cross-sectional and longitudinal relationships between cerebrospinal fluid biomarkers and cognitive function in people without cognitive impairment from across the adult life span. JAMA Neurol. 71, 742–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YY, Ellis KA, Harrington K, Kamer A, Pietrzak RH, Bush AI, Darby D, Martins RN, Masters CL, Rowe CC, 2013. Cognitive consequences of high Aβ amyloid in mild cognitive impairment and healthy older adults: implications for early detection of Alzheimer’s disease. Neuropsychology 27, 322. [DOI] [PubMed] [Google Scholar]
- Loewenstein DA, Curiel RE, Greig MT, Bauer RM, Rosado M, Bowers D, Wicklund M, Crocco E, Pontecorvo M, Joshi AD, Rodriguez R, Barker WW, Hidalgo J, Duara R, 2016. A novel cognitive stress test for the detection of preclinical alzheimer disease: discriminative properties and relation to amyloid load. Am. J. Geriatr. Psychiatry 24, 804–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra C, Ferraccioli M, Gainotti G, 2007. Gender-related dissociations of categorical fluency in normal subjects and in subjects with Alzheimer’s disease. Neuropsychology 21, 207. [DOI] [PubMed] [Google Scholar]
- Mathis CA, Kuller LH, Klunk WE, Snitz BE, Price JC, Weissfeld LA, Rosario BL, Lopresti BJ, Saxton JA, Aizenstein HJ, McDade EM, Kamboh MI, Dekosky ST, Lopez OL, 2013. In vivo assessment of amyloid-β deposition in nondemented very elderly subjects. Ann. Neurol 73, 751–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Machulda MM, Hagen CE, Christianson TJ, Roberts RO, Knopman DS, Vemuri P, Lowe VJ, Kremers WK, Jack CR, Petersen RC, 2016. Influence of amyloid and APOE on cognitive performance in a late middle-aged cohort. Alzheimer’s Dementia 12, 281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P, 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6, e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Martínez FJ, Laws KR, 2007. An attenuation of the ‘normal’category effect in patients with Alzheimer’s disease: a review and bootstrap analysis. Brain Cogn. 63, 167–173. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, McKeel DW Jr, Rubin EH, Price JL, Grant EA, Berg L, 1996. Cerebral amyloid deposition and diffuse plaques in’ normal’ aging: evidence for presymptomatic and very mild Alzheimer’s disease. Neurology 46, 707–719. [DOI] [PubMed] [Google Scholar]
- Murphy KJ, Rich JB, Troyer AK, 2006. Verbal fluency patterns in amnestic mild cognitive impairment are characteristic of Alzheimer’s type dementia. J. Int. Neuropsychol. Soc 12, 570–574. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ, Davies P, Tredici KD, 2012. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J. Neuropathol. Exp. Neurol 71, 362–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Mormino EC, Madison C, Hayenga A, Smiljic A, Jagust WJ, 2011. β-Amyloid affects frontal and posterior brain networks in normal aging. NeuroImage 54, 1887–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Madison C, Haight TJ, Markley C, Jagust WJ, 2012. Effects of age and β-amyloid on cognitive changes in normal elderly people. Neurobiol. Aging 33, 2746–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele R, Jansen WJ, Rabinovici GD, Knol DL, van der Flier WM, van Berckel BN, Scheltens P, Visser PJ, Verfaillie SC, Zwan MD, 2015. Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. Jama 313, 1939–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A, 2016. Rayyan—a web and mobile app for systematic reviews. Syst. Rev 5, 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp KV, Mormino EC, Amariglio RE, Munro C, Dagley A, Schultz AP, Johnson KA, Sperling RA, Rentz DM, 2016. Biomarker validation of a decline in semantic processing in preclinical Alzheimer’s disease. Neuropsychology 30, 624–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp KV, Rentz DM, Orlovsky I, Sperling RA, Mormino EC, 2017. Optimizing the preclinical Alzheimer’s cognitive composite with semantic processing: the PACC5. Alzheimer’s Dementia 3, 668–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK, 2002. Models of visuospatial and verbal memory across the adult life span. Psychol. Aging 17, 299–320. [PubMed] [Google Scholar]
- Payoux P, Delrieu J, Gallini A, Adel D, Salabert AS, Hitzel A, Cantet C, Tafani M, De Verbizier D, Darcourt J, Fernandez P, Monteil J, Carrié I, Voisin T, Gillette-Guyonnet S, Pontecorvo M, Vellas B, Andrieu S, 2015. Cognitive and functional patterns of nondemented subjects with equivocal visual amyloid PET findings. Eur. J. Nucl. Med. Mol. Imaging 42, 1459–1468. [DOI] [PubMed] [Google Scholar]
- Pereira TV, Patsopoulos NA, Salanti G, Ioannidis JP, 2010. Critical interpretation of Cochran’s Q test depends on power and prior assumptions about heterogeneity. Res. Synth. Methods 1, 149–161. [DOI] [PubMed] [Google Scholar]
- Perrotin A, Mormino EC, Madison CM, Hayenga AO, Jagust WJ, 2012. Subjective cognition and amyloid deposition imaging: a Pittsburgh compound B positron emission tomography study in normal elderly individuals. Arch. Neurol 69, 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J, Welch V, Losos M, Tugwell P, 2011. The Newcastle-ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. Ottawa Hospital Research Institute, Ottawa. [Google Scholar]
- Price JL, McKeel DW Jr, Buckles VD, Roe CM, Xiong C, Grundman M, Hansen LA, Petersen RC, Parisi JE, Dickson DW, Smith CD, Davis DG, Schmitt FA, Markesbery WR, Kaye J, Kurlan R, Hulette C, Kurland BF, Higdon R, Kukull W, Morris JC, 2009. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol. Aging 30, 1026–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2018. R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Rami L, Fortea J, Bosch B, Solé-Padullés C, Lladó A, Iranzo A, Sánchez-Valle R, Molinuevo JL, 2011. Cerebrospinal fluid biomarkers and memory present distinct associations along the continuum from healthy subjects to AD patients. J. Alzheimer Dis 23, 319–326. [DOI] [PubMed] [Google Scholar]
- Rao SM, Bonner-Jackson A, Nielson KA, Seidenberg M, Smith JC, Woodard JL, Durgerian S, 2015. Genetic risk for Alzheimer’s disease alters the five-year trajectory of semantic memory activation in cognitively intact elders. Neuroimage 111, 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roostaei T, Nazeri A, Felsky D, De Jager PL, Schneider JA, Pollock BG, Bennett DA, Voineskos AN, 2017. Genome-wide interaction study of brain beta-amyloid burden and cognitive impairment in Alzheimer’s disease. Mol. Psychiatry 22, 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg PB, Wong DF, Edell SL, Ross JS, Joshi AD, Brašíc JR, Zhou Y, Raymont V, Kumar A, Ravert HT, Dannals RF, Pontecorvo MJ, Skovronsky DM, Lyketsos CG, 2013. Cognition and amyloid load in Alzheimer disease imaged with florbetapir F 18 (AV-45) positron emission tomography. Am. J. Geriatr. Psychiatry 21, 272–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala-Llonch R, Idland AV, Borza T, Watne LO, Wyller TB, Brækhus A, Zetterberg H, Blennow K, Walhovd KB, Fjell AM, 2017. Inflammation, amyloid, and atrophy in the aging brain: relationships with longitudinal changes in cognition. J. Alzheimer Dis 58, 829–840. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, 2010. Selective review of cognitive aging. J. Int. Neuropsychol. Soc 16, 754–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Janse E, Visser K, Meyer AS, 2014. What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Front. Psychol 5, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snitz BE, Weissfeld LA, Lopez OL, Kuller LH, Saxton J, Singhabahu DM, Klunk WE, Mathis CA, Price JC, Ives DG, 2013. Cognitive trajectories associated with β-amyloid deposition in the oldest-old without dementia. Neurology 80, 1378–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snitz BE, Tudorascu DL, Yu Z, Campbell E, Lopresti BJ, Laymon CM, Minhas DS, Nadkarni NK, Aizenstein HJ, Klunk WE, Weintraub S, Gershon RC, Cohen AD, 2020. Associations between NIH Toolbox Cognition Battery and in vivo brain amyloid and tau pathology in non-demented older adults. Alzheimers Dement. (Amst) 12, e12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldan A, Pettigrew C, Cai Q, Wang MC, Moghekar AR, O’Brien RJ, Selnes OA, Albert MS, Rodzon B, Gottesman R, Sacktor N, McKhann G, Turner S, Farrington L, Grega M, Rudow G, D’Agostino D, Rudow S, Miller M, Mori S, Ratnanather T, Brown T, Chi H, Kolasny A, Oishi K, Reigel T, Younes L, Spangler A, Scherer R, Shade D, Ervin A, Jones J, Toepfner M, Parlett L, Patterson A, Mohammed A, Lu D, Troncoso J, Crain B, Pletnikova O, Fisher K, 2016. Hypothetical preclinical Alzheimer disease groups and longitudinal cognitive change. JAMA Neurol. 73, 698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Johnson KA, Doraiswamy PM, Reiman EM, Fleisher AS, Sabbagh MN, Sadowsky CH, Carpenter A, Davis MD, Lu M, Flitter M, Joshi AD, Clark CM, Grundman M, Mintun MA, Skovronsky DM, Pontecorvo MJ, 2013. Amyloid deposition detected with florbetapir F 18 (18F-AV-45) is related to lower episodic memory performance in clinically normal older individuals. Neurobiol. Aging 34, 822–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosun D, Landau S, Aisen PS, Petersen RC, Mintun M, Jagust W, Weiner MW, Initiative, As.D.N., 2017. Association between tau deposition and antecedent amyloid-β accumulation rates in normal and early symptomatic individuals. Brain 140, 1499–1512. [DOI] [PubMed] [Google Scholar]
- Um YH, Choi WH, Jung WS, Park YH, Lee C-U, Lim HK, 2017. Whole brain voxel-wise analysis of cerebral retention of Beta-amyloid in cognitively normal older adults using 18F-florbetaben. Psychiatry Investig. 14, 883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini P, Hedden T, Huijbers W, Ward A, Johnson KA, Sperling RA, 2013. The ups and downs of the posteromedial cortex: age- and amyloid-related functional alterations of the encoding/retrieval flip in cognitively normal older adults. Cereb. Cortex 23, 1317–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneri A, Mitolo M, De Marco M, 2016. Paradigm shift: semantic memory decline as a biomarker of preclinical Alzheimer’s disease. Future Med. [DOI] [PubMed] [Google Scholar]
- Venneri A, Jahn-Carta C, de Marco M, Quaranta D, Marra C, 2018. Diagnostic and prognostic role of semantic processing in preclinical Alzheimer’s disease. Biomark. Med 12, 637–651. [DOI] [PubMed] [Google Scholar]
- Venneri A, Mitolo M, Beltrachini L, Varma S, Della Pieta C, Jahn-Carta C, Frangi AF, De Marco M, 2019. Beyond episodic memory: semantic processing as independent predictor of hippocampal/perirhinal volume in aging and mild cognitive impairment due to Alzheimer’s disease. Neuropsychology 33, 523–533. [DOI] [PubMed] [Google Scholar]
- Verberk IMW, Hendriksen HMA, van Harten AC, Wesselman LMP, Verfaillie SCJ, van den Bosch KA, Slot RER, Prins ND, Scheltens P, Teunissen CE, Van der Flier WM, 2020. Plasma amyloid is associated with the rate of cognitive decline in cognitively normal elderly: the SCIENCe project. Neurobiol. Aging 89, 99–107. [DOI] [PubMed] [Google Scholar]
- Verfaillie SCJ, Witteman J, Slot RER, Pruis IJ, Vermaat LEW, Prins ND, Schiller NO, van de Wiel M, Scheltens P, van Berckel BNM, van der Flier WM, Sikkes SAM, 2019. High amyloid burden is associated with fewer specific words during spontaneous speech in individuals with subjective cognitive decline. Neuropsychologia 131, 184–192. [DOI] [PubMed] [Google Scholar]
- Verhage F, 1965. Intelligence and age in a Dutch sample. Hum. Dev 8, 238–245. [Google Scholar]
- Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, Szoeke C, Macaulay SL, Martins R, Maruff P, 2013. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 12, 357–367. [DOI] [PubMed] [Google Scholar]
- Vonk JMJ, Flores RJ, Rosado D, Qian C, Cabo R, Habegger J, Louie K, Allocco E, Brickman AM, Manly JJ, 2019a. Semantic network function captured by word frequency in nondemented APOE ε4 carriers. Neuropsychology 33 (2), 256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonk JMJ, Rizvi B, Lao PJ, Budge M, Manly JJ, Mayeux R, Brickman AM, 2019b. Letter and category fluency performance correlates with distinct patterns of cortical thickness in older adults. Cereb. Cortex 29, 2694–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonk JMJ, Higby E, Nikolaev A, Cahana-Amitay D, Spiro R I.I.I., Albert ML, Obler LK, 2020. Demographic effects on longitudinal semantic processing, working memory, and cognitive speed. J. Gerontol. B Psychol. Sci. Soc. Sci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigand AJ, Bangen KJ, Thomas KR, Delano-Wood L, Gilbert PE, Brickman AM, Bondi MW, Initiative, As.D.N., 2020. Is tau in the absence of amyloid on the Alzheimer’s continuum?: a study of discordant PET positivity. Brain communications 2, fcz046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney C, Kirk M, O’Sullivan J, Lambon Ralph MA, Jefferies E, 2011. The neural organization of semantic control: TMS evidence for a distributed network in left inferior frontal and posterior middle temporal gyrus. Cereb. Cortex 21, 1066–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth M, Oh H, Mormino EC, Markley C, Landau SM, Jagust WJ, 2013. The effect of amyloid β on cognitive decline is modulated by neural integrity in cognitively normal elderly. Alzheimer’s & Dementia 9 (687–698), e681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischik C, Novak M, Edwards P, Klug A, Tichelaar W, Crowther R, 1988. Structural characterization of the core of the paired helical filament of Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A 85, 4884–4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, 2019. Is semantic learning strategy an early clinical marker for amnestic mild cognitive impairment and Alzheimer’s disease? Int. Psychogeriatr 31, 1695–1697. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


