Abstract
Cigarette smoke is a potent proinflammatory trigger contributing to acute lung injury and the development of chronic lung diseases via mechanisms that include the impairment of inflammation resolution. We have previously demonstrated that secondhand smoke (SHS)-exposure exacerbates bacterial infection-induced pulmonary inflammation and suppresses immune responses. It is now recognized that resolution of inflammation is a bioactive process mediated by lipid-derived specialized proresolving mediators (SPM), that counter-regulate proinflammatory signaling and promote resolution pathways. We therefore hypothesized that pro-resolving mediators could reduce the burden of inflammation due to chronic lung infection following SHS exposure, and restore normal immune responses to respiratory pathogens. To address this question, we exposed mice to SHS followed by chronic infection with non-typeable Haemophilus influenzae (NTHI). Some groups of mice were treated with aspirin-triggered resolvin D1 (AT-RvD1) during the latter half of the smoke exposure period or during a period of smoking-cessation and before infection. Treatment with AT-RvD1 markedly reduced the recruitment of neutrophils, macrophages and T-cells in lung tissue and bronchoalveolar lavage (BAL) and levels of proinflammatory cytokines in the BAL. Additionally, treatment with AT-RvD1 improved antibody titers against the NTHI outer-membrane lipoprotein antigen P6 following infection. Furthermore, treatment with AT-RvD1 prior to classically adjuvanted immunization with P6 increased antigen-specific antibody titers resulting in rapid clearance of NTHI from the lungs after acute challenge. Collectively, we have demonstrated that AT-RvD1 potently reverses the detrimental effects of SHS on pulmonary inflammation and immunity, and thus could be beneficial in reducing lung injury associated with smoke exposure and infection.
Introduction
Cigarette smoke (CS) in the form of mainstream tobacco smoke (MTS) or secondhand smoke (SHS) globally kills around 8 million people annually (1). Smoking is a major risk factor in the development and progression of cancer, cardiovascular disorders and respiratory diseases like chronic obstructive pulmonary disease (COPD) (2,3,4). Chronic respiratory diseases are among the leading causes of death worldwide (2–6). CS as a proinflammatory trigger is immunosuppressive, enhancing the risk of infections associated with respiratory diseases like COPD (4, 7–15). In the lung, many cells including macrophages and fibroblasts respond to CS by producing multiple pro-inflammatory mediators resulting in an inflammatory microenvironment and inducing tissue damage (4, 9–14). Importantly, long term smokers with and without COPD, and people chronically exposed to SHS, are at increased risk from acute exacerbations due to infections that are responsible for the majority of the morbidity, mortality, and costs of smoking-related lung diseases, which can persist even long after smoking cessation (7, 8, 14–19). Nontypeable Haemophilus influenzae (NTHI) is an opportunistic gram-negative bacterium commonly found in the upper respiratory tract. It causes otitis media in children, bronchitis in adults and the majority of invasive H. influenzae disease in all age groups (20). It is a major cause of acute exacerbations and worsening of symptoms in individuals with COPD (21). Vaccination trials against NTHI in individuals with recurrent exacerbations of chronic bronchitis or COPD have not been successful (22). Moreover, NTHI infections are typically persistent in COPD patients with some strains acquiring drug resistance and potential to avoid phagocytosis, thus leading to airway bacterial colonization (7, 23–25). NTHI infections induce a potent and prolonged inflammatory response in COPD patients, thus repeated infections could further worsen the inflammatory microenvironment and lead to extensive lung tissue destruction (7, 8, 13, 26). We have demonstrated that MTS and SHS worsen NTHI-induced pulmonary inflammation and suppress antibacterial immunity (9,10). Importantly, SHS exposure significantly impaired the protective antibody response to immunization with NTHI P6 protein in Freund’s adjuvant. We have also reported that SHS exposure has long-term consequences on the lung microenvironment (10), and we thus reasoned that SHS may also induce defects in resolution of inflammation.
Timely resolution of inflammation is critical to tissue remodeling and wound healing to minimize tissue damage (27–29). Resolution of inflammation, previously thought to occur passively, is an active process mediated by a family of endogenous lipid-derived mediators, termed specialized pro-resolving mediators (SPMs) (30–35). SPMs offer both anti-inflammatory and pro-resolving actions without inducing immunosuppression (34, 36–40). There is growing interest in evaluating the efficacy of SPMs in resolving CS- and infection-associated pulmonary inflammation to allow the design of strategies to manage lung diseases having an inflammatory component as a causal factor. SPMs play two distinct roles that are of special interest when considering the problem of respiratory infections in smokers and people exposed to SHS. First, SPMs can mitigate acute and chronic inflammation. There is evidence that smokers with COPD have lower levels of RvD1 in their serum and BAL fluid (41) and higher levels of eicosanoid oxidoreductase (EOR), an enzyme that degrades SPMs, in their lung tissue (42). Aspirin-triggered RvD1 is a stereoisomer of RvD1 that is formed when the enzyme cyclooxygenase-2 is acetylated by aspirin. AT-RvD1 exhibits increased biological activity and a longer in vivo half-life than RvD1, due in part to its resistance to degrading enzymes (43, 44), and is generally preferred for in vivo experiments for this reason.
Second, SPMs have important properties in modulating adaptive immune responses. SPMs inhibit maturation of DCs, inhibit Th17 responses and promote Tregs, all of which tend to promote a return to homeostasis (reviewed in 45). SPMs can either up- or downregulate antibody production, depending on the specific SPM and the cellular context. We recently reported that AT-RvD1 or its precursor 17-hydroxydocosahexaenoic acid (17-HDHA) as vaccine adjuvants could restore antibody responses to NTHI P6 antigen in mice exposed to SHS, resulting in improved clearance of NTHI following an acute infectious challenge (46). This raises the exciting possibility that SPMs could be used as a novel class of adjuvants to enhance vaccine responses to common respiratory pathogens and mitigate the effects of chronic SHS exposure.
However, improving vaccine efficacy does not address the underlying cause of the increased susceptibility to respiratory infection, which is chronic inflammation resulting from SHS exposure. Therefore, we set out to determine whether proresolving AT-RvD1, given during smoke exposure or during cessation, could mitigate lung inflammation due to chronic infection and improve the adaptive immune response to the classically adjuvanted antigen vaccination. We report here that AT-RvD1 given during SHS exposure or during cessation, reduced lung inflammation and inflammatory cytokines, and blocked the pathological Th17 response, while enhancing the antibody response to NTHI. Treatment with AT-RvD1 during smoke exposure or after cessation also improved antibody responses to vaccination using conventional adjuvants that began after the final treatment with AT-RvD1. Collectively these results demonstrate that AT-RvD1 mitigates lung inflammation and improves immune responses to NTHI, suggesting that AT-RvD1 has a high translational potential in people formerly exposed to SHS.
Materials and Methods
Mice
Eight week old female C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were used in all experiments. Animals were maintained under specific pathogen-free conditions. Number of animals per group in each experiment was n=10, unless mentioned otherwise in the figure legends. All procedures performed on animals were approved by the Animal Care and Use Committees of both institutions (Roswell Park Comprehensive Cancer Center, Buffalo, NY and University of Rochester, Rochester NY), and complied with all state, federal, and National Institutes of Health regulations.
Secondhand smoke (SHS) exposure
Animals were housed in the Inhalation Core Facility at the University of Rochester and exposed to SHS 5 h per day, 5 days per week for 8 wks as described previously (10). Briefly, 3R4F research cigarettes were combusted using an automated smoking machine (TE-10, Teague Enterprises, Woodland CA). Mainstream tobacco smoke was generated in a puff volume of 35 ml in 2 sec duration once per minute (the Federal Trade Commission (FTC) protocol). SHS was generated by collecting and mixing sidestream smoke with the mainstream tobacco smoke at a ratio of 89%/11% (47). Total particulate matter (TPM) concentration of 99±3 mg/m3 was achieved for these experiments by adjusting the number of cigarettes loaded and the flow rate of the dilution air. Control mice were exposed to filtered air using the same exposure protocol.
AT-RvD1 treatment Regimen
AT-RvD1 (Cayman Chemical) was dissolved in ethanol at 200μg/ml, aliquoted, and stored frozen at −20°C. Immediately prior to administration, the AT-RvD1 was diluted with 7 volumes of saline, and 40μl per mouse was administered by oropharyngeal aspiration under isoflurane anesthesia (48). Thus, each mouse received 1μg AT-RvD1 in 40μl of 12.5% ethanol in saline. Control animals received 40μl of 12.5% ethanol in saline. Mice were treated twice per week (Monday and Thursday or Tuesday and Friday), 1 h prior to the start of the smoke exposure. For one experiment, mice were treated on wks 5–8 of an 8-wk smoke exposure (Figure 1). For a second experiment, mice were treated on wks 5–12, where wks 5–8 were the second half of smoke exposure and wks 9–12 were a 4-wk smoke-free cessation period (Supplemental Figure 1).
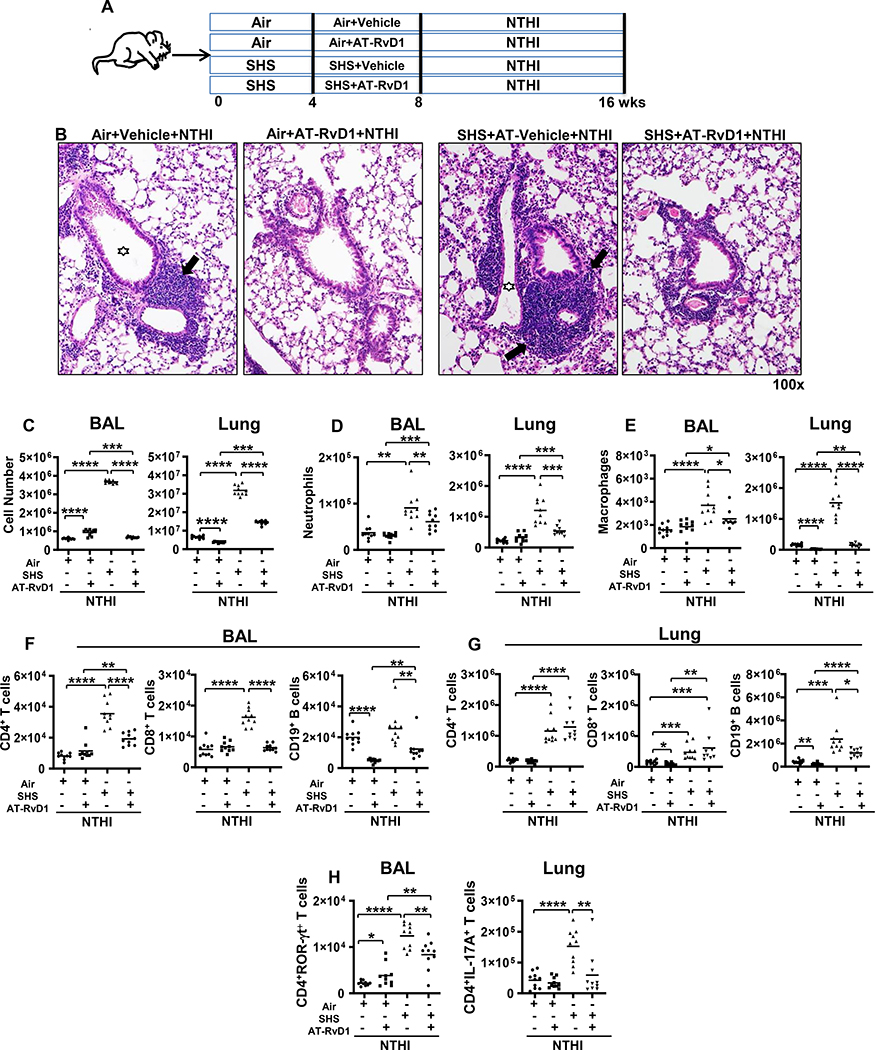
FIGURE 1. AT-RvD1 treatment reduces SHS-exacerbated, NTHI-mediated pulmonary inflammation.
(A) C57BL/6J mice were exposed to air or SHS for 8 wks (5 days/wk; 5 h/day) with AT-RvD1 or vehicle given during the last 4 wks of exposure, and were then chronically infected by intratracheal (i.t.) NTHI instillation for an additional 8 wks. Animals were euthanized 48 h after the final instillation. (B) Representative H&E stained histological sections of lung from each group showing resolution of SHS-exacerbated pulmonary inflammation as a consequence of AT-RvD1 treatment. Large areas of lymphocytic inflammation around bronchovascular bundles are depicted by arrows and airway lumen are shown with stars. Original magnification x100. (C) Count of total white blood cells in the BAL (left) and lungs (right) of air or SHS-exposed mice that were treated with vehicle or AT-RvD1 and then infected with NTHI by i.t. administration for 8 wks (n = 10 per group). Innate (D-E), adaptive (F-G) and inflammatory (H) immune cell composition in the BAL and lungs of treated mice were evaluated by flow cytometry using cell type-specific markers as described in materials and methods. Our gating strategy is shown in Supplemental Figure 2. Line represents mean value for the group. All treatment groups were performed at the same time, and data represent results generated from a single experiment using a total of n=10 mice/group. The results are depicted as mean ± SE. Statistical significance between the treatment groups was determined using two-tailed unpaired students t test, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Chronic and acute pulmonary infection with NTHI
Chronic and acute pulmonary infection experiments with NTHI were done similarly as described previously (9, 10). A frozen glycerol stock of NTHI bacterial strain 1479 (clinical isolate from a COPD exacerbation) was streaked onto chocolate-agar plates and incubated overnight at 35°C under 5% CO2 conditions. A loop-full of NTHI colonies was grown in a liquid culture of brain-heart infusion media supplemented with 10 mg/ml hemin and 10 mg/ml β-nicotinamide adenine dinucleotide (Sigma, St. Louis, Missouri) for 3–4 h in a 35°C shaking incubator. OD600nm was measured to dilute the required number of CFUs in PBS to 2 × 107 CFUs/ml. Bacteria were pelleted by centrifugation at 13,000 x g for 5 minutes and washed twice in PBS. When air or SHS exposures and AT-RvD1 treatments were completed, NTHI was administered by intratracheal route as described previously (10). To achieve NTHI-induced chronic pulmonary inflammation, mice were given 1 × 106 live bacteria twice a week for 8 consecutive weeks. For acute challenge, mice were given a single intratracheal instillation with 1 × 106 live NTHI bacteria and euthanized 4 and 24 h later to collect the BAL and whole lungs for evaluating the clearance of bacteria from the lungs of mice and quantifying albumin leak as a marker of lung epithelial-cell damage.
P6 immunization
When air or SHS exposures and AT-RvD1 treatments were completed, mice were vaccinated with 40 μg purified native P6 emulsified in CFA, boosted 1 wk later with P6 in IFA and 2 wks later with P6 alone as described previously (9, 10, 49). To quantify the titers and levels of antigen-specific antibodies by ELISA, mice were bled retro-orbitally on a weekly basis to collect immune sera.
Lung histology
At the end of the chronic pulmonary infection, animals were euthanized, and lungs harvested. Lungs were fixed overnight in 10% neutral buffered formalin (Merck, Gibbstown, NJ), then transferred to ethanol, embedded in paraffin, sectioned, and stained with H&E by the Mouse Tumor Model Resources (MTMR) at Roswell Park Comprehensive Cancer Center. Images were taken on an Olympus light microscope equipped with a CCD camera and Spot image analysis software (v25.4; Diagnostics Instruments, Sterling Heights, MI). Lung pathology was evaluated by a pathologist (P.N.B.) who was blinded to treatment groups, as described previously (10).
Bronchoalveolar lavage (BAL) collection
To determine total leukocyte counts, numbers of specific immune cells, the levels of various cytokines, albumin and antigen-specific antibody titers in the lung lavage, we harvested the BAL from the lungs of mice as described previously (9,10). Briefly, after treatments were completed, mice were euthanized by injecting intra-peritoneally 1 ml of warmed 2.5% avertin solution (2,2,2-tribromethanol). After no pinch reflex was observed in the animals, the thoracic cavity was opened, and the trachea cannulated. Lungs were gently lavaged twice by injecting 750 μl of ice cold 1% BSA solution in PBS as described previously (10). Total leukocyte counts in BAL samples were calculated by hemocytometer before staining with cell type-specific antibodies for FACS analysis. Cell-free BAL fluid was stored at −80°C till used to quantitate the levels of cytokines and albumin and also to measure the titers of antigen-specific antibodies by ELISA.
Isolation of lung lymphocytes, splenocytes and bone marrow cells
After mice were euthanized, lungs were excised and minced into small pieces in a 6 cm petri-dish on ice. The resulting lung slurry was mixed with 1 mg/ml Type IA-S collagenase solution containing 50 U/mL DNase I (Sigma) and placed for 1 h on a rotator at 37°C. Single-cell suspension was passed through a 40 μm filter to remove debris and undigested tissue, and then centrifuged for 5 minutes and resuspended in complete RPMI-1640 culture media containing 10% FBS. The cell suspension was then gently overlaid on top of Ficoll-Paque and centrifuged at 700 x g for 30 minutes with brake-off. Immune cells at the interface were collected, washed twice in cell culture media to remove residual Ficoll-Paque and counted. Single cell suspensions of splenocytes were isolated in complete cell culture media. Bone marrow was collected by flushing femur and tibia with sterile ice-cold PBS. Splenocytes and bone marrow cells were centrifuged for 5 minutes at 500 x g at room temperature, resuspended in complete cell culture media and counted using hemocytometer.
Flow cytometry
Immune cell populations in the BAL and lungs of mice were determined by flow cytometry as described previously (10). Briefly, 0.5 million BAL cells and lung lymphocytes from each sample were stained with specific fluorophore-tagged antibodies in 100 μl of FACS staining buffer (1% BSA in PBS) for 30 minutes at 4○C and then washed in FACS buffer before fixing with cytofix (BD Biosciences, CA, USA). To stain intracellular markers, cells were first treated with permeabilizing solution (BD Biosciences) and then stained with cell-type specific fluorophore-tagged antibodies. All the samples were acquired on LSRII-A flow cytometer and data were analyzed by FlowJo software.
ELISA
To quantitate the cytokine levels, albumin leak into the BAL and antigen-specific Ab titers, ELISA assays were performed as described previously (9, 10). Briefly, the levels of cytokines in the BAL fluid of mice were quantified by ELISA using the eBioscience kits (San Diego, CA, USA) following the manufacturer’s instructions: IL-1β (cat # 88–7013-77), IL-6 (cat # 88–7064-77), IL-17 (cat # 88–7371-77), TNF-α (cat # 88–7324-77), IFN-γ (cat # 88–7314-77). Albumin levels in BAL fluid (as a surrogate marker of lung-epithelial damage) were quantified by a mouse albumin-specific kit from Bethyl Laboratories (cat # E90–134; Montgomery, TX, USA). Titers of antigen-specific antibodies in mouse serum and BAL fluid were measured using ELISA plates coated with 3 μg/ml of purified P6 protein as described previously (9, 10). Specific dilutions of weekly mouse serum and endpoint BAL fluid were incubated with P6-coated, BSA-blocked ELISA plates at room temperature, and bound anti-P6 immunoglobulins were detected with HRP-conjugated goat anti-mouse Ig(H+L), IgA, IgG1, and IgG2b antibodies (Southern Biotech, Birmingham, AL, USA). All the ELISA plates were developed with eBioscience 3,3’,5,5’-tetramethylbenzidine (TMB; for HRP) solution, and absorbance was read at 450 nm on an ELISA plate reader (Synergy HTX Multi-Mode Reader, Winooski, VT, USA).
ELISPOTS
ELISPOT assays were performed to quantitate the frequency of antigen-specific, cytokine-secreting T and antigen-specific antibody-secreting B cells as described previously (10). Briefly, for T cell ELISPOTS multiscreen HA plates (Millipore, Carrigtwohill, Co. Cork, Ireland) were coated with 3 μg/ml anti–IL-17A, anti–IL-4 or anti–IFN-γ antibodies in ELISPOT coating buffer overnight at 4○C. Next day, lung or splenic lymphocytes were co-cultured with 1 μM P641–55 peptide-pulsed APCs (49). After 48 h co-culture, ELISPOT plates were washed and cytokines detected with cytokine-specific biotinylated antibodies (anti–IL-17A, anti–IL-4 or anti–IFN-γ) followed by addition of streptavidin-HRP (all reagents from ThermoFisher Scientific, GA, USA). To quantify the frequency of antigen-specific antibody-secreting B cells in bone marrow and spleen, ELISPOT plates were coated with 3 μg/ml native P6 protein overnight at 4°C. Next day, B cells were incubated in P6-coated, BSA-blocked ELISPOT plates at 37°C and 5% CO2 for 48 hr. After plates were gently washed, bound anti-P6 antibodies were detected with HRP-conjugated secondary antibodies to mouse IgG1 and IgG2a (Southern Biotech, Birmingham, AL, USA). After washing plates, spots were developed with TMB substrate (Vector Labs, CA, USA), air-dried and enumerated. Spots were enumerated using an ImmunoSpot® Reader (Cellular Technology Limited, Shaker Hts, OH, USA).
Assessment of NTHI clearance
NTHI bacterial burden in the lungs of mice following acute infection was assessed as described previously (10). Briefly, the mice were euthanized 4 and 24 h after acute bacterial infection and whole lungs were excised. Lungs were then gently homogenized in 1 ml PBS on ice. Under sterile conditions, serial dilutions of lung homogenates were plated on chocolate agar plates and incubated for 16 h at 35oC at 5% CO2 conditions. The next day, NTHI colonies were counted and bacterial burden in the lungs determined and data was expressed as the number of bacterial CFUs recovered compared to 1 × 106 CFUs instilled initially.
Statistical analysis
Results are expressed as mean ± SEM. Testing for differences between mean values was determined using a two-tailed unpaired Student t test or two-way ANOVA with a Tukey’s post-test comparisons using GraphPad Prism 8 software. The difference between two groups was considered to be significant at P≤0.05.
Results
AT-RvD1 diminishes SHS exposure-induced NTHI-mediated pulmonary inflammation
Since SPMs are considered to be potent anti-inflammatory and pro-resolving mediators, we evaluated the impact of AT-RvD1 on SHS-augmented infection-induced pulmonary inflammation. Air- or SHS-exposed mice treated with AT-RvD1 were chronically infected in the lungs with NTHI (Figure 1A). Histopathological examination of lung sections revealed characteristic leukocytic accumulation surrounding airways and bronchovasculature in all the groups. We found that prior exposure to chronic SHS aggravated NTHI-induced pulmonary inflammation (Figure 1B, panel 3 vs panel 1), which agreed with our previous findings (10). Notably we observed that when mice were treated with AT-RvD1 during the second half of the smoke exposure period (wks 4–8), the extent of immune-cell infiltration into the lungs markedly decreased compared to untreated mice (Figure 1B, panel 4 vs panel 3). The histological data were additionally supported by quantitation of inflammatory cell infiltrates in the BAL and lung. Chronic SHS exposure increased total leukocyte infiltrate in the BAL and lungs after chronic infection, and AT-RvD1 treatment resulted in significant decreases (Figure 1C). Of note, infiltration in the BAL of SHS-exposed, AT-RvD1-treated mice was reduced to the level comparable to that observed in mice exposed to air, demonstrating that SPM AT-RvD1 potently ameliorates pulmonary inflammation (Figure 1C).
We also evaluated the impact of AT-RvD1treatment in SHS-exposed mice on the phenotype of immune cell infiltrates in the BAL and lungs. The numbers of neutrophils and macrophages in the BAL and lungs of mice exposed to chronic SHS were significantly elevated compared to air-exposure. AT-RvD1 treatment of SHS-exposed mice significantly reduced the numbers of these cells compared to SHS-exposed, vehicle treated mice (Figure 1D, E). Furthermore, chronic SHS augmented the accumulation of CD4+ T, CD8+ T and CD19+ B cells in the BAL and lungs, and AT-RvD1 treatment of SHS-exposed mice significantly reduced this lymphocytic accumulation in the BAL (Figure 1F) and also decreased the numbers of B cells in the lungs (Figure 1G). Elevated numbers of proinflammatory CD4+RORγt+ and CD4+IL-17A+ Th17 T cells in the BAL and lungs were also significantly reduced by AT-RvD1-treatment in SHS-exposed mice (Figure 1H). We also assessed the effect of smoking cessation by interposing a 4 wk smoke-free cessation period between the final smoke exposure and first bacterial infection (Supplemental Figure 1A). We found that even following a 4 wk period of cessation of exposure, mice previously exposed to SHS had significantly elevated lung inflammation compared with air-exposed mice. (Supplemental Figure 1B, C). However, AT-RvD1 treatment lead to marked reduction in the total numbers of immune cells, numbers of neutrophils, macrophages, lymphocytes (CD4+ T and CD19+B cells) and proinflammatory IL-17A+ Th17 and RORγ t+ Th17 T cells infiltrating the lungs after chronic NTHI infection (Supplemental Figure 1B–E). Our gating strategy is shown in Supplemental Figure 2.
AT-RvD1 treatment is effective in reducing proinflammatory cytokine levels in the BAL and diminishing SHS-induced lung-epithelial damage
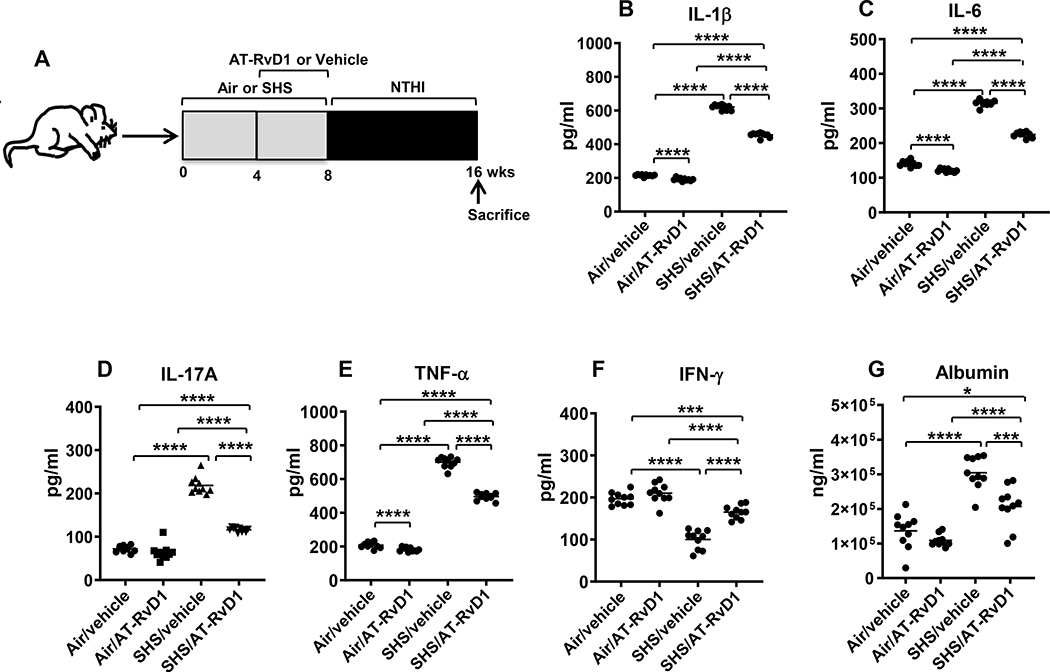
We have previously shown that SHS exposure induces augmented levels of proinflammatory cytokines in response to pulmonary infection (10). Since AT-RvD1 reduced chronic SHS-induced pulmonary inflammation and suppressed accumulation of proinflammatory immune cells in the lungs, we next evaluated its influence on the levels of proinflammatory cytokines (Figure 2A). AT-RvD1 co-treatment markedly reduced levels of proinflammatory cytokines IL-1β, IL-6, IL-17A and TNF-α in the BAL of mice, although they were still above the levels seen in air-exposed mice (Figure 2B-E). Notably, the decrease in IFN-γ levels seen in the BAL of SHS-exposed mice was reversed when these mice were treated with AT-RvD1 (Figure 2F). The efficacy of AT-RvD1 was enhanced by giving the mice a 4 wk smoke-free period prior to the first bacterial infection, as proinflammatory cytokine levels were restored to the baseline levels seen in vehicle-treated, air-exposed mice (Supplemental Table 1 and Supplemental Figure 3). Additionally, we observed that IFN-γ levels in the BAL of SHS-exposed mice after a period of cessation were augmented compared to without cessation, irrespective of RvD1 treatment (Supplemental Table 1). Albumin leak, a measure of epithelial barrier integrity, was significantly reduced in SHS-exposed mice co-treated with AT-RvD1 (Figure 2G), and the efficacy of AT-RvD1 at reducing alveolar leakage was also enhanced by a 4 wk smoking cessation period (Supplemental Figure 3G and Supplemental Table 1).
FIGURE 2. AT-RvD1 treatment markedly diminishes SHS-augmented, NTHI-induced proinflammatory cytokine production, reducing SHS-enhanced lung epithelial damage.
(A) C57BL/6J mice were exposed to air or SHS for a total of 8 wks, with or without AT-RvD1 treatment given in the last 4 wks of exposure, and then subjected to chronic infection for an additional 8 wks, as described in material and methods. (B-F) After mice were euthanized, lungs were lavaged and cytokine levels in the BAL were determined by ELISA (n = 10 mice per group). (G) The levels of mouse albumin in the BAL were determined by ELISA as described in methods. Line represents mean value for the group. All treatment groups were performed at the same time, and data show results generated from a single experiment using a total of n=10 mice/group. The results are shown as mean ± SE. Statistical significance between the treatment groups was determined by two-tailed unpaired students t test, *p<0.05, ***p<0.001, ****p<0.0001.
AT-RvD1 alleviates immuno-suppressive effects of SHS exposure on the generation of Ab responses
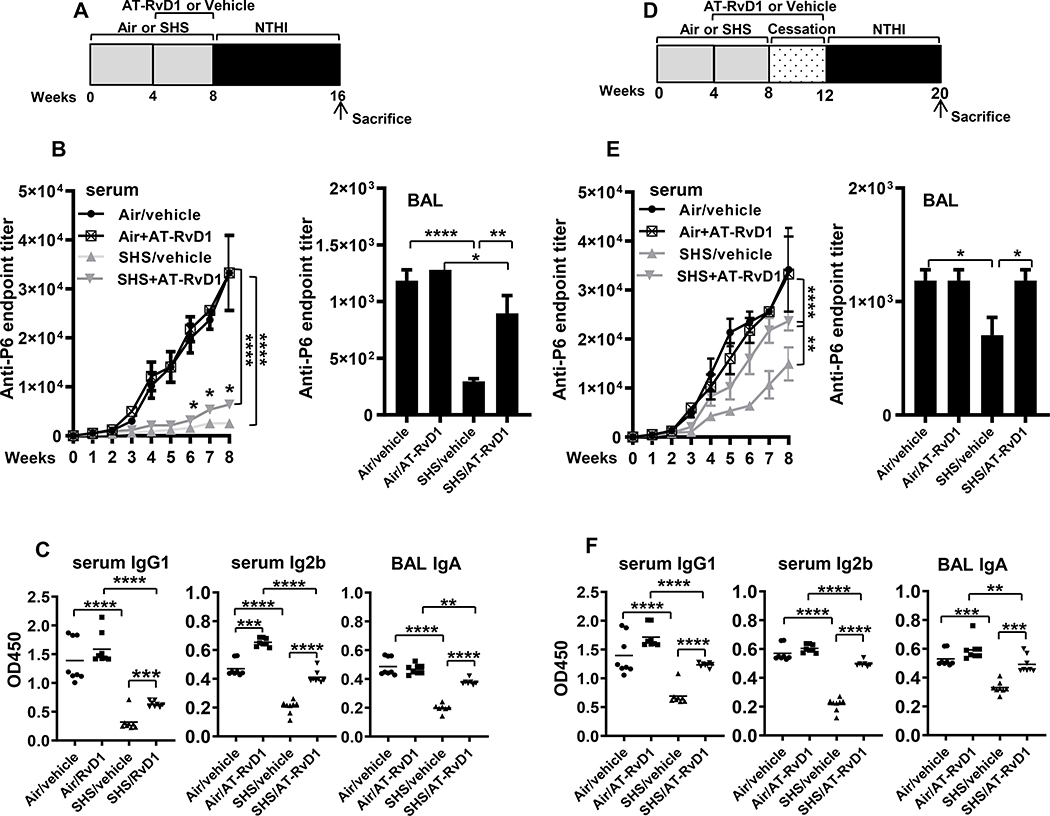
We have previously reported that chronic exposure to SHS diminishes the development of adaptive immunity to chronic respiratory infection (10). We therefore examined if AT-RvD1 treatment (schema in Figure 3A) could augment antigen-specific Ab responses in the serum and BAL of mice chronically infected with NTHI. We measured antibodies against the immunodominant P6 antigen of NTHI in the serum and BAL. While total anti-P6 antibody levels were considerably reduced in the serum and BAL of SHS-exposed mice compared with air-exposed mice, AT-RvD1 treatment modestly increased anti-P6 Ab levels in the serum and markedly restored anti-P6 antibodies in the BAL Figure 3B). Furthermore, while the levels of antibody subclasses IgG1 and IgG2b in serum and IgA in the BAL were significantly reduced in SHS-exposed mice, treatment with AT-RvD1 markedly elevated these levels (Figure 3C). When AT-RvD1 treatment was continued during a 4 wk smoke-free cessation period, it was even more effective at improving anti-P6 antibody levels (SHS+RvD1 vs SHS+Vehicle in Figure 3D-F), which were now similar to the responses observed in air-exposed controls (SHS+RvD1 vs Air+Vehicle in Figure 3D-F). This clearly indicates that although the effect of SHS persists despite cessation (SHS+Vehicle vs Air+Vehicle Figure 3D-F), continued SPM treatment significantly rescues and boosts the antibody responses (SHS+RvD1 vs Air+Vehicle Figure 3D-F).
FIGURE 3. AT-RvD1 treatment of SHS-exposed mice enhances antigen-specific antibody responses, which further are augmented by a period of cessation.
(A) C57BL/6J mice were exposed to air or SHS for 8 wks, with or without AT-RvD1 treatment given in the last 4 wks of exposure, and then subjected to the chronic infection model for 8 wks, as described. (B) Total anti-P6 IgG antibodies were measured by ELISA in serum (left panel) collected weekly during the period of chronic NTHI infection and in end-point BAL (right panel) collected at the time of euthanasia. (C) Levels of antigen-specific Ab subclasses IgG1 and IgG2b in end-point (wk 8) serum (left and middle panels) and IgA in BAL collected at euthanasia (right panel) were measured by determining OD450 values at dilutions of 1:1600 for serum and 1:400 for the BAL by ELISA assays as described in methods. (D) C57BL/6J mice were exposed to air or SHS for 8 wks, followed by a 4-wk period of cessation. Mice were treated with AT-RvD1 or vehicle between wks 4–12, encompassing part of the period of SHS exposure and period of cessation. All mice were chronically infected with NTHI by i.t. instillations starting at wk 12 as described in methods. (E) Total anti-P6 IgG antibodies were measured in weekly serum (left panel) and end-point BAL (right panel) by ELISA. (F) Levels of antigen-specific Ab subclasses IgG1 and IgG2b in end-point serum (left and middle panels) and IgA in end-point BAL (right panel) were determined by measuring the OD450 values for serum at 1:1600 dilution and for the BAL at 1:400 dilution by ELISA assays as described in methods. Line represents mean value for the group. All treatment groups were performed at the same time, and data represent results generated from a single experiment using a total of n=7–10 mice/group. The results are shown as mean ± SE. Statistical significance was determined by either two-way ANOVA with Tukey’s posttest for multiple comparisons (B and E, left panels) or by two-tailed unpaired students t test (all other figures), **p<0.01, ***p<0.001, ****p<0.0001.
AT-RvD1 treatment rescues SHS-induced impairment in antigen-specific T and B cell responses
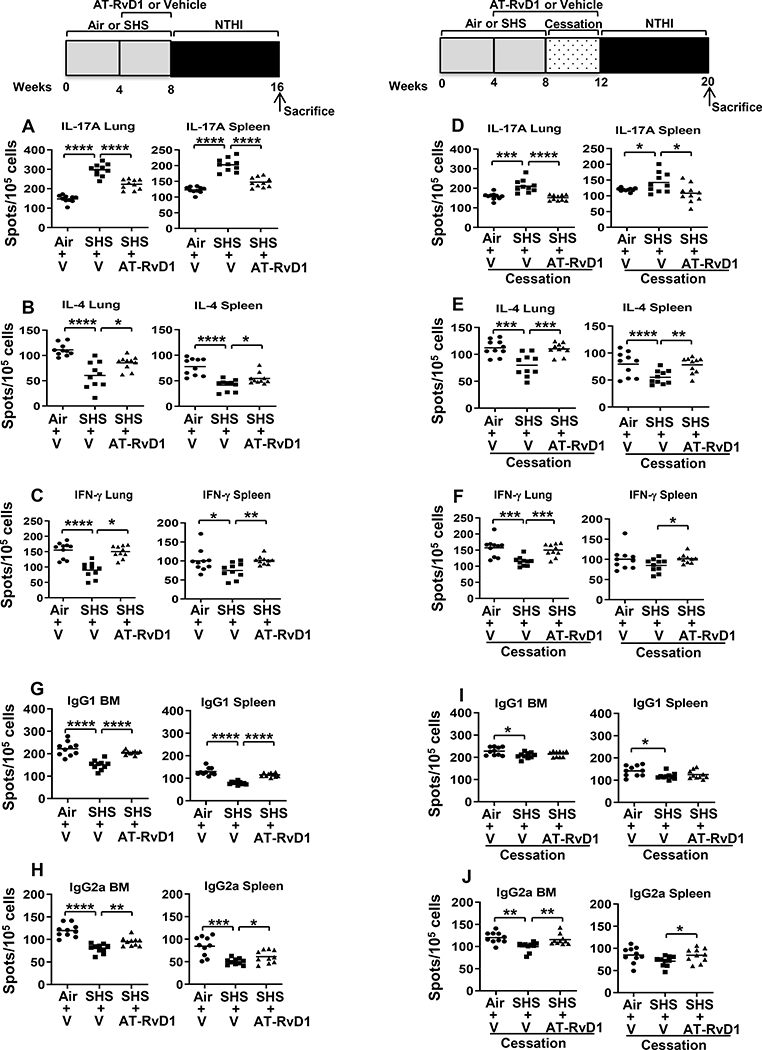
T cell responses are essential for antibacterial immunity and critical to the generation of a robust antibody response against the pathogen. Since AT-RvD1 alleviated the effects of SHS on the antibody response to NTHI, we therefore assessed the impact of AT-RvD1 on T cell responses using the ELISpot assay. We observed that SHS exposure caused marked increase in the frequencies of P6-specific Th17 cells. AT-RvD1 treatment in SHS-exposed mice decreased the frequencies of IL-17A- secreting inflammatory Th17 T cells both in the lung and spleen (Figure 4A). Furthermore, treatment with AT-RvD1 increased the frequency of IL-4- and IFN-γ-producing T cells both in the lungs and spleen of mice exposed to chronic SHS (Figure 4B-C). Continued treatment with AT-RvD1 for another 4 wks following cessation, resulted in a small additional beneficial effect, with further reduction in the frequency of IL-17A-secreting Th17 T cells and increase in the frequency of IL-4-producing T cells in both organs (Figure 4D, E). A period of cessation plus treatment with AT-RvD1 did not provide additional enhancement in the numbers of IFN-γ-producing T cells in the lungs and spleen when compared to treatment with AT-RvD1 (Figure 4F vs 4C).
FIGURE 4. AT-RvD1 improves SHS-impaired frequency of antigen-specific cytokine-secreting T cells, thereby augmenting antigen-specific antibody-secreting B cell frequency.
(A-F) Lung lymphocytes (Left panel) and splenocytes (Right panel) were isolated from air- or SHS-exposed, AT-RvD1 or vehicle treated mice after 8 wks of chronic NTHI infection. Cells were incubated with P641–55 peptide-pulsed APCs to determine the frequency of IL-17A-, IL-4- and IFN-γ-cytokine-secreting T cells by ELISPOT assays as described in methods. (G-J) B cell ELISPOTs were performed to quantify the frequency of P6-specific IgG1-secreting (upper panels) and IgG2a-secreting (bottom panels) B cells, from BM (left panels) and spleens (right panels) of air- or SHS-exposed, AT-RvD1 or vehicle treated mice. Spots were enumerated using a CTL ELISPOT reader. All treatment groups were performed at the same time, and data depict results generated from a single experiment using a total of n=10 mice/group. The results are depicted as mean ± SE. Line represents mean value for the group. Two-tailed unpaired students t test was utilized to determine the statistical significance between the treatment groups. *p≤0.05, **p≤0.01, ***p≤0.005, ****p≤0.0001,
We also evaluated the impact of At-RvD1 in modulating the frequency of antigen-specific B cell responses following chronic infection. While SHS exposure suppressed NTHI-specific IgG1 and IgG2a secreting B cell frequency in bone marrow and spleen, AT-RvD1 treatment enhanced their frequencies (Figure 4G, H). Furthermore, we observed that SHS exposure followed by a period of cessation and continuation of treatment with AT-RvD1 during this interval was at least equally as effective as cessation alone in augmenting the NTHI-specific IgG1-secreting B cell frequency (Figure 4I); however this treatment strategy was beneficial in further augmenting the NTHI-specific IgG2a-secreting B cell frequency in both bone marrow and spleen (Figure 4J).
AT-RvD1 treatment augments efficacy of immunization in SHS-exposed mice
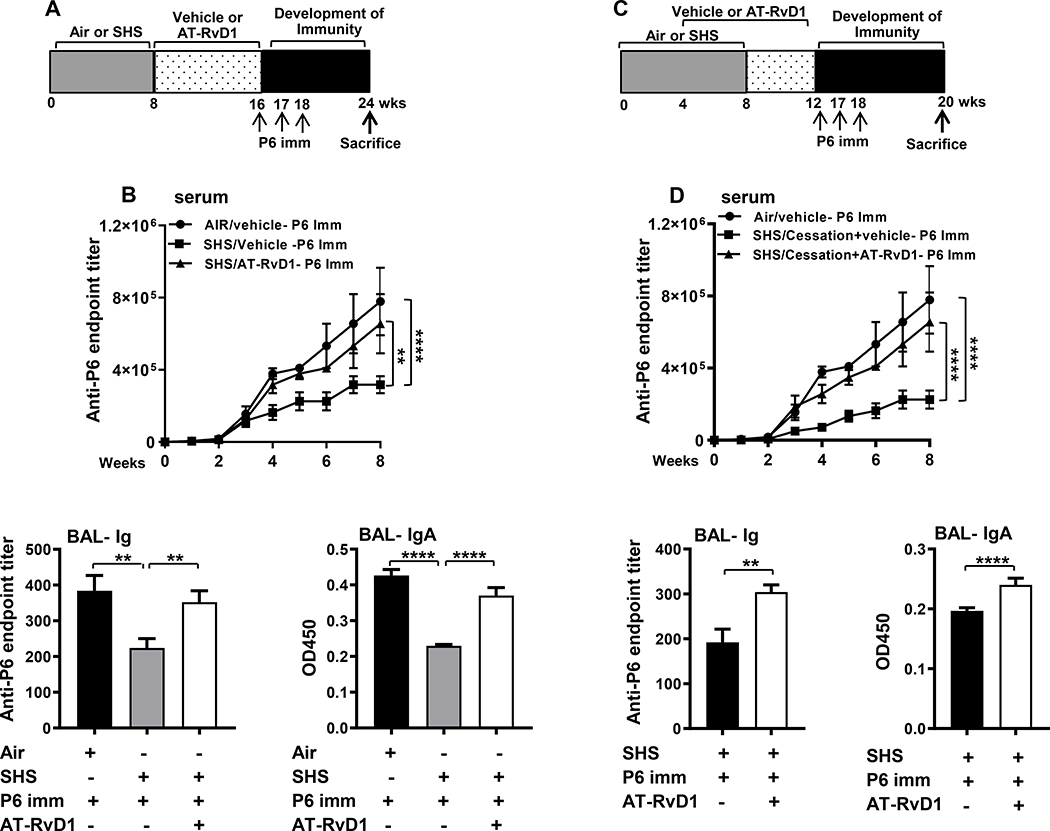
Having established that AT-RvD1 treatment reduced inflammation and augmented immunity to infection, we evaluated whether AT-RvD1 treatment in SHS-exposed mice could enhance the efficacy of P6 vaccination (schema in Figure 5A, C). Our results, in agreement with our previous findings (10), showed that prior chronic SHS exposure suppresses the generation of Ab responses to P6 vaccination in mice compared to air-exposed mice (Figure 5 B, D). AT-RvD1 treatment either started immediately after the end of the SHS exposure (Schema Figure 5A) or midway into the SHS exposure and continued for 8 wks (Schema Figure 5C), significantly enhanced the production of both systemic (serum Ig) as well as mucosal (BAL total Ig and IgA) antigen-specific antibodies following vaccination compared to vehicle-treated, SHS-exposed mice (Figure 5 B, D).
FIGURE 5. AT-RvD1 treatment of SHS-exposed mice augments the efficacy of P6 vaccination.
(A) Mice exposed to air or SHS for 8 wks and then treated with AT-RvD1 or vehicle for an additional 8 wks were vaccinated with purified P6 lipoprotein as described in methods. (B) Total P6-specific IgG Ab titers were quantified by ELISA. in weekly serum (upper panel) collected after the start of vaccination and in end-point BAL (lower left panel) collected at the time of euthanasia Levels of mucosal anti-P6 IgA (lower right panel) were quantified by measuring OD450 values in the BAL at dilutions of 1:400 in ELISA assays as described in methods. (C) Mice were exposed to air or SHS for a total of 8 wks, with or without AT-RvD1 treatment between wks 4–12, and then vaccinated with NTHI P6 antigen as described in methods. (D) Total P6-specific IgG Ab titers were measured by ELISA in serum (upper panel) collected weekly after P6 vaccination and in end-point BAL (lower left panel). Mucosal anti-P6 specific IgA levels (lower right panel) were determined by measuring OD450 values in the BAL at 1:400 dilutions by ELISA as described in methods. All treatment groups were performed at the same time, and data represent results generated from a single experiment using a total of n=10 mice/group at each time point. The results are depicted as mean ± SE. Statistical significance was determined either by two-way ANOVA with Tukey’s posttest for multiple comparisons (B & D) or two-tailed unpaired students t test (**p<0.01, ****p<0.0001).
Enhanced efficacy of P6 vaccination and AT-RvD1 treatment translates into rapid bacterial clearance in SHS-exposed mice
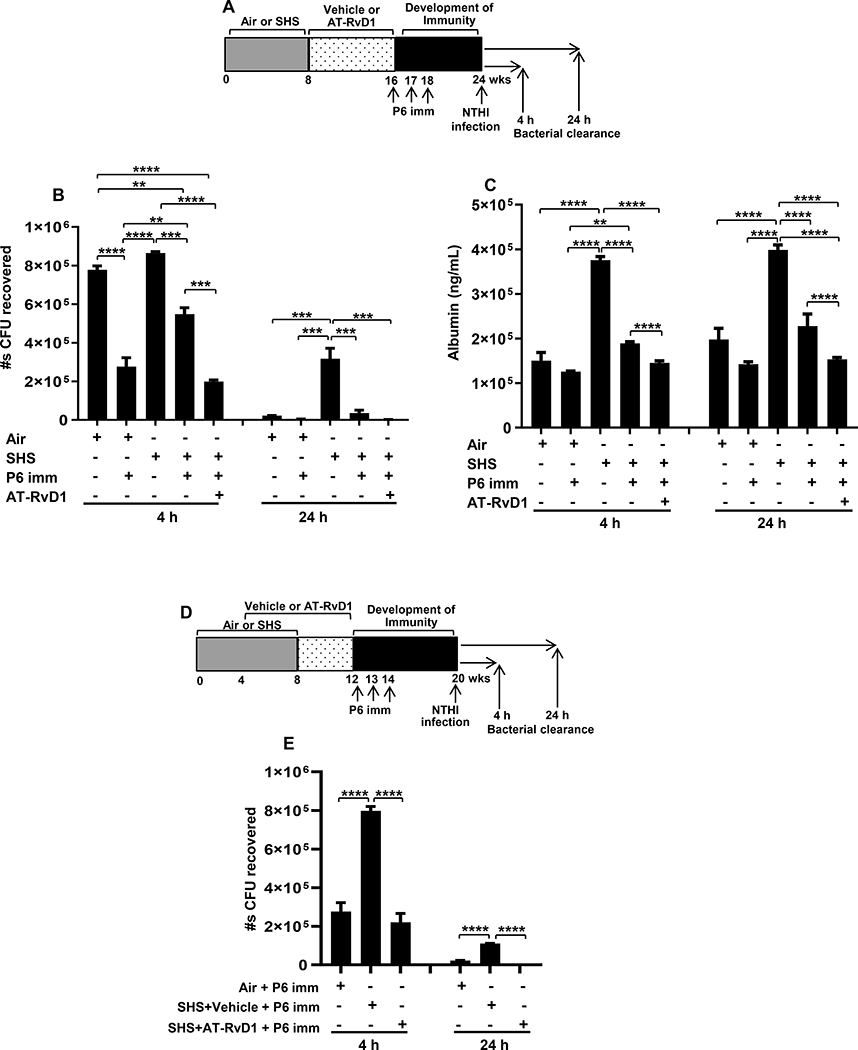
Sham-immunized air or sham-immunized SHS-exposed mice served as controls to evaluate the effectiveness of vaccination in decreasing the bacterial burden measured 4 and 24 h after acute NTHI infection (Figure 6A). While the bacterial clearance from the lungs of sham-immunized, SHS-exposed mice was non-significantly reduced at 4 h time point, it was significantly inhibited compared to sham-immunized, air-exposed mice at 24 h after acute NTHI infection, even though this challenge infection was given 16 wks after the last smoke exposure (3rd bar versus 1st bar in both the panels in Figure 6B). P6 vaccination enhanced NTHI clearance in both air- and SHS-exposed mice compared with their respective sham-immunized controls but was not as effective at the 4 h time point in SHS-exposed mice relative to air-exposed mice (Figure 6B). However, clearance was markedly enhanced when mice were treated with AT-RvD1 prior to vaccination (Figure 6B). With a physiological milieu of rapid bacterial clearance in the lungs of mice, we predicted less lung-epithelial damage. Albumin leak into BAL of mice served as a surrogate marker of lung-epithelial integrity. SHS-exposed, P6 vaccinated mice that were treated with SPM AT-RvD1 had significantly lower levels of albumin in the BAL at 4 and 24 h following acute NTHI infection (Figure 6C).
FIGURE 6. AT-RvD1 treatment-enhanced vaccination efficacy translates into marked decrease in SHS-augmented pulmonary bacterial burden and lung epithelial-cell damage following acute infection.
(A) Mice exposed to air or SHS for 8 wks were then treated with AT-RvD1 or vehicle for another 8 wks. All mice were vaccinated i.p. with 40 μg purified native P6 emulsified in CFA at wk16 and boosted 1 wk later with Ag in IFA and 2 wks later with Ag alone and bacterial challenge was done at wk24 as described in methods. Bacterial clearance was measured at 4 and 24 h following bacterial challenge. (B) Bacterial burden in the lungs of mice was measured by bacterial colony-plating assay and data are represented as total number of CFUs recovered from the lungs. (C) The levels of albumin in the BAL were measured by ELISA. (D-E) A group of SHS-exposed mice were given AT-RvD1- or vehicle-treatment that started immediately at week 4 and continued till week 12. Treated mice were vaccinated i.p. with 40 μg purified native P6 emulsified in CFA at wk12 and boosted 1 wk later with Ag in IFA and 2 wks later with Ag alone and then challenged with acute bacterial infection 8 wks after immunization at wk20. Bacterial burden in the lungs of this group of mice was measured at 4 and 24 h after bacterial challenge and data represented as total number of CFUs recovered from the lungs. All treatment groups were performed at the same time, and data represent results generated from a single experiment using a total of n=3 mice/group (B & E) or n=5 mice/group (C) at each time point. The results are depicted as mean ± SE. Statistical significance was determined by two-way ANOVA with Tukey’s posttest for multiple comparisons (**p≤0.01; ***p≤0.001; ****p≤0.0001).
We also evaluated the model in which mice were treated with AT-RvD1 during the second half of the smoke exposure period and treatment continued during a 4 wk period of cessation to smoke exposure (Figure 6D). We again observed that AT-RvD1 treatment prior to vaccination resulted in enhanced bacterial clearance compared with SHS-exposed, vehicle-treated, P6 vaccinated mice (Figure 6E). These results also indicate that AT-RvD1 treatment started either during SHS exposure or at the end of the exposure has similar beneficial effects in enhancing bacterial clearance.
Discussion
CS is a powerful inducer of inflammatory mediators in the lung that play a critical role in pulmonary inflammation and damage (4,9,10). Persistence of inflammatory mediators induces lung damage, impaired lung function and increased susceptibility to infections (4, 9, 10, 50, 51). In fact, there is evidence that the pathology of COPD is driven by chronic unresolved inflammation, even in the absence of ongoing smoke exposure, as activated inflammatory cells produce reactive oxygen and nitrogen species, cytokines and chemokines and enzymes that cause progressive lung tissue destruction, airway remodeling, and impaired lung function (4,7,8). Additionally, CS impairs the function of innate immune cells like neutrophils and macrophages by prolonging inflammatory signaling, thus accelerating tissue damage (4, 13, 52–54). Smoking-related disorders resolve poorly, with the inflammatory damage to the lung persisting long after smoking cessation (10, 55, 56). Individuals like flight attendants, hospitality workers and children exposed to chronic SHS display an increased risk of smoking-related complications including respiratory infections (57, 58). Indeed, we have recently demonstrated that chronic SHS exposure exacerbates pulmonary inflammation and diminishes immunity to pulmonary bacterial infections (10).
We recently demonstrated the ability of SPMs to function as vaccine adjuvants (46). We now show that treatment with the pro-resolving mediator AT-RvD1 during smoke exposure or immediately after initiation of smoking cessation, decreases SHS-exacerbated, infection-induced lung inflammation, with decreased recruitment of inflammatory cells and cytokine production. Furthermore, AT-RvD1 treatment alleviated smoke-induced impairment of T cell and B cell function and generation of anti-NTHI antibodies. Importantly, although the efficacy of AT-RvD1 was improved by smoking cessation, it was also effective when given concurrently with smoke exposure. This suggests that pro-resolving mediators could be developed as new clinical therapies to reduce the burden of infectious diseases in smokers, ex-smokers, and children and other vulnerable populations exposed unwillingly to SHS.
Anti-inflammatory drugs, cytokine receptor antagonists, antioxidants and oxidative stress response modifiers have been shown to reduce CS-induced inflammation (59–61). However, anti-inflammatory agents that do not simultaneously induce immunosuppression are highly desired. SPMs not only diminish production of proinflammatory factors, but also induce a cellular phenotypic shift to promote anti-inflammatory effects, thus resetting the balance between pro- and anti-inflammatory processes to promote resolution (45, 62).
It is known that CS-induced macrophage and neutrophil dysfunction impairs bacterial killing and increases apoptotic cell-accumulation, thus impairing resolution of inflammation (4, 63, 64). RvD1 has been reported to inhibit neutrophilic inflammation and promote efferocytosis in experimental colitis and allergic airway models (65–67). Macrophages assist inflammation resolution via tissue healing process and are recruited to ward-off exacerbated lung inflammation (68). However, CS is shown to alter the phenotype of alveolar macrophages and inhibit bacterial phagocytosis that might be implicated in SHS-induced suppression of antigen-specific immunity and resolution of inflammation (19, 63, 69, 70). RvD1 is reported to enhance macrophage phagocytic activity, thus promoting inflammation resolution (71, 72–74). Collectively these observations suggest that AT-RvD1 might reduce SHS-exacerbated pulmonary inflammation by diminishing neutrophilic influx to the lungs and modulating macrophage function, thus promoting inflammation resolution and augmenting immunity.
Based on epidemiological findings, inflammatory diseases in smokers are associated with a Th17 phenotype. Chronic CS promotes Th17 polarized immunity, that is associated with lung damage during emphysema, and is characterized by increased ROR-γt, IL-17, and IL-6 expression with concomitant decline in Foxp3 and IL-10 expression (75–79). Such an induction of proinflammatory IL-17 signaling and differentiation to a Th17 cellular profile is attributed to the presence of multiple aryl hydrocarbon receptor (AhR) ligands in CS (80, 81). Our studies show that SHS causes an imbalance in Th1, Th2, and Th17 responses against NTHI infection, resulting in an augmentation of the IL-17 response. Furthermore, SHS-induced augmentation of IL-17A cytokine in the BAL, CD4+IL17A+ and CD4+RORγt+ T cell subsets in the lung supports the involvement of the IL-17 axis in the sustenance of SHS-exacerbated infection-induced chronic inflammation. Our findings are supported by an earlier report that IL-17A is required for CS-augmented, NTHI-induced pulmonary neutrophilia in acute exacerbation of COPD (82). Reports also show that RvD1 suppresses lipopolysaccharide -induced lung injury (83) and that D-series resolvins abrogate IL-17-induced inflammatory effects (84). From these perspectives, we propose that AT-RvD1-mediated amelioration of SHS-exacerbated, infection-induced pulmonary inflammation might be in part due to suppression of IL-17-dependent mechanisms.
While CS-induced oxidative stress is reported to increase immune cell recruitment to cause pulmonary inflammation and damage associated with emphysema (85–87), however augmented immune cell infiltration in the lungs itself is one of the critical mechanisms involved in oxidative stress induction in airway diseases (88). Although AT- RvD1 is not an antioxidant at the concentration used, it could contribute to the overall antioxidant activity via an indirect effect of decreased pulmonary recruitment of activated neutrophils and macrophages, immune cells that are a major source of oxidative stress during inflammation (50, 88, 89). Additionally, while CS-induced oxidative stress suppresses dendritic cell (DC) function (90), putting smokers at a higher risk of mucosal infections and lung damage, thus resolution of inflammation could reduce the detrimental impact. Indeed, RvDs decrease CS-generated, ROS-induced levels of carbonylated proteins (41), and thus could help reverse CS-mediated, oxidative stress-induced immune dysfunction and improve anti-pathogen host responses.
Along with the improvement in immune responses to NTHI infection, AT-RvD1 also enhanced antibody responses after immunization with NTHI P6 antigen that lead to increased bacterial clearance following bacterial challenge. Vaccination to prevent acute exacerbations in people with smoking-related diseases has limited effectiveness, likely due to the immunosuppressive effects of CS exposure. Our results show that a short course of AT-RvD1 treatment given after SHS exposure but prior to immunization can augment the immune response (Figure 5), leading to higher antibody titers and more rapid clearance of bacteria following acute infection. This is consistent with the earlier and recent results from our group showing that RvD1 or its precursor 17-HDHA are effective adjuvants when given concurrently with the antigen (39, 45, 46, 91). We found that AT-RvD1-augmented antibacterial Ab responses could be due to restoration of antigen-specific B cell responses that were suppressed in BM and spleen of SHS-exposed mice. This immune-enhancing effect could be a direct translation of AT-RvD1-induced increased frequency of antigen-specific T cells secreting IL-4 and IFN-γ, cytokines that are required for Ab class switching to IgG1 and IgG2a respectively (92–94). This immune-enhancing impact of AT-RvD1 on antibody production was further improved with a period of SHS cessation.
AT-RvD1 thus appears to have multiple beneficial effects in the context of bacterial infection following cigarette smoke exposure. AT-RvD1 diminishes SHS-exacerbated, infection-induced pulmonary inflammatory cells and cytokines, while enhancing anti-bacterial adaptive immunity and augmenting antigen-specific systemic and mucosal Ab responses after vaccination. This enhanced efficacy of vaccination translated into decreased pulmonary bacterial burden with the induction of less lung epithelial-cell damage following infection. Additionally, AT-RvD1 was effective when given during an ongoing SHS exposure, as well as after smoking cessation.
RvD1 is only one of a large family of SPMs, and it is possible that other SPMs may also have significant utility in smoke-induced inflammation and respiratory illness. However, there are several lines of research supporting the hypothesis that endogenous SPMs in general, and RvD1 in specific, act to mitigate inflammation and regulate immunity, and that excess inflammation is associated with deficiencies in SPMs (62). For example, in mice, RvD1 and AT-RvD1 signal via the G protein-coupled receptor ALX/FPR2. Using a self-limiting model of peritonitis, in which the role of endogenous SPMs is well-described, ALX/FPR2 overexpressing mice were more responsive to the effect of exogenous RvD1 treatment in reducing inflammation, while ALX/FPR2 KO mice exhibited excess inflammation that could not be controlled with exogenous RvD1 (44). Lung tissue from COPD patients had elevated levels of EOR, an enzyme that degrades RvD1 (42), and RvD1 was decreased in the serum and BAL fluid of COPD patients relative to non-COPD subjects (41). Interestingly, CS also downregulates the RvD1 target miR-208a (95). Other SPMs implicated in the resolution of CS-induced inflammation include protectin D1, lipoxin A4, and RvD2 (62). Future work to explore the cellular sources and role of endogenous RvD1 could be performed using inhibitors of the lipoxygenase synthesis enzymes, soluble epoxide hydrolase inhibitors that block degradation of RvD1, and using ALX/FPR2 knockout and knock-in mice (96, 97), which include the possibility of creating cell or tissue specific knockouts using the Cre-Lox system.
SPMs may represent an important new therapeutic tool for preventing CS-related diseases in vulnerable populations, including children, the elderly and immunocompromised individuals who have been previously exposed to mainstream or secondhand smoke. SPMs can both reduce the burden of chronic smoking-related lung inflammation, and also increase the efficacy of antibody responses to pathogens or vaccines, thereby potentially leading to an overall decrease in morbidity and mortality. This may be especially important in the elderly, because even though the rate of active smoking has decreased, there remains a large population of elderly individuals who are former smokers or were formerly exposed to secondhand smoke in the home or workplace, in whom smoke exposure has lifelong consequences. The elderly and immunocompromised individuals are also less proficient at mounting adaptive immune responses to infection and vaccination (98, 99). Thus, the elderly in general, and specifically persons with smoking or SHS exposure history, may benefit from therapeutic use of SPMs to reduce lung inflammation and increase adaptive immune responses.
In conclusion, our current findings demonstrate that SPM AT-RvD1 helps resolve SHS-exacerbated, chronic infection-induced pulmonary inflammation and might represent one of the mechanisms through which AT-RvD1 enhances adaptive immunity to infection and efficacy to vaccination in SHS-exposed mice. Importantly, pro-resolving and anti-inflammatory effects of AT-RvD1 contrast with classical anti-inflammatory therapies such as non-steroidal anti-inflammatory drugs and corticosteroids and underscore its potential use as a therapeutic agent to manage or treat infectious co-morbidities of disorders having chronic inflammation as a causal factor.
Supplementary Material
Key points:
SPM AT-RvD1 diminishes SHS-exacerbated, infection-induced pulmonary inflammation
SHS-exposed mice treated with AT-RvD1 induce augmented anti-bacterial immunity
AT-RvD1 treated, SHS-exposed, vaccinated mice rapidly clear acute bacterial challenge
Acknowledgments
This work was supported by Flight Attendant Medical Research Institute (FAMRI) Clinical Innovators Award to Y.T. and P.J.S. This work was supported in part by National Institutes of Health Grant R01 HL120908 (to P.J.S.). This work was supported by National Cancer Institute Grant P30CA016056 involving the use of Roswell Park Core facilities: Division of Laboratory Animal Resources, Mouse Tumor Model Resource, and Flow Cytometry.
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References:
- 1.World Health Organization. 2016. Tobacco – fact sheet. Available at: http://www.who.int/mediacentre/factsheets/fs339/en/. Accessed: July 26, 2019.
- 2.U.S. Department of Health and Human Services. 2014. The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Atlanta, GA. [Google Scholar]
- 3.Eisner MD, Balmes J, Katz PP, Trupin L, Yelin EH, and Blanc PD. 2005. Lifetime environmental tobacco smoke exposure and the risk of chronic obstructive pulmonary disease. Environ. Health 4: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stampfli MR, and Anderson GP. 2009. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat. Rev. Immunol. 9: 377–384. [DOI] [PubMed] [Google Scholar]
- 5.Oberg M, Jaakkola MS, Woodward A, Peruga A, and Pruss-Ustun A. 2011. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet. 377: 139–146. [DOI] [PubMed] [Google Scholar]
- 6.Thun MJ, Carter BD, Feskanich D, Freedman ND, Prentice R, Lopez AD, Hartge P, and Gapstur SM. 2013. 50-year trends in smoking-related mortality in the United States. N. Engl. J. Med. 368: 351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sethi S, Evans N, Grant GJ, Murphy TF. 2002. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 347:465–71. [DOI] [PubMed] [Google Scholar]
- 8.Sethi S Infection as a comorbidity of COPD. 2010. Eur. Res. J. 35: 1209–1215. [DOI] [PubMed] [Google Scholar]
- 9.Lugade AA, Bogner PN, Thatcher TH, Sime PJ, Phipps RP, and Thanavala Y. 2014. Cigarette smoke exposure exacerbates lung inflammation and compromises immunity to bacterial infection. J. Immunol. 192: 5226–5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhat TA, Kalathil SG, Bogner PN, Miller A, Lehmann PV, Thatcher TH, Phipps RP, Sime PJ, Thanavala Y. 2018. Secondhand smoke induces inflammation and impairs immunity to respiratory infections. J. Immunol. 200:2927–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J, Taneja V, and Vassallo R. 2012. Cigarette smoking and inflammation: cellular and molecular mechanisms. J. Dent. Res. 91: 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung KF, and Adcock IM. 2008. Multifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destruction. Eur. Res. J 31: 1334–1356. [DOI] [PubMed] [Google Scholar]
- 13.Bhat TA, Panzica L, Kalathil SG, and Thanavala Y. 2015. Immune dysfunction in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 12 :S169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strzelak A, Ratajczak A, Adamiec A, and Feleszko W. 2018. Tobacco smoke induces and alters immune responses in the lung triggering inflammation, allergy, asthma and other lung diseases: a mechanistic review. Int J Environ Res Public Health. 15. pii: E1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshida T, and Tuder RM. 2007. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol Rev. 87:1047–82. [DOI] [PubMed] [Google Scholar]
- 16.Sethi S, Wrona C, Eschberger K, Lobbins P, Cai X, and Murphy TF. 2008. Inflammatory profile of new bacterial strain exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 177(5):491–497. [DOI] [PubMed] [Google Scholar]
- 17.Sethi S, and Murphy TF. 2008. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 359(22):2355–2365. [DOI] [PubMed] [Google Scholar]
- 18.Anzueto 2010. A. Impact of exacerbations on COPD. Eur Respir Rev. 19(116):113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terzano C, Colamesta V, Unim B, Romani S, Meneghini A, Volpe G, and La Torre G. 2017. Chronic obstructive pulmonary disease (COPD) exacerbation: impact of comorbidities on length and costs during hospitalization. Eur Rev Med Pharmacol Sci. 21(16):3680–9. [PubMed] [Google Scholar]
- 20.Centers for disease control and prevention. Haemophilus influenzae Disease (Including Hib). February 13, 2020. https://www.cdc.gov/hi-disease/about/types-infection.html.
- 21.Sullivan SD, Ramsey SD, and Lee TA. 2000. The economic burden of COPD. Chest. 117: 5S–9S. [DOI] [PubMed] [Google Scholar]
- 22.Teo E, Lockhart K, Purchuri SN, Pushparajah J, Cripps AW, and van Driel ML. 2017. Haemophilus influenzae oral vaccination for preventing acute exacerbations of chronic bronchitis and chronic obstructive pulmonary disease. Cochrane Database Syst Rev.;6:CD010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berenson CS, Garlipp MA, Grove LJ, Maloney J, and Sethi S. 2006. Impaired phagocytosis of nontypeable Haemophilus influenzae by human alveolar macrophages in chronic obstructive pulmonary disease. J. Infect. Dis. 194: 1375–1384 [DOI] [PubMed] [Google Scholar]
- 24.Maddi S, Kolsum U, Jackson S, Barraclough R, Maschera B, Simpson KD, Pascal TG, Durviaux S, Hessel EM, and Singh D. 2017. Ampicillin resistance in Haemophilus influenzae from COPD patients in the UK. Int J Chron Obstruct Pulmon Dis. 12: 1507–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jalalvand F, and Riesbeck K. 2018. Update on non-typeable Haemophilus influenzae-mediated disease and vaccine development. Exp. Rev. Vacc. 17: 503–512. [DOI] [PubMed] [Google Scholar]
- 26.Janeway CA Jr, Travers P, Walport M, Shlomchik MJ. 2001. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science. Part V, The Immune System in Health and Disease. Available from: https://www.ncbi.nlm.nih.gov/books/NBK10775/. [Google Scholar]
- 27.Freire MO, Van Dyke TE. 2013. Natural resolution of inflammation. Periodontol. 2000. 63(1):149–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King PT, and Sharma R. 2015. The Lung Immune Response to Nontypeable Haemophilus influenzae (Lung Immunity to NTHI). J Imm. Res 2015:706376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medzhitov R 2008. Origin and physiological roles of inflammation. Nature. 454: 428–435. [DOI] [PubMed] [Google Scholar]
- 30.Serhan CN. 2007. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Ann. Rev. Imm. 25: 101–137. [DOI] [PubMed] [Google Scholar]
- 31.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. 2002. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 196(8):1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serhan CN. 2010. Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am J Pathol. 177(4):1576–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serhan CN. 2014. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 510(7503):92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serhan CN, Chiang N, and Van Dyke TE. 2008. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. rev. Imm. 8: 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Basil MC, Levy BD. 2016. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat Rev Imm. 2016 January;16(1):51–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR. 2000. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol. 164:1663–1667. [DOI] [PubMed] [Google Scholar]
- 37.Fierro IM, Colgan SP, Bernasconi G, A Petasis N, Clish CB, Arita M, and Serhan CN. 2003. Lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 inhibit human neutrophil migration: comparisons between synthetic 15 epimers in chemotaxis and transmigration with microvessel endothelial cells and epithelial cells. J Immunol. 170:2688–2694. [DOI] [PubMed] [Google Scholar]
- 38.Souza DG, Fagundes CT, Amaral FA, Cisalpino D, Sousa LP, Vieira AT, Pinho V, Nicoli JR, Vieira LQ, Fierro IM, and Teixeira MM. 2007. The required role of endogenously produced lipoxin A4 and annexin-1 for the production of IL-10 and inflammatory hyporesponsiveness in mice. J Immunol. 179:8533–8543. [DOI] [PubMed] [Google Scholar]
- 39.Ramon S, Baker SF, Sahler JM, Kim N, Feldsott EA, Serhan CN, Martínez-Sobrido L, Topham DJ, and Phipps RP. 2014. The specialized proresolving mediator 17-HDHA enhances the antibody-mediated immune response against influenza virus: a new class of adjuvant? J Immunol. 193(12):6031–6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmid M, Gemperle C, Rimann N and Hersberger M. 2016. Resolvin D1 polarizes primary human macrophages toward a proresolution phenotype through GPR32. J. Immunol. 196 (8): 3429–3437. [DOI] [PubMed] [Google Scholar]
- 41.Croasdell A, Thatcher TH, Kottmann RM, Colas RA, Dalli J, Serhan CN, Sime PJ, Phipps RP. 2015. Resolvins attenuate inflammation and promote resolution in cigarette smoke-exposed human macrophages. Am J Physiol Lung Cell Mol Physiol. 309(8): L888–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsiao HM, Thatcher TH, Colas RA, Serhan CN, Phipps RP, and Sime PJ. 2015. Resolvin D1 reduces emphysema and chronic inflammation. Am J Pathol. 185(12):3189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun YP, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, Colgan SP, Petasis NA, Serhan CN. 2007. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J Biol Chem. 282(13): 9323–34. [DOI] [PubMed] [Google Scholar]
- 44.Krishnamoorthy S, Recchiuti A, Chiang N, Fredman G, Serhan CN. 2012. Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am J Pathol. 180(5): 2018–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duffney PF, Falsetta ML, Rackow AR, Thatcher TH, Phipps RP, Sime PJ. Key roles for lipid mediators in the adaptive immune response. 2018. J Clin Invest. 128(7): 2724–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhat TA, Kalathil SG, Miller A, Thatcher TH, Sime PJ, Thanavala Y. 2020. Specialized proresolving mediators overcome immune suppression induced by exposure to secondhand smoke. J Immunol. doi: 10.4049/jimmunol.2000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hwang JW, Sundar IK, Yao H, Sellix MT, and Rahman I. 2014. Circadian clock function is disrupted by environmental tobacco/cigarette smoke, leading to lung inflammation and injury via a SIRT1-BMAL1 pathway. FASEB J. 28: 176–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lakatos HF, Burgess HA, Thatcher TH, Redonnet MR, Hernady E, Williams JP, Sime PJ. 2006. Oropharyngeal aspiration of a silica suspension produces a superior model of silicosis in the mouse when compared to intratracheal instillation. Exp Lung Res. 32(5):181–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McMahon M, Murphy TF, Kyd J, and Thanavala Y. 2005. Role of an immunodominant T cell epitope of the P6 protein of nontypeable Haemophilus influenzae in murine protective immunity. Vaccine. 23: 3590–3596. [DOI] [PubMed] [Google Scholar]
- 50.Barnes PJ 2016. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 138(1): 16–27. [DOI] [PubMed] [Google Scholar]
- 51.Agustí A, Edwards LD, Rennard SI, MacNee W, Tal-Singer R, Miller BE, Vestbo J, Lomas DA, Calverley PM, Wouters E, Crim C, Yates JC, Silverman EK, Coxson HO, Bakke P, Mayer RJ, and Celli B. 2012. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One. 7:e37483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu Y, Li H, Bajrami B, Kwak H, Cao S, Liu P, Zhou J, Zhou Y, Zhu H, Ye K, and Luo HR. 2013. Cigarette smoke (CS) and nicotine delay neutrophil spontaneous death via suppressing production of diphosphoinositol pentakisphosphate. Proc. Natl. Acad. Sci. USA 110: 7726–7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petrusca DN, Gu Y, Adamowicz JJ, Rush NI, Hubbard WC, Smith PA, Berdyshev EV, Birukov KG, Lee CH, Tuder RM, Twigg HL, Vandivier RW, and Petrache I. 2010. Sphingolipid-mediated inhibition of apoptotic cell clearance by alveolar macrophages. J Biol Chem. 285: 40322–40332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Monick MM, Powers LS, Walters K, Lovan N, Zhang M, Gerke A, Hansdottir S, and Hunninghake GW. 2010. Identification of an autophagy defect in smokers’ alveolar macrophages. J Immunol.185: 5425–5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turato G, Di Stefano P, Maestrelli P, Mapp M, Ruggieri A, Roggeri A, Fabbri L. 1995. Effect of smoking cessation on airway inflammation in chronic bronchitis. Am J Respir Crit Care Med.152:1262–1267. [DOI] [PubMed] [Google Scholar]
- 56.Domagala-Kulawik J, Maskey-Warzechowska M, Kraszewska I, Chazan R. 2003. The cellular composition and macrophage phenotype in induced sputum in smokers and ex-smokers. Chest.123:1054–1059. [DOI] [PubMed] [Google Scholar]
- 57.Ebbert JO, Croghan IT, Schroeder DR, Murawski J, and Hurt RD. 2007. Association between respiratory tract diseases and secondhand smoke exposure among never smoking flight attendants: A cross-sectional survey. Environmental Health: A Global Access Science Source, 6, [28]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vanker A, Gie RP, and Zar HJ. 2017. The association between environmental tobacco smoke exposure and childhood respiratory disease: a review. Expert Rev Respir Med. 11:661–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sezer M, Sahin O, Solak O, Fidan F, Kara Z, and Unlu M. 2007. Effects of caffeic acid phenethyl ester on the histopathological changes in the lungs of cigarette smoke-exposed rabbits. Basic Clin Pharmacol Toxicol. 101:187–91. [DOI] [PubMed] [Google Scholar]
- 60.Thatcher TH, Hsiao HM, Pinner E, Laudon M, Pollock SJ, Sime PJ, and Phipps RP. 2013. Neu-164 and Neu-107, two novel antioxidant and anti-myeloperoxidase compounds, inhibit acute cigarette smoke-induced lung inflammation. Am J Physiol Lung Cell Mol Physiol. 305: L165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dianat M, Radan M, Badavi M, Mard SA, Bayati V, and Ahmadizadeh M. 2018. Crocin attenuates cigarette smoke-induced lung injury and cardiac dysfunction by anti-oxidative effects: the role of Nrf2 antioxidant system in preventing oxidative stress. Respir Res. 19:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thatcher TH, Woeller CF, McCarthy CE, Sime PJ. 2019. Quenching the fires: Pro-resolving mediators, air pollution, and smoking. Pharmacol Ther. 197: 212–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Phipps JC, Aronoff DM, Curtis JL, Goel D, O’Brien E, and Mancuso P. 2010. Cigarette smoke exposure impairs pulmonary bacterial clearance and alveolar macrophage complement-mediated phagocytosis of Streptococcus pneumoniae. Infect Immun. 78:1214–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gualano RC, Hansen MJ, Vlahos R, Jones JE, Park-Jones RA, Deliyannis G, Turner SJ, Duca KA, and Anderson GP. 2008. Cigarette smoke worsens lung inflammation and impairs resolution of influenza infection in mice. Respir. Res. 9: 53–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bento AF, Claudino RF, Dutra RC, Marcon R, and Calixto JB. 2011. Omega-3 fatty acid-derived mediators 17(R)-hydroxy docosahexaenoic acid, aspirin-triggered resolvin D1 and resolvin D2 prevent experimental colitis in mice. J Immunol. 187: 1957–1969. [DOI] [PubMed] [Google Scholar]
- 66.Eickmeier O, Seki H, Haworth O, Hilberath JN, Gao F, Uddin M, Croze RH, Carlo T, Pfeffer MA, and Levy BD. 2012. Aspirin-triggered resolvin D1 reduces mucosal inflammation and promotes resolution in a murine model of acute lung injury. Mucosal Immunol. 6: 256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rogerio AP, Haworth O, Croze R, Oh SF, Uddin M, Carlo T, Pfeffer MA, Priluck R, Serhan CN, and Levy BD. 2012. Resolvin D1 and aspirin-triggered resolvin D1 promote resolution of allergic airways responses. J. Immunol. 189: 1983–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Allard B, Panariti A, and Martin JG. 2018. Alveolar Macrophages in the resolution of inflammation, tissue repair, and tolerance to infection. Front Immunol. 9:1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Droemann D, Goldmann T, Tiedje T, Zabel P, Dalhoff K, and Schaaf B. 2005. Toll-like receptor 2 expression is decreased on alveolar macrophages in cigarette smokers and COPD patients. Respir. Res. 6: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lugade AA, Bogner PN, Murphy TF, and Thanavala Y. 2011. The role of TLR2 and bacterial lipoprotein in enhancing airway inflammation and immunity. Front. Immunol. 2: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hsiao HM, Sapinoro RE, Thatcher TH, Croasdell A, Levy EP, Fulton RA, Olsen KC, Pollock SJ, Serhan CN, Phipps RP, and Sime PJ. 2013. A novel anti-inflammatory and pro-resolving role for resolvin D1 in acute cigarette smoke-induced lung inflammation. PloS one. 8: e58258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Porcheray F, Viaud S, Rimaniol AC, Leone C, Samah B, Dereuddre-Bosquet N, Dormont D, and Gras G. 2005. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol 142: 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gordon S, and Martinez FO. 2010. Alternative activation of macrophages: mechanism and functions. Immunity. 32: 593–604. [DOI] [PubMed] [Google Scholar]
- 74.Recchiuti A, Krishnamoorthy S, Fredman G, Chiang N, and Serhan CN. 2011. MicroRNAs in resolution of acute inflammation: identification of novel resolvin D1-miRNA circuits. FASEB J. 25: 544–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gaschler GJ, Skrtic M, Zavitz CC, Lindahl M, Onnervik PO, Murphy TF, Sethi S, and Stämpfli MR. 2009. Bacteria challenge in smoke-exposed mice exacerbates inflammation and skews the inflammatory profile. Am. J. Respir. Crit. Care Med. 179: 666–675. [DOI] [PubMed] [Google Scholar]
- 76.Miossec P, and Kolls JK. 2012. Targeting IL-17 and TH17 cells in chronic inflammation. Nat. Rev. Drug Discov. 11: 763–776. [DOI] [PubMed] [Google Scholar]
- 77.Ramirez-Velazquez C, Castillo EC, Guido-Bayardo L, and Ortiz-Navarrete V. 2013. IL-17-producing peripheral blood CD177+ neutrophils increase in allergic asthmatic subjects. Allergy Asthma Clin. Immunol. 9: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang H, Peng W, Weng Y, Ying H, Li H, Xia D, and Yu W. 2012. Imbalance of Th17/Treg cells in mice with chronic cigarette smoke exposure. Int. Immunopharmacol. 14: 504–512. [DOI] [PubMed] [Google Scholar]
- 79.Zhang J, Chu S, Zhong X, Lao Q, He Z, and Liang Y. 2013. Increased expression of CD4+IL-17+ cells in the lung tissue of patients with stable chronic obstructive pulmonary disease (COPD) and smokers. Int. Immunopharmacol. 15: 58–66. [DOI] [PubMed] [Google Scholar]
- 80.Martey CA, Baglole CJ, Gasiewicz TA, Sime PJ, and Phipps RP. 2005. The aryl hydrocarbon receptor is a regulator of cigarette smoke induction of the cyclooxygenase and prostaglandin pathways in human lung fibroblasts. Am. J. Physiol. Lung Cell. Mol. Physiol 289: L391–L399. [DOI] [PubMed] [Google Scholar]
- 81.Chen K, Pociask DA, McAleer JP, Chan YR, Alcorn JF, Kreindler JL, Keyser MR, Shapiro SD, Houghton AM, Kolls JK, and Zheng M. 2011. IL-17RA is required for CCL2 expression, macrophage recruitment, and emphysema in response to cigarette smoke. PLoS ONE. 6: e20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roos AB, Sethi S, Nikota J, Wrona CT, Dorrington MG, Sandén C, Baue CM, Shen P, Bowdish D, Stevenson CS, Erjefält JS, and Stampfli MR. 2015. IL-17A and the promotion of neutrophilia in acute exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 192: 428–37. [DOI] [PubMed] [Google Scholar]
- 83.Liao Z, Dong J, Wu W, Yang T, Wang T, Guo L, Chen L, Xu D, Wen F. 2012. Resolvin D1 attenuates inflammation in lipopolysaccharide-induced acute lung injury through a process involving the PPARγ/NF-κB pathway. Respir Res. 13:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maekawa T, Hosur K, Abe T, Kantarci A, Ziogas A, Wang B, Van Dyke TE, Chavakis T, and Hajishengallis G. 2015. Antagonistic effects of IL-17 and D-resolvins on endothelial Del-1 expression through a GSK-3β-C/EBPβ pathway. Nat. comm. 6: 8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Biswas SK, and Rahman I. 2009. Environmental toxicity, redox signaling and lung inflammation: The role of glutathione. Mol. Asp. Med. 30: 60–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zuo L, He F, Sergakis GG, Koozehchian MS, Stimpfl JN, Rong Y, Diaz PT, and Best TM. 2014. Interrelated role of cigarette smoking, oxidative stress, and immune response in COPD and corresponding treatments. Am J Physiol Lung Cell Mol Physiol. 307:L205–218. [DOI] [PubMed] [Google Scholar]
- 87.Morse D, and Rosas IO. 2014. Tobacco smoke-induced lung fibrosis and emphysema. Annu Rev Physiol. 76: 493–513. [DOI] [PubMed] [Google Scholar]
- 88.Holguin F 2013. Oxidative stress in airway diseases. Ann Am Thorac Soc. 10: S150–157. [DOI] [PubMed] [Google Scholar]
- 89.Beavers WN, and Skaar EP. 2016. Neutrophil-generated oxidative stress and protein damage in Staphylococcus aureus. Pathog Dis. 74: ftw060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kroening PR, Barnes TW, Pease L, Limper A, Kita H, Vassallo R. 2008. Cigarette smoke-induced oxidative stress suppresses generation of dendritic cell IL-12 and IL-23 through ERK-dependent pathways. J Immunol. 181(2): 1536–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ramon S, Gao F, Serhan CN, and Phipps RP. 2012. Specialized proresolving mediators enhance human B cell differentiation to antibody-secreting cells. J Immunol.189:1036–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sideras P, Bergstedt-Lindqvist S, Severinson E. 1985. Partial biochemical characterization of IgG1-inducing factor. Eur J Immunol. 15(6): 593–598. [DOI] [PubMed] [Google Scholar]
- 93.Vitetta ES, Ohara J, Myers CD, Layton JE, Krammer PH, and Paul WE. 1985. Serological, biochemical, and functional identity of B cell-stimulatory factor-I and B cell differentiation factor for IgG1. J Exp Med. 162(5): 1726–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Snapper CM, Paul WE. 1987. Interferon-γ and B-cell stimulatory factor-I reciprocally regulate Ig isotype production. Science. 236(4804): 944–947. [DOI] [PubMed] [Google Scholar]
- 95.Seike M, Goto A, Okano T, Bowman ED, Schetter AJ, Horikawa I, Mathe EA, Jen J, Yang P, Sugimura H, Gemma A, Kudoh S, Croce CM, Harris CC. 2009. MiR-21 is an EGFR-regulated anti-apoptotic factor in lung cancer in never-smokers. Proc Natl Acad Sci USA. 106(29): 12085–12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang J, Bratt J, Franzi L, Liu JY, Zhang G, Zeki AA, Vogel CF, Williams K, Dong H, Lin Y, Hwang SH, Kenyon NJ, Hammock BD. 2015. Soluble epoxide hydrolase inhibitor attenuates inflammation and airway hyperresponsiveness in mice. Am J Respir Cell Mol Biol. 52(1): 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Podolin PL, Bolognese BJ, Foley JF, Long E 3rd, Peck B, Umbrecht S, Zhang X, Zhu P, Schwartz B, Xie W, Quinn C, Qi H, Sweitzer S, Chen S, Galop M, Ding Y, Belyanskaya SL, Israel DI, Morgan BA, Behm DJ, Marino JP Jr., Kurali E, Barnette MS, Mayer RJ, Booth-Genthe CL, Callahan JF. 2013. In vitro and in vivo characterization of a novel soluble epoxide hydrolase inhibitor. Prostaglandins Other Lipid Mediat. 104–105(): 25–31. [DOI] [PubMed] [Google Scholar]
- 98.Weinberger B 2018. Vaccines for the elderly: current use and future challenges. Immun Ageing. 15: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Löbermann M, Boršo D, HilgendorfI I, Fritzsche C, Zettl UK, and Reisinger EC. 2012. Immunization in the adult immunocompromised host. Autoimmun Rev. 11(3): 212–218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.