Abstract
Objective:
Amyotrophic lateral sclerosis (ALS) is a complex neurodegenerative disease that causes the progressive loss of voluntary muscle control. Recent studies have reported conflicting results on alterations in resting-state functional brain networks in ALS by adopting unimodal techniques that measure either electrophysiological or vascular-hemodynamic neural functions. However, no study to date has explored simultaneous electrical and vascular-hemodynamic changes in the resting-state brain in ALS. Using complementary multimodal electroencephalography (EEG) and functional near-infrared spectroscopy (fNIRS) recording and analysis techniques, we explored the underlying multidimensional neural contributions to altered oscillations and functional connectivity in people with ALS.
Methods:
10 ALS patients and 9 age-matched controls underwent multimodal EEG-fNIRS recording in the resting state. Resting-state functional connectivity (RSFC) and power spectra of both modalities in both groups were analyzed and compared statistically.
Results:
Increased fronto-parietal EEG connectivity in the alpha and beta bands and increased interhemispheric and right intra-hemispheric fNIRS connectivity in the frontal and prefrontal regions were observed in ALS. Frontal, central, and temporal theta and alpha EEG power decreased in ALS, as did parietal and occipital alpha EEG power, while frontal and parietal hemodynamic spectral power increased in ALS.
Significance:
These results suggest that electro-vascular disruption in neuronal networks extends to the extra-motor regions in ALS patients, which can ultimately introduce novel neural markers of ALS that can be exploited further as diagnostic and prognostic tools.
Index Terms—: Amyotrophic lateral sclerosis (ALS), electroencephalography (EEG), functional near-infrared spectroscopy (fNIRS), resting-state functional connectivity (RSFC)
I. Introduction
AMYOTROPHIC lateral sclerosis (ALS) is a complex neurodegenerative disease fundamentally characterized by the progressive loss of voluntary muscle control attributable to motor neuron degeneration. Neuroimaging studies have consistently provided growing evidence of extra-motor neural involvement in addition to the motor involvement known well in ALS pathophysiology [1]–[5]. Underlying interconnected neural networks of motor and extra-motor regions has been explored during cognitive tasks, motor functions, and the resting-state to identify the potential effects of ALS disease on functional cortical networks and further clarify pathophysiology and clinically established disease markers for a large group of patients. Resting-state studies may be central to information processing as they provide insight into alterations in spontaneous cognition associated with the disease. Although these resting-state studies do not reflect conscious mental activity exclusively, they may reflect more intrinsic properties of functional brain organization and represent the spontaneous coherent fluctuations in functionally connected brain regions [4].
Resting-state functional connectivity (RSFC) has been widely studied in ALS patients as these patients are often not able to perform other experimental protocols, which include motor or cognitive tasks due to the impairments caused by the disease. However, conflicting RSFC findings have led to a lack of consistent functional connectivity markers for ALS patients [6], and the way functional cortical networks are altered in ALS patients is not yet understood clearly [3], [7]. One study reported decreased RSFC in both the right and left prefrontal cortex [8], while others have reported increased RSFC in prefrontal regions in ALS patients [5], [9], [10]. However, among these studies, the prior selection of seeds (either by direct selection or by source localization) varied, which indicates the important effect of channel/source location on results and shows the essential need for high spatial resolution recordings in frontal and prefrontal regions. In another study, Fraschini et al. [2] reported overall decreased electrical RSFC in ALS patients using electroencephalography (EEG) recordings, while Kopitzki et al. [3] reported overall preserved hemodynamic RSFC using functional near-infrared spectroscopy (fNIRS) to evaluate hemodynamic-based connectivity. Using functional magnetic resonance imaging (fMRI), Verstraete et al. [11] reported overall preserved RSFC in the motor and sensorimotor network, while other groups also have reported increased RSFC in the motor [9], [6], premotor [10], and sensorimotor [9], [10] networks. Further, these studies have different views on the way their findings are related to the underlying neural dynamics of the disease. For example, increased functional connectivity in ALS patients has been interpreted both as a reflection of impaired inhibitory neural functions and as a physiological compensation for reduced structural integrity [10]. These divergent findings might be attributable to differences in methodological approaches and/or neuroimaging techniques that may affect RSFC estimation [6], [7], which corroborates the essential need to utilize complementary multimodal approaches to explore underlying neural alterations comprehensively.
Several neuroimaging modalities have been used to measure the neural and hemodynamic alterations of functional cortical networks in ALS, but there are methodological issues that may affect the reliability of these findings [7]. fMRI is an established method used widely to investigate hemodynamic activities in ALS, but it is costly and many patients with ALS have body positioning constraints that affect scanning. Alternatively, fNIRS measures vascular dynamics and is quite portable and simple to set up for clinical application even in patients with severe motor impairment, for whom fMRI is contraindicated [3]. Moreover, fNIRS is less sensitive to potential motion artifacts, which eliminates motion-induced spurious functional relations between cortical regions and does not influence measurement differences in patient studies. More recently, fNIRS has been used in ALS neural investigation studies [3], [12], and as an input to brain-computer interface (BCI) systems to help patients with severe motor disabilities, including those with ALS, communicate [13]. However, fNIRS use has its own limitations. The individual channel-wise functional connection fNIRS measures have raised reliability issues, and thus, cluster-wise measurements are recommended instead for reliable interpretations [14], which requires a large number of fNIRS optodes to analyze different functional clusters. This causes a decay in temporal resolution attributable to the one-by-one light emission queue of the optodes, which affects the suitability of fNIRS for studying larger numbers of clusters. EEG is another alternative neuroimaging method with a high temporal resolution that allows analysis of functional connections in different specific frequency bands, each of which has characteristic biological and pathophysiological significance. EEG can directly measure the electrical activity of neurons, while fNIRS and fMRI both measure the vascular dynamics that serve only as an indirect measure of neural activity. As the functional states of neurons affect both their electrical and vascular-hemodynamic properties, many studies have explored the fundamental electrical and hemodynamic activities of neurological functions using multimodal techniques [15], [16]. Accordingly, our recent studies and others have suggested the important role of multimodal measures (electrical-EEG and hemodynamic-fNIRS) in discovering cognitive neural markers, including those for attention and memory [12], [17] and mental distress [16]. However, to our knowledge, no study has characterized alterations in resting-state electrical and vascular-hemodynamic functional neural networks in patients with ALS. Such a study is of particular interest in functional network investigations of ALS, as there is no fundamental understanding of the heterogeneous pathological effects of this disease on the brain network connectivity of these patients.
The goal of this study is to explore RSFC alterations in ALS using complementary multimodal EEG-fNIRS recording and analysis techniques. While fNIRS allows the examination of correlated low oscillatory hemodynamic fluctuations on the metabolic level, EEG allows investigation of the temporal dynamics of precise band-specific electrical activity affected directly by underlying neural interactions. Therefore, band-specific hemodynamic and electrical power analyses were conducted. Using coherence and correlation analysis, RSFC network analysis across different cortical regions, including prefrontal, frontal, parietal, and occipital were further performed. Also, the associations between the significant findings in EEG and fNIRS were further explored to investigate how ALS affects the interrelations between electrical (EEG) and vascular (fNIRS) dynamics.
II. Methods
A. Subjects
Ten participants diagnosed with definite ALS (age 58.2±11.6 years, two females) using the El Escorial diagnostic criteria, and nine healthy controls (age 61.0±3.8 years, six females) were recruited for this study (see Tables I and 1S in the supplementary file). ALS patients had functional rating scale-revised (ALSFRS-R) scores of 23.2±13.7 (Mean±SD) on a 48-point scale, on which 48 represents a normal function in activities of daily living (ADL) and 0 represents a complete loss of function [18]. Age-matched control subjects had no reported history of visual, mental, or substance-related disorders that could potentially affect the results or affect their performance during data recording. The study protocol was approved by the Institutional Review Board (IRB) of the University of Rhode Island (URI), and all subjects provided informed consent or assent for the study and received financial compensation.
TABLE I.
Patients’ Demographic Information
| Subject No. | Age | Sex | ALSFRS-R (max 48) |
|---|---|---|---|
| ALS-1 | 55 | M | 4 |
| ALS-2 | 67 | M | 7 |
| ALS-3 | 69 | F | 23 |
| ALS-4 | 52 | M | 22 |
| ALS-5 | 72 | M | 36 |
| ALS-6 | 61 | M | 39 |
| ALS-7 | 33 | M | - |
| ALS-8 | 52 | M | 10 |
| ALS-9 | 54 | F | 39 |
| ALS-10 | 67 | M | 29 |
| Mean±SD | 58.2±11.6 | - | 23.2±13.7 |
B. Experimental Protocol
Subjects participated in two sessions with one run per session. All subjects were instructed to close their eyes and remain awake during the resting state recording. The subjects were also asked to relax and try not to think about any particular matter. In each run, five minutes of resting-state EEG-fNIRS data were acquired.
C. Data Acquisition
Both signals were recorded simultaneously using a single cap mounted with both EEG electrodes and fNIRS optodes. fNIRS data were recorded using NIRScout (NIRx Inc.) with two NIR lights (760 nm and 850 nm wavelengths) and digitized at 7.81 Hz. EEG data were recorded using the g.USBamp amplifier (g.tec Medical Tech., Schiedlberg, Austria) and digitized at 256 Hz. Figure 1 shows a schematic head montage model of the fNIRS-EEG sensors. EEG was recorded from fourteen channels: F1*, Fz*, F2*, Cz, P3, P7, Pz, P4, P8, PO7, PO8, T7, T8, and Oz covering all of the frontal, central, parietal, temporal and occipital areas, which are investigated commonly in whole head surface ALS studies [2], [3], [5] (note: F1*, Fz*, and F2* respectively, were the nearest electrode placements to fNIRS-occupied F1, Fz, and F2 according to the 128-channel montage). As depicted in figure 1, most of the fNIRS channels were mounted on the frontal and prefrontal areas that cover the regions in which the extra-motor ALS alterations and cognitive impairments are reported most often [1]. Moreover, as prefrontal eyeblink artifacts are reported to be one of the greatest sources of distortion of EEG in the prefrontal and frontal regions [19], fNIRS was employed as an outperforming modality for those regions. To achieve this purpose, six emitters and five detectors acquired fourteen fNIRS channels that covered the frontal and prefrontal regions primarily, together with two emitters and two detectors that formed two channels in the parietal lobe. Following the modified combinatorial nomenclature (MCN) montage, emitters were placed at FPZ, AF3, AF4, Fz, F3, F4, CP5, and CP6, and detectors at FP1, FP2, AFZ, F1, F2, P5 and P6. Each fNIRS channel used an emitter-detector pair with the optimal 3-cm. This multimodal montage follows standards closely and is convenient to mount, making it an appropriate candidate for future multimodal applications. All experimental protocols, and data acquisition were controlled using BCI2000 and NIRStar software.
Fig. 1.

Schematic head model of the fNIRS-EEG sensors.
D. Signal Processing
EEG data were bandpass filtered at 0.5–30 Hz and detrended to remove baseline drift and out of band artifacts. Then, the data were checked for extreme values and outliers which led to exclusion of the signals of one channel each from two runs (one from the ALS group and one from the Healthy group). The power spectra were computed for the delta (0.5–3.5 Hz), theta (3.5–8.5 Hz), alpha (8.5–12.5 Hz), and beta (12.5–30 Hz) frequency bands by applying a Hanning window 1.5 seconds long to reduce spectral leakage and with a 50% overlap for spectral smoothing, and then using a Fast Fourier Transform (FFT). Magnitude squared coherency referred to as coherence was used as the measure of EEG functional connectivity between two regions [20]. Briefly, the coherence between two signals represents their linear relation at a specific frequency by quantifying the phase and amplitude synchrony between them. It is one of the most commonly used methods to analyze functionally correlated cortical neuronal networks. Coherence as a function of frequency is the squared value of complex coherency and is a normalized quantity bounded by 0 and 1. It is the frequency domain equivalent to the time domain cross-correlation function and is computed mathematically as:
where Sxy(ω) is the cross-spectral density between x and y, and Sxx(ω) and Syy(ω) are the respective auto-spectral densities of x and y. First, for each signal, the same windowing procedure was used to obtain spectral density. Then, the coherence was computed through the modulus of the cross-spectrum of the signals normalized to the product of their auto-spectra, after which the mean coherence was obtained for each specific frequency band across all the frequency bands of interest. All of the measures obtained were averaged further over all runs.
fNIRS data were bandpass-filtered at 0.009–0.1 Hz as is done commonly in resting-state fNIRS studies to remove higher frequency physiological artifacts such as respiratory and cardiac signals, and long-term baseline drift [21]. Oxygenated hemoglobin (HbO2) and deoxygenated hemoglobin (HbR) concentration changes were extracted from raw optical intensity data using the modified Beer–Lambert law [22]. All time series were checked for outliers, including poorly-connected channels detected during the initial recording calibration and time series with sudden sharp peaks. After the data were preprocessed, the power spectra were computed for two frequency bands: very low-frequency oscillations (VLFO) (0.009–0.04 Hz) and low frequency oscillations (LFO) (0.04–0.1 Hz) to investigate possible frequency-specific hemodynamic organizations across different regions of the resting-state brain [23]. For the fNIRS functional connectivity analysis, we calculated Pearson’s correlation coefficient for all pairs of channels. According to figure 1, channel numbers 1 to 5 were considered as prefrontal, channel numbers 6 to 14 as frontal, and channel numbers 15 and 16 as parietal channels. Finally, all the connectivity measures were averaged over runs and across all subjects within each group for the between-group comparison. Given that the HbO2 signal has shown more implications in characterizing resting-state blood flow dynamics than has the HbR signal, and significant connectivity results in similar studies [3], [21] are related primarily to HbO2, our results focused largely on HbO2, while the HbR results are shown in the supplementary section.
Spearman correlation analysis was conducted to explore the relations between EEG and fNIRS measures that had significant findings in both groups of subjects. To do so, correlations were calculated between EEG measures (i.e., theta, alpha, and beta band power and RSFC) and fNIRS measures (VLFO and LFO band power, and RSFC). Using the Spearman correlation analysis, we also investigated the interrelations between EEG/fNIRS measures and ALSFRS-R scores of the patients.
E. Statistical Analysis
To statistically compare the results between groups, a non-parametric permutation testing procedure was used for both power spectral and RSFC results. To do so, all of the data points from healthy control and ALS patients (19 points) for each measure of power and RSFC in each related frequency band separately were combined, and nine data points (equal to the size of the smaller group) were selected randomly. Then, the mean of this group was subtracted from the mean of the remainder of the data points to generate a surrogate difference between two groups selected randomly. This procedure was iterated 1000 times to create a null histogram (probability distribution) of the group differences. The proportion of the histogram points less or greater than the difference observed between the ALS and healthy group, depending on which tail of the histogram met the observation point, determined the p-values. The difference in the means was statistically significant if p < 0.05. Finally, to account for multiple comparisons, a method suggested by Cohen [24] for non-parametric statistical analysis which compares between-group differences to a distribution of family-wise maxima, similar to Tukey’s HSD method which also accounts for family-wise error correction, was conducted. To do so, a distribution of individual differences from each iteration of permutation testing was created with the most extreme statistical value among all the channels (or connections between channels in the RSFC analysis). For example, for EEG power analysis, at each iteration a difference between two group means was calculated for fourteen EEG channels. Then the maximum and minimum differences between the channels were stored for each iteration. After all iterations were completed, the distribution of all stored values was determined, and all the significant findings were compared to the distribution with a threshold p-value of 0.05.
III. Results
A. Power Results
Figure 2 shows the channel map of the mean EEG power within the four aforementioned frequency bands for the healthy controls and ALS patients. We observed an overall power decrease in the ALS cohort relative to the control group. Permutation testing with correction for multiple comparisons revealed a significantly decreased theta power in patients at channels F2, Fz, T7, T8 and Cz with a maximum difference in channel T8 (Healthy 2.01±0.91 µV2, ALS 1.13±0.84 µV2, p = 0.006). Alpha power was also significantly decreased in patients at all channels except F1 and F2, with a maximum difference in channel PO8 (Healthy 5.84±0.91 µV2, ALS 3.02±0.75 µV2, p = 0.005) (See Table II). No significant power difference was observed in the delta and beta frequency bands.
Fig. 2.

Channel map of averaged EEG power within the delta, theta, alpha and beta frequency bands for healthy controls and ALS patients.
TABLE II.
Significant EEG Spectral Power Changes in ALS Patients Compared to the Healthy Control Group
| EEG CH. | Freq. Band | Healthy Averaged Power±STD (µV2) | ALS Averaged Power±STD (µV2) | p-value |
|---|---|---|---|---|
| F2 | Theta | 2.91±0.43 | 2.16±0.38 | 0.004 |
| Fz | Theta | 2.93±0.38 | 2.37±0.34 | 0.02 |
| T7 | Theta | 2.23±0.85 | 1.61±0.6 | 0.007 |
| T8 | Theta | 2.01±0.91 | 1.13±0.84 | 0.006 |
| Cz | Theta | 2.71±0.56 | 2.25±0.36 | 0.03 |
| Fz | Alpha | 4.13±0.37 | 2.67±0.72 | 0.01 |
| T7 | Alpha | 3.42±0.73 | 1.81±0.52 | <0.001 |
| T8 | Alpha | 3.21±0.77 | 1.42±0.83 | 0.005 |
| Cz | Alpha | 4.44±0.82 | 3.18±0.72 | 0.008 |
| P7 | Alpha | 4.56±0.82 | 2.53±0.58 | 0.03 |
| P3 | Alpha | 5.63±0.84 | 3.37±0.42 | 0.004 |
| Pz | Alpha | 6.23±1.12 | 3.84±0.52 | 0.005 |
| P4 | Alpha | 5.71±1.12 | 3.36±0.81 | 0.02 |
| P8 | Alpha | 5.4±0.94 | 2.92±0.57 | 0.01 |
| PO7 | Alpha | 5.61±0.72 | 2.94±0.75 | 0.006 |
| PO8 | Alpha | 5.84±0.91 | 3.02±0.75 | 0.005 |
| Oz | Alpha | 4.43±0.76 | 2.23±0.72 | <0.001 |
Figure 3 shows the channel map of the mean HbO2 power within the VLFO and LFO ranges for healthy controls and ALS patients. Despite an overall EEG power decrease in patients, a general HbO2 power increase in patients was observed. Our statistical analysis revealed a significant power increase in LFO in ALS patients in both the left parietal (CH15) and right parietal (CH16) channels. In addition, significant increases in VLFO power were found in patients across all frontal channels, including AF3-F1 (CH8), Fz-AFz (CH9), AF4-F2 (CH10), F3-F1 (CH11), Fz-F1 (CH12), Fz-F2 (CH13), F4-F2 (CH14), and in left parietal (CH15) and right parietal (CH16) channels, with the maximum difference at CH16 (Healthy 0.56±0.43 µmol2, ALS 0.98±0.61 µmol2, p < 0.001) (See Table III).
Fig. 3.

Channel map of averaged HbO2 power for very low frequency oscillations (VLFO) and low frequency oscillations (LFO) for healthy controls and ALS patients.
TABLE III.
Significant fNIRS Spectral Power Changes in ALS Patients Compared to the Healthy Control Group
| fNIRS CH. | Frequency Band | Healthy Averaged Power±STD (µmol2) | ALS Averaged Power±STD (µmol2) | p-value |
|---|---|---|---|---|
| CH15 | LFO | 0.41±0.08 | 0.48±0.08 | 0.03 |
| CH16 | LFO | 0.40±0.12 | 0.49±0.1 | 0.03 |
| CH8 | VLF() | 0.79±0.45 | 1.02±0.52 | 0.02 |
| CH9 | VLFO | 0.72±0.57 | 0.95±0.52 | 0.02 |
| CH10 | VLF() | 0.78±0.49 | 0.98±0.61 | 0.02 |
| CH11 | VLFO | 0.64±0.54 | 0.88±0.53 | 0.01 |
| CH12 | VLFO | 0.59±0.45 | 0.95±0.51 | 0.005 |
| CH13 | VLFO | 0.59±0.57 | 0.93±0.50 | 0.005 |
| CH14 | VLF() | 0.66±0.43 | 0.93±0.63 | 0.006 |
| CH15 | VLFO | 0.59±0.40 | 0.89±0.62 | 0.002 |
| CH16 | VLFO | 0.56±0.43 | 0.98±0.61 | <0.001 |
B. RSFC Results
Figure 4 illustrates the negative logarithm of the p-values, or “activation index” that was obtained from the statistical comparison of the mean EEG coherence between ALS patients and healthy controls in four frequency bands (delta, theta, alpha and beta) and for three channels (Fz, Cz, Pz) as seed channels (note: results from other seed channels are reported here but not shown in the figure). The significant p-values after correction for multiple comparisons are illustrated by dashed lines between the corresponding connections. Statistical analysis revealed a significant alpha band RSFC increase in patients over fronto- and central-parietal connections, specifically F1-P3, F2-P4, and Cz-P3. More importantly, a significant beta RSFC increase was found in patients over fronto-parietal connections, including the F1-P3, F1-Pz, F1-P4, Fz-P3, Fz-Pz, Fz-P4, F2-P3, F2-Pz, and F2-P4, cenral-parietal connection of Cz-P3, along with an inter-hemispheric parietal connection of P3-P4, with the most profound increase in Fz-Pz (Healthy 0.21±0.03, ALS 0.44±0.04, p < 0.001) (See Table IV).
Fig. 4.

Head plots of RSFC activation indices (negative logarithm of the p-values) for EEG showing the difference of averaged magnitude squared coherence between ALS patients and healthy controls (increased RSFC in ALS patients) in four frequency bands (delta, theta, alpha, and beta) and for five seed channels (Fz, Cz, Pz, P3, and P4). The significant p-values after multiple comparisons correction are illustrated with dashed lines between the seed channel (highlighted in blue) and the significant region at the other end (highlighted in red based on the activation index of the connection).
TABLE IV.
Significant EEG RSFC Changes in ALS Patients Compared to the Healthy Control Group
| EEG Connection | Frequency Band | Healthy Averaged RSFC±STD | ALS Averaged RSFC±STD | p-value |
|---|---|---|---|---|
| F1-P3 | Alpha | 0.24±0.01 | 0.41±0.03 | 0.009 |
| F2-P4 | Alpha | 0.20±0.03 | 0.36±0.06 | 0.007 |
| Cz-P3 | Alpha | 0.22±0.03 | 0.38±0.08 | 0.02 |
| F1-P3 | Beta | 0.21±0.04 | 0.44±0.04 | 0.002 |
| F1-Pz | Beta | 0.21±0.06 | 0.42±0.04 | 0.002 |
| F1-P4 | Beta | 0.16±0.02 | 0.33±0.02 | 0.004 |
| Fz-P3 | Beta | 0.19±0.08 | 0.42±0.05 | <0.001 |
| Fz-Pz | Beta | 0.21±0.03 | 0.44±0.04 | <0.001 |
| Fz-P4 | Beta | 0.18±0.03 | 0.37±0.06 | <0.001 |
| F2-P3 | Beta | 0.16±0.03 | 0.37±0.03 | <0.001 |
| F2-Pz | Beta | 0.2±0.04 | 0.4±0.04 | <0.001 |
| F2-P4 | Beta | 0.18±0.07 | 0.36±0.04 | <0.001 |
| Cz-P3 | Beta | 0.19±0.08 | 0.39±0.05 | 0.002 |
| P3-P4 | Beta | 0.38±0.05 | 0.58±0.07 | 0.01 |
Figure 5 illustrates the activation index based on obtained p-values for different regions calculated from the statistical comparison of the averaged HbO2 correlation between ALS patients and healthy controls for eight seed channels that had significantly altered connections with other channels. The significant p-values after multiple comparisons are illustrated with dashed lines between the corresponding regions. The magnitude squared correlation of HbO2 channel pairs revealed a significant RSFC increase in patients within both the right prefrontal (CH2-CH4) and right frontal regions (CH13-CH4, CH13-CH7). Investigating interhemispheric RSFC also revealed a significant increase in patients over various within-frontal connections, including: (CH6-CH7, CH6-CH10, CH6-CH13, CH11-CH14) and between the frontal and prefrontal regions (CH6-CH2) (See Table V).
Fig. 5.

Frontal head plots illustrating activation indices (negative logarithms of p-values) showing the difference of averaged HbO2 correlation between ALS and healthy controls (i.e., increased RSFC in ALS patients) for eight seed channels. The significant p-values after multiple comparisons are illustrated by dashed lines between the seed channel (highlighted in blue) and the significant region at the other end (highlighted in red based on the activation index of the connection). The numbers in the figure indicate channel numbers.
TABLE V.
Significant fNIRS RSFC changes in ALS Patients Compared to the Healthy Control Group
| fIVIRS Connection | Healthy Average RSFC±STD | ALS Average RSFC±STD | p-value |
|---|---|---|---|
| CH2-CH4 | 0.56±0.09 | 0.8110.10 | 0.02 |
| CH13-CH4 | 0.56±0.09 | 0.81±0.1 | 0.02 |
| CH13-CH7 | 0.61±0.08 | 0.73±0.05 | 0.03 |
| CH6-CH7 | 0.74±0.05 | 0.89±0.07 | 0.04 |
| CH6-CH10 | 0.61±0.07 | 0.79±0.09 | 0.02 |
| CH6-CH13 | 0.47±0.06 | 0.79±0.07 | 0.01 |
| CH11-CH14 | 0.48±0.08 | 0.74±0.11 | 0.02 |
| CH6-CH2 | 0.71±0.05 | 0.83±0.06 | 0.02 |
C. Correlation Results
Figure 6 illustrates correlations between EEG and fNIRS measures, which correlation analysis found to be significant in either of healthy or ALS groups. As the figure shows, only the healthy group had significant (or marginally significant) correlations between EEG and fNIRS powers in three pairs of adjacent channels for both modalities as follows: EEG theta at channel P4 and fNIRS LFO at channel 16 (p-value = 0.03), EEG alpha at channel P4 and fNIRS LFO band at channel 16 (p-value = 0.05), EEG theta at channel F2 and fNIRS VLFO band at channel 13 (p-value = 0.04). No significant correlations were found between EEG and fNIRS measures in the ALS group.
Fig. 6.
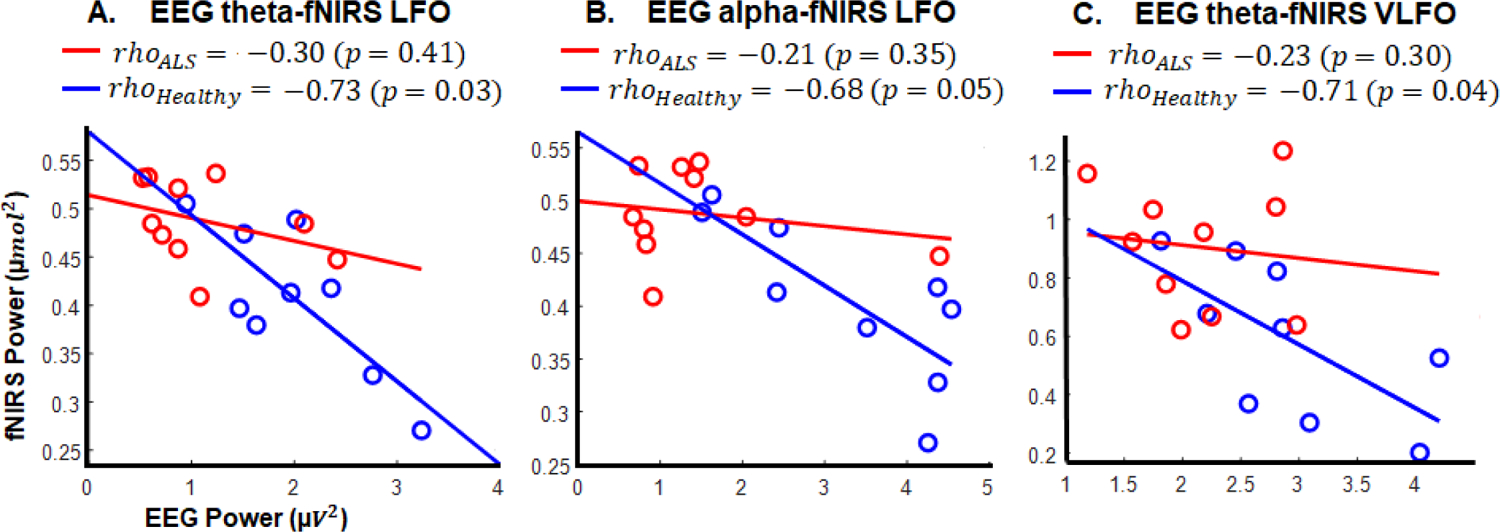
Correlation results between EEG and fNIRS measures for healthy and ALS groups: A) between EEG theta in P4 and fNIRS LFO in CH16; B) between EEG alpha in P4 and fNIRS LFO in CH16; C) between EEG theta in F2 and fNIRS VLFO in CH13. Note: only correlations which were significant (or marginally significant) in at least one group are shown.
No significant correlations were found between EEG/fNIRS measures and ALSFRS-R scores in patients.
IV. Discussion
In this study, the electrical and vascular-hemodynamic functional neural alterations of ALS were characterized by integrating EEG and fNIRS in a multimodal framework of recording and analyses. Functional network organizational impairments specific to ALS were characterized using frequency-band specific RSFC measures and spectral power in both hemodynamic and electrical activities during the resting-state. Our comparative group analysis demonstrated significantly increased fronto-parietal EEG connectivity in the alpha and beta bands, along with increased interhemispheric and intraright hemisphere fNIRS connectivity in the frontal and prefrontal regions in the ALS group. Furthermore, we observed an overall reduction in alpha and theta EEG spectral power in the frontal, central, and temporal regions, and alpha power reduction in the parietal and occipital regions, as well as increased hemodynamic spectral power in the frontal (VLFO) and parietal regions (VLFO and LFO) in ALS patients. We also found that only the healthy group had a significant correlation between EEG and fNIRS measures, specifically in the right parietal frontal region between theta and alpha EEG powers and fNIRS LFO power along with theta EEG power and fNIRS VLFO power in the frontal region.
Our scalp-level findings of increased functional connectivity in ALS are consistent with various hemodynamic and electrophysiological resting-state functional connectivity studies, across various cortical networks in ALS patients, including the Default Mode network (DMN), the Fronto-Parietal network (FPN), Dorsal Attention network (DAN), and the salience network. For example, increased functional connectivity in ALS has been consistently reported in resting-state fMRI studies during closed eyes [8]–[11], [25], [26] primarily in the DMN and FPN which involve the prefrontal, frontal, and parietal regions. This increased functional connectivity has been shown to be associated with clinical and cognitive deficits in ALS patients [8], disease progression rates, and regions of decreased structural connectivity [9], [10]. Functional connectivity increases have been suggested to primarily reflect the extensive involvement of extra-motor networks in ALS rather than simply a physiological compensation mechanism for the reduced structural integrity or a reflection of a progressive loss of cortical inhibitory influence as an element of the pathophysiology of ALS.
Incongruently, other resting-state fMRI studies during closed eyes have reported contradictory findings of reduced functional connectivity in ALS and in networks involved in cognitive and behavioral functions [4], [27], [28]. Thus, the characteristic signatures of RSFC impairments in ALS remain incongruent in the literature, as there is no clear agreement whether ALS-specific functional connectivity impairments represent an increased or decreased synchronization. Variations in the underlying structural degeneration, as well as methodological differences, including instability of independent component analysis (ICA)-based resting-state analysis compared to other methods such as structural imaging-derived network-guided component analysis used in the functional connectivity analysis and seed region-based functional connectivity analysis [29], may greatly contribute to variations in these observed signatures. Despite these incongruent results, alterations in the functional organization of the extra-motor networks have been interpreted generally as correlates of cognitive dysfunctions in ALS.
Resting-state electrophysiological studies have provided additional evidence that points to extensive involvement of extra-motor networks in ALS based on functional connectivity findings. However, the precise neuroelectric signatures of the altered cortical communication mechanisms have not been characterized fully to date [30]. Increased functional connectivity over the alpha and beta bands in scalp-level areas corresponding to the DMN and FPN in ALS patients with closed eyes have been identified in [5] using nodal connectivity measures among localized sources of EEG recordings, which is consistent with our results. Although there was no clear association between frequency band-specific findings and ALS pathological changes, the authors linked overall increased functional connectivity to enhanced cortical network recruitment as compensation for structural neuronal loss or alternatively, as a result of the loss of inhibitory control over network regions, which suggests a biomarker for early cortical changes in ALS. Fraschini et al. [7] reported significant network topology alterations in the beta band, similar to our findings for ALS patients during closed eyes recordings. They linked their findings of frequency-specific beta band network alterations to reports that beta band connectivity is associated with maintaining the current cognitive state (i.e., status quo) [31]. In [2], reduced bilateral central and temporal alpha band functional connectivity estimated at the source level was reported in ALS patients compared with healthy controls during closed eyes recordings, suggesting the hypothesis of widespread alterations in synchronization to extra-motor connections. In a high-density longitudinal resting-state EEG study of ALS during open eyes recordings [32], characteristic patterns of increased EEG-gamma coherence between frontoparietal regions and EEG-theta coherence between bilateral regions over motor areas have also been identified. Based on correlations with the structural MRIs of the patients, the authors also suggested this increased neural communication reflects the extensive involvement of extra-motor pathways.
To date, multimodal investigations of ALS functional neural alterations have not characterized electrical-vascular functions of the underlying altered network in these cohorts. However, a few studies conducted combined structural and functional explorations of multidimensional connectivity in ALS. For example, in a study conducted by Verstraete et al. [11] using combined fMRI and diffusion tensor imaging (DTI), the authors reported no significant functional change in ALS. Similarly, Kopitzki et al. [3] obtained the same results for functional connectivity in ALS during closed eyes recordings using DTI and fNIRS. In this study, the authors placed eight individual fNIRS optodes apart from each other all over the head. Because individual channel-wise RSFC measured by fNIRS has reliability issues [14] due to difficulty matching channel-to-channel for both RSFC strength and location precisely, this may explain the contrast with our fNIRS findings.
Our complementary electrical and vascular-hemodynamic functional connectivity results of increased fronto-parietal connectivity using EEG and increased frontal and prefrontal connectivity using fNIRS are consistent with many aforementioned resting-state studies that employed unimodal neuroimaging techniques including EEG or fMRI. Consistent with these previous studies, the prefrontal, frontal, and parietal brain regions are well-defined functionally coherent areas during the resting-state and overlap with the DMN, FPN, and DAN. The observed alterations in these regions point to the extensive role of cognitive and extra-motor networks in addition to motor pathways identified conventionally in ALS. The DMN is known widely to provide a baseline state of the brain that represents memory, emotional processing, self-reference, spontaneous cognition, and aspects of consciousness [33]. Increased resting-state connectivity in the DMN has been consistently reported in ALS and was often significantly associated with greater disability and faster progression rates [4], [27]. Moreover, executive functioning impairments in ALS have been reported to be associated with the FPN and DAN networks, which are believed to act as control systems for various cognitive activities, including attention and executive processing [34]–[36]. Furthermore, we observed increased connectivity in hemodynamic activities in the frontal and right prefrontal regions, which is consistent with the findings of [26]. As the left frontal and prefrontal regions including the left lateral and left anterior prefrontal areas, are highly responsible for task and stimulus oriented control processes, such as response planning and stimulus-response relations [37], this might explain why these areas did not demonstrate significant activation or connectivity changes in ALS during our resting-state analysis when there was no specific task or stimulus. On the other hand, constant monitoring for upcoming stimuli as a non-task oriented activity is controlled largely by the right frontal and prefrontal regions, including the right lateral frontal and rostral prefrontal areas [37]. As activityrelated to constant monitoring for upcoming stimuli has been reported to occur in the resting-state [38], we speculate that the increased connectivity and power of hemodynamic activities in ALS patients is likely a compensatory mechanism for monitoring deficits. This is also consistent with the findings of Hammer et al. [39] on ALS patients in a dual spatial-working memory processing task, implying altered processing in the right dorsolateral prefrontal cortex. All of these can be associated with the previous ALS findings that have suggested that executive dysfunctions, including issues with information maintenance and monitoring, attentional processing, working memory, language, and social cognition, are present in people with ALS [40], [41]. The alterations in functional connectivity observed in the extra-motor network provide further evidence that ALS is a multisystem disease that might have special markers in addition to its characteristic motor dysfunctions.
Notably, our results of significant increased fronto-parietal connectivity were found primarily in the EEG-beta frequency band, which supports our previous work [42]. As beta band neural coupling has been reported to be expressed more strongly if the maintenance of the current status is intended or predicted [31], the increased fronto-parietal connectivity in this frequency band in the patients can be interpreted as a compensatory mechanism for maintenance and monitoring deficits of the fronto-parietal control system. In [43], the increased functional connectivity in ALS has been hypothesized to result from the loss of intracortical inhibitory influence supported by neurophysiological findings of altered cortical betadesynchronization in motor execution in ALS patients during movement preparation and post-movement beta-rebound [44].
In this study, the alterations in functional communication patterns in ALS were also characterized by spectral power analysis. Our EEG results are consistent with several studies that have reported a decrease in neural spectral power in ALS. For example, a recent study [30] observed reductions in EEG spectral power in the prefrontal region in the delta and theta bands, the sensorimotor region in the beta band, and in the occipital and temporal regions in the delta, alpha, and beta bands in ALS patients during open eyes recordings. Our finding of overall reduced alpha band power also supports the findings in [45] and [46] of which were conducted with closed eyes and the authors reported that the decrease in alpha power was associated with reduced neural activity correlated with the disease-specific structural degeneration that results from the structural loss of pyramidal neurons in ALS. Decreases in theta band spectral power have also been reported in resting-state studies of ALS patients with open eyes [47], similar to our findings in the central and temporal regions of the brain.
In contrast to reduced EEG spectral power, our band-specific hemodynamic results indicated increased VLFO and LFO activities in the frontal, prefrontal, and parietal regions in our ALS patients. The interaction between these opposite effects of ALS on EEG and fNIRS was further investigated by correlation analysis. Interestingly, the correlation between these two modalities in the healthy group was disrupted in ALS patients, particularly in the frontal and right parietal regions. This lack of correlation between EEG and fNIRS is consistent with our previous study [12] where we found similar results in a task-based analysis for ALS patients. The increased hemodynamic activity in extra-motor networks has been interpreted in [8] to reflect compensatory processes for fronto-parietal network dysfunctions, which was supported with negative correlations with disease progression rates in their study. These interpretations are also consistent with [11], in which the authors suggested increased VLFO and LFO activity relates to cognitive impairment in ALS. However, the hypothesis of the compensatory mechanism and the spatial characteristics of these low-frequency power alterations in ALS needs to be investigated in future studies.
There are several limitations in this study, including its small sample size and heterogeneous characteristics of ALS patients. If a larger number of patients is recruited in future studies, it will be possible to classify them into subgroups based on the onset of clinical symptoms, involvement level of motor degeneration, and cognitive deficits to discriminate between different patterns rather than considering putative patterns of altered networks for all ALS patients. In addition, quantitative assessment of cognitive profiles and psychometric behavioral screens, along with other biological information, including respiration, peripheral capillary oxygen saturation, and electromyography in ALS patients, should be provided in future studies for more precise interpretations of the results. Furthermore, future studies can exploit a higher density head montage to investigate the between-group differences in all the brain regions in which the volume conduction effect has to be considered and removed by proper spatial filtering methods, including Laplacian filtering or explore possible altered connectivity patterns by focusing on more direct phase-based connectivity measures, including the imaginary part of coherency, which avoids spurious connectivity resulting from volume conduction effects on EEG signals. This consideration might be especially useful for a scalp-level EEG connectivity analysis, as it is assumed that an observed scalp potential has approximately zero time-lag to the underlying source activity. It is worth noting that further investigations should focus as well on exploring more detailed relations between electrical and hemodynamic aspects of altered neural networks, although caution is needed when interpreting the findings from multimodal measures and comparing them to other modalities that measure different properties of the underlying neural networks. Moreover, these techniques differ with respect to their temporal and spatial resolutions, and it is not straightforward to compare their findings. Thus, they should be used instead in a complementary way to improve a comprehensive understanding of multimodal findings.
V. Conclusion
Overall, our EEG-fNIRS multimodal resting state recording could capture functional neural and hemodynamic alterations in ALS, supporting the findings in several previous studies that employed unimodal EEG or fNIRS/fMRI techniques. We observed spectral power alterations in the VLFO and LFO ranges of hemodynamic responses primarily in the frontal and prefrontal regions and in the theta and alpha bands of electrophysiological responses in ALS. These observations were complemented by the identification of additional functional electrophysiological alterations in the fronto-parietal connections in higher frequency bands (primarily beta) and functional hemodynamic alterations in the frontal and prefrontal connections in this cohort. Our proposed multimodal recording and analysis framework permits multidimensional investigations of functional network alterations underlying heterogeneous ALS pathologies. The outcomes can potentially be expanded further as a tool for non-invasive diagnosis and prognosis of the disease in clinical environments. Our findings highlight the importance of integrative recording and analysis techniques in capturing broader ranges of disease-specific functional alterations that can potentially provide quantitative biomarkers of ALS pathogenesis.
Supplementary Material
Acknowledgment
The authors would like to thank the participants who took part in this study, without whom this study would not have been possible. We would also like to thank the ALS Association Rhode Island Chapter and the National Center for Adaptive Neurotechnologies for their continuous support. This study was supported by the National Science Foundation (NSF-1913492) and the Institutional Development Award (IDeA) Network for Biomedical Research Excellence from the NIGMS of the NIH (P20GM103430).
Contributor Information
R. J. Deligani, Department of Electrical, Computer, and Biomedical Engineering, URI, RI 02881, USA.
S. I. Hosni, Department of Electrical, Computer, and Biomedical Engineering, URI, RI 02881, USA.
S. B. Borgheai, Department of Electrical, Computer, and Biomedical Engineering, URI, RI 02881, USA..
J. McLinden, Department of Electrical, Computer, and Biomedical Engineering, URI, RI 02881, USA..
A. H. Zisk, Interdisciplinary Neuroscience Program, URI, RI, 02881, USA.
K. Mankodiya, Department of Electrical, Computer, and Biomedical Engineering, URI, RI 02881, USA..
Y. Shahriari, Department of Electrical, Computer & Biomedical Engineering, University of Rhode Island (URI), RI 02881, USA..
References
- [1].Christidi F, Karavasilis E, Rentzos M, Kelekis N, Evdokimidis I, and Bede P, “Clinical and radiological markers of extra-motor deficits in amyotrophic lateral sclerosis,” Frontiers in Neurology 2018, doi: 10.3389/fneur.2018.01005. [DOI] [PMC free article] [PubMed]
- [2].Fraschini M et al. , “Functional brain connectivity analysis in amyotrophic lateral sclerosis: An EEG source-space study,” Biomed. Phys. Eng. Express, 2018, doi: 10.1088/2057-1976/aa9c64. [DOI]
- [3].Kopitzki K et al. , “Interhemispheric connectivity in amyotrophic lateral sclerosis: A near-infrared spectroscopy and diffusion tensor imaging study,” NeuroImage Clin, 2016. [DOI] [PMC free article] [PubMed]
- [4].Mohammadi B, Kollewe K, Samii A, Krampfl K, Dengler R, and Münte TF, “Changes of resting state brain networks in amyotrophic lateral sclerosis,” Exp. Neurol, 2009. [DOI] [PubMed]
- [5].Iyer PM et al. , “Functional connectivity changes in resting-state EEG as potential biomarker for Amyotrophic Lateral Sclerosis,” PLoS One, 2015, doi: 10.1371/journal.pone.0128682. [DOI] [PMC free article] [PubMed]
- [6].Pievani M, Filippini N, Van Den Heuvel MP, Cappa SF, and Frisoni GB, “Brain connectivity in neurodegenerative diseases - From phenotype to proteinopathy,” Nature Reviews Neurology 2014, doi: 10.1038/nrneurol.2014.178. [DOI] [PubMed]
- [7].Fraschini M et al. , “EEG functional network topology is associated with disability in patients with amyotrophic lateral sclerosis,” Sci. Rep, 2016, doi: 10.1038/srep38653. [DOI] [PMC free article] [PubMed]
- [8].Agosta F et al. , “Divergent brain network connectivity in amyotrophic lateral sclerosis,” Neurobiol. Aging, 2013, doi: 10.1016/j.neurobiolaging.2012.04.015. [DOI] [PubMed]
- [9].Luo CY et al. , “Patterns of Spontaneous Brain Activity in Amyotrophic Lateral Sclerosis: A Resting-State fMRI Study,” PLoS One, 2012, doi: 10.1371/journal.pone.0045470. [DOI] [PMC free article] [PubMed]
- [10].Douaud G, Filippini N, Knight S, Talbot K, and Turner MR, “Integration of structural and functional magnetic resonance imaging in amyotrophic lateral sclerosis,” 2011, doi: 10.1093/brain/awr279. [DOI] [PubMed]
- [11].Verstraete E et al. , “Motor network degeneration in amyotrophic lateral sclerosis: A structural and functional connectivity study,” PLoS One, 2010, doi: 10.1371/journal.pone.0013664. [DOI] [PMC free article] [PubMed]
- [12].Borgheai SB et al. , “Multimodal exploration of non-motor neural functions in ALS patients using simultaneous EEG-fNIRS recording,” J. Neural Eng, vol. 16, no. 6, 2019, doi: 10.1088/1741-2552/ab456c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Borgheai SB et al. , “Enhancing Communication for People in Late-Stage ALS Using an fNIRS-Based BCI System,” IEEE Trans. Neural Syst. Rehabil. Eng, 2020, doi: 10.1109/TNSRE.2020.2980772. [DOI] [PMC free article] [PubMed]
- [14].Zhang H, Zhang Y-J, Duan L, Ma S-Y, Lu C-M, and Zhu C-Z, “Is resting-state functional connectivity revealed by functional near-infrared spectroscopy test-retest reliable?,” J. Biomed. Opt, 2011, doi: 10.1117/1.3591020. [DOI] [PubMed]
- [15].Nguyen DK et al. , “Non-invasive continuous EEG-fNIRS recording of temporal lobe seizures,” Epilepsy Res, 2012, doi: 10.1016/j.eplepsyres.2011.10.035. [DOI] [PubMed]
- [16].Al-Shargie F, Kiguchi M, Badruddin N, Dass SC, Hani AFM, and Tang TB, “Mental stress assessment using simultaneous measurement of EEG and fNIRS,” Biomed. Opt. Express, 2016, doi: 10.1364/boe.7.003882. [DOI] [PMC free article] [PubMed]
- [17].Borgheai SB et al. , “Multimodal Evaluation of Mental Workload Using a Hybrid EEGfNIRS Brain-Computer Interface System,” 2019, doi: 10.1109/NER.2019.8717118. [DOI]
- [18].Cedarbaum JM et al. , “The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function,” J. Neurol. Sci, 1999, doi: 10.1016/S0022-510X(99)00210–5. [DOI] [PubMed]
- [19].Du Chang W, Cha HS, Kim K, and Im CH, “Detection of eye blink artifacts from single prefrontal channel electroencephalogram,” Comput. Methods Programs Biomed, 2016, doi: 10.1016/j.cmpb.2015.10.011. [DOI] [PubMed]
- [20].Nolte G, Bai O, Wheaton L, Mari Z, Vorbach S, and Hallett M, “Identifying true brain interaction from EEG data using the imaginary part of coherency,” Clin. Neurophysiol, 2004. [DOI] [PubMed]
- [21].Sasai S, Homae F, Watanabe H, and Taga G, “Frequency-specific functional connectivity in the brain during resting state revealed by NIRS,” Neuroimage, 2011, doi: 10.1016/j.neuroimage.2010.12.075. [DOI] [PubMed]
- [22].Kocsis L, Herman P, and Eke A, “The modified Beer-Lambert law revisited,” Phys. Med. Biol, 2006, doi: 10.1088/0031-9155/51/5/N02. [DOI] [PubMed]
- [23].Fernandez Rojas R, Huang X, Hernandez-Juarez J, and Ou KL, “Physiological fluctuations show frequency-specific networks in fNIRS signals during resting state,” 2017, doi: 10.1109/EMBC.2017.8037377. [DOI] [PubMed]
- [24].Cohen MX, “Analyzing Neural Time Series Data: Theory and Practice,” MIT Press. 2014, doi: 10.1017/CBO9781107415324.004. [DOI]
- [25].Menke RAL et al. , “Increased functional connectivity common to symptomatic amyotrophic lateral sclerosis and those at genetic risk,” J. Neurol. Neurosurg. Psychiatry, 2016. [DOI] [PMC free article] [PubMed]
- [26].Ma X et al. , “Altered cortical hubs in functional brain networks in amyotrophic lateral sclerosis,” Neurol. Sci, 2015. [DOI] [PubMed]
- [27].Tedeschi G et al. , “Interaction between aging and neurodegeneration in amyotrophic lateral sclerosis,” Neurobiol. Aging, 2012, doi: 10.1016/j.neurobiolaging.2010.07.011. [DOI] [PubMed]
- [28].Li F, Zhou F, Huang M, Gong H, and Xu R, “Frequency-specific abnormalities of intrinsic functional connectivity strength among patients with amyotrophic lateral sclerosis: A resting-state fMRI study,” Front. Aging Neurosci, 2017. [DOI] [PMC free article] [PubMed]
- [29].Zhou F et al. , “Altered motor network functional connectivity in amyotrophic lateral sclerosis: A resting-state functional magnetic resonance imaging study,” Neuroreport, 2013. [DOI] [PubMed]
- [30].Dukic S et al. , “Patterned functional network disruption in amyotrophic lateral sclerosis,” Hum. Brain Mapp, 2019. [DOI] [PMC free article] [PubMed]
- [31].Engel AK and Fries P, “Beta-band oscillations-signalling the status quo?,” Current Opinion in Neurobiology. 2010. [DOI] [PubMed]
- [32].Nasseroleslami B et al. , “Characteristic Increases in EEG Connectivity Correlate with Changes of Structural MRI in Amyotrophic Lateral Sclerosis,” Cereb. Cortex, 2019. [DOI] [PubMed]
- [33].Raichle ME, “The Brain’s Default Mode Network,” Annu. Rev. Neurosci, 2015, doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed]
- [34].Vincent JL, Kahn I, Snyder AZ, Raichle ME, and Buckner RL, “Evidence for a frontoparietal control system revealed by intrinsic functional connectivity,” J. Neurophysiol, 2008. [DOI] [PMC free article] [PubMed]
- [35].Corbetta M and Shulman GL, “Control of goal-directed and stimulus-driven attention in the brain,” Nat. Rev. Neurosci, 2002. [DOI] [PubMed]
- [36].Christidi F, Zalonis I, Smyrnis N, and Evdokimidis I, “Selective attention and the three-process memory model for the interpretation of verbal free recall in amyotrophic lateral sclerosis,” J. Int. Neuropsychol. Soc, 2012, doi: 10.1017/S1355617712000562. [DOI] [PubMed]
- [37].Yunusova Y et al. , “Frontal Anatomical Correlates of Cognitive and Speech Motor Deficits in Amyotrophic Lateral Sclerosis,” Behav. Neurol, 2019, doi: 10.1155/2019/9518309. [DOI] [PMC free article] [PubMed]
- [38].Francis MM et al. , “Association of medial prefrontal resting state functional connectivity and metacognitive capacity in early phase psychosis,” Psychiatry Res. - Neuroimaging, 2017. [DOI] [PubMed]
- [39].Hammer A, Vielhaber S, Rodriguez-Fornells A, Mohammadi B, and Münte TF, “A neurophysiological analysis of working memory in amyotrophic lateral sclerosis,” Brain Res, 2011, doi: 10.1016/j.brainres.2011.09.010. [DOI] [PubMed]
- [40].Raaphorst J, De Visser M, Linssen WHJP, De Haan RJ, and Schmand B, “The cognitive profile of amyotrophic lateral sclerosis: A meta-analysis,” Amyotrophic Lateral Sclerosis 2010. [DOI] [PubMed]
- [41].Volpato C et al. , “Working memory in amyotrophic lateral sclerosis: Auditory event-related potentials and neuropsychological evidence,” J. Clin. Neurophysiol, 2010, doi: 10.1097/WNP.0b013e3181e0aa14. [DOI] [PubMed]
- [42].Shahriari Y; Sellers EW; McCane LM; Vaughan TM; Krusienski DJ, “Directional brain functional interaction analysis in patients with amyotrophic lateral sclerosis,” 7th Int. IEEE/EMBS Conf. Neural Eng., 2015, doi: 10.1109/ner.2015.7146788. [DOI] [Google Scholar]
- [43].Clark R, Blizzard C, and Dickson T, “Inhibitory dysfunction in amyotrophic lateral sclerosis: future therapeutic opportunities,” Neurode-generative disease management 2015. [DOI] [PubMed]
- [44].Bizovičar N, Dreo J, Koritnik B, and Zidar J, “Decreased movement-related beta desynchronization and impaired post-movement beta rebound in amyotrophic lateral sclerosis,” Clin. Neurophysiol, 2014, doi: 10.1016/j.clinph.2013.12.108. [DOI] [PubMed]
- [45].Mai R, Facchetti D, Micheli A, and Poloni M, “Quantitative electroencephalography in amyotrophic lateral sclerosis,” Electroencephalogr. Clin. Neurophysiol, 1998, doi: 10.1016/S0013-4694(97)00159-4. [DOI] [PubMed]
- [46].Santhosh J, Bhatia M, Sahu S, and Anand S, “Decreased electroencephalogram alpha band [8–13 Hz] power in amyotrophic lateral sclerosis patients: A study of alpha activity in an awake relaxed state,” Neurol. India, vol. 53, no. 1, p. 99, 2005, doi: 10.4103/0028-3886.15071. [DOI] [PubMed] [Google Scholar]
- [47].Jayaram V et al. , “Brain-computer interfacing in amyotrophic lateral sclerosis: Implications of a resting-state EEG analysis,” 2015, doi: 10.1109/EMBC.2015.7319998. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


