Abstract
Locally advanced basal cell carcinoma (laBCC) represents a rare but possible occurrence in the vast scenario of dermatological diseases. It is well known that most BCC has a pathological activation of the hedgehog pathway, making them susceptible to targeted therapy with selective inhibitors. Sonidegib, approved for the treatment of laBCC on the basis of the results of the basal cell carcinoma outcomes with LDE225 treatment study, demonstrated rapid efficacy and a manageable safety profile. Here, we describe the case of a patient affected by multiple laBCC treated with Sonidegib. The patient experienced an important regression of tumors after only 2 months of therapy, with few side effects. This result confirms the role of Sonidegib as a valid and well-tolerated therapeutic option for laBCC.
Keywords: hedgehog pathway inhibitors, locally advanced basal cell carcinoma, nonmelanoma skin cancer, sonidegib, treatment
Introduction
Basal cell carcinoma (BCC) is the most common form of skin cancer, making up approximately 80% of all nonmelanoma skin cancers [1]. The vast majority of BCCs can be effectively cured by complete histopathology controlled excision, which is the gold standard for BCC treatment. However, about 1–10% of lesions evolve towards advanced forms, including laBCC and Metastatic BCC [2]; in these cases, surgery may not represent a possible therapeutic option, because curing is unlikely and surgery might result in substantial deformity [3].
In order to avoid unreasonable postsurgical cosmetic changes, alternative approaches such as radiation, electrochemotherapy or systemic treatment [4] can be required, as specified by the European guidelines for the management of BCC [5]. Particularly, these approaches are indicated in patient affected by multiple comorbidities, numerous lesions or Gorlin–Goltz syndrome.
However, about 90% of BCCs have a pathologic activation of the Sonic hedgehog pathway, making them susceptible to targeted therapy with selective hedgehog pathway inhibitors (HPI) [6,7]. Notably, Sonidegib (Odomzo) demonstrated a sustained and clinically relevant efficacy and a manageable safety profile in phase II randomized, double-blind basal cell carcinoma outcomes with LDE225 treatment (BOLT) study [8,9].
Herein, we describe a rapid and exceptional response to Sonidegib in a patient affected by multiple disfiguring laBCC.
Case report
An otherwise healthy 71-year-old male patient comes to our attention for the sudden bleeding of an ulcerated abdominal lesion of 8 cm × 5 cm (Fig. 1a). Complete physical examination allowed us to identify about 30 other pink or reddish papules and plaques of different shapes and sizes (up to 15 cm) located on the face, trunk and limb; clinical and dermoscopic characteristics were compatible with BCCs (Fig. 1b–d).
Fig. 1.
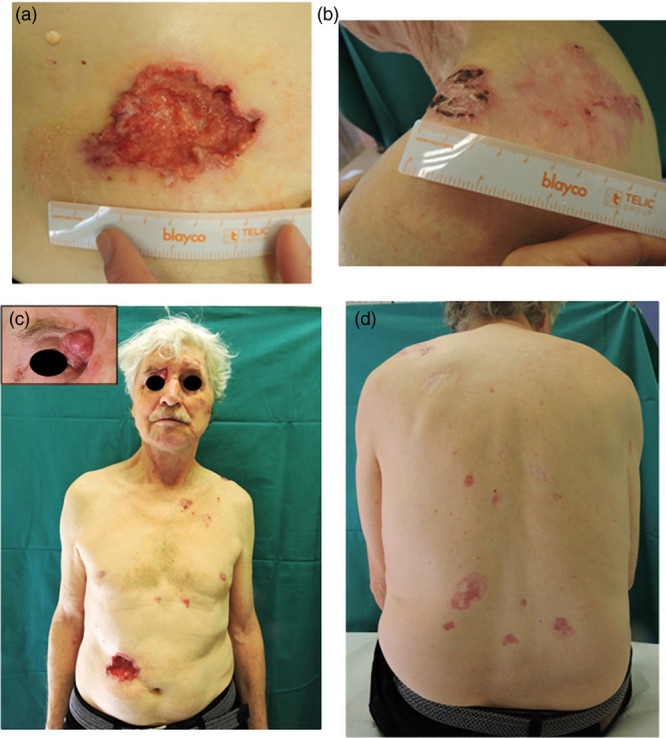
(a) Deep ulcerated abdominal lesion; (b) Basal cell carcinoma (BCC) of 15 cm × 8 cm located on the left shoulder; (c) multiple BCCs of different shapes and sizes on the anterior trunk, with a detail of the voluminous nodule at the internal cantus; (d) multiple BCC on the posterior trunk.
We collected the patient’s medical history, which was negative for comorbidity, chronic therapy or radiotherapy treatments; he reported a previous surgical treatment to remove a BCC on nasal dorsum without any dermatological follow-up for over 10 years. A skin biopsy was performed on the abdominal lesion, and the histological examination confirmed the diagnosis of BCC. The skull X-ray and the orthopantomography showed no evidence of calcification of the falx cerebri or odontogenic keratocysts; the patient did not appear to have skeletal anomalies or intellectual deficit; he also denies family history of skin cancers. All other criteria for a possible Gorlin–Goltz syndrome have been excluded.
Considering the extensive dimensions of the lesions on the abdomen and back, the numerousness of tumors, and the involvement of critical sites, we excluded the possibility of a surgical approach and decided, on the basis of the results of the BOLT study [8] and the evidence reported by Dummer et al. [10], to start systemic therapy with Sonidegib 200 mg, 1 cp per day.
Therapy was well-tolerated by the patient, except for a transient CTCAE grade I increase in the creatine phosphokinase, less than 2.5 × ULN, observed at the end of the first month of treatment, which did not require a dose adjustment. The other grades 1–2 side effects observed were dysgeusia and nocturnal muscle cramps resolving in a few minutes. Already at the end of the second month of treatment, the abdominal lesion appeared considerably reduced in size and depth (Fig. 2a). Furthermore, a similar clinical improvement was observed in other lesions; notably, the lesion on the back was less infiltrated and the size of the nodule at the internal cantus decreased significantly (Fig. 2b).
Fig. 2.
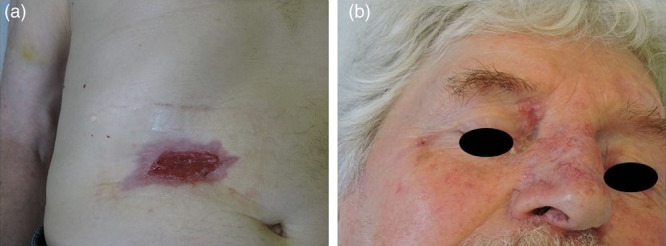
(a) Abdominal basal cell carcinoma (BCC) considerably reduced in size and depth; (b) internal cantus BCC, significantly decreased.
At the end of the sixth month, there was no clinical or dermatoscopic evidence of BCC: the abdominal ulcerated lesion was healed (Fig. 3a), the nodule in the inner corner of the eye was not palpable and the lesions localized in the trunk assumed a scar-like appearance (Fig. 3b–d).
Fig. 3.
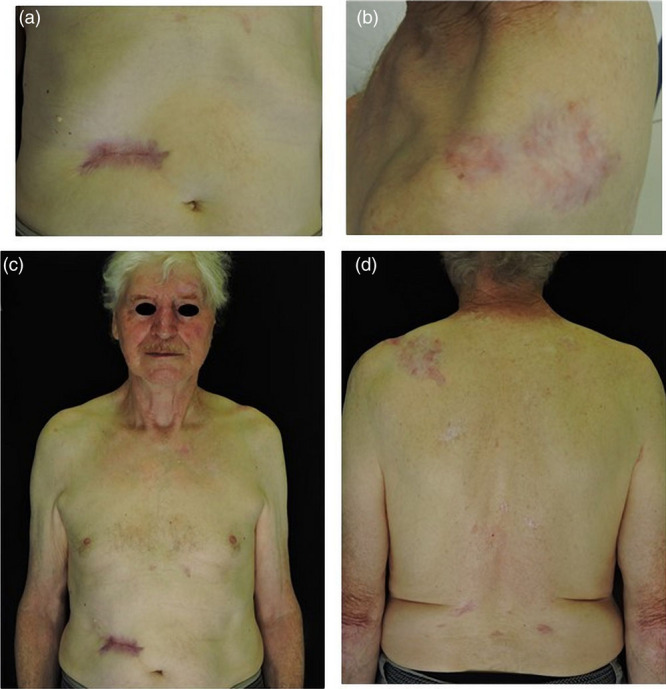
(a) Ulcerated abdominal lesion healed; (b) scar appearance of the lesion localized in the left shoulder; (c) appearance of the anterior trunk and face lesions after 6 months of therapy; (d) appearance of the posterior trunk lesions after 6 months of therapy.
Currently, the treatment is ongoing, and the excellent clinical response persists.
Discussion
Although BCC is the most common skin cancer, the finding of laBCC is rather infrequent, often related to tumors with a long-term course, located in midface or on ears, with aggressive histopathologic subtype, perivascular or perineural infiltration, history of radiation exposure or previous surgical treatment failure. Patients with immunosuppressive status or multiple comorbidities are more affected [11]. In particular, neglected patients are one of the major contributing factors for the development of mutilating and aggressive BCC.
BCC occurs in the head area in 85–90% of cases [12] and can cause, when the tumor reaches a considerable size, social isolation in affected people. Here, the use of alternative therapeutic strategies is mandatory, to avoid cosmetically unacceptable postsurgical outcomes.
In phase II randomized double-blind BOLT study, which led to the approval of Sonidegib [8], the median time of response assessed by investigators was 1.9 months. Even in our patient, we observed a quick response to the treatment, with a significant improvement of all skin lesions after only 2 months. Already during the first weeks of therapy, in addition to an obvious reduction in the size of the tumors, the patient experienced a significative improvement of symptoms; in particular, the bleeding stopped and the patient reported a progressive reduction in the visual discomfort given by the BCC located at the inner corner of the eye. The rapidity of these events is very important as it promotes patient adherence to treatment, limiting the risk of spontaneous drop-out.
The good tolerability profile of Sonidegib detected by the BOLT study [8] was also confirmed in the long-term observations [10,13,14]. Also, in our experience, dysgeusia appeared as the first and main adverse event, whereas muscle cramps (reported as the first side effect in the patients received the 200 mg dose) arose later. However, also a slight spontaneously resolved increase in the CK value was observed.
Nevertheless, this value should be monitored, to evaluate any dose adjustment as required by the drug data sheet [8,9,13,14].
Even if the usefulness of HPI in the treatment of laBCC is widely confirmed [15], there are still few real-life experiences on Sonidegib [16,17] and anecdotal reports on multiple laBCC.
Our observations confirm the efficacy and safety of Sonidegib in this setting, suggesting it as an excellent therapeutic choice in neglected and laBCC.
Conclusion
In our real-life experience, Sonidegib has fulfilled its main goal, which is to obtain a rapid improvement of lesions and related symptoms, to avoid the risk of infection and anemia, to promote patient compliance towards the treatment and to reduce their social isolation improving his quality of life. The safety and effectiveness of the treatment will be confirmed by the long-term follow-up.
Acknowledgements
The authors are grateful to the patient for agreeing to share photos contributing to extend medical knowledge on this topic. The study received unrestricted grant from Sun Pharmaceutical Industries.
Conflicts of interest
We thank SunPharma for the non-conditioning contribution. There are no conflicts of interest.
References
- 1.Rubin AI, Chen EH, Ratner D. Basal-cell carcinoma. N Engl J Med. 2005; 353:2262–2269. [DOI] [PubMed] [Google Scholar]
- 2.Migden M, Xie J, Wei J, Tang W, Herrera V, Palmer JB. Burden and treatment patterns of advanced basal cell carcinoma among commercially insured patients in a United States database from 2010 to 2014. J Am Acad Dermatol. 2017; 77:55–62.e3. [DOI] [PubMed] [Google Scholar]
- 3.Peris K, Licitra L, Ascierto PA, Corvò R, Simonacci M, Picciotto F, et al. Identifying locally advanced basal cell carcinoma eligible for treatment with vismodegib: an expert panel consensus. Future Oncol. 2015; 11:703–712. [DOI] [PubMed] [Google Scholar]
- 4.Espeli V, Ruegg E, Hottinger AF, Modarressi A, Dietrich PY. Weekly multi-agent chemotherapy (CMF-b) for advanced non-melanoma skin cancer. Anticancer Res. 2016; 36:2359–2364. [PubMed] [Google Scholar]
- 5.Peris K, Fargnoli MC, Garbe C, Kaufmann R, Bastholt L, Seguin NB, et al. ; European Dermatology Forum (EDF), the European Association of Dermato-Oncology (EADO) and the European Organization for Research and Treatment of Cancer (EORTC). Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur J Cancer. 2019; 118:10–34. [DOI] [PubMed] [Google Scholar]
- 6.Daya-Grosjean L, Couvé-Privat S. Sonic hedgehog signaling in basal cell carcinomas. Cancer Lett. 2005; 225:181–192. [DOI] [PubMed] [Google Scholar]
- 7.Campione E, Di Prete M, Lozzi F, Lanna C, Spallone G, Mazzeo M, et al. High-risk recurrence basal cell carcinoma: focus on hedgehog pathway inhibitors and review of the literature. Chemotherapy. 2020; 65:2–10. [DOI] [PubMed] [Google Scholar]
- 8.Migden MR, Guminski A, Gutzmer R, Dirix L, Lewis KD, Combemale P, et al. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): a multicentre, randomised, double-blind phase 2 trial. Lancet Oncol. 2015; 16:716–728. [DOI] [PubMed] [Google Scholar]
- 9.Dummer R, Guminksi A, Gutzmer R, Lear JT, Lewis KD, Chang ALS, et al. Long-term efficacy and safety of sonidegib in patients with advanced basal cell carcinoma: 42-month analysis of the phase II randomized, double-blind BOLT study. Br J Dermatol. 2020; 182:1369–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dummer R, Ascierto PA, Basset-Seguin N, Dréno B, Garbe C, Gutzmer R, et al. Sonidegib and vismodegib in the treatment of patients with locally advanced basal cell carcinoma: a joint expert. J Eur Acad Dermatol Venereol. 2020; 34:1944–1956. [DOI] [PubMed] [Google Scholar]
- 11.Wollina U, Pabst F, Krönert C, Schorcht J, Haroske G, Klemm E, et al. High-risk basal cell carcinoma: an update. Expert Rev Dermatol. 2010; 5:357–368. [Google Scholar]
- 12.Lovatt TJ, Lear JT, Bastrilles J, Wong C, Griffiths CE, Samarasinghe V, et al. Associations between ultraviolet radiation, basal cell carcinoma site and histology, host characteristics, and rate of development of further tumors. J Am Acad Dermatol. 2005; 52:468–473. [DOI] [PubMed] [Google Scholar]
- 13.Dummer R, Guminski A, Gutzmer R, Dirix L, Lewis KD, Combemale P, et al. The 12-month analysis from Basal Cell Carcinoma Outcomes with LDE225 Treatment (BOLT): a phase II, randomized, double-blind study of sonidegib in patients with advanced basal cell carcinoma. J Am Acad Dermatol. 2016; 75:113–125.e5. [DOI] [PubMed] [Google Scholar]
- 14.Lear JT, Migden MR, Lewis KD, Chang ALS, Guminski A, Gutzmer R, et al. Long-term efficacy and safety of sonidegib in patients with locally advanced and metastatic basal cell carcinoma: 30-month analysis of the randomized phase 2 BOLT study. J Eur Acad Dermatol Venereol. 2018; 32:372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodon J, Tawbi HA, Thomas AL, Stoller RG, Turtschi CP, Baselga J, et al. A phase I, multicenter, open-label, first-in-human, dose-escalation study of the oral smoothened inhibitor Sonidegib (LDE225) in patients with advanced solid tumors. Clin Cancer Res. 2014; 20:1900–1909. [DOI] [PubMed] [Google Scholar]
- 16.Hou X, Rokohl AC, Ortmann M, Heindl LM. Effective treatment of locally advanced periocular basal cell carcinoma with oral hedgehog pathway inhibitor? Graefes Arch Clin Exp Ophthalmol. 2020; 258:2335–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon J, Apicelli AJ, 3rd, Pavlopoulos TV. Intracranial regression of an advanced basal cell carcinoma using sonidegib and itraconazole after failure with vismodegib. JAAD Case Rep. 2018; 4:10–12. [DOI] [PMC free article] [PubMed] [Google Scholar]


