Abstract
Objective: To examine the prevalence of postpartum depression (PPD) and postpartum anxiety (PPA) in mothers with spinal cord injury (SCI).
Design: Retrospective, cross-sectional study.
Setting: Online multi-national study.
Participants: We surveyed an international sample of 102 women who gave birth following cervical SCI (C1–C8, n = 30), upper thoracic SCI (T1–T6, n = 12) or lower level SCI (T7 & below, n = 60). Participants were primarily from Canada and Sweden, and mean age at childbirth was 30 ± 6 years.
Outcome Measures: Subscales from the Pregnancy Risk Assessment Monitoring System (PRAMS) were used to measure PPD (PRAMS-3D) and PPA (PRAMS-2A).
Results: PPD and PPA were most prevalent in women with cervical SCI, followed by upper thoracic SCI then lower SCI. Self-reported PPD was more prevalent than clinically diagnosed PPD in women with cervical SCI (P = 0.03) and upper thoracic SCI (P = 0.03). With cervical SCI, 75% of women diagnosed with MDD before pregnancy scored >9 on the PRAMS PPD subscale, indicating clinically relevant PPD. However, only 10% were diagnosed with PPD. Of women with lower SCI diagnosed with MDD before pregnancy, 25% had a clinically relevant score for self-reported PPD; 7% were diagnosed.
Conclusions: This is currently the largest study examining PPD and PPA after SCI. Clinicians should be aware that mothers with SCI (particularly high-level SCI) may have increased risk of PPD and PPA. PPD is poorly understood in women with SCI and may even be underdiagnosed. SCI-related risk factors for PPD and PPA should be explored.
Keywords: Spinal cord injury, Postpartum depression, Postpartum anxiety, Depression, Motherhood
Introduction
Compared to the general population, major depressive disorder (MDD) is significantly more prevalent in individuals with spinal cord injury (SCI), with estimates of prevalence ranging from 9.8 to 38%.1 Globally, traumatic SCI impacts between 250,000–500,000 individuals every year, with the ratio of men to women being approximately 3.8–1.2,3 The majority of individuals sustain traumatic SCI during their reproductive years,4,5 with the mean age at onset of injury recently estimated to be 39.8 ± 12.2 years.6 Hence, it is perhaps unsurprising that individuals with SCI (particularly females) have reported sexual health/function to be one of the highest priorities to target for improved quality of life.7 Indeed, an increasing proportion of women are choosing to have children after SCI. Despite the tendency of the general public to overlook the possibility of motherhood after physical disability,8 one of the largest studies on reproductive health in women with SCI reported that nearly 14% of women became pregnant after their injuries, with 101 pregnancies across 472 participants.9 As a history of depression (including diagnosis of MDD) is a substantial risk factor for postpartum depression (PPD), it is important to examine maternal psychiatric health in women with SCI. Although it is suggested that PPD symptoms are more prevalent in women with physical disability, this highly relevant and crucial topic remains understudied and no studies to date have assessed the incidence or rates of PPD in women with SCI.10
Left untreated, PPD has been linked to adverse childhood outcomes including developmental delay, lower IQ and behavioral problems.11 One study of 41 women whose pregnancies after SCI resulted in delivery showed that 35% had experienced PPD,12 which is nearly three times higher than the general maternal population (13%).13
While the majority of research on postpartum psychiatric disorders is focused on PPD, postpartum anxiety (PPA) is also a widespread condition that affects up to 18% of women in the general population and is often described as being more common than PPD.14 Like PPD, children of mothers with untreated PPA have been found to develop more behavioral, emotional and conduct disorders.11 Women with SCI have been reported to experience more anxiety, including fear related to childbirth and care. However, clinically diagnosed PPA has not been examined in this population.12
In light of the lack of knowledge surrounding maternal psychiatric health in the SCI population, it is imperative to understand the extent to which mothers with SCI experience PPD and PPA compared to the general population, as well as to examine discrepancies between clinically-diagnosed and self-reported prevalence, so as to guide management strategies. The aim of this novel study was therefore to examine the prevalence of PPD and PPA in women with SCI, which has not previously been established.
Methods
Study setting and design
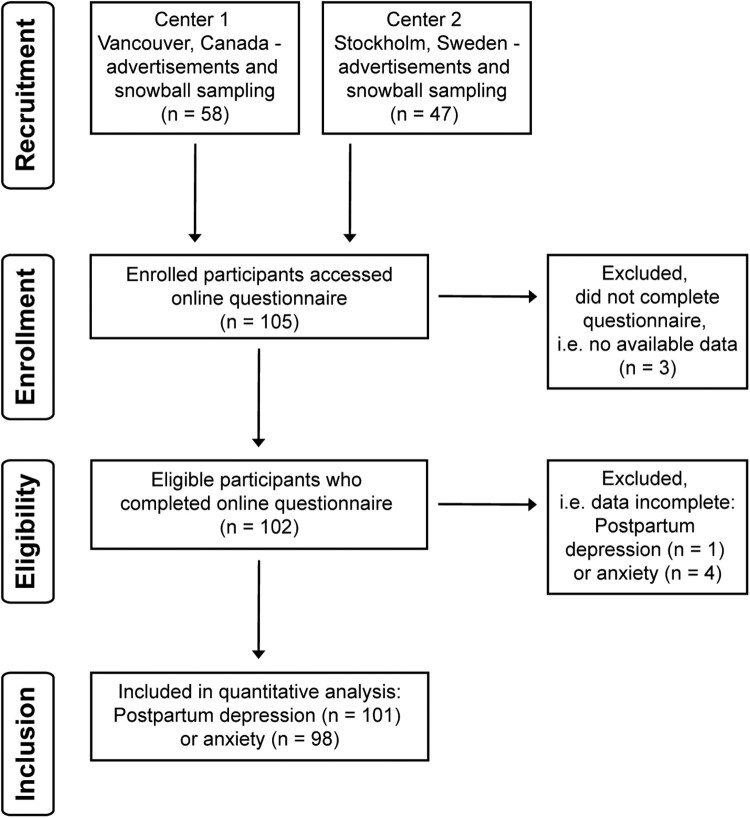
In this retrospective, multi-center study, an international cohort of 105 women who had given birth after SCI were recruited primarily through two institutions: (1) International Collaboration on Repair Discoveries (ICORD) in Vancouver, BC, Canada and (2) Karolinska Institute in Stockholm, Sweden. Each center contributed to recruitment via institution websites, snowball sampling and social media through advocacy group partners. A series of web-based questionnaires on motherhood after SCI (including pregnancy, breastfeeding and other postpartum experiences) was hosted on FluidSurveys from February through November 2017. Questionnaire domains pertaining to this study included demographics, injury characteristics and postpartum emotional wellbeing. Participants detailed the neurological level and completeness of their SCI (i.e. sensorimotor impairment) by reporting their grade on the American Spinal Injury Association (ASIA) Impairment Scale (AIS), according to the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI).15 All participants provided written informed consent according to the Helsinki II Declaration using an online informed consent form on the first page of the questionnaire. Four individuals with SCI completed a test run of the survey and provided feedback on length, content, website functionality and clarity of language. This feedback was incorporated to create the final version of the survey. Of the 105 respondents, three women started the survey but did not provide sufficient data to be included in analysis, resulting in a final study sample of 102 participants (Fig. 1). The study was approved by the University of British Columbia Research Ethics Board and the Karolinska Institute Research Ethics Board.
Figure 1.
Flow chart indicating recruitment and inclusion of participants. Three participants completed the consent form and accessed the questionnaire but did not provide the minimum amount of information needed for inclusion into data analysis (i.e. demographics).
Materials
The first section of the questionnaire was comprised of 15 questions regarding the following demographics: age (current, when SCI was sustained, when participant gave birth), injury characteristics (cause, neurological level and completeness of injury, mobility aids). This section took an average of 22 min to complete.
The 10-question section on postpartum emotional wellbeing took an average of 16 min to complete and included validated scales for maternal confidence, breastfeeding self-efficacy, self-esteem for persons with SCI and positive affect & wellbeing after SCI. Participants were also asked to report whether they had received clinical diagnoses of MDD prior to childbirth as well as PPD. All questions referred to participants’ first child after SCI. For the primary outcome measures, we used two subscales from the Centers for Disease Control and Prevention Pregnancy Risk Assessment Monitoring System (PRAMS) questionnaire.
PRAMS 3-D scale: postpartum depression
Self-reported PPD was evaluated using the PRAMS-3D, which is a 3-item scale assessing the incidence of PPD based on frequency of depressive symptoms postpartum. Participants are asked to rate how frequently they experience each of the following emotions after giving birth: (1) down/depressed/sad, (2) hopeless, (3) slowed down. Scores range from 3–15, with higher scores indicating more PPD symptoms. Using a cut-off score of 9, the PRAMS-3D is 80% sensitive and has a positive predictive value of 70% for detecting PPD.16
PRAMS 2-A scale: postpartum anxiety
Self-reported PPA was evaluated using the PRAMS-2A, a 2-item scale with scores ranging from 2–10 and higher scores implicating the presence of more PPA symptoms. Participants are asked to rate how frequently they experience each of the following emotions after giving birth: (1) panicky and (2) restless. A cut-off score of 6 is 75% sensitive and has a 37% positive predictive value for detecting PPA.17
Participants who completed all PPD scale items (n = 101, completion rate: 99%) or all PPA scale items (n = 98, completion rate: 96%) were included in the respective prevalence calculations. As the objective of this study was not to compare prevalence of PPD vs. PPA, we did not exclude the three participants who completed the PPD scales but not the PPA scales in full.
Statistical analysis
Reliability analysis was conducted for the PRAMS-3D and PRAMS-2A scales using Cronbach’s alpha coefficient. Positive screens for PPD (indicated by a total PRAMS-3D score ≥9) and PPA (indicated by a total PRAMS-2A score ≥6) were determined for each participant. The prevalence of positive screens of each condition was compared to the prevalence of clinical diagnosis using a χ2 test with continuity correction, with a significance level set at P < 0.05. All statistical analysis was completed using IBM SPSS Statistics (V25).
Results
High reliability was demonstrated in this study for the PRAMS-3D scale for PPD (Cronbach’s alpha coefficient = 0.86). The PRAMS-2A scale for PPA also exhibited good reliability (Cronbach’s alpha coefficient = 0.77).
Participant demographics are shown in Table 1 with ages expressed as mean ± standard deviation (SD). There were large discrepancies between the prevalence of clinically diagnosed and self-reported PPD, with self-reported PPD being up to 4 times more prevalent overall (Table 2). Compared to clinically diagnosed PPD, the proportion of women who self-reported PPD was 3.7 times greater in the cervical SCI group, 4.1 times greater in the upper thoracic group and 3.6 times greater in the lower SCI group. The rates of self-reported PPD compared to rates of clinically diagnosed PPD were significantly higher in the cervical (P = 0.033) and lower SCI (P = 0.012) groups.
Table 1. Participant demographics.
| Characteristic | Total participants (n = 102) |
|---|---|
| Years ± SD (range) | |
| Age at time of survey | 41 ± 10 (22–65) |
| Years since SCI | 22 ± 12 (2–64) |
| Age at child birth | 29 ± 6 (18–49) |
| Time between SCI and child birth | 10 ± 8 (8–37) |
| Time since child birth | 12 ± 9 (1–47) |
| Plegia | n (%) |
| Tetraplegia | 31 (30) |
| Paraplegia | 71 (70) |
| Completeness of injury (AIS) | n (%) |
| AIS A | 35 (34) |
| AIS B | 23 (22) |
| AIS C | 24 (24) |
| AIS D | 12 (12) |
| AIS E | 8 (8) |
| Neurological level of injury | n (%) |
| Cervical, C1–C8 | 30 (29) |
| Upper thoracic, T1–T6 | 12 (12) |
| Lower thoracic & lumbar, T7 and below | 60 (59) |
SCI = spinal cord injury, SD = standard deviation, AIS = American Spinal Injury Association (ASIA) Impairment Scale. Percentages may not add to 100% due to missing responses.
Table 2. Prevalence of postpartum depression among mothers with spinal cord injury.
| PPD | MDD | ||||||
|---|---|---|---|---|---|---|---|
| SCI Level | Clinical diagnosis N (%) | Self-reported N (%) | P-value | Clinical diagnosis of MDD before pregnancy N (%) | Clinical diagnosis of PPD in mothers with previous MDD†N (%) | Self-reported PPD†N (%) | P-value |
| Cervical, C1–C8 (n = 30) | 3 (10) | 11 (37) | 0.033* | 4 (13) | 3 (75) | 3 (75) | 1.00 |
| Upper thoracic, T1–T6 (n = 12) | 1 (8) | 4 (33) | 0.121 | 1 (8) | 0 (0) | 0 (0) | - |
| Lower SCI, T7 and below (n = 59) | 4 (7) | 15 (25) | 0.012* | 10 (17) | 0 (0) | 7 (70) | 0.005* |
SCI = spinal cord injury, PPD = postpartum depression, MDD = major depressive disorder. Data is presented as number of individuals and percentage (%). Significance level at P < 0.05, where P-values reported are derived from χ2 test with continuity correction and * indicates statistical significance.
†Percentage given for mothers with a history of MDD in each SCI group who developed PPD.
Prevalence of PPD (Table 2) and PPA (Table 3) was greatest in the cervical SCI group, followed by the upper thoracic (T1–T6) then lower SCI (T7–L5) groups. Within the cervical and lower SCI groups, at least 70% of women who had been clinically diagnosed with MDD prior to pregnancy met criteria for self-reported PPD. Additionally, 16% of all participants (n = 16) had both self-reported PPD and PPA. Among women who had a history of MDD before pregnancy, self-reported PPD was significantly more frequent than clinically diagnosed PPD (P = 0.005) and women with cervical SCI were more likely to be clinically diagnosed with PPD (P = 0.006). In terms of group differences, there were no statistically significant differences for PPD prevalence (for either clinically diagnosed or self-reported).
Table 3. Prevalence of postpartum anxiety among mothers with spinal cord injury.
| SCI Level | Self-reported PPA N (%) |
|---|---|
| Cervical, C1–C8 (n = 29) | 9 (31) |
| Upper thoracic, T1–T6 (n = 12) | 3 (25) |
| Lower SCI, T7 and below (n = 57) | 10 (18) |
| P-value | 0.378 |
SCI = spinal cord injury, PPA = postpartum anxiety. Data is presented as number of individuals and percentage (%).
Discussion
Our findings point to a higher prevalence of PPD (25–37%) and PPA (18–33%) in women with SCI compared to the general maternal population, in which PPD and PPA are estimated to affect 13% and 18% of women, respectively.13,14 Women with disability are reported to have greater incidence of perinatal and PPD symptoms compared to able-bodied women. For example, a cross-sectional survey revealed that compared to women without disabilities (n = 3,440), women who self-reported having a disability (n = 287) were diagnosed more frequently with depression before (39% vs 16%) and after (30% vs. 10%) pregnancy. This discrepancy in diagnosed PPD occurrence persisted even after controlling for diagnosis of depression before and during pregnancy.10 However, it must be acknowledged that the data on number of subjects with SCI compared to other disabilities was not reported, and this study was not SCI-specific.
Furthermore, this study did not determine whether factors related to physical disability contributed to mothers’ subjective experiences of depression or negative affect. It has been reported that women with physical disability may encounter a variety of reactions to their new status as a mother, including disbelief, intrusive questions or even condescension.8,10 Certain protective factors against PPD may also be impeded by the unique set of challenges faced by mothers with SCI. For example, able-bodied mothers who breastfed for six months postpartum experienced reduced rates of PPD.18 However, mothers with SCI may not reach their goals for breastfeeding duration due to factors such as mobility limitations and lactation dysfunction.19 Indeed, these barriers were reported by many of our study participants (all of whom did breastfeed their infants) and preliminary results for this study have been published.19
External stressors and a history of depression are well-known risk factors for the onset of PPD.20 Approximately 70% of participants in our study who met PRAMS-3D criteria for PPD had also been diagnosed with MDD prior to pregnancy. Although not examined specifically in patients with SCI, a prior history of depression has been established as a risk factor for PPD. One study ascertained that 65% of women with a history of MDD (n = 26) developed PPD compared to 35% of women with no prior MDD (n = 183).21 A more recent Canadian study cited history of depression as a major variable that increased the risk of both sub-clinical PPD [odds ratio (OR) = 2.27, 95% confidence interval (95% CI) = 1.42–3.63) and major PPD (OR = 2.78, 95% CI = 1.56–4.97)].22 In our study, women previously diagnosed with MDD were more likely to be clinically diagnosed with PPD if they had a cervical SCI. It is possible that clinicians were more vigilant in screening for PPD for women with more severe SCI and previous psychiatric diagnosis. However, exploring clinicians’ attitudes and priorities regarding maternal care and psychiatric health must be examined.
The SCI population exhibits a relatively high prevalence of general depression: this is especially true for women who sustain a traumatic SCI or are in the acute phase post-injury.1,23,24 New mothers who have not yet adjusted to life after SCI may be at greater risk, and it is difficult to ascertain the extent to which depressive symptoms are attributed to SCI, PPD or PPA. Future studies should consider pre- and post-injury depression history, time elapsed between SCI and childbirth, and should use validated scales to account for psychosocial wellbeing. Currently, there are existing scales for aspects of psychosocial health (i.e. depression, self-esteem, positive affect and well-being) that have been validated for use in SCI populations.25
In this study, a major discrepancy was observed between clinically diagnosed and self-reported PPD, which was significant in the cervical and lower SCI groups. This difference implicates a need for more clinical support and attention regarding postpartum mental health in women with SCI. It is currently unknown whether the discrepancy we found is due to PPD being underdiagnosed in mothers with SCI, and future research should be conducted to elucidate this finding. As a result of mobility changes following SCI that necessitate the use of aids such as wheelchairs, clinician offices often become physically inaccessible to individuals with SCI. Mothers may require assistance from personnel or transportation services to get to a clinic, and they may also avoid seeking healthcare services due to concerns about stigmatization. SCI also results in a multitude of medical sequelae, including spasticity,26 neuropathic pain,26,27 bladder and bowel dysfunction,28 and life-threatening hypertensive episodes termed autonomic dysreflexia.29 Management of these conditions may take precedence over recognition of mental health problems, which are relatively less visible. There is a paucity of research on maternal health and SCI, leading to a lack of satisfactory SCI-specific knowledge among clinicians. As such, mothers with SCI may not feel confident approaching their healthcare providers.
The main limitations of the study are the retrospective design and use of self-reported PPD symptoms by participants. Although this is the largest study to date that evaluates PPD and SCI, it must be acknowledged that our sample is not representative of the entire global population of SCI. Obtaining an adequate sample size is a well-documented challenge in SCI research: survey studies recruit an average range of 35–100 participants with SCI.30 Although our sample size (n = 102) exceeds this range, we encountered the added complexity of recruiting women (a minority of the SCI population) who also gave birth. Future studies should strive to obtain a larger sample and screen for depression and anxiety in the immediate postpartum.
In light of these novel findings, it is necessary for clinicians and allied health professionals to be aware of the high prevalence of PPD and PPA among mothers with SCI, particularly those with cervical-level injuries. Early screening and initiation of evidence-based treatments are recommended to prevent adverse maternal and child health outcomes associated with PPD and PPA. Easily administered screening questionnaires such as the PRAMS subscales are available and validation of such tools in mothers with SCI should be considered.
Disclaimer Statement
Contributors None.
Funding This work was supported by the Craig H. Neilsen Foundation under [grant number 431713]; the Spinalis Foundation under [grant number 117]; the Canadian Institutes of Health Research under [award number 6556] (Frederick Banting and Charles Best Canada Graduate Scholarship-Master’s (CGS M) to Amanda H. X. Lee); the Michael Smith Foundation for Health Research in partnership with the Rick Hansen Foundation under Grant number 17110 (Research Trainee Award to Matthias Walter).
Conflict of Interest The authors have no conflicts of interest to declare.
Acknowledgements
The authors would like to acknowledge all the participants of this study.
References
- 1.Williams R, Murray A.. Prevalence of depression after spinal cord injury: A meta-analysis. Arch Phys Med Rehabil 2015;96(1):133–40. doi: 10.1016/j.apmr.2014.08.016 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . Spinal Cord Injury [Internet]. 2013. Available from: http://www.who.int/news-room/fact-sheets/detail/spinal-cord-injury.
- 3.Wyndaele M, Wyndaele J-J.. Incidence, prevalence and epidemiology of spinal cord injury: what learns a worldwide literature survey? Spinal Cord 2006;44(9):523–9. doi: 10.1038/sj.sc.3101893 [DOI] [PubMed] [Google Scholar]
- 4.Singh A, Tetreault L, Kalsi-Ryan S, Nouri A, Fehlings MG.. Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol 2014;6:309–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellucci CHS, Castro Filho Jd, Gomes CM, Bessa Junior Jd, Battistella LR, Souza Dd, et al. Contemporary trends in the epidemiology of traumatic spinal cord injury: changes in age and etiology. Neuroepidemiology 2015;44(2):85–90. doi: 10.1159/000371519 [DOI] [PubMed] [Google Scholar]
- 6.Kumar R, Lim J, Mekary RA, Rattani A, Dewan MC, Sharif SY, et al. Traumatic spinal injury: global epidemiology and worldwide volume. World Neurosurg 2018;113:e345–63. doi: 10.1016/j.wneu.2018.02.033 [DOI] [PubMed] [Google Scholar]
- 7.Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma 2004;21(10):1371–83. doi: 10.1089/neu.2004.21.1371 [DOI] [PubMed] [Google Scholar]
- 8.Iezzoni LI, Wint AJ, Smeltzer SC, Ecker JL.. How did that happen?” Public responses to women with mobility disability during pregnancy. Disabil Health J 2015;8(3):380–7. doi: 10.1016/j.dhjo.2015.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson AB, Wadley V.. A multicenter study of women’s self-reported reproductive health after spinal cord injury. Arch Phys Med Rehabil 1999;80(11):1420–8. doi: 10.1016/S0003-9993(99)90253-8 [DOI] [PubMed] [Google Scholar]
- 10.Mitra M, Iezzoni LI, Zhang J, Long-Bellil LM, Smeltzer SC, Barton BA.. Prevalence and risk factors for postpartum depression symptoms among women with disabilities. Matern Child Health J 2015;19(2):362–72. doi: 10.1007/s10995-014-1518-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brand SR, Brennan PA.. Impact of antenatal and postpartum maternal mental illness: How are the children? Clin Obstet Gynecol 2009;52(3):441–55. doi: 10.1097/GRF.0b013e3181b52930 [DOI] [PubMed] [Google Scholar]
- 12.Ghidini A, Simonson M.. Pregnancy after spinal cord injury: a review of the literature. Top Spinal Cord Inj Rehabil 2011;16(3):93–103. doi: 10.1310/sci1603-93 [DOI] [Google Scholar]
- 13.Gavin NI, Gaynes BN, Lorh KN, Meltzer-Brody S, Gartlehner G, Swinson T.. Perinatal depression: A systematic review of prevalence and incidence. Obstet Gynecol 2005;106(5):1071–83. doi: 10.1097/01.AOG.0000183597.31630.db [DOI] [PubMed] [Google Scholar]
- 14.Dennis CL, Falah-Hassani K, Shiri R.. Prevalence of antenatal and postnatal anxiety: Systematic review and meta-analysis. Br J Psychiatry 2017;210(5):315–23. doi: 10.1192/bjp.bp.116.187179 [DOI] [PubMed] [Google Scholar]
- 15.Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (Revised 2011). J Spinal Cord Med 2011;34(6):535–46. doi: 10.1179/204577211X13207446293695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis K, Pearlstein T, Stuart S, O’Hara M, Zlotnick C.. Analysis of brief screening tools for the detection of postpartum depression: Comparisons of the PRAMS 6-item instrument, PHQ-9, and structured interviews. Arch Womens Ment Health 2013;16(4):271–7. doi: 10.1007/s00737-013-0345-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Hara MW, Stuart S, Watson D, Dietz PM, Farr SL, D’Angelo D.. Brief scales to detect postpartum depression and anxiety symptoms. J Women’s Heal 2012;21(12):1237–43. doi: 10.1089/jwh.2012.3612 [DOI] [PubMed] [Google Scholar]
- 18.Mohamad Yusuff AS, Tang L, Binns CW, Lee AH.. Prevalence and risk factors for postnatal depression in Sabah, Malaysia: A cohort study. Women Birth 2015;28(1):25–9. doi: 10.1016/j.wombi.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 19.Holmgren T, Lee AHX, Hocaloski S, Hamilton LJ, Hellsing I, Elliott S, et al. The influence of spinal cord injury on breastfeeding ability and behavior. J Hum Lact 2018;34(3):556–65. doi: 10.1177/0890334418774014 [DOI] [PubMed] [Google Scholar]
- 20.Robertson E, Grace S, Wallington T, Stewart DE.. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry 2004;26(4):289–95. doi: 10.1016/j.genhosppsych.2004.02.006 [DOI] [PubMed] [Google Scholar]
- 21.McCoy SJB, Beal JM, Shipman SBM, Payton ME, Watson GH.. Risk factors for postpartum depression: A retrospective investigation at 4-weeks postnatal and a review of the literature. J Am Osteopath Assoc 2006;106(4):193–8. [PubMed] [Google Scholar]
- 22.Davey HL, Tough SC, Adair CE, Benzies KM.. Risk factors for sub-clinical and major postpartum depression among a community cohort of Canadian women. Matern Child Health J 2011;15(7):866–75. doi: 10.1007/s10995-008-0314-8 [DOI] [PubMed] [Google Scholar]
- 23.Krause JS, Kemp B, Coker J.. Depression after spinal cord injury: relation to gender, ethnicity, aging, and socioeconomic indicators. Arch Phys Med Rehabil 2000;81(8):1099–109. doi: 10.1053/apmr.2000.7167 [DOI] [PubMed] [Google Scholar]
- 24.Hoffman JM, Bombardier CH, Graves DE, Kalpakjian CZ, Krause JS.. A longitudinal study of depression from 1 to 5 years after spinal cord injury. Arch Phys Med Rehabil 2011;92(3):411–8. doi: 10.1016/j.apmr.2010.10.036 [DOI] [PubMed] [Google Scholar]
- 25.Tulsky DS, Kisala PA, Victorson D, Tate DG, Heinemann AW, Charlifue S, et al. Overview of the Spinal Cord Injury–Quality of Life (SCI-QOL) measurement system. J Spinal Cord Med 2015;38(3):257–69. doi: 10.1179/2045772315Y.0000000023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finnerup NB. Neuropathic pain and spasticity: intricate consequences of spinal cord injury. Spinal Cord 2017;55(12):1046–50. doi: 10.1038/sc.2017.70 [DOI] [PubMed] [Google Scholar]
- 27.Adriaansen JJE, Ruijs LEM, van Koppenhagen CF, van Asbeck FWA, Snoek GJ, van Kuppevelt D, et al. Secondary health conditions and quality of life in persons living with spinal cord injury for at least ten years. J Rehabil Med 2016;48(10):853–60. doi: 10.2340/16501977-2166 [DOI] [PubMed] [Google Scholar]
- 28.Park SE, Elliott S, Noonan VK, Thorogood NP, Fallah N, Aludino A, et al. Impact of bladder, bowel and sexual dysfunction on health status of people with thoracolumbar spinal cord injuries living in the community. J Spinal Cord Med 2017; 40(5):548–559. doi: 10.1080/10790268.2016.1213554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krassioukov A, Warburton DE, Teasell R, Eng JJ, Cord S, Rehabilitation I.. A systematic review of the management of autonomic dysreflexia after spinal cord injury. Arch Phys Med Rehabil 2009;90(4):682–95. doi: 10.1016/j.apmr.2008.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Craven BC, Balioussis C, Hitzig SL, Moore C, Verrier MC, Giangregorio LM, et al. Use of screening to recruitment ratios as a tool for planning and implementing spinal cord injury rehabilitation research. Spinal Cord 2014;52(10):764–8. doi: 10.1038/sc.2014.126 [DOI] [PubMed] [Google Scholar]