Abstract
Context
Cardiovascular disease is one of the leading causes of mortality in individuals with spinal cord injury (SCI), highlighting the need for targeted risk minimization interventions.
Objective
To determine the effect of dietary interventions on CVD risk in adults with SCI.
Methods
A systematic literature review of studies investigating the impact of dietary intervention on CVD risk in SCI individuals was conducted according to the PRISMA statement. CASP checklists were used for critical appraisal, Academy of Nutrition and Dietetics Quality criteria checklist (QCC) for determining risk of bias and the GRADE approach to ascertain the quality of evidence of the outcomes. The results were reported descriptively.
Results
A total of eight studies were included from the identified 862 articles. Dietary intervention strategies varied across all studies, as did the outcome measures. Adult learning theories were not considered. The lack of controlled trials (two only) meant that while some interventions proved useful, risk of bias was high. Outcome measures were assessed as low to very low quality again identifying that this area is highly under-researched.
Conclusion
Despite documented evidence of the benefits of diet on CVD risk reduction, this review has identified a dearth of research in SCI. Nonetheless, the review emphasizes the potential of diet in conjunction with exercise in minimizing CVD risk in SCI. Further good quality research backed by robust data collection, simple, actionable strategies and knowledge translation techniques are essential to ascertain the effects of dietary intervention in lowering CVD risk in SCI.
Introduction
Individuals with spinal cord injury (SCI) are profoundly impacted physically1, socially,2,3 emotionally4 and physiologically.5,6 A myriad of factors and complications commonly encountered by people with SCI include neurogenic bowel,7 pressure injuries8 and osteoporosis.9 However, less obviously these individuals are at greater risk of cardiovascular disease (CVD) comparatively to individuals with no spinal trauma.10–13 With SCI, also comes a cascade of health conditions which are both directly as a result of the injury itself (like sensory and motor functional limitations) and secondary complications as a sequela of changes in the metabolic profile and body composition leading to increased incidence of dyslipidemia, overweight, Type 2 Diabetes Mellitus and CVD.14–18
The increased risk of CVD in SCI is attributable to a variety of causes. Alterations in the body composition occur with a decline in fat free mass due to skeletal muscle atrophy and a resultant increase in adipose tissue,19 causing several metabolic constellations such as glucose intolerance, insulin resistance and altered lipid profile20–24 along with a low basal metabolic rate (BMR) and consequently a low energy expenditure.25,26 Bauman et al.27 found that men with a SCI had higher homocysteine levels, which are further elevated with increasing age. The inflammatory marker, C-reactive protein (CRP) was also found to be high in individuals with chronic SCI in a cross-sectional study, positively correlated with the level of injury.28
CVD in the form of ischemic heart disease (IHD) is one of the leading causes of mortality in Australia.29 CVD is also one of the leading causes of mortality in individuals with SCI, with data identifying a significantly higher risk of heart disease and stroke in individuals with SCI.30 This study also highlighted the need for targeted interventions to minimize modifiable CVD risk factors. Furthermore, another prospective study31 has shown an increased prevalence of hypertension, dyslipidaemia and overweight echoing the need for early CVD screening and targeted risk reduction programs for primary and secondary prevention of CVD in individuals with SCI. Chopra et al.32 highlighted the gap between the high prevalence of CVD in SCI and lack of screening and monitoring of treatment adherence. Therefore there is a need for proactive strategies to mitigate this risk.
Nutrition has long been accepted and identified as being beneficial in both prevention and management of CVD.33–35 The nutritional cascade post SCI is due to the combined effect of metabolic changes and also lifestyle choices and practices.36 Pellicane et al.37 found that the mean energy intake was significantly higher in subjects with SCI in comparison to those with traumatic brain injury, stroke and Parkinson’s disease. They also found that sex and age were significant predictors of energy and protein intake, with higher intakes seen in males and younger age groups which is also supported by another observational study.38 A lower energy intake in SCI compared to general population was reported by Groah et al. (2009).39 The difference may be attributed to primarily the variable study population in these studies with the latter reviewing intake of community dwelling with a mean age of 38 years and predominantly male (84%) compared to the former study37 assessing rehabilitation inpatients of average age 56 years on average with 64% male. Other factors such as food accessibility, barriers to self-feeding along with other socio-economic constraints cannot be excluded.
In the general population, it is shown that a consumption of whole grains rich in fiber positively affects the lipid profile,40 and Type 2 Diabetes Mellitus attributed to its effect on the glycemic index.41 There is also evidence that for primary prevention of CVD in the general population a simple reduction in sodium intake is beneficial to lower CVD risk.42 The Australian Guidelines for the Management of Absolute Cardiovascular Disease Risk (2012)43 recommends lifestyle intervention including dietary and physical activity for identified risks from low, moderate to high. While many factors influence food choices and dietary practices of individuals with SCI including age, social and economic factors, food and nutrition knowledge and access, there is limited data on nutrition practices, intervention and its impact on health outcomes.
Given the increased prevalence of CVD in SCI with premature risk development as outlined, and known benefits of diet in CVD prevention, this review aimed to examine the available evidence on the effectiveness of dietary interventions on CVD risk in adults with SCI.
Methods
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement44 and registered with PROSPERO International Prospective Register of Systematic Reviews (Registration # CRD42017078890).
Studies with adults suffering SCI of any level and duration, traumatic or non-traumatic reporting on the efficacy of dietary interventions (either as a monotherapy or part of a multi-factorial intervention) were included. Dietary intervention including dietary supplementation, manipulation of dietary intake and nutrition education with or without behavior modification were included. Given the paucity of information as ascertained from a preliminary search with a lack of randomized control trials (RCT), other observational, intervention studies with pre-post design, cohort, quasi-experimental case series were included.
Outcomes related to at least one of the following primary measures were included: CVD, CVD risk factor as an overall measure, Type 2 Diabetes Mellitus, chronic inflammation, autonomic dysreflexia, dyslipidemia, obesity (including weight, body mass index (BMI) and waist-circumference), hypertension, non-alcoholic fatty liver disease, C-reactive protein, HbA1c, fasting glucose, and insulin resistance. Dietary behavior change was included as a secondary outcome measure where available.
Search strategy
The electronic database search was carried out by the primary reviewer (PI) using MEDLINE (via Ovid), PROQUEST, PubMed, Cochrane library, CINAHL (via EBSCO), and Web of Science. Additionally, the reference lists from the literature retrieved were reviewed. A hand-search of relevant journals was also performed using grey literature. Search was limited to human subjects only, adults >18years and English language, and with no date limits set with articles up to October 2017 retrieved. Keywords, combinations and MeSH headings used were: (spinal* OR neuro trauma “ OR central cord syndrome” OR paraplegi* OR quadriplegi* OR tetraplegi*) AND (food OR nutrition* OR diet* OR healthy eating “ OR healthy food choices”) AND (CVD OR cardio* disease “ OR vascular disease” OR heart disease “ OR IHD OR coronary* OR CHD OR CAD OR hypertension OR diabetes OR obesity OR overweight OR hypercholesterol* OR cholesterol* OR lipidemia OR lipidaemia OR hyperglycaemi* OR hyperglycemi* OR stroke OR CVA OR cerebrovascular accident” OR triglyceride OR atherosclerosis OR metabolic syndrome “ OR HbA1c OR glycosylated haemoglobin” OR “glycosylated hemoglobin”).
Study selection and data extraction
Initial search results were imported into EndNote reference management system and duplicates were removed. The primary reviewer (PI) screened the title and abstract and tabulated results with reasons for exclusion using a PICO model.45 The full paper was retrieved, if meeting the inclusion criteria. A selection of full-text articles reviewed initially by the primary reviewer (PI) was reassessed independently by the second (EB) and third reviewer (KW). Disagreements were resolved through panel discussion.
The data extraction template for this review included the following: authors, setting, study design, population characteristics, sample size, intervention and comparator, outcome measures and findings. Lack of homogeneity of the studies meant the inclusion of meta-analyses were not possible. Therefore, relevant information from the studies including dietary interventions, control, outcome measures and results were scrutinized, compared and reported narratively using descriptive synthesis. The studies were also critically appraised by the primary reviewer using the Critical Appraisal Skills Program (CASP) checklist and tools.46–49
None of the studies were excluded based on the quality criteria. Individual studies included in the review were assessed to identify the risk of bias using the Academy of Nutrition and Dietetics Quality Criteria Checklist (QCC) for Primary Research50 which determines the quality by assessing ten validity questions: research question, sample selection/bias, control and confounders, intervention assignment and outcome measures’ reliability, statistical relevance and analyses. The tool allows the rating of the question as positive, negative or neutral whilst also looking at the applicability of the findings to practice. The outcomes’ quality of evidence was determined using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system51 with the aid of computer software GRADEproGDT (https://gradepro.org).52 GRADE approach51 considers the risk of bias, inconsistency, indirectness, imprecision and other factors applicable (publication bias, large effect, plausible confounding effect change and dose-gradient effect) to arrive at a certainty of evidence for the outcomes as very low, low, moderate or high.
Results
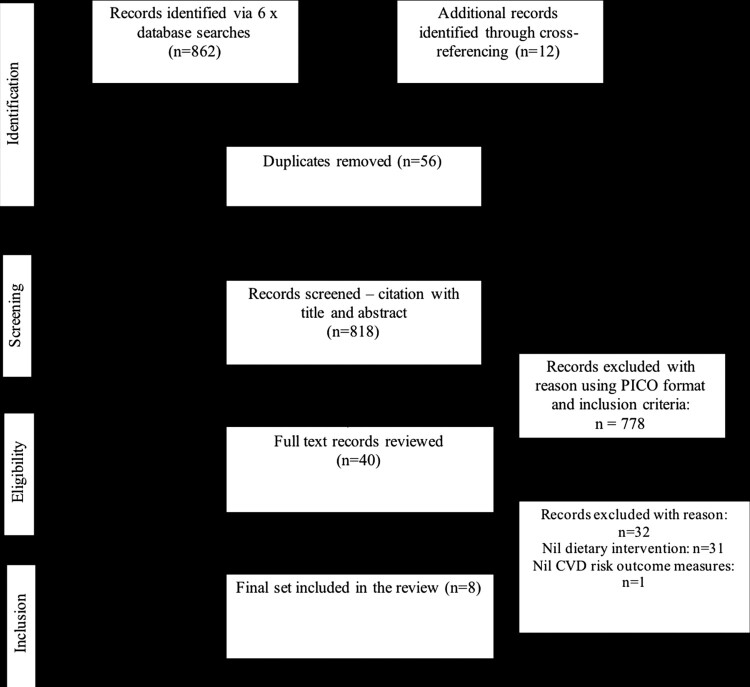
The preliminary search from six electronic databases identified 862 titles with cross-referencing retrieving an additional 12 studies (Fig. 1). Following removal of duplicates (via EndNote and manual checking), initial screening and review of full texts for eligibility, eight studies were finally included in this review.
Figure 1.
PRISMA flow diagram.
All included studies (n = 8) included dietary intervention and a CVD risk related outcome measure in SCI subjects (Table 1). Studies included randomized controlled trials (n = 2),53,54 a non-randomized clinical controlled trial (n = 1),55 pre-post study (n = 2),56,57 prospective case series (n = 1),58 cohort (n = 1)59 and quasi-experimental single-group pre-post study (n = 1).60 The majority of the studies were conducted in the United States (n = 6),53,55,56,58–60 with one study in Spain57 and one randomized controlled trial from Iran.54
Table 1. Characteristics of included studies (dietary interventions and relevant CVD outcome measures with findings outlined).
| Author, year, setting, Country, study design & sample size | Subject Characteristics | Intervention | Control | Outcome measures and findings | Study quality1 |
|---|---|---|---|---|---|
| Szlachcic et al. 2001; Outpatient Center, CA; USA; Non-randomized, clinical controlled trial (n = 222)55 | 198 men, 24 women, 86 in treatment group & 136 in control. Age 38.5+/−11.1years, duration post injury 12.8+/−8.3yrs. 22% Caucasian, 21% African-American, 54% Hispanic, 2% Asian and 1% other. 38% complete paraplegia, 34% complete tetraplegia, 12% incomplete paraplegia and 16% incomplete tetraplegia. | Group 1 (n = 86) with >200 mg/dL referred to staff dietitian who completed food intake recall to review fat and cholesterol consumed in day. Dietary intervention based on American Heart Association and American dietetic association guidelines – nutritionally adequate, varied diet, ↓ intake of SFA & cholesterol, achieving and maintaining ideal body weight, ↓sodium intake, limiting alcohol. To ↓ CVD, subjects advised to ↓ daily fat intake to <30% and SFA to <10% of total calories, ↓ cholesterol to <300 mg and ↑ CHO to 60% of calories. Subjects were reviewed twice. | 136 subjects (group 2) with no dietary intervention. | Intervention group n = 86 (42.8+/−11.3yrs of age, 11.1+/−7.5yrs post injury), control n = 136 (35.7+/−10.2yrs, post injury 15.6+/−8.8yrs), (F = 16.9; df = 1.220; P < .0001). Multivariate analysis showed overall group difference and a significant group-by-examination (pre-post) interaction (F = 7.12; df = 4.214; P < .0001). Univariate analyses showed similar for lipids, not significant for HDL-C. Group 1 – significant ↓ in TC 234+/−31–224+/−31 mg/dL (F = 14.7; df = 1,85; P < 0.001). Group 2 – significant ↑ TC (162+/−23–166+/−30 mg/dL, F = 7.94; df = 1,135; P = .006). 69% group 1 ↓ cholesterol vs 43% group 2 (x2 = 14.2; df = 1; P < .0002). LDL ↓159+/−28–151+/−28 mg/dL in group 1 (F = 8.63; df = 1.85; P = .004); group 2 ↑ from 101+/−21–104+/−27 mg/dL (not significant).67% had LDL-C ↓ in group 1, 47% in group 2 (x2 = 7.28; df = 1; P = .007). Significant group-by-examination interaction for mean triglyceride values but univariate analyses – no significant difference in group 1. ↓ TG – 60% group 1, 45% group 2. HDL-C – nil significant effects. | Neutral (Ø) |
| Javierre et al. 2005; Community, Spain; Pre-post study (n = 19)57 | 19 adult males, 17 paraplegia & 2 tetraplegia, 64% ASIA A, 21% ASIA B, 10% ASIA C & 5% ASIA D, 12 months post injury. Fairly constant physical activity. | Total daily dose of 1.5 g of DHA and 0.75 g EPA given for 6 months in the form of gelatin pearls; 6 a day taken as 2 with each meal. | Evaluation protocol: control 1 – baseline; control 2 – review at 3 months; control 3 – evaluation at 6 months | Statistically significant ↑ in plasma EPA – 7.86 times between control 1 (basal) & control 2 (3 months), 10.01 times between control 1 & 3 – (F = 30.556, P < 0.05). DHA – doubled the level – control 1 at control 2 and ↑ 125% (F = 106.6, P < 0.05) between control 1 and 3. Plasma concentrations of glucose, total cholesterol, HDL-C, LDL-C, VLDL-C, TG showed no difference between controls. | Negative (−) |
| Chen et al. 2006; Rehabilitation Center outpatients; USA; Pre-post study (n = 16)56 | Chronic SCI community-dwelling, overweight or obese; average weight 97.4 kg & BMI 34.3. >19years; 9 male, 7 female; White (n = 13), African American (n = 3); paraplegia (n = 12), tetraplegia (n = 4); 17.5yrs (mean) since SCI. | Face to face interview to collect weight/other. FFQ collected. Time-calorie displacement diet developed at the center in 1976 was utilized. This pattern promotes high bulk, low energy dense foods prolonging eating time; reducing energy density. 1200 kcal for women and 1400 kcal for men with number of servings from 5 food groups prescribed. Specific nutrients for SCI was taken into account. Once a week, the subjects with spouses attended a 90 min education class on nutrition and weight management. From week 6, 30 min exercise (of home-based activities) segment was introduced. | NA | 6 month program – average weight gain post injury was 20.7 kg (n = 13). Over the 12-week program intervention, 14 subjects lost weight (4.2 +/− 2.7 kg), one subject maintained his initial weight, one gained 2.3 kg. Overall weight loss = 3.5 kg, (3.8% of the initial weight). Significant ↓ in BMI, WC, neck circumference, and skinfold thickness seen. Among 12 subjects with DXA scan, total body fat significantly ↓, unlike lean mass & bone mineral. The HDL-C ↓ 3.2 mg/dl from 43.1 mg/dl, LDL-C – not significant. Nil significant change in blood pressure, Hb, albumin. Average energy intake ↓ 219.5 kcal/d, increased fiber and ↓ SFA. Moderate to strong correlation with the weight loss and WC, cholesterol and increase in diet’s nutritional quality. Not statistically significant due to sample size. | Neutral (Ø) |
| Radomski et al., 2011; Community; USA; Quasi-experimental, single-group pre-post study (n = 15)60 | SCI individuals 1 year post injury, no upper extremity pathology limiting exercise, BMI >20, complete SCI below cervical level. Paraplegia subjects only enrolled for same diagnostic group. 15 out of 38 people met criteria. | "Take Action”, a 12-week program including assessment by a nutritionist, exercise physiologist, physical therapist for individualized diet and exercise program. Exercise 3/7. Nutrition component included individualized meal plan. Weekly exercise classes with discussion about nutrition, behavior management, mind-body connection, motivation, life-long change and stress mastery. | NA | Outcomes measured at baseline, 12 and 24 weeks. Only 12 weeks results reported in the study secondary health issues seen in subjects at 24 week follow-up. Of 15 individuals – 2 failed screening at pre-test evaluation; one due to cardiac issues, second was at ideal bodyweight. 3 subjects withdrew in week 5 - 1 due to unrelated personal reasons, 1 due to schedule conflicts. 12 week data is reported for 10 subjects (6 male and 4 female); median age 53yrs (25–64yrs), median years with SCI 8.5 (1.5–37); all had complete injuries; SCI levels T12–4, T3–2, 1 each for T1, T5–T6, T7 & T9. Significant improvement in skinfold body fat percent (P = .013). Median improvement of 8% (>2.5% goal). Other anthropometric measures also showed significant improvements. Changes in physiological measures not statistically significant. Median weight reduction of 6% seen (P = .037). | Neutral (Ø) |
| Gorgey et al. 2012; Community; USA; Randomized controlled trial (n = 9)53 | SCI Paraplegia adults (n = 9); Group 1 (n = 5; RT and diet) and group 2 (n = 4; diet only group) | Intervention group (n = 5): diet and RT group. Anthropometric measurements taken; follow a standard diet (45% CHO, 30% fat and 25% protein) protocol. RMR using indirect calorimetry. Food diaries recorded for 12 weeks. RT twice weekly. DXA for FFM and FM was used. Screening was done 1 week pre-intervention and 1 week post-intervention. Dietitian devised the diet protocol. | Diet only group n = 4. Diet adherence was checked in both groups by the dietitian via interview, telephone call, email. Daily Food diary kept. | Groups not significantly different in age or time since injury. Mean caloric intake = 1781 +/− 228 and 1731 +/− 127 kcal/day for the RT + diet and diet groups, respectively (P < 0.05). % energy distribution similar across groups. Body weight and BMI not significantly different. For the RT + diet group, skeletal muscle of the whole thigh (28%), knee extensor (35%), and flexor (16%) muscle groups ↑ significantly. Post intervention, there was a difference in the ratio of leg FFM to whole body FFM in the RT + diet group compared with the diet group (0.15 T 0.01 vs. 0.12 T 0.02, P = 0.043), a significant interaction effect (P = 0.01, partial G2 = 0.74). Plasma insulin to plasma glucose ratio ↓ in RT + diet group with nil effect on HOMA-IR in both groups. Significant ↓ in TG (P = 0.028), TC (P = 0.017) was seen in RT + diet group. Plasma FFA significantly ↓ in both groups. | Positive (+) |
| Myers et al. 2012; Outpatient Center; USA; Cohort (n = 26)59 | 26 male with SCI (57+/− 6yrs of age) with ↑ CVD risk. Cervical injury (10), thoracic (13) & lumbar (3). Non-ambulatory and patients, Framingham Risk Score (FRS) associated with age-adjusted >20% 10 yr absolute CVD risk with no overt CVD. | Pilot 2-year risk intervention program. At baseline, blood tests, diet, lifestyle & physical activity questionnaires completed with a maximal exercise test. Individualized exercise and nutrition recommendations given. Weekly phone contacts using case manager model in first 6 weeks, at 8 weeks, at 3, 4, 5 and 6 months. Cardiac risk – lipid profile, homocysteine, high sensitivity CRP, fasting glucose, insulin, homeostasis model of assessment-insulin resistance (HOMA-IR) determined at every study visit. BMI and blood pressure, exercise testing and activity monitoring done. Dietary intake using Block FFQ with intake of 3 days, including one weekend day. Total calorie intake, intake of macronutrients (fat, CHO, protein), % calories from fat, saturated fat, polyunsaturated to saturated fat ratio, fiber and cholesterol intake measured. Program – physician supervised, managed by non-physicians. Verbal and written material given at initial visit tailored to the risk profile. Nutritional counseling, smoking intervention and lipid lowering therapy provided individually. EBP interventions using American Heart Association Dietary Guidelines, National cholesterol educational program were used. Pharmacologic therapy revised during visits with additional diet and exercise assistance. | Each subject is own control | Baseline – 73% hyperlipidemia, 15% past or current smokers, 23% T2DM, & 81% overweight or obese. Dropouts due to medical issues, followed by personal reasons, inability to comply, travel and time barriers. 22 people completed 6mo, 18-12mo, 15-18mo & 10-24mo respectively. Medical reasons were pressure sores (2), back pain (2), hand surgery (1), uncontrolled dyslipidemia (1). Two subjects died between 12 & 18mo, from reasons unrelated to the study. Weight ↓ at each visit but significant at 6mo (∼4 lb, P = 0.004). Fasting glucose – no change, mean for both insulin and HOMA-IR significantly lower at each comparison from baseline. 90-94% – reduction in insulin & 85-88% of subjects showed reduced HOMA-IR at 6mo and 12mo (P < 0.01 for all). TC/HDL ratio lower at 6mo (P = 0.05), TG lower (∼10–20%). TC was 3-7% lower but, not statistically significant. Strong effect only seen for insulin and HOMA-IR (effect size ∼0.80–0.90 at each evaluation). Small effect size for weight and lipids (<0.20). No difference in subjective and objective physical activity patterns. No significant differences seen in total calories, % of fat, CHO, protein or macronutrient intake. However, average total calories considerably lower than US general population average. Caloric and macronutrient intake lower than RDI for age and sex. Fat and cholesterol intake higher at the end than baseline. Fiber lower at 24 months than baseline (17.3+/−8 g baseline and 11.1+/−5 g at 24mo). Protein lower at 24 months (47.6g+/−30 in comparison to 62.5+/−27 g at baseline). | Neutral (Ø) |
| Sabour et al. 2016; Rehabilitation center; Iran; Randomized Controlled Trial (n = 60)54 | SCI adults >18yrs referred to Brain and Spine Center, with BMI 22 or more, SCI >2yrs and both male and female (n = 45 were male). | Nutrition education – Standard brochures with general recommendations about healthy diets & maintaining weight provided to all. Intervention group attended a monthly educational session for the first 3 months; then every 2 months as 5 sessions in a 7 month period. Also, received a tailored diet plan with energy and recommended foods. Total energy ∼1200–1800 kcal with ∼20% from fat. Allowed to be modified by clients as long as the total calorie restriction is adhered to. Weekly phone follow-up was done as 28 calls in 7 months. | 30; 3 failed to return for review at the end. n = 27, received only standard nutrition brochures. | TC = 186.01 ± 36.48 mg/dL and 195.24 ± 41.88 mg/dL in the education group at the beginning of the trial and after 7 months. In the control group, TC levels were 179.69 ± 38.37 mg/dL and 178.68 ± 39.54 mg/dL at the beginning and end of the trial. Changes in TC were not statistically different between groups (P = 0.224). Also, nutrition education program showed no significant effect on the levels of TG, LDL-C and HDL-C (P = 0.172, 107 & 0.081). No significant difference in the anthropometric measurements between groups at the beginning of the trial (P = 0.64, 0.10, 0.92 and 0.71). After 7 months, no significant changes in weight and WC observed (P = 0.970 and 0.361). |
Neutral (Ø) |
| Bigford et al. 2017; Community; USA; Prospective Case Series (n = 3)58 | Chronic complete SCI 3 males 42-56yrs; chronic neurologically complete SCI T3-T7 (1.5-29 years duration). | 6mo program of circuit resistance exercise, dietitian assisted nutrition plan using Mediterranean diet and behavior support followed by 6mo of maintenance phase with minimal support. | NA | Subject 1 – BMI 28.9, insulin resistance (HOMA-IR = 3.42), T2DM. Post 6mo – ↓ BMI by 6.8 kg (8.3%), fBGL from 138 to 123 mg/dL, insulin resistance by 0.45. Post 1 year – BMI ↓ 7.9 kg (9.7%), fBGL ↓ to 114 mg/dL. Calorie intake <553 kcal/d at intervention and ↑ 148 kcal by the end. Subject 2 – baseline BMI 44, insulin resistance (HOMA-IR = .03), ↑ TG (158 mg/dL), low HDL (30 mg/dl). Post 6mo – BMI ↓ 18.2 kg (7.3%), fBGL <10 mg/dL, TG <97 mg/dL, insulin resistance <2.61. HDL-C ↑ 5 mg/dL. Post 1 year – fBGL 6 mg/dL ↓ than 6mo, HDL-C stable at 36 mg/dL. HOMA-IR ↓ & was normal post 1 year. Caloric intake ↑ 40 kcal per day at 6mo, another 148 kcal/d at the end. Subject 3 – BMI 29.6 at baseline, insulin resistance (HOMA-IR = 4.34), ↑ TG (205 mg/dL), ↓ HDL (35 mg/dL). After 6mo – BMI ↓ by 5.7 kg (6.8%), fBGL by 10 mg/dL, TG by 9 mg/dL and insulin resistance by 0.33. HDL-C ↑ 5 mg/dL. Post 1 year – fBGL ↓ 6 mg/dL, HDL ↓ 2 mg/dL. HOMA-IR significantly ↓ at 6mo but ↑ past baseline at end. Calorie intake ↑ by 250 kcal/d at 6mo and remained as is. | Negative (−) |
1Assessment of Quality and risk of bias using the Quality Criteria Checklist.50
SFA, Saturated fatty acid; BMI, Body mass index; CVD, Cardiovascular disease; ↓ – decrease; ↑ – increase; SCI, spinal cord injury; CHO, Carbohydrates; HDL-C, High density lipoprotein cholesterol; LDL-C, Low density lipoprotein cholesterol; VLDL, Very low density lipoprotein; HOMA-IR, Homeostatic model assessment of insulin resistance; BGL, Blood glucose level; GAS, Goal attainment scale; TG, Triglycerides; TC, Total cholesterol; fBGL, fasting blood glucose level; FFM, Fat free mass; FM, Fat mass; Hb, Haemoglobin; DHA, Docosahexaenoic acid; EPA, Eicosapentaenoic acid; NA: Not applicable; FFQ: Food frequency questionnaire; RT, Resistance training; WC, Waist circumference/circumflex; RMR, Resting metabolic rate; FFA, Free fatty acid; EBP, Evidence based practice; T2DM, Type 2 Diabetes Mellitus.
Characteristics of study subjects
The studies included a total of 368 subjects (3–222) comprising of 315 male and 47 female with six lost in missing data due to withdrawals.54,60 Subjects were aged 21–66 years (n = 6)53,55,56,58–60 with body mass index (BMI) ranging from 19 to 44 kg m−2 (n = 7)53–56,58–60 and duration post-SCI being one year to 60.3 years (n = 6).53,55,56,58–60 All studies (n = 8)53–60 reported the level of injury (though not the completeness), a majority with Paraplegia (61%) compared to Tetraplegia (39%). Ethnicity of the population was only reported in two studies55,56 (Table 1).
Characteristics of dietary interventions
Dietary interventions varied in all the studies (Table 2) with two studies investigating dietary intervention as the only mode of intervention on CVD risk outcomes,55,57 whilst one study54 assessed nutrition education as the sole intervention. The remaining studies (n = 5)53,56,58–60 included a dietary component as part of a multicomponent intervention such as exercise, behavior management and education. The duration of interventions ranged from 12 weeks to 2 years across the studies.
Table 2. Comparison of characteristics of dietary interventions and outcome measures.
| Reference | Baseline nutrition assessment | Estimation of energy/nutrient requirements | Diet history | Dietary manipulation | Education & behavior modification | Intervention duration | Outcome measures |
|---|---|---|---|---|---|---|---|
| Szlachcic et al.55 | None. Annual checks and lipid profile screening | No | Food recall by dietitian (average day) for amount of fat and cholesterol initially. Nil intake data during intervention period. | Recommendations based on American Heart Association61 and American dietary guidelines62,63 for nutritionally adequate, varied diet; low in sodium, moderate to less in alcohol, <30% fat and <10% of calories from saturated fat & <300 mg cholesterol/day. 60% of calories from carbohydrates | One on one dietitian consult, twice during intervention. Nil behavior modification. | 16 months | Lipid profile |
| Javierre et al.57 | Nil. Recruitment data collection of demographics, lifestyle (physical activity, diet), injury duration & level, locomotion | No | No | Daily supplement of 1.5 g DHA and 0.75 g EPA as 6 capsules, taken as 2 per main meal | Nil | 6 months | Lipid profile Plasma EPA & DHA levels Plasma glucose and atherogenic index |
| Chen et al.56 | None. Data collected – socio-demographics, height, weight, BMI & medical history, medications, anthropometry, DXA, psychosocial, diet behaviors, lipids, blood pressure, Hb and albumin | No | Health Habits and History Questionnaire66 over past month at initial, week 12 and week 24. DietSys67 for nutrient calculation. Rated nutrition knowledge, quality and habits at baseline & week 12 | Time-calorie displacement diet by University of Alabama (1976)65 with high fiber and moderated energy dense foods like meats, cheese, fat and sugars. From 1200 kcal for women and 1400 kcal for men, specific portions of 5 food groups given | Once a week, 90mins group class run by a dietician covering nutrition and exercise. Subjects and spouses or attendants partake Skills training for behavior change and Psychologist consulted “where needed” | 12 weeks | Body weight, BMI. Body composition, waist & neck circumference, skinfold thickness, BP, lipids, Hb and albumin, diet behavior (calories, saturated fat, cholesterol and fiber intake per day and rating) |
| Radomski et al.60 | No. Data – BMI, Resting metabolic rate using BodyGem, body composition and skinfold thickness,, weight, peak oxygen uptake, readiness to change scale, % attendance, goal attainment scale, general wellbeing schedule, biochemistry, 1 year post SCI, paraplegia only | No BodyGem for resting metabolic rate | Nil | Individualized meal plan to meet nutritional and weight management goals, by a nutritionist with no details | Weekly classes includes nutrition, behavior management, motivation and others. Nil details regarding nutrition component covered | 12 weeks and a follow-up at 24 weeks not reported | Skinfold body fat, weight, BMI, BodPod, Girth at waist and hips, Blood pressure, Hb, glucose, lipids and HbA1c. Fitness outcomes and readiness to change and general wellbeing scale |
| Gorgey et al.53 | No. Data – demographics, body composition, weight and height using bed scale, DXA and MRI, BMI, OGTT, Plasma IGF-1, lipids, 1 yr post SCI, aged 18-50yrs, BMI</=30, AIS A or B, inclusion criteria | No. Indirect Calorimetry used to measure resting metabolic rate | Nil diet history. Daily food diary analyzed using Nutrition data system software for research program by a dietitian to assess calorie intake and % calories from carbohydrates, fat and protein. | Standard diet recommended by the dietitian who followed up with food diaries, telephone calls & emails weekly | Nil details. Weekly email and telephone support from dietitian mentioned. Standard diet advised but nil details regarding how it was conveyed | 12 weeks | Lipid profile, body composition and fitness markers, insulin levels, plasma IGF-1, glucose, free fatty acid concentration |
| Myers et al.59 | Nil. Initial data – demographics, height, weight, time since injury & level, smoking, HT, T2DM, exercise, overweight, obesity using BMI, FRS, lipids, HOMA-IR, CRP, homocysteine, fasting glucose, insulin levels, hyperlipidemia, ASIA classification, diet & activity questionnaires | No | Block Food Frequency Questionnaire64 for 3 days – one weekend day. Nutrient analysis – total calories, fat, protein and carbohydrates, % calories from fat, saturated fat, polyunsaturated to saturated fat ratio, fiber & cholesterol intake. Done for the duration of intervention. | Individualized nutrition counseling with verbal and written materials based on risk profile with nil specifics. In line with National Cholesterol Educational Program, National Institutes of Health and American Heart Association dietary guidelines61–63 & NIH consensus statement for High BP | Nil specifics provided. Just stated as individualized nutrition counseling provided with no details about the person delivering, duration and frequency | 2 years | Weight, lipids, glucose, insulin, HOMA-IR, macronutrient intake, fitness parameters, BP |
| Sabour et al.54 | No. Preliminary data – body weight, height, waist circumflex, demographics, lipid profile, physical activity. Inclusion criteria >18yrs age, SCI > 2yrs, BMI > 22 | No | Nil | Individualized based on anthropometry to have total energy intake between 1200 and 1800kCal. Allowed to modify keeping to the target energy. Emphasized low fried foods and high fat food choices, fast foods, desserts and dairy. Increase fruits and vegetables. Nil references | 5 sessions in 7 months – monthly in the first quarter, bi-monthly thereafter. Movies, slides and face-to-face consults – not specified if it is a dietitian. Brochures given. Nil behavior modification specified.?face to face sessions and movies supported it | 7 months | Lipid profile, weight, waist circumflex, BMI |
| Bigford et al.58 | No. Data – demographics with height, weight, age, sex, injury details, CMS risk markers – abdominal obesity as BMI>/=22 in SCI, Triglycerides >/=150 mg, HDL-C <40 mg, hyperglycemia as FPG >/=100 mg and HOMA-IR >/=2.3, upper extremity strength 1-RM & peak oxygen consumption (VO2peak) | No | 4-day dietary recall done by the registered dietitian (2 weekdays and 2 weekend days). Daily food log kept during intervention | Nutrition plan by a registered dietitian as one-on-one consult initially – 24 week Mediterranean diet with energy restriction. Daily energy target was determined by the dietitian using the total energy expenditure to reduce 500–1000kcal a day to lose 0.45–0.91 kg a week as per Diabetes Prevention Program. Mediterranean diet with 50% calories from carbohydrates, 15% protein, 35% fat. < 7% saturated fat, <18-20% total fat intake mainly from monounsaturated fat | One-on-one nutrition session, and 16 sessions of behavioral intervention with education in the 24 week period. 16 sessions of behavioral intervention with education in the 24 week period with goals and motivation training. A photo of the subject changed as a virtual image 7% lighter using the internet freeware Weight Mirror as a tool | 6 months intervention and 6 months of maintenance as an extension | Weight, BMI, calorie intake, fasting glucose, fasting TG, HOMA-IR, HDL-C, 1-RM & VO2peak |
Note: Outcomes in Italics are assessed using GRADE criteria51 and certainty of evidence reported.
CMS, Cardiometabolic syndrome; BMI, Body mass index; TG, Triglycerides; HDL-C, High density lipoprotein cholesterol; HOMA-IRm, Homeostatic Model Assessment of Insulin Resistance; BP, Blood pressure; FRS, Framingham Risk Score; SCI, Spinal cord injury; CRP, C-reactive protein; Hb, Hemoglobin; OGTT, Oral glucose tolerance test; FPG, Fasting plasma glucose; HbA1c, Glycated hemoglobin; EPA, Eicosapentaenoic acid; DHA, Docosahexaenoic acid; IGF-1, Insulin-like growth factor 1;T2DM, Type 2 Diabetes Mellitus; HT, Hypertension; AIS, American Spinal Injury Association Impairment Scale.
Characteristics of outcome measures
The outcome measures related to CVD risk reported in the studies (Table 2) were lipid profile, CVD risk score, atherogenic index, body weight and BMI, body composition using DXA scan and/or MRI or BodyGem and skinfold thickness, blood pressure, insulin and HOMA-IR for insulin resistance, homocysteine, high sensitivity CRP and plasma glucose. Other indirect measures of relevance included readiness to change, nutritional quality of the diet outlined as energy, fat and saturated fat intake, cholesterol and fiber intake. None of the studies used nutrition knowledge and behaviors as a measure with correlation to clinical outcome measures. Compliance with the diet plan was only included in one study60 and a secondary outcome measure of dietary behavior was only referred to in one other study.56
Dietary interventions and CVD risk in SCI
Several dietary interventions used nutrition education either as a key or one of the many strategies.54,56,58,60 Whilst a few studies did not exemplify the details, it was inferred from the protocol outlined.53,55,59 Seven studies53–56,58–60 investigated the use of dietary intervention in the form of either a diet plan by manipulating the macronutrient profile or nutrition counseling in line with the national guidance systems whilst one study57 used dietary supplementation of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA).
Two of these studies55,59 based their dietary intervention at the time on the American Heart Association (AHA)61 and National Dietary Guidelines62 and additionally Myers et al.59 also made reference to the National Cholesterol Lowering Education Program.63 The Block Food Frequency Questionnaire (FFQ)64 was used to gather intake data over three days to analyze macronutrient intake. This was the only study59 which used a validated CVD risk score for recruitment, the Framingham Risk Score.63 There was a significant reduction (P < 0.01) in insulin levels and HOMA-IR with a strong effect size at every periodic evaluation (∼0.8–0.90) in the 2-year period despite poor compliance and high dropout (40%). Although weight and lipids showed a small effect size (<0.20) with their targeted individualized counseling, weight reduction at six months was significant (∼1.8 kg, P = 0.004). Whilst total cholesterol and triglycerides were lower at each review, they were not considered clinically significant. Notably, fat intake was higher at 24 months whilst the fiber and protein intake was lower at the end compared to baseline.
Szlachcic et al.55 also based the dietary intervention on AHA61 which included specific nutrient targets (Table 2). The intervention group (n = 86) showed a significant decrease in total cholesterol (F = 14.7; df = 1,85; P < 0.001) whilst the control (n = 136) showed an increase (F = 7.94; df = 1,135; P = .006) with a 60% decline in triglycerides and LDL also declining from 159+/−28 to 151+/−28 mg/dL (F = 8.63; df = 1.85; P = .004). No details of dietary intake were provided from the diet recall nor any correlation of intake with outcomes confirmed.
It is noteworthy to mention that both these studies, using similar dietary approaches showed clinically significant reductions in important CVD risk markers of insulin and HOMA-IR and lipid profile. However, the Myers et al. study59 used dietary intervention with an exercise component and the study by Szlachcic55 used only dietary intervention. Both studies had methodological flaws and high risk of bias (neutral QCC).
Gorgey et al.53 investigated dietary intervention (n = 4), and diet in combination with resistance training (n = 5). A dietitian consult was provided to follow a standard diet in both groups which was essentially 45% of total energy intake from carbohydrates, 30% fat and 25% protein. Improvements in central adiposity, body composition, insulin resistance and lipids profile were noted in the diet and exercise group. This was the only study identified to be of good quality meeting validity criteria (+ QCC rating). However, the small sample size (imprecision) together with the clustered level of injury in one group limits the generalizability.
Three studies54,56,58 used daily energy intake targets as part of their dietary intervention strategy. Sabour et al.54 used a diet-only approach in two ways; namely, education using an individualized diet plan, and inferred behavior modification. This study used monthly education sessions recommending a diet with total energy intake from fat restricted to ∼20%, and daily energy limited to 1200 kcal to 1800 kcal based on anthropometric measurements. However, details of any pre-post evaluation of the education sessions and whether the session was designed and delivered by a qualified individual were absent. The education session duration is unknown but included movies, information on healthy cooking and lowering high fat foods, desserts and dairy whilst encouraging fruit and vegetables. Lipid profile and anthropometric (weight and waist circumference) indices showed no significant changes. Despite being a RCT, the study had many flaws and was limited by a small sample size. Confounding by physical activity, although data was collected, was not considered. Though the risk of bias was neutral for this study, there were several limitations such as, no compliance monitoring, no CVD risk score as inclusion criteria and inadequate study design (for example, no blinding of outcomes assessment).
Another study56 also used daily energy restriction with a target of 1200 kcal for women and 1400 kcal for men who were either overweight or obese with no other rationale provided. To support the daily energy targets, a “Time-calorie displacement diet”65 was utilized, which increases satiety with low energy, high fiber bulk prolonging eating time. The weekly education sessions facilitated by a dietitian were of 90 min duration. This is the only study that highlighted the participation of the subjects along with their spouses or attendants. This six-month program with a component of exercise introduced at week 6, showed an overall weight loss of 3.8% of initial weight. A moderate to strong correlation between weight loss and waist circumference, cholesterol and nutritional quality was noticed, though statistically insignificant due to small sample size.
A prospective case series58 evaluated a 6-month program with a dietitian assisted nutrition plan using a Mediterranean diet and exercise program followed by a 6-month maintenance phase. The subjects (n = 3) followed a Mediterranean style diet with calorie restriction of 500–1000 kcal a day. Energy distribution recommended was 50% from carbohydrates, 15% from protein and 35% from fat and the daily energy limit was set using the resting energy expenditure, BMI and adjustments for physical activity. Decrease in BMI (exceeding the 7% loss target), lipids plasma glucose and HOMA-IR was seen, though sample size reduced power. The risk of bias was high given the design of case series with a small sample size but the clinical significance of the outcomes in conjunction with the compliance and sustenance at maintenance is of note.
Another single group pre-post study60 evaluated a 12-week program with a nutrition and exercise component in SCI subjects with BMI >20 kg m−2. The nutrition component incorporated an individualized meal plan with limited details. The improvement in skinfold body fat percent by 3.2% at 12 weeks (P = .013) with a median weight reduction of 6% (P = .037) was clinically significant. It should be noted that although the study intervention was for 24 weeks, selective reporting meant only the 12-week data were reported due to a significant dropout rate.
Three studies53,55,59 using dietary intervention as part of the treatment strategy either on its own or in combination with exercise provided limited detail on the education component. However, it was inferred from two of these studies55,59 that one-on-one individualized nutrition counseling was provided whilst the third study53 reported email and telephone support from the dietitian with no further details. It is interesting to note that all three of these studies reviewed lipid profile as one of the outcome markers which also showed marked improvements with the above dietary interventions.
One prospective study57 investigated the supplementation of a total daily dose of 1.5 g DHA and 0.75 g EPA for 6 months in 19 adults (>12 months post SCI). Despite a statistically significant rise in plasma EPA in 3 months, plasma concentrations of glucose, total cholesterol, HDL-C, LDL-C, VLDL-C, TG showed no difference between intervention and control subjects. This study showed high risk of bias with poorly addressed validity and other quality criteria including selection bias and study design (QCC rating – negative).
As noted, seven studies53–56,58–60 implemented a dietary modification as part of their intervention strategy with the criteria of total energy intake being a common factor. Only two studies assessed the resting metabolic rate at baseline, one53 using Indirect Calorimetry, the other using BodyGem.60 None of the studies estimated energy or macronutrient requirements at baseline to tailor intervention nor delve into pre-existing nutrition knowledge and behaviors to inform education strategy, though one study56 obtained subjects’ self-rating of their nutrition knowledge initially.
A diet history was obtained from the subjects in only four studies with each utilizing different method. One study55 used food recall conducted by a dietitian for one average day focusing on the amount of fat and cholesterol intake at baseline with nil further intake assessment. Another study58 also incorporated this method but, data were collected over four days by a dietitian at baseline followed by a daily food log for the intervention period. Chen et al.56 administered the Health Habits and History Questionnaire66 collating data over the past month which is also inherently flawed with recall bias. However, this was also administered throughout the intervention session and the DietSys computer program67 used to calculate nutrients. Similarly, the Block FFQ64 was administered over 3 days including one weekend day in another pilot evaluation study59 with macronutrients analyzed during the intervention. All of these methods of data collection attract recall errors due to the retrospective nature of the instrument.
Finally, all of the dietary intake data analyses in the studies were limited to selected macronutrients such as energy, protein and fat and not the overall nutritional quality of food groups. The American Dietary Guidelines68 promote dietary patterns based recommendations emphasizing their benefit in lowering risk of chronic diseases like CVD. A prospective cohort study69 assessing the changes over time with adherence of diet quality scores (Alterative Healthy Eating Index, Alternative Mediterranean Diet Score and Dietary Approach to Stop Hypertension) has shown a significantly lower CVD risk in adults. Despite the evidence70 on the role of overall diet quality on CVD risk and availability of validated diet quality measuring instruments, none of these studies measured the overall diet quality.
Risk of bias and quality of included studies
Only one randomized controlled trial53 showed a low risk of bias with a positive score whilst other studies were rated as either neutral (n = 5)54–56,59,60 or negative (n = 2)57,58 (Table 1). The rating of high risk and poor quality was attributed to non-randomized controlled study design, absence of a power calculation and adjustment factors, incomplete outcome data and selection bias in some studies.
Outcome measures and assessment of evidence
The quality of evidence ascertained using GRADE51 via computer software GRADEproGDT52 indicated that relevant outcome measures were of low to very low quality (Table 3).
Table 3. Certainty of evidence using GRADE51,52 for key outcome measures.
| Outcomes | Effects/results | Studies: subjects (n) | GRADE rating of evidence | Additional comments |
|---|---|---|---|---|
| Lipid profile53–60 | ↓LDL ↓TG ↓Total Cholesterol ↑HDL-C |
8(2RCT):368 |
○○○ ○○ |
Diet and exercise intervention may lower lipid profile. But, poor quality studies with high variability makes evidence very uncertain. All studies were rated very low (risk of bias, inconsistency, imprecision), except for 1 RCT,53 which was graded as low. |
| Body Mass Index (BMI)54,56,58,60 | ↓BMI | 4(1RCT):94 | ○○○ | BMI confounded in SCI due to metabolic changes. Significant reduction in BMI was seen in 1 case series58 with a small sample of 3. One RCT54 showed nil change. The outcomes were deemed very low to make a strong recommendation though clinically significant in the case series.58 |
| Waist Circumference (WC)54,56,60 | ↓ WC | 3 (1RCT):91 | ○○○ | Significant decrease seen in one study correlated with weight loss56 whilst nil change was seen in the RCT.54 WC can see a decline with diet and exercise interventions based on 2 studies with a very low level of evidence. Clinical relevance is high with WC used as a predictor of obesity in comparison to BMI in SCI. |
| Body weight54,56,58–60 | ↓ weight | 5(1RCT):120 | ○○○ | One study showed significant reduction in weight at a 6 month review (∼4 lb, P = 0.004).59 Targeted exercise and diet program may result in weight loss in SCI based on 4 studies with low evidence. |
| Plasma Glucose53,57–60 | ↓ glucose | 5(1RCT):72 | ○○○ | Evidence is uncertain from the 5 studies in reducing plasma glucose in SCI individuals with diet and exercise interventions. |
| Insulin resistance (insulin levels & HOMA-IR)53,58,59 | ↓ insulin levels ↓ HOMA-IR |
3(1RCT):38 | ○○○ | Large effect size of ∼0.80 to 0.90 was seen consistently for insulin and HOMA-IR in one study59 but rated very low due to high lost numbers, study design, imprecision and inconsistency. Improved insulin resistance may be seen with exercise and diet intervention. |
| Blood Pressure56,59,60 | ↓ Systolic ↓ Diastolic |
3:57 | ○○○ | An individualized exercise and diet intervention program in SCI can assist in lowering blood pressure based on 3 pre-post studies. Resting systolic pressure decline was significant at P = .013 in one study,60 but all studies were of very low quality due to high risk of bias, dropouts, imprecision, indirectness, inconsistency & small sample. |
| Dietary behavior56 | ↓ SFA intake ↑ self-rating of nutrition knowledge |
1:16 | ○○○ | Only one study56 reviewed the effect of intervention on dietary behaviors and showed a significant decrease in SFA intake and increased self-rating of nutrition knowledge at 12 weeks follow-up. The study is of a very low quality due to lost numbers with follow-up, imprecision due to small sample size and absence of control. |
BMI, Body mass index; SCI, Spinal cord injury; RCT, Randomized controlled trial; ↓ – Decreased; ↑ – Increased; HDL-C, High density lipoprotein cholesterol; LDL, Low density lipoprotein; TG, Triglycerides; SFA, Saturated fatty acid; WC, Waist circumference; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; ○○○ – Very Low; ○○ – Low.
Lipid profile
All studies in this review reported on this outcome measure. All interventions53,55,56,58–60 with diet and exercise combination showed a reduction in the lipid profile; unlike two studies54,57 with diet as a monotherapy. One study59 indicated revision of pharmacotherapy as part of the intervention with limited details and was unaccounted for interpreting the results. Significant reduction was seen in two studies.53,55 The certainty of evidence was rated to be very low for all54–60 except one RCT which was low.53
Glycemic indices
Five studies53,57–60 reported on fasting glucose measure but showed nil significant change. The evidence was determined to be very low due to design with risk of bias, sample size, indirectness and imprecision and prognostic imbalance. Insulin levels and HOMA-IR were reported either individually or a combination of both measures in three studies.53,58,59 A large effect size for HOMA-IR was seen in one cohort study.59 The overall evidence was assessed to be very low due to high dropout affecting reporting and outcome confidence, inconsistency, small sample and study design with high risk of bias. HbA1c was reported in only one study60 which had a very low level of evidence.
Blood pressure
Only three studies56,59,60 outlined blood pressure as an outcome measure with a very low certainty of evidence.
Obesity measures
BMI, body weight, waist circumference, body composition were reported in six studies53,54,56,58–60 individually or in combination, though measurement methods and instruments varied. A significant decline in total body fat, BMI and waist circumference was demonstrated in a single-group uncontrolled trial56 similarly to another pre-post study.60 The certainty of evidence was very low.
Dietary behaviors
One study56 identified dietary behaviors as an outcome measure but, the evidence was rated as very low. Almost all studies (n = 6)53,55,56,58–60 tended to show that nutrition or dietary intervention (especially in combination with physical activity) resulted in improved CVD risk markers. Of interest, although nutrition education was found to be a key element in the majority (n = 7)53–56,58–60 of studies, details were scant on the type of education, with none making reference to nor incorporating adult learning principles or theories.
Discussion
This review aimed to explicate evidence regarding the effect of dietary interventions on CVD risk factors in adults with SCI. To the best of our knowledge, this is the first review to assess evidence in this population. Dietary interventions in the studies show increased variability impeding the ability to compare and assess the cumulative effects. Most (n = 6)53,55,56,58–60 studies showed some elements of positive correlation between the dietary intervention strategy and the CVD outcome measures used despite the identified poor quality and very low level of evidence.
It is worth considering the results in the context of dietary changes which are associated with decreased CVD risk or amelioration of risk factors. For example, the two studies55,59 which referred to published and approved guidelines for the dietary recommendations based on evidenced-based guidelines,61–63 showed reduction in cholesterol levels. Similarly, the case series58 using the evidence-based Mediterranean diet demonstrated clinically significant outcomes in weight reduction and other markers. The Mediterranean diet has gained a lot of attention in the recent years with evidence of its favorable effects on reducing cardiovascular risk.33,71,72
Sabour et al.54 advised increasing fiber intake as part of nutrition education, which was a common recommendation across most studies. However, this study promoted lowering dairy intake which is not supported by evidence. This is the only study where the outcomes were not positively correlated and it may be argued that this could be attributed partly to poor evidence-based dietary recommendations in addition to the identified methodological flaws. Fruit and vegetable intake is known to reduce CVD risk significantly33,73 and it is noteworthy that almost all studies (n = 7)53–56,58–60 incorporated this recommendation. A pilot study74 has also shown that SCI individuals do not meet their recommended servings for fruits, whole grains and dairy.
All of the studies that reported the macronutrient distribution, used different percentages of total calories from carbohydrates, fats and protein. These dissonances can be ascribed to the variation in the reference guidelines used. This adds to the challenge of comparison of outcomes and limits generalizability. Some differences in dietary recommendations from various guidelines have also been elucidated by Stewart et al.75 in his review of contemporary guidance and evidence in primary prevention of CVD. The INTERHEART study (2004)76 across 52 countries highlighting the cardioprotective role of fruits and vegetables asserts use of simple messages to achieve same outcome, regardless of the population. Similar good quality studies across wide demographics, not limited to the geographical area alone but extended to special groups like SCI are required to inform practice.
A further limitation to measuring dietary relevance in CVD prevention is the variation noted in the collection of dietary intake data. The methods were highly variable with different tools used across the studies. Use of validated tools to collect intake data is a pre-requisite to good quality studies. And, despite collating dietary intake data and reviewing the macronutrient distribution, none of the studies assessed the overall diet quality as a measure. There is promising evidence regarding the benefits of sustained high diet quality’s association with lower CVD risk.33,68–72
If dietary interventions are to be successfully measured, then it is important to measure relevant outcomes. Typically, these should be those markers associated with risk including lipid profiles, blood pressure, waist circumference and markers of glycemic control.
Lipid profile is the most predominant and common measure used across all the studies in the review which is not surprising, given its sensitivity in determining CVD risk;77,78 being a modifiable risk factor with diet being a key influencer.79 A cross-sectional study in community-dwelling SCI adults showed that 40% of the subjects had high total cholesterol and LDL with low HDL.80 While weight gain is a common issue in SCI,81–83 BMI is inherently fraught with poor validity in SCI population.84–87 Waist circumference is a reliable predictor of obesity in SCI85–87 and has been used in a few studies in this review. Future studies examining weight in CVD risk should include waist circumference. In this review, very few studies measured HOMA IR, HbA1c and glucose with low levels of evidence.
Dietary behaviors can also be pivotal as outcome measures in dietary interventions in view of the impact of diet on many CVD risk factors.69–73 One study56 reported on dietary behaviors, however no standard tools of evaluation were used nor its correlation with other outcomes assessed. It has been recommended that knowledge translation effecting behavior change is backed by a theoretical framework.88 Dietary behaviors are complex due to multiple factors influencing practices and habits such as cultural background, personal preferences, age, cooking skills, knowledge and location. None of the studies broached the effect of any of these factors on compliance of dietary intervention nor outcome measures.
Regardless of the variability seen in these dietary interventions, the majority of the studies in this review (n = 6)53,55,56,58–60 posit a favorable outcome signifying the positive association between dietary intervention, particularly in combination with an exercise component and CVD risk reduction. WHO (2007)89 recognizes the role of dietary modification in minimizing CVD risk and recommends the use of multifaceted risk reduction strategies to prevent CVD. Poor dietary practices and behaviors are key influencers of health and wellbeing and its consideration as one risk factor is important.
There is limited literature reporting on the effect of dietary behaviors on CVD risk, and on the long-term benefits of modifying dietary behaviors in improving CVD risk, both in the general population and specifically in people with SCI, as highlighted in our review. More research is needed in this area which can extend to explore the causal relationship between food behaviors and CVD risk in SCI.
This is the first review examining the effect of dietary interventions on CVD risk in the SCI population which is a strength as it lends itself to evidence collation. Another strength of this review is the use of a standard quality assessment tool, the QCC,50 used often in nutrition and dietary studies which ensures consistency. The GRADE approach51 for rating relevant outcomes support translation into practice by confirming certainty of evidence.
Several limitations should also to be noted in this review. The review was limited to English language and published data only contributing to publication bias. The search strategy as stipulated by the set inclusion criteria could have excluded potential articles with non-CVD risk outcomes or associated outcomes like dietary habits or behaviors and others. Future reviews should expand the inclusion criteria to various outcome measures to prevent the dearth of information. Also, data extraction, grading of evidence and risk of bias was completed by one reviewer which potentially could have introduced human error and bias.
Conclusion
Despite convincing evidence on the cardio-protective benefits of diets, the research is sparse in the area of dietary interventions to lower CVD risk in SCI. Due to the variability in intervention and outcome measures, lack of adequate rigor in the studies resulting in very low-quality evidence, the findings from this review are equivocal to make definitive practice recommendations.
Notwithstanding, this review underscores the potential for dietary interventions with other lifestyle modifications to ameliorate CVD risk in the SCI population. Future research should also incorporate healthy dietary behaviors as outcome measures, which could translate into improved clinical outcomes as reduced CVD risk and/or CVD events. Most critically, collection of dietary data needs to be robust to inform specific nutritional practice encompassing assessment of overall diet quality, not just limited to a few nutrients. Hence, there exists an unmet need for further well-planned research to facilitate practice changes and develop universal guidelines on nutrition in CVD prevention in SCI.
Disclaimer statements
Contributors None.
Funding None.
Conflicts of interest This research was conducted using personal time and resources, and authors report no conflict of interest.
References
- 1.Murray RF, Asghari A, Egorov DD, Rutkowski SB, Siddall PJ, Soden RJ, et al. Impact of spinal cord injury on self-perceived pre- and postmorbid cognitive, emotional and physical functioning. Spinal Cord. 2007;45:429–36. [ONLINE: Accessed 07/10/18]. https://www.nature.com/articles/3102022 [DOI] [PubMed] [Google Scholar]
- 2.Tsai IH, Graves DE, Chan W, Darkoh C, Lee MS, Pompeii LA.. Environmental barriers and social participation in individuals with spinal cord injury. Rehabil Psychol. 2017;62(1):36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Craig A, Nicholson Perry K, Guest R, Tran Y, Middleton J.. Adjustment following chronic spinal cord injury: determining factors that contribute to social participation. J Health Psychol. 2015;20(4):807–23. [DOI] [PubMed] [Google Scholar]
- 4.North NT. The psychological effects of spinal cord injury: a review. Spinal Cord. 1999;37:671–9. [DOI] [PubMed] [Google Scholar]
- 5.Scott JM, Warburton DE, Williams D, Whelan S, Krassioukov A.. Challenges, concerns and common problems: physiological consequences of spinal cord injury and microgravity. Spinal Cord. 2011;49:4–16. [DOI] [PubMed] [Google Scholar]
- 6.Post MW, van Leeuwen CM.. Psychosocial issues in spinal cord injury: a review. Spinal Cord. 2012;50(5):382–9. [DOI] [PubMed] [Google Scholar]
- 7.Awad RA. Neurogenic bowel dysfunction in patients with spinal cord injury, myelomeningocele, multiple sclerosis and Parkinson’s disease. World J Gastroenterol. 2011;17(46):5035–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheel-Sailer A, Wyss A, Boldt C, Post MW, Lay V.. Prevalence, location, grade of pressure ulcers and association with specific patient characteristics in adult spinal cord injury patients during the hospital stay: a prospective cohort study. Spinal Cord. 2013;51(11):828–33. [DOI] [PubMed] [Google Scholar]
- 9.Bauman WA, Cardozo CP.. Osteoporosis in individuals with spinal cord injury. PM R. 2015;7(2):188–201. [DOI] [PubMed] [Google Scholar]
- 10.Garshick E, Kelley A, Cohen SA, Garrison A, Tun CG, Gagnon D, et al. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord. 2005;43(7):408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demirel S, Demirel G, Tükek T, Erk O, Yilmaz H.. Risk factors for coronary heart disease in patients with spinal cord injury in Turkey. Spinal Cord. 2001;39(3):134–8. [DOI] [PubMed] [Google Scholar]
- 12.Bauman WA, Kahn NN, Grimm DR, Spungen AM.. Risk factors for atherogenesis and cardiovascular autonomic function in persons with spinal cord injury. Spinal Cord. 1999;37(9):601–16. [DOI] [PubMed] [Google Scholar]
- 13.Lee MY, Myers J, Hayes A, Madan S, Froelicher VF, Perkash I, et al. C-reactive protein, metabolic syndrome, and insulin resistance in individuals with spinal cord injury. J Spinal Cord Med. 2005;28(1):20–5. [DOI] [PubMed] [Google Scholar]
- 14.Myers J, Lee M, Kiratli J.. Cardiovascular disease in spinal cord injury - an overview of prevalence, risk, evaluation, and management. Am J Phys Med Rehabil. 2007;86:142–52. [DOI] [PubMed] [Google Scholar]
- 15.Graupensperger S, Sweet SN, Evans MB.. Multimorbidity of overweight and obesity alongside anxiety and depressive disorders in individuals with spinal cord injury. J Spinal Cord Med. 2018;(Sep 05): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Köseoğlu BF, Safer VB, Öken Ö, Akselim S.. Cardiovascular disease risk in people with spinal cord injury: is there a possible association between reduced lung function and increased risk of diabetes and hypertension? Spinal Cord. 2017;55(1):87–93. [ONLINE: Accessed 07/10/18] [DOI] [PubMed] [Google Scholar]
- 17.Cragg JJ, Noonan VK, Krassioukov A, Borisoff J.. Cardiovascular disease and spinal cord injury. Neurology. 2013;81(8):723–8. [ONLINE Accessed 2/5/17]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3776463/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groah SL, Nash MS, Ward EA, Libin A, Mendez AJ, Burns P, et al. Cardiometabolic risk in community-dwelling persons with chronic spinal cord injury. J Cardiopulm Rehabil Prev. 2011;31(2):73–80. [DOI] [PubMed] [Google Scholar]
- 19.Bauman WA, Spungen AM.. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia and quadriplegia: a model of premature aging. Metabolism. 1994;43(6):749–56. [DOI] [PubMed] [Google Scholar]
- 20.Spungen AM, Adkins RH, Stewart CA, Wang J, Pierson RN, Waters RL, et al. Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol. 2003;95:2398–407. [ONLINE: Accessed 29/3/18] https://www.physiology.org/doi/pdf/10.1152/japplphysiol.00729.2002 [DOI] [PubMed] [Google Scholar]
- 21.Gorgey AS, Dudley GA.. Skeletal muscular atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord. 2007;45(4):304–9. [ONLINE: Accessed 29/3/18] https://www.nature.com/articles/3101968.pdf [DOI] [PubMed] [Google Scholar]
- 22.Elder CP, Apple DF, Bickel CS, Meyer RA, Dudley GA.. Intramuscular fat and glucose tolerance after spinal cord injury – a cross-sectional study. Spinal Cord. 2004;42:711–6. [DOI] [PubMed] [Google Scholar]
- 23.Bauman WA, Spungen AM.. Carbohydrate and lipid metabolism in chronic spinal cord injury. J Spinal Cord Med. 2001;24(4):266–77. [DOI] [PubMed] [Google Scholar]
- 24.Bauman WA, Adkins RH, Spungen AM, Kemp BJ, Waters RL.. The effect of residual neurological deficit on serum lipoproteins in individuals with chronic spinal cord injury. Spinal Cord. 1998;36(1):13–17. [DOI] [PubMed] [Google Scholar]
- 25.Gorgey AS, Dolbow DR, Dolbow JD, Khalil RK, Castillo C, Gater DR.. Effects of spinal cord injury on body composition and metabolic profile – part 1. J Spinal Cord Med. 2014;37(6):693–702. [ONLINE: Accessed 29/3/18] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4231957/#C2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buchholz AC, Pencharz PB.. Energy expenditure in chronic spinal cord injury. Curr Opin Clin Nutr Metab Care. 2004;7(6):635–9. [DOI] [PubMed] [Google Scholar]
- 27.Bauman WA, Adkins RH, Spungen AM, Waters RL, Kemp B, Herbert V.. Levels of plasma homocysteine in persons with spinal cord injury. J Spinal Cord Med. 2001;24(2):81–6. [DOI] [PubMed] [Google Scholar]
- 28.Gibson AE, Buchholz AC, Martin Ginis KA, Research Group SHAPE-SCI . C-Reactive protein in adults with chronic spinal cord injury: increased chronic inflammation in tetraplegia vs paraplegia. Spinal Cord. 2008;46(9):616–21. [DOI] [PubMed] [Google Scholar]
- 29.Nichols M, Peterson K, Herbert J, Alston L, Allender S, Australian Heart Disease Statistics . 2015. Melbourne: National Heart Foundation of Australia, 2016. [ONLINE: Accessed 29/3/18] https://www.heartfoundation.org.au/images/uploads/publications/RES-115-Aust_heart_disease_statstics_2015_WEB.PDF
- 30.Rabadi MH, Mayanna SK, Vincent AS.. Predictors of mortality in veterans with traumatic spinal cord injury. Spinal Cord. 2013;51(10):784–8. [DOI] [PubMed] [Google Scholar]
- 31.Wahman K, Nash MS, Westgren N, Lewis JE, Seiger A, Levi R.. Cardiovascular disease risk factors in person with paraplegia: The Stockholm spinal cord injury study. J Rehabil Med. 2010;42(3):272–8. [DOI] [PubMed] [Google Scholar]
- 32.Chopra A, Miyatani M, Craven BC.. Cardiovascular disease risk in individuals with chronic spinal cord injury: prevalence of untreated risk factors and poor adherence to treatment guidelines. J Spinal Cord Med. 2018;41(1):2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collins C, Burrows T, Rollo M. Dietary Patterns and Cardiovascular Disease Outcomes: an Evidence Check Review brokered by the Sax Institute ( www.saxinstitute.org.au) for the National Heart Foundation of Australia, 2017. [ONLINE: Accessed 30/3/18] https://www.heartfoundation.org.au/images/uploads/main/For_professionals/Dietary_patterns_and_cardiovascular_disease_outcomes.pdf.
- 34.Weichselbaum E. Dietary patterns and the heart: evidence paper. New Zealand Heart Foundation, 2013. [ONLINE: Accessed 30/3/18] https://www.heartfoundation.org.nz/shop/submissions/dietary-patterns-evidence-paper.pdf.
- 35.National Health and Medical Research Council (NHMRC) . Healthy eating for adults. 2013. Canberra: NHMRC. [ONLINE: Accessed 11/12/16] https://www.eatforhealth.gov.au/sites/default/files/content/The%20Guidelines/n55g_adult_brochure.pdf
- 36.Fraser C, Teasell RW, Foulon BL, Mehta S. Nutrition issues following spinal cord injury. Spinal cord injury rehabilitation evidence, version 3.0. Vancouver: 1-19. (ONLINE: Accessed 20/3/18). https://www.researchgate.net/publication/265627275_Nutrition_Issues_Following_Spinal_Cord_Injury.
- 37.Pellicane AJ, Millis SR, Zimmerman SE, Roth EJ.. Calorie and protein intake in acute rehabilitation inpatients with traumatic spinal cord injury versus other diagnoses. Top Spinal Cord Inj Rehabil. 2013;19(3):229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sabour H, Javidan AN, Vafa MR, Shidfar F, Nazari M, Saberi H, et al. Calorie and macronutrients intake in people with spinal cord injuries: an analysis by sex and injury- related variables. Nutrition. 2012;28:143–7. [DOI] [PubMed] [Google Scholar]
- 39.Groah SL, Nash MS, Ljungberg IH, Kibin A, Hamm LF, Ward E, et al. Nutrient intake and body habitus after spinal cord injury: an analysis by sex and level of injury. J Spinal Cord Med. 2009;32(1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ross AB, Bruce SJ, Blondel-Lubrano A, Oguey-Araymon S, Beaumont M, Bourgeois A, et al. A whole- grain cereal-rich diet increases plasma betaine, and tends to decrease total and LDL-cholesterol compared with a re ned-grain diet in healthy subjects. Br J Nutr. 2011;105(10):1492–502. [DOI] [PubMed] [Google Scholar]
- 41.Fung TT, Hu FB, Pereira MA, Liu S, Stampfer MJ, Colditz GA, et al. Whole-grain intake and the risk of type 2 diabetes: a prospective study in men. Am J Clin Nutr. 2002;76(3):535–40. [DOI] [PubMed] [Google Scholar]
- 42.Price HC, Simmons RK.. Primary prevention of CVD: diet, clinical evidence. Br Med J. 2011;1–11. [ONLINE: Accessed 22/8/18] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3217739/pdf/2011-0219.pdf [PMC free article] [PubMed] [Google Scholar]
- 43.National Vascular Disease Prevention Alliance . Guidelines for the management of absolute cardiovascular disease risk. 2012. [ONLINE: Accessed 28/8/18] https://www.heartfoundation.org.au/images/uploads/publications/Absolute-CVD-Risk-Full-Guidelines.pdf
- 44.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Connor D, Green S, Higgins JPT.. Chapter 5: Defining the review question and developing criteria for including studies. In: Higgins JPT, Green S, (eds.) Cochrane handbook for systematic reviews of interventions. Chichester: John Wiley & Sons; 2008. [Google Scholar]
- 46.CASP Randomised Controlled Trial Checklist . Critical Appraisal Skills Programme. 2017 [ONLINE: Accessed 25/2/2018]. https://casp-uk.net/casp-tools-checklists/
- 47.CASP Case Control Checklist . Critical Appraisal Skills Programme. 2017. [ONLINE: Accessed 25/2/2018]. https://casp-uk.net/casp-tools-checklists/
- 48.CASP Qualitative Checklist . Critical Appraisal Skills Programme. 2017. [ONLINE: Accessed 25/2/2018]. https://casp-uk.net/casp-tools-checklists/
- 49.CASP Economic Evaluation Checklist . Critical Appraisal Skills Programme. 2017. [ONLINE: Accessed 25/2/2018]. https://casp-uk.net/casp-tools-checklists/
- 50.Academy of Nutrition and Dietetics . Evidence Analysis Manual: Steps in Academy Evidence Analysis Process. 2016. [ONLINE: Accessed 28/8/18] https://www.andeal.org/vault/2440/web/files/2016_April_EA_Manual.pdf [DOI] [PubMed]
- 51.Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from guidelinedevelopment.org/handbook.
- 52.GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University, 2015 (developed by Evidence Prime, Inc.). Available from gradepro.org.
- 53.Gorgey AS, Mather KJ, Cupp HR, Gater DR.. Effects of resistance training on adiposity and metabolism after spinal cord injury. Med Sci Sports Exerc. 2012;44(1):165–74. [DOI] [PubMed] [Google Scholar]
- 54.Sabour H, Javidan AN, Soltani Z, Pakpour AH, Yekaninejad MS, Mousavifar SA.. The effect of behavioral intervention and nutrition education program on serum lipid profile, body weight and blood pressure in Iranian individuals with spinal cord injury: a randomized clinical trial. J Spinal Cord Med. 2016: 1–9. doi: 10.1080/10790268.2016.1209890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szlachcic Y, Adkins RH, Adal T, Yee F, Bauman W, Waters RL.. The effect of dietary intervention on lipid profiles in individuals with spinal cord injury. J Spinal Cord Med. 2001;24(1):26–9. doi: 10.1080/10790268.2001.11753551. [DOI] [PubMed] [Google Scholar]
- 56.Chen Y, Henson S, Jackson AB, Richards JS.. Obesity intervention in persons with spinal cord injury. Spinal Cord. 2006;44(2):82–91. [DOI] [PubMed] [Google Scholar]
- 57.Javierre C, Vidal J, Segura R, Medina J, Garrido E.. Continual supplementation of n-3 fatty acids does not modify plasma lipid profile in spinal cord injury patients. Spinal Cord. 2005;43:527–30. [DOI] [PubMed] [Google Scholar]
- 58.Bigford GE, Mendez AJ, Betancourt L, Burns-Drecq P, Backus D, Nash MS.. A lifestyle intervention program for successfully addressing major cardiometabolic risks in persons with SCI: a three-subject case series. Spinal Cord Ser Cases. 2017;3:17007. doi: 10.1038/scsandc.2017.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Myers J, Gopalan R, Shahoumian T, Kiratli BJ.. Effects of customized risk reduction program on cardiovascular risk in males with spinal cord injury. J Rehabil Res and Dev. 2012;49(9):1355–64. [DOI] [PubMed] [Google Scholar]
- 60.Radomski M, Finkelstein M, Hagel S, Masemer S, Theis J, Thompson M.. A pilot wellness and weight management program for individuals with spinal cord injury: Participants’ goals and outcome. Top Spinal Cord Inj Rehabil. 2011;17(2):59–69. [Google Scholar]
- 61.Cardiovascular Health Branch , Division of Chronic Disease Control and Community Intervention , National Center for Chronic Disease Prevention and Health Promotion , CDC . Trends in ischemic heart disease mortality-United States, 1980-1988. Morb Mortal Wkly Rep. 1992;41:548–56. Cited in Szlachcic Y et al., 2001. [Google Scholar]
- 62.Krauss RM, Deckelbaum RJ, Ernst N, et al. Dietary guidelines for healthy American adults: a statement for health professionals from the nutrition Committee, American Heart Association. Circulation. 1996;94:1795–800. Cited in Szlachcic Y et al., 2001. [DOI] [PubMed] [Google Scholar]
- 63.Grundy SM, Pasternak R, Greenland P, Jr Smith S, Fuster V.. Assessment of cardiovascular risk by use of multiple- risk-factor assessment equations: a statement for healthcare professionals from the American Heart Association and the American College of Cardiology. Circulation. 1999;100(13):1481–92. 10.1161/01.CIR.100.13.1481. Cited in Myers J et al ., 2012. [DOI] [PubMed] [Google Scholar]
- 64.Block G, Woods M, Potosky A, Clifford C.. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43(12):1327–35. 10.1016/0895-4356(90)90099-B. Cited in Myers J et al ., 2012. [DOI] [PubMed] [Google Scholar]
- 65.Weinsier RL, Bacon JA, Birch R.. Time–calorie displacement diet for weight control: a prospective evaluation of its adequacy for maintaining normal nutritional status. Int J Obes. 1983;7:539–48. Cited in Chen et al., 2006. [PubMed] [Google Scholar]
- 66.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L.. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–69. Cited in Chen et al., 2006. [DOI] [PubMed] [Google Scholar]
- 67.National Cancer Institute (U.S.) , Information Management Services, & Block Dietary Data Systems (FIRM) . DIETSYS version 3.0 user's guide: health habits and history questionnaire, diet history and other risk factors: dietary analysis system. Bethesda, MD: The Institute; 1994. [Google Scholar]
- 68.U.S. Department of Health and Human Services and U.S. Department of Agriculture . 2015–2020 Dietary Guidelines for Americans. 8th Edition. December 2015. [ONLINE: Accessed 11/10/18]. https://health.gov/dietaryguidelines/2015/guidelines/.
- 69.Sotos-Prieto M, Bhupathiraju SN, Mattei J, Fung TT, Li Y, Pan A, et al. . Changes in diet quality scores and risk of cardiovascular disease among US men and women. Circulation. 2015;132:2212–9. [ONLINE: Accessed 11/10/18] https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.115.017158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van Horn L, Carson JA, Appel LJ, Burke LE, Economos C, Karmally W, et al. Recommended dietary pattern to achieve adherence to the American Heart Association/American College of Cardiology (AHA/ACC) guidelines a scientific statement from the American Heart Association. Circulation. 2016;134(22):e505–29. [DOI] [PubMed] [Google Scholar]
- 71.Anand SS, Hawkes C, de Souza RJ, Mente A, Dehghan M, Nugent R, et al. Food consumption and its impact on cardiovascular disease: importance of solutions focused on the globalized food system: a report from the workshop convened by the World Heart Federation. J Am Coll Cardiol. 2015;66(14):1590–614. doi: 10.1016/j.jacc.2015.07.050. [ONLINE: Accessed 24/9/18] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4597475/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Widmer RJ, Flammer AJ, Lerman LO, Lerman A.. The Mediterranean diet, its components, and cardiovascular disease. Am J Med. 2015;128(3): 229–38. [ONLINE: Accessed 24/9/18] https://www.amjmed.com/article/S0002-9343(14)00913-9/pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhan J, Liu Y, Cai L, Xu F, Xie T, He Q.. Fruit and vegetable consumption and risk of cardiovascular disease: a meta-analysis of prospective cohort studies. Crit Rev Food Sci Nutr. 2017;57(8):1650–63. doi: 10.1080/10408398.2015.1008980. [DOI] [PubMed] [Google Scholar]
- 74.Lieberman J, Goff Jr D, Hammond F, Schreiner P, Norton HJ, Dulin M, et al. Dietary intake and adherence to the 2010 dietary guidelines for Americans among individuals with chronic spinal cord injury: A pilot study. J Spinal Cord Med. 2014;37(6):751–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stewart J, Manmathan G, Wilkinson P.. Primary prevention of cardiovascular disease: a review of contemporary guidance and literature. JRSM Cardiovasc Dis 2017;6:2048004016687211. doi: 10.1177/2048004016687211 [ONLINE: Accessed 24/9/18]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5331469/#bibr7-2048004016687211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case–control study. Lancet. 2004;364:937–52. [DOI] [PubMed] [Google Scholar]
- 77.Orozco-Beltran D, Gil-Guillen VF, Redon J, Martin-Moreno JM, Pallares-Carratala V, Navarro-Perez J, et al. Lipid profile, cardiovascular disease and mortality in a Mediterranean high-risk population: The ESCARVAL-RISK study. PLoS ONE. 2017;12(10):e0186196. doi: 10.1371/journal.pone.0186196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/ American Heart Association Task Force on practice guidelines. Circulation. 2014;129(2):S1–S45. [ONLINE: Accessed 28/8/18] https://www.ahajournals.org/doi/pdf/10.1161/01.cir.0000437738.63853.7a [DOI] [PubMed] [Google Scholar]
- 79.National Heart Foundation of Australia . Dietary fats and dietary cholesterol for cardiovascular health: Summary of Evidence. 2009:1–44. [ONLINE: Accessed 11/10/18] https://www.heartfoundation.org.au/images/uploads/publications/Dietary-fats-summary-evidence.pdf
- 80.Moussavi RM, Ribas-Cardus F, Rintala DH, Rodriguez GP.. Dietary and serum lipids in individuals with spinal cord injury living in the community. J Rehabil Res Dev. 2001;38(2):225–33. [PubMed] [Google Scholar]
- 81.Crane DA, Little JW, Burns SP.. Weight gain following spinal cord injury: a pilot study. J Spinal Cord Med. 2011;34(2):227–32. doi: 10.1179/2045772311Y.0000000001. [ONLINE: Accessed 24/9/18]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3066508/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gupta N, White KT, Sandford PR.. Body mass index in spinal cord injury – a retrospective study. Spinal Cord. 2006;44(2):92–4. [ONLINE: Accessed 11/10/18] https://www.nature.com/articles/3101790 [DOI] [PubMed] [Google Scholar]
- 83.Powell D, Affuso O, Chen Y.. Weight change after spinal cord injury. J Spinal Cord Med. 2017;40(2):130–7. doi: 10.1179/2045772314Y.0000000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yarar-Fisher C, Chen Y, Jackson AB, Hunter GR.. Body mass index underestimates adiposity in women with spinal cord injury. Obesity (Silver Spring). 2013;21(6):1223–5. doi: 10.1002/oby.20199. [ONLINE: Accessed 11/10/18] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3740452/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Buchholz AC, Bugaresti JM.. A review of body mass index and waist circumference as markers of obesity and coronary heart disease risk in persons with chronic spinal cord injury. Spinal Cord. 2005;43(9):513–8. [DOI] [PubMed] [Google Scholar]
- 86.Eriks-Hoogland I, Hilfiker R, Baumberger M, Balk S, Stucki G, Perret C.. Clinical assessment of obesity in persons with spinal cord injury: validity of waist circumference, body mass index, and anthropometric index. J Spinal Cord Med 2011;34(4):416–22. doi: 10.1179/2045772311Y.0000000014. [ONLINE: Accessed 28/8/18] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3152814/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cragg JJ, Ravensbergen HJ, Borisoff JF, Claydon VE.. Optimal scaling of weight and waist circumference to height for adiposity and cardiovascular disease risk in individuals with spinal cord injury. Spinal Cord. 2015;53(1):64–8. [DOI] [PubMed] [Google Scholar]
- 88.Graham ID, Tetroe J.. KT theories Research Group. Some theoretical underpinnings of knowledge translation. Acad Emerg Med. 2007;14(11):936–41. [DOI] [PubMed] [Google Scholar]
- 89.World Health Organisation . Prevention of cardiovascular disease: guidelines for assessment and management of cardiovascular risk. 2007. [ONLINE: Accessed 24/9/18] http://apps.who.int/iris/bitstream/handle/10665/43685/9789241547178_eng.pdf;jsessionid = DFF2874528CA9E41F8DE863787B1E7A0?sequence = 1