Supplemental Digital Content is available in the text.
Keywords: Perinatal, Particulate matter, Wheezing, Asthma, Meta-analysis
Background:
This systematic review aimed to summarize epidemiologic evidence regarding long-term effects of prenatal and infant particulate matter with an aerodynamic diameter less than 2.5 µm (PM2.5) exposure on wheezing and asthma.
Methods:
Epidemiologic data investigating the associations between ambient PM2.5 exposures during prenatal or the first 2 years of life and wheezing or asthma throughout life were extracted from five databases. All included studies were assessed according to the Critical Appraisal Skills Programme checklists. We performed meta-analyses if ≥2 studies estimated the effects of continuous PM2.5.
Results:
Nine of 18 eligible studies were suitable for meta-analyses. For prenatal PM2.5 exposure and asthma by 10 years of age (n = 4), the overall risk estimate per 10-unit increase (95% confidence interval) was 1.12 (1.00, 1.26). Although meta-analysis of prenatal exposure and wheezing by 4 years of age (n = 5) was not possible due to inconsistent exposure and outcome assessments, four studies found strong positive associations with wheeze by 2 years of age. The overall risk of developing asthma (n = 5) and wheezing (n = 3) by 8 years of age for infant PM2.5 exposure was 1.14 (0.96, 1.35) and 1.49 (0.99, 2.26), respectively. One large high-quality study reporting risk differences not suitable for meta-analysis demonstrated significant associations between prenatal or infant PM2.5 exposure and childhood asthma. High heterogeneity was present among studies of prenatal exposures and asthma, whereas studies of other associations showed low heterogeneity. There was insufficient evidence about susceptible subgroups.
Conclusions:
The limited and inconsistent evidence is suggestive of an association between early life PM2.5 exposure and wheezing/asthma. Large standardized studies are needed to explore the associations and identify vulnerable populations.
What this study adds
This systematic review provided synthesized results on long-term impacts of prenatal and infant fine particulate matter exposure and development of wheezing or asthma based on the existing evidence. It also highlighted limitations to current research in this rapidly developing field and recommended future epidemiologic studies to use standardized designs and evaluate susceptible populations to assist policy makers in improving public health.
Introduction
Exposure to particulate matter with an aerodynamic diameter less than 2.5 µm (PM2.5) is a well-recognized global public health issue. It has been estimated that mortality from PM2.5 exposure increased from approximately 3.5 million in 1990 to 4.2 million in 2015.1 Globally, the association among PM2.5 exposure, wheezing, and asthma has been widely studied.2–4 Short-term (e.g., daily) increases in PM2.5 have a well-established association with worsening asthma symptoms and increases in hospital attendance rates,5 whereas long-term exposure has been shown to increase the risk of developing asthma.6 However, few studies have evaluated the long-term impacts of exposure during early life.
The period from in utero to the first 2 years of life is a critical window for lung development and growth.7,8 Increasingly, studies have suggested that exposure to air pollution during this period could increase the risk of developing wheezing and/or asthma in later life. For example, a systematic review has found a significant association between prenatal exposure and particulate matter with an aerodynamic diameter <10 µm (PM10) and childhood asthma9 with in vivo laboratory models suggesting that this relationship is causal.10,11
However, the identified associations in the literature between PM2.5 exposure during this critical period and the long-term risk of wheezing and asthma are inconsistent. For example, an American study suggested that childhood asthma was significantly associated with prenatal PM2.5 exposure as estimated by a land use regression (LUR) model (odds ratio [OR], 1.17; 95% confidence interval [CI] = 1.04, 1.30),12 whereas a Canadian study using a similar methodologic approach did not observe associations.13 These inconsistencies might be explained by differences in PM2.5 sources, exposure and outcome measurements, and analytic approaches in different studies, making further analysis necessary to better assess this relationship.
Previous systematic reviews have focused on the effects of either prenatal exposure alone9 or many years of exposure to traffic-related air pollution.3,6 The aim of this systematic review was to identity and summarize the available epidemiologic evidence for the association between prenatal or infant (younger than 2 years of age) exposure to PM2.5 and the subsequent development of wheezing and asthma.
Methods
We followed the Cochrane guidelines14 and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist15 (PRISMA 2009 Checklist; http://links.lww.com/EE/A35, which provides details of the checklist).
Search strategy
We initially searched PubMed, Scopus, Web of Science core collection, ProQuest, and Cochrane library on 5 November 2016 for scientific articles. We used a combination of free-text words found in the title, abstract, and keywords (Table 1).
Table 1.
Items for database search
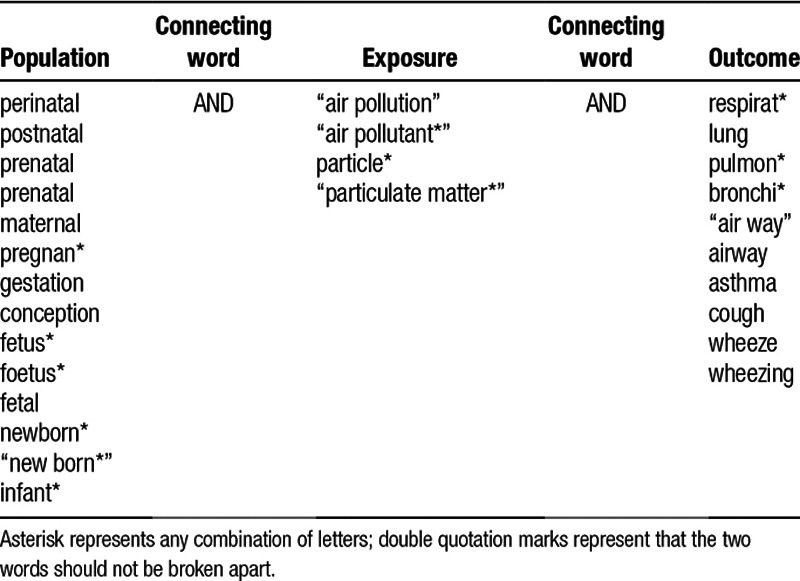
We included all respiratory outcomes in the search terms to reduce the loss of potentially relevant articles. There was no restriction on publication date. Articles that were not written in English were excluded. We updated the database search and searched the reference lists of all included studies by 12 April 2017.
Study screening
We screened titles and abstracts of all included articles for potential relevance. After that, full texts of all relevant studies were reviewed based on the following inclusion and exclusion criteria. We included all epidemiologic studies which:
were peer-reviewed journal articles, conference proceedings, theses and official reports using a cohort, case-control or cross-sectional design;
evaluated the effects of exposure to PM2.5 prenatally or during the first 2 years of life;
assessed the impact of prenatal and infant PM2.5 exposure on wheezing and asthma incidence or prevalence ≥1 year after the exposure period investigated.
Studies were excluded if they:
were experimental studies, reviews, meeting abstracts, book sections, blogs, newspaper articles, editorials, or nonresearch letters;
only assessed maternal PM2.5 exposure before conception or childhood exposure after 2 years of age;
only assessed indoor air pollution, tobacco smoke, or other air pollution exposure metrics;
only assessed other respiratory illnesses or symptoms;
assessed acute effects of PM2.5 exposure.
Data extraction
Data were extracted manually from all eligible studies for information on study design, location, population characteristics, exposure, outcomes, confounding factors, and effect estimates with 95% CIs. We contacted the corresponding authors of studies with important data missing.
Critical appraisal
We examined the quality of all included studies using the Critical Appraisal Skills Programme (CASP) checklists16,17 (CASP checklist for cohort study and CASP checklist for case-control study; http://links.lww.com/EE/A35, which provides details of these checklists).
Analysis
We employed random-effects meta-analyses to calculate the weighted effect estimates and 95% CIs for every 10 µg/m3 increase in PM2.5 concentrations. Meta-analysis was conducted if ≥2 studies reporting Odds Ratios (ORs), Risk Ratios (RRs) or Hazard Ratios (HRs) using continuous PM2.5 concentrations as an independent variable. Studies reporting ORs, RRs, or HRs were combined in a single meta-analysis as this is acceptable for common outcomes with a small effect size18 and is a well-established approach.6,19 All meta-analyses were performed on Review Manager 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark, 2014) using the generic inverse variance method. Heterogeneity was assessed using the I2 statistic and P value from the chi-squared test. Publication bias was visually evaluated using funnel plots. We conducted sensitivity analyses by employing fixed-effects models, excluding case-control studies, and excluding studies estimating exposure using techniques other than the most common approach of LUR. Because one study20 used both LUR and inverse distance weighted (IDW) approaches to estimate PM2.5 exposure, we included LUR in the primary meta-analysis and used IDW in the sensitivity analysis.
Results
Study screening
Our search strategy initially identified 8,031 articles (Figure 1). After removing duplicates (n = 3,326) and conducting the first screening of titles and abstracts (n = 4,705), we reviewed 111 full-text articles which yielded 13 relevant studies. We added five more articles by further searching for new publications and reference lists of all the included articles. Eighteen studies were included in our final review consisting of 17 peer-reviewed journal articles and 1 thesis (eTable 1; http://links.lww.com/EE/A35, which provides details).
Figure 1.
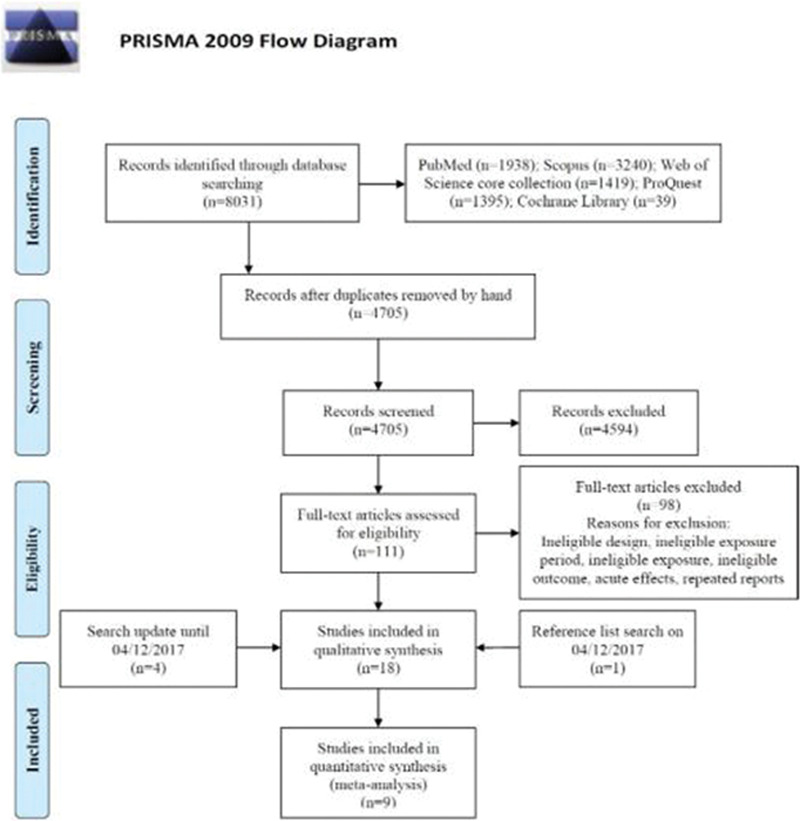
PRISMA flow diagram describing the database search and study screening process.
Study setting
All 18 studies were published between October 2002 and January 2018. The majority of the studies were conducted in North American and European countries, including five in America,12,21–24 three in Canada,13,20,25 three in Poland,26–28 two in Germany,29,30 one in the Netherlands,31 and one in the Czech Republic.32 One study was conducted in Mexico.33 The remaining two studies were pooled analyses of multicenter cohorts conducted in Canada, Germany, and the Netherlands.34,35 Sample sizes ranged from 184 to 41,569 and follow-up periods ranged from 2 to 10 years. Most of the studies (n = 16) focused on the general population (2–21 years of age), except one study of high-risk children (i.e., ≥1 first-degree asthmatic relative or ≥2 first-degree relatives with other Immunoglobulin E (IgE)-mediated allergic disease)25 and another on ethnic minorities.23
Study design
Most of the studies were pregnancy or birth cohort studies (n = 15) including two pooled analyses of multiple birth cohorts from different locations.34,35 The remaining three13,20,23 were matched case-control studies in which two were nested within birth cohorts.13,20
PM2.5 sources and measurements
There were 11 studies evaluating outdoor PM2.5 from traffic-related sources,12,13,20,22,24,25,29–31,34,35 woodsmoke,20 industrial points,20 or other sources,12,22 whereas three investigated PM2.5 from both outdoor and indoor sources.26–28 The remaining four studies did not specify the source of ambient PM2.5.21,23,32,33
Various methods were used for estimating prenatal and infant PM2.5 exposure. The LUR model was mostly based on Geographic Information Systems (GIS)13,20,25,29–31,34,35 or satellite data.12,21,22,33 Studies estimating prenatal PM2.5 exposure12,13,20–22,33 have taken into account participants’ residential histories, whereas studies estimating postnatal exposure25,29–31,34,35 only used birth address. Other studies employed an IDW approach20,23 or a dispersion model24 based on individual’s residential histories, personal environmental monitoring samplers (PEMSs),26–28 and data from the central monitoring sites.32
Outcome definition
The majority of the included studies (n = 13) relied on questionnaires or interviews to define doctor-diagnosed wheezing and asthma (Table 1). There were four studies defining asthma from medical records as different combinations of physician diagnoses, hospital admissions, and asthma-related medication use.13,20,24,32 One study diagnosed asthma by a blinded pediatric allergist based on the presence of asthmatic symptoms.25 We included parental reports of doctor-diagnosed asthmatic/spastic/obstructive bronchitis as an indication of asthma in two German studies29,30 due to the relatively low asthma frequency and the strict diagnostic criteria for preschool asthma.34
Quality assessment
According to the CASP checklists, all the studies were highly13,20–22,24,32 or moderately qualified12,23,25–31,33–35 (eTables 2 and 3; http://links.lww.com/EE/A35, which provides details). The major concerns for the validity of the studies were potential for information bias (n = 13), selection bias (n = 10), short follow-up duration (n = 9). and not accounting for important confounding factors (n = 8) (eTables 2 and 3 and notes for CASP quality assessment of all included studies; http://links.lww.com/EE/A35, which provides details).
PM2.5 exposure and wheezing/asthma
Prenatal PM2.5 exposure and asthma
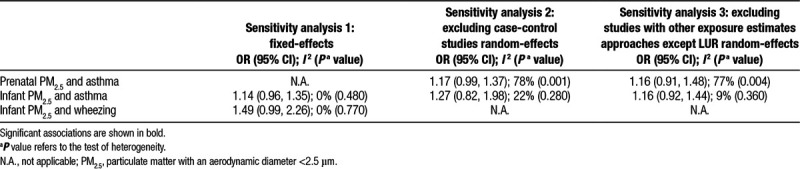
Of the six studies assessing prenatal PM2.5 exposure and asthma development, four were included in the meta-analysis,12,13,20,32 whereas the other two either contained overlapping data22 or investigated the RDs,24 respectively. The overall risk of developing childhood asthma for a 10 µg/m3 increase in prenatal PM2.5 exposure was 1.12 (95% CI = 1.00, 1.26), with borderline significance (P = 0.050) (Figure 2). We found high heterogeneity among those studies (I2 = 73%; P = 0.0005). Sensitivity analyses all found similar but nonsignificant associations between prenatal PM2.5 exposure and asthma development (Table 2; eFigure 1; http://links.lww.com/EE/A35, which provides details).
Figure 2.
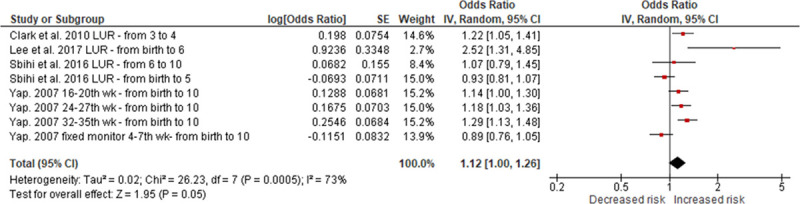
Random-effects meta-analysis of the association between prenatal PM2.5 exposure (per 10 µg/m3) and asthma. df indicates degrees of freedom. SE, standard error; IV, inverse variance.
Table 2.
Prenatal and infant PM2.5 exposure (per 10 µg/m3 increase) on wheezing/asthma from the sensitivity meta-analyses
The meta-analyses did not include a recent study using RDs to estimate the effect of prenatal PM2.5 exposure on asthma development of nearly 20,000 American children.24 In this study, the authors found significant positive associations between log-transformed prenatal PM2.5 exposure (per 2.7-fold increase) and cumulative asthma incidences from 2 to 6 years of age with RDs ranging from 0.015 to 0.035 after adjustment for confounders. Sensitivity analysis of modeling exposure by quintiles also revealed significant associations between prenatal PM2.5 exposure and asthma incidence and persistence by 5 years of age. However, modeling PM2.5 linearly resulted in positive associations but with no statistical significance (eTable 4; http://links.lww.com/EE/A35, which provides details).
Infant PM2.5 exposure and asthma
There were nine studies evaluating the associations between infant PM2.5 exposure and asthma. These included one for birth year exposure,25 four for exposure during the first of life,20,23,24,35 and four for exposure during first 2 years of life.29–31,34 After excluding four studies either with repeated data25,29,31 or estimating the effect by RDs,24 five remained in the meta-analyses.20,23,30,34,35 Our meta-analyses showed a trend toward a positive association that was not statistically significant (overall OR, 1.14; 95% CI = 0.96, 1.35) with low heterogeneity (I2 = 0%; P = 0.480) (Figure 3). The results were robust to multiple sensitivity analyses (Table 2; eFigures 2 and 3; http://links.lww.com/EE/A35, which provides details).
Figure 3.
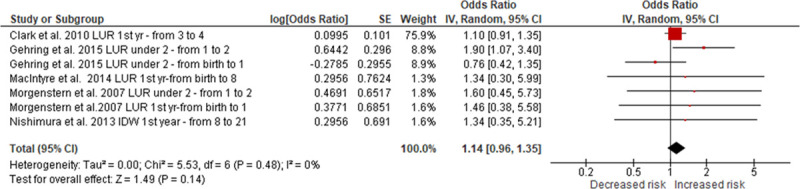
Random-effects meta-analysis of the association between infant PM2.5 exposure (per 10 µg/m3) and asthma. df indicates degrees of freedom. SE, standard error; IV, inverse variance.
One study also analyzed the outcomes as current asthma or ever asthma plus current wheeze in their regression models,35 which was not included in the meta-analyses. According to the results of those analyses, infant PM2.5 exposure was found to be significantly associated with an increased risk of current asthma of 35% (95% CI = 7%, 70%) at 6–8 years of age, whereas ever asthma plus current wheeze did not show statistically significant associations (eTable 4; http://links.lww.com/EE/A35, which provides details).
In the study assessing RDs,24 significant associations were observed for PM2.5 exposure during the first year of life and incident or persistent asthma when modeling exposure as a log-transformed continuous variable and by quintiles. Similar with the results of prenatal PM2.5 exposure, modeling the PM2.5 as a continuous variable without log-transformation revealed nonsignificant associations. However, goodness-of-fit analyses suggested that the log-transformed modeling was better than the linear continuous modeling. Other sensitivity analyses all suggested significant associations (eTable 4; http://links.lww.com/EE/A35, which provides details).
Prenatal PM2.5 exposure and wheezing
Meta-analysis was not applicable for the five studies of prenatal PM2.5 exposure and wheezing because most of the studies categorized PM2.5 exposure by median and had different outcome definitions.21,26–28
There was only one study that modeled PM2.5 as a continuous variable using regression analyses.33 The authors evaluated the effect of PM2.5 exposure during different trimesters of pregnancy on ever or current wheeze (wheeze in the past year) in five hundred fifty-two 4-year-old children. No significant association was observed in any trimester PM2.5 exposure and wheezing outcomes.
Another study suggested that higher prenatal PM2.5 exposure (>11.22 µg/m3) was significantly associated with a 102% increase (95% CI = 20%, 240%) in the risk of repeated wheezing in children from birth to 2 years of age compared with the lower exposure group (≤11.22 µg/m3), with consistent results from multiple sensitivity analyses.21
The other three studies were from the same project—the Krakow study26–28 which used PEMS to measure PM2.5 exposure during the second trimester of pregnancy. All studies suggested significant associations between prenatal PM2.5 exposure and wheezing duration in the first 2 years of life; however, although the association for 3–4 years of age was also positive, it was not statistically significant (eTable 4; http://links.lww.com/EE/A35, which provides details).
Infant PM2.5 exposure and wheezing
Meta-analyses included three of the four studies investigating the association between infant PM2.5 and wheezing,30,31,35 whereas the other one containing repeated data was excluded.29 Infant PM2.5 exposure was not associated with wheezing development in either random- or fixed-effects models (overall OR, 1.49; 95% CI = 0.99, 2.26) (Figure 4; eFigure 4; http://links.lww.com/EE/A35, which provides details). Low heterogeneity was found in the three studies as indicated by an I2 = 0% and a P value = 0.770. PM2.5 was also not significantly associated with current wheeze at 6–8 years of age35 [eTable 4; http://links.lww.com/EE/A35, which provides details).
Figure 4.
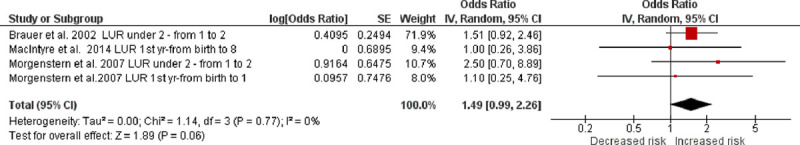
Random-effects meta-analysis of the association between infant PM2.5 exposure (per 10 µg/m3) and wheezing. df indicates degrees of freedom. SE, standard error; IV, inverse variance.
Publication bias
Small studies with negative findings have not been published on the associations between prenatal or infant PM2.5 exposure and asthma. The distribution was symmetrical in the funnel plot of infant exposure and wheezing, despite the small number of studies included in the meta-analysis (eFigures 5–7; http://links.lww.com/EE/A35, which provides details).
Outcomes by specific characteristics
There were nine studies including stratified analyses by gender,12,13,20,22–24,29 heredity,23,24 maternal stress during pregnancy,12,33 race,24 atopic status,23 and other characteristics including birth weight, gestational length, maternal age, parity, neighborhood socioeconomic status (SES),13 and genotype35 (eTable 4; http://links.lww.com/EE/A35, which provides details).
The differences of effects by gender were inconsistent among the seven studies. To illustrate, two studies suggested larger magnitudes of effects in males compared with females,12,22 whereas the other five suggested stronger effects in females.13,20,23,24,29 Of those studies, Hsu and colleagues22 reported significant associations in men exposed to PM2.5 during the 12–26th gestational weeks with asthma development, whereas Pennington and colleagues24 reported significant associations between infant PM2.5 exposure and asthma development in women. Other studies did not show significant results among different genders.
Higher risk was shown for children with a family history of asthma than those without in one study,23 whereas the other one24 only found significantly increased risks of asthma in children of mothers without asthma, but not in children of mothers with asthma.
Stratified analyses by maternal stress during pregnancy revealed a consistently significant and increased risk in children whose mothers were highly stressed during pregnancy compared with those slightly stressed.12,33
Only one study24 tested for potential effect modification by race or ethnicity and found no statistical differences between groups described as “white” or “black.”
Studies that evaluated atopic status23 and other characteristics including birth weight, maternal age, parity, gestational length, and SES13 did not find any significant associations with asthma. However, evidence of effect modification was seen with birth weight. Children with a birth weight <2,500 g were at a higher risk of developing asthma associated with prenatal PM2.5 exposure. Children with the Glutathione S-transferase P1 (GSTP1) rs1138272 (Ala114Val) or rs1695 (IIe105Val) minor alleles were more susceptible to developing asthma associated with infant PM2.5 exposure.35
Discussion
Our meta-analyses demonstrated positive associations between prenatal PM2.5 exposure and asthma and infant PM2.5 exposure, and both wheezing and asthma; however, there were a limited number of relevant studies, and the results were inconsistent. There was high heterogeneity among the studies for prenatal PM2.5 exposure and asthma. This might be due to the variability in children’s ages, exposure measurement methods, sources of particulate matter, outcome definitions, and adjustment of confounding factors. Studies investigating prenatal PM2.5 exposure and subsequent wheezing were not amenable to meta-analysis but consistently reported significant associations, especially in infants (2 years or younger).
This is the largest review assessing long-term effects of prenatal and infant PM2.5 exposure on subsequent wheezing or asthma. We added three more studies12,32,33 to a previous systematic review and meta-analysis of the effects of prenatal exposure to all types of air pollutants including PM2.5 on the development of wheezing and asthma.9 Our results of meta-analyses of the association between prenatal PM2.5 exposure and asthma were similar to this previous review, observing no significant associations and high heterogeneity. In contrast, the other new study not included in meta-analysis reported significantly increased risk of asthma by 2–6 years of age after prenatal exposure to PM2.5.24 However, the evidence was mixed, with more significant associations seen in children followed to school age12,24,32 than preschool age.20,24 This phenomenon might be explained by the difficulties in the diagnosis of asthma among young children,36 leading to the underestimation of physician-diagnosed asthma in this population. The significant associations between prenatal PM2.5 exposure and wheezing in infants26–28 rather than in older children28,33 could indirectly support this explanation. However, some researchers argue that it is difficult to predict asthma based solely on early life wheezing as less than half of children with episodes of preschool wheezing will have continuing childhood asthma.37
For infant PM2.5 exposure and the subsequent development of wheezing or asthma, our meta-analyses did not demonstrate an association. However, these studies were of higher risk of bias due to potential for selection bias,23,30,31,34,35 recall bias,23,30,31,34,35 not adjusted for important confounding factors,20,35 and a case-control design.20,23 In contrast, a recent large, high-quality cohort study of nearly 20,000 children revealed positive associations between PM2.5 exposure during the first year of life and asthma incidence by 6 years of age, despite not adjusting for important confounders.24 This result was robust to different asthma definitions but sensitive to PM2.5 modeling decisions and covariate controls. Overall, the small number of studies identified in this systematic review limited our confidence in conclusively suggesting the presence or absence of associations. Studies with a larger sample size, a standardized exposure estimate method, more accurate outcome assessment approaches, and greater statistical power are needed to further explore the long-term effects of prenatal and infant PM2.5 exposure on asthma or wheeze development.
Our review also highlights the limited evidence of susceptible populations to prenatal and infant PM2.5 exposure. Children whose mothers were exposed to negative life events during pregnancy were more likely to develop wheezing or asthma after prenatal and infant PM2.5 exposure than those not exposed. The different effects of PM2.5 exposure by gender and heredity were inconsistent between studies. There was insufficient evidence to suggest that race, low birth weight, and specific genotypes could increase the risk of wheezing or asthma development after PM2.5 exposure, whereas the effects of atopic status, gestational length, maternal age, parity, and SES require further investigation.
The main strength of our systematic review was the comprehensive search strategy and reproducible evaluation of current evidence. Our findings provide a timely contribution to the rapidly developing field, which could highlight limitations and guide future studies. However, some limitations should also be acknowledged. First, evidence of prenatal and infant PM2.5 exposure and wheezing or asthma is still limited. In addition, publication bias might be present in studies evaluating early life PM2.5 exposure and asthma. Therefore, any conclusions should be made with caution and confirmed by further investigations. Second, high variability was found between studies in study design, exposure estimating methods, outcome assessment approaches, participants’ ages at assessment, and adjustment of confounders, especially in those evaluating prenatal PM2.5 exposure and asthma. Future syntheses of evidence in this area will benefit from more studies using standardized designs and methods. In addition, diagnosis of asthma in young children is difficult, and outcome misclassification is inevitable in this population. Finally, the major source of PM2.5 in this systematic review was traffic, with scarce evidence regarding the long-term respiratory effects of early life PM2.5 exposure from other sources such as wildfire smoke, which is an increasing global concern due to climate change.38,39 More research on PM2.5 from other sources is needed to guide public health responses.
Conclusions
Prenatal and infant PM2.5 exposure was not clearly associated with subsequent development of wheezing or asthma in our review of the literature. The strongest evidence was for an association between prenatal PM2.5 exposure and wheezing in infants, whereas in utero exposure and asthma had a borderline positive overall effect estimate. However, evidence was insufficient and mixed, indicated by a small number of studies included in the meta-analyses and inconsistent results. Further research is necessary to explore the associations using harmonized exposure methods and appropriate statistical analyses controlling for important covariates. Furthermore, studies of susceptible populations and other sources of PM2.5 are needed to help policy makers improving public health.
Conflicts of interest statement
The authors declares that they have no conflicts of interest with regard to the content of this report.
Acknowledgments
We thank Ulrike Gehring for providing their unpublished data.
Supplementary Material
Footnotes
Published online 22 February 2019
Sponsorships or competing interests that may be relevant to content are disclosed at the end of the article.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
References
- 1.Cohen AJ, Brauer M, Burnett R, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet 20173891907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castner J, Guo L, Yin Y. Ambient air pollution and emergency department visits for asthma in Erie County, New York 2007-2012. Int Arch Occup Environ Health 201891205–214. [DOI] [PubMed] [Google Scholar]
- 3.Bowatte G, Lodge C, Lowe AJ, et al. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: a systematic review and a meta-analysis of birth cohort studies. Allergy 201570245–256. [DOI] [PubMed] [Google Scholar]
- 4.Yang SI. Particulate matter and childhood allergic diseases. Korean J Pediatr 20196222–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan J, Li S, Fan C, Bai Z, Yang K. The impact of PM2.5 on asthma emergency department visits: a systematic review and meta-analysis. Environ Sci Pollut Res Int 201623843–850. [DOI] [PubMed] [Google Scholar]
- 6.Khreis H, Kelly C, Tate J, Parslow R, Lucas K, Nieuwenhuijsen M. Exposure to traffic-related air pollution and risk of development of childhood asthma: a systematic review and meta-analysis. Environ Int 20171001–31. [DOI] [PubMed] [Google Scholar]
- 7.Burri PH. Structural aspects of postnatal lung development: alveolar formation and growth. Biol Neonate 200689313–322. [DOI] [PubMed] [Google Scholar]
- 8.Dietert RR, Etzel RA, Chen D, et al. Workshop to identify critical windows of exposure for children’s health: immune and respiratory systems work group summary. Environ Health Perspect 2000108suppl 3483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hehua Z, Qing C, Shanyan G, Qijun W, Yuhong Z. The impact of prenatal exposure to air pollution on childhood wheezing and asthma: a systematic review. Environ Res 2017159519–530. [DOI] [PubMed] [Google Scholar]
- 10.Herbert C, Siegle JS, Shadie AM, et al. Development of asthmatic inflammation in mice following early-life exposure to ambient environmental particulates and chronic allergen challenge. Dis Model Mech 20136479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang PL, You DH, Saravia J, Shen HH, Cormier SA. Maternal exposure to combustion generated PM inhibits pulmonary Th1 maturation and concomitantly enhances postnatal asthma development in offspring. Part Fibre Toxicol 20131029.doi: 10.1186/1743-8977-10–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee A, Leon Hsu HH, Mathilda Chiu YH, et al. Prenatal fine particulate exposure and early childhood asthma: effect of maternal stress and fetal sex. J Allergy Clin Immunol 20181411880–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sbihi H, Tamburic L, Koehoorn M, Brauer M. Perinatal air pollution exposure and development of asthma from birth to age 10 years. Eur Respir J 2016471062–1071. [DOI] [PubMed] [Google Scholar]
- 14.Clarke M, Oxman AD, Paulsen E, Higgins JPT, Green Se. Higgins JPT, Green S. Appendix A: guide to the contents of a Cochrane methodology protocol and review. In: Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Updated March 2011) 2011The Cochrane Collaboration; Available at: www.handbook.cochrane.org [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med 20096e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Critical Appraisal Skills Programme. CASP Cohort Study Checklist. Available at: http://docs.wixstatic.com/ugd/dded87_5ad0ece77a3f4fc9bcd3665a7d1fa91f.pdf. Accessed 11 June 2017.
- 17.Critical Appraisal Skills Programme. CASP Case-Control Study Checklist. http://docs.wixstatic.com/ugd/dded87_afbfc99848f64537a53826e1f5b30b5c.pdf. Accessed 11 June 2017.
- 18.Davies HT, Crombie IK, Tavakoli M. When can odds ratios mislead? BMJ 1998316989–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson HR, Favarato G, Atkinson RW. Long-term exposure to air pollution and the incidence of asthma: meta-analysis of cohort studies. Air Qual Atmos Health 2013647–56. [Google Scholar]
- 20.Clark NA, Demers PA, Karr CJ, et al. Effect of early life exposure to air pollution on development of childhood asthma. Environ Health Perspect 2010118284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiu YH, Coull BA, Sternthal MJ, et al. Effects of prenatal community violence and ambient air pollution on childhood wheeze in an urban population. J Allergy Clin Immunol 2014133713–722.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu HH, Chiu YH, Coull BA, et al. Prenatal particulate air pollution and asthma onset in Urban children. Identifying sensitive windows and sex differences. Am J Respir Crit Care Med 20151921052–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishimura KK, Galanter JM, Roth LA, et al. Early-life air pollution and asthma risk in minority children. The GALA II and SAGE II studies. Am J Respir Crit Care Med 2013188309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pennington AF, Strickland MJ, Klein M, et al. Exposure to mobile source air pollution in early-life and childhood asthma incidence: the Kaiser Air Pollution and Pediatric Asthma Study. Epidemiology 20182922–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlsten C, Dybuncio A, Becker A, Chan-Yeung M, Brauer M. Traffic-related air pollution and incident asthma in a high-risk birth cohort. Occup Environ Med 201168291–295. [DOI] [PubMed] [Google Scholar]
- 26.Jedrychowski W, Flak E, Mroz E, et al. Modulating effects of maternal fish consumption on the occurrence of respiratory symptoms in early infancy attributed to prenatal exposure to fine particles. Ann Nutr Metab 2008528–16. [DOI] [PubMed] [Google Scholar]
- 27.Jedrychowski W, Perera F, Maugeri U, et al. Effect of prenatal exposure to fine particles and postnatal indoor air quality on the occurrence of respiratory symptoms in the first two years of life. Int J Environ Health 20082314–329. [Google Scholar]
- 28.Jedrychowski WA, Perera FP, Maugeri U, et al. Intrauterine exposure to polycyclic aromatic hydrocarbons, fine particulate matter and early wheeze. Prospective birth cohort study in 4-year olds. Pediatr Allergy Immunol 2010214 pt 2e723–e732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gehring U, Cyrys J, Sedlmeir G, et al. Traffic-related air pollution and respiratory health during the first 2 yrs of life. Eur Respir J 200219690–698. [DOI] [PubMed] [Google Scholar]
- 30.Morgenstern V, Zutavern A, Cyrys J, et al. Respiratory health and individual estimated exposure to traffic-related air pollutants in a cohort of young children. Occup Environ Med 2007648–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brauer M, Hoek G, Van Vliet P, et al. Air pollution from traffic and the development of respiratory infections and asthmatic and allergic symptoms in children. Am J Respir Crit Care Med 20021661092–1098. [DOI] [PubMed] [Google Scholar]
- 32.Yap PS. . Risk Factors for Atopic Diseases in a Czech Birth Cohort [Ph.D.] 2007Ann Arbor, MI: University of California, Davis [Google Scholar]
- 33.Rosa MJ, Just AC, Kloog I, et al. Prenatal particulate matter exposure and wheeze in Mexican children: effect modification by prenatal psychosocial stress. Ann Allergy Asthma Immunol 2017119232–237.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gehring U, Wijga AH, Hoek G, et al. Exposure to air pollution and development of asthma and rhinoconjunctivitis throughout childhood and adolescence: a population-based birth cohort study. Lancet Respir Med 20153933–942. [DOI] [PubMed] [Google Scholar]
- 35.MacIntyre EA, Brauer M, Melén E, et al. ; TAG Study Group GSTP1 and TNF Gene variants and associations between air pollution and incident childhood asthma: the traffic, asthma and genetics (TAG) study. Environ Health Perspect 2014122418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cave AJ, Atkinson LL. Asthma in preschool children: a review of the diagnostic challenges. J Am Board Fam Med 201427538–548. [DOI] [PubMed] [Google Scholar]
- 37.Sears MR. Predicting asthma outcomes. J Allergy Clin Immunol 2015136829–836.quiz 837 [DOI] [PubMed] [Google Scholar]
- 38.Flannigan M, Cantin AS, de Groot WJ, Wotton M, Newbery A, Gowman LM. Global wildland fire season severity in the 21st century. For Ecol Manage 201329454–61. [Google Scholar]
- 39.Westerling AL, Hidalgo HG, Cayan DR, Swetnam TW. Warming and earlier spring increase western U.S. forest wildfire activity. Science 2006313940–943. [DOI] [PubMed] [Google Scholar]


