Abstract
Asthma is a chronic inflammatory disease of the airways related to epithelial damage, bronchial hyperresponsiveness to contractile agents, tissue remodeling, and luminal narrowing. Currently, there are many data about the pathophysiology of asthma; however, a new aspect has emerged related to the influence of reactive oxygen and nitrogen species (ROS and RNS) on the origin of this disease. Several studies have shown that an imbalance between the production of ROS and RNS and the antioxidant enzymatic and nonenzymatic systems plays an important role in the pathogenesis of this disease. Considering this aspect, this study is aimed at gathering data from the scientific literature on the role of oxidative distress in the development of inflammatory airway and lung diseases, especially bronchial asthma. For that, articles related to these themes were selected from scientific databases, including human and animal studies. The main findings of this work showed that the respiratory system works as a highly propitious place for the formation of ROS and RNS, especially superoxide anion, hydrogen peroxide, and peroxynitrite, and the epithelial damage is reflected in an important loss of antioxidant defenses that, in turn, culminates in an imbalance and formation of inflammatory and contractile mediators, such as isoprostanes, changes in the activity of protein kinases, and activation of cell proliferation signalling pathways, such as the MAP kinase pathway. Thus, the oxidative imbalance appears as a promising path for future investigations as a therapeutic target for the treatment of asthmatic patients, especially those resistant to currently available therapies.
1. Introduction
Asthma is a chronic inflammatory disease of the airways in which many cells of the innate and adaptive immune system act together with epithelial cells to cause bronchial hyperreactivity, overproduction of mucus, remodeling of the wall, and narrowing of the airways. In this inflammatory context, a series of reactive species are produced, leading to changes in the function of the epithelium, smooth muscle cells, and the immune system and in the structure of the airway wall, leading to the pulmonary limitations that characterize this disease [1].
The oxidative stress resulting from an imbalance between oxidants, such as reactive oxygen and nitrogen species (ROS and RNS), and antioxidants, in favor of oxidants, has been called oxidative distress, a condition known to lead to biological damage. ROS refer to radicals derived from O2 metabolism, as well as to nonradical derivatives reactive to O2 (for example, hydrogen peroxide), and the term RNS refer to radicals of nitrogen reactive to other molecules in which the reactive center is nitrogen [2].
The most common ROS and RNS, in the order of reactive capacity, are as follows: superoxide anion (O2-•), hydrogen peroxide (H2O2), hydroxyl radical (HO•), singlet oxygen (1O2), peroxyl radical (HO2•), nitric oxide (•NO), peroxynitrite (ONOO−), perhydroxy radical (HO2•), hydroperoxyl radical (ROOH•), hypochlorous acid (HClO), ozone (O3), and nitric dioxide (NO2) [3].
Although the generation of oxidative molecules is part of normal metabolism, the intracellular levels of ROS and RNS are kept at low levels of concentrations under normal physiological conditions, acting as important mediators involved in the regulation of some cellular processes, such as growth, adhesion, cell differentiation and death, modulation of gene expression, and signalling of cellular transduction pathways [4, 5].
The involvement of reactive species in the regulation of intracellular signalling occurs through posttranslational modification of proteins sensitive to redox alteration, including several receptor and ion channels, kinases and phosphatases, caspases, and transcription factors, which have functionally significant cysteine residues, liable of undergoing oxidation [6]. Thus, ROS can oxidize cysteine sulfhydryl groups (Cys-SH) with the formation of sulfenic (Cys-SOH) and then sulfinic (Cys-SO2H) and sulfonic acids (Cys-SO3H), resulting in the alteration of activity and functioning of the protein participating in signal transduction pathways [7].
The lungs are continuously exposed to a variety of oxidants that differ in type and degree, and can easily overcome the resulting oxidative stress with the help of an enzymatic and nonenzymatic antioxidant network. The main antioxidant enzymes include superoxide dismutase (SOD), catalase (CAT), and glutathione-dependent enzymes, such as glutathione peroxidase (GSH-Px), glutathione S-transferase (GST), glutathione reductase (GSH), and glutathione synthetase [8].
While airway inflammation and asthma tend to increase the production of ROS and RNS, mainly through the activity of eosinophils and neutrophils [9], accumulated evidences suggest the participation of oxidative stress in asthma genesis and modulation [10, 11].
Thus, this work is aimed at exploring the respiratory system as a place for the production of reactive species. It also explores the role of pulmonary antioxidant systems in relation to the origin of inflammatory diseases of the respiratory system, particularly bronchial asthma. In addition, it explores the mechanisms involved with these effects mediated by oxidative imbalance.
2. Origin of ROS in the Respiratory System and Its Antioxidant Barrier
The respiratory system is an environment that presents an increased risk of suffering from damage caused by oxidative stress due to the high susceptibility to interactions with environmental factors. The lungs are exposed to exogenous ROS, originating from pollutants and cigarette smoke, and endogenous ROS, produced diffusely as a by-product of cell metabolism [12], mainly by inflammatory cells, such as eosinophils, macrophages, and neutrophils [13], as well as by fibroblasts and epithelial cells [2, 12]).
The production of ROS in the respiratory system occurs at cellular sites, such as mitochondria, microsomes, and enzymes (such as xanthine oxidase, monooxygenase P450, cyclooxygenase, lipoxygenase, indolamine dioxygenase, and monoamine oxidase) [14, 15]. Of these, mitochondria are the major contributors to the formation of ROS; it is estimated that 1 to 3% of the electron flow in this organelle forms a superoxide anion [16].
The initial step for the formation of these free radicals is the activation of the enzyme complex of nicotinamide adenine dinucleotide reduced phosphate (NADPH) oxidase, present in the cell membrane, and the generation of the superoxide anion [17]. This compound can be spontaneously or enzymatically dismuted to H2O2 and molecular oxygen (O2). Both O2− and H2O2 can react, in the presence of iron or other metals, to form the most potent OH° radical [18].
In the context of asthma, inflammatory cells are the main sources of ROS. Granulocytes contain peroxidases (such as myeloperoxidase and eosinophilic peroxidase), which catalyze the reaction of H2O2 with halides, leading to the formation of hypohalides, such as hypochlorous acid (HClO) [19]. Antigenic challenges in asthmatic patients increase the formation of ROS by eosinophils [20]. In addition, leukocytes circulating in the blood increase the production of superoxide anion, indicating that both pulmonary and intravascular inflammatory cells contribute to oxidative stress in asthma [21]. In this case, the airway epithelium of asthmatics produces few antioxidants, which explains the loss of the epithelium effectiveness in maintaining airway homeostasis [22].
To counterbalance the formation of these ROS, the respiratory system has enzymatic and nonenzymatic antioxidant systems, in order to maintain normal levels of these free radicals, necessary for the perfect cellular functioning. The major enzyme systems in the airways are the SOD complex, which converts the superoxide anion into hydrogen peroxide; catalase, which converts hydrogen peroxide into water and molecular oxygen; and glutathione peroxidase (GSH-Px) and reductase (GR), which inactivate hydrogen peroxide and other hydroperoxides [23–26]. Among these antioxidant systems, SOD is highly expressed extracellularly in the lungs, around the airways and smooth muscle, being abundant in the epithelium [27], and plays an important role in combating oxidative stress in asthma. In this context, asthmatic patients are deficient in antioxidant defenses, with the resulting burden of oxidative stress contributing to pulmonary dysfunction [28].
The main aspects related to the formation of reactive species in the respiratory system associated with diseases such as asthma are shown in Figure 1.
Figure 1.
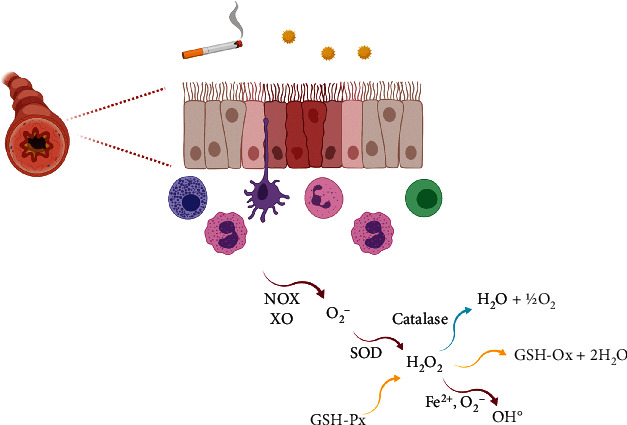
Formation of ROS by inflammatory cells and the antioxidant defenses on airways. Created with BioRender.com.
3. Nitric Oxide: Good Guy or Villain in the Airways?
Nitric oxide (NO) has been well described in the literature as an important signalling molecule involved in the regulation of many mammalian physiological and pathophysiological processes, particularly in the lung [29]. It plays a role in regulating both pulmonary vascular tone and bronchomotor tone in the airways. In addition, NO participates in the host's inflammation and defense against infection through changes in vascular permeability, changes in epithelial barrier function and repair, cytotoxicity, ciliary motility regulation, mucus secretion, and inflammatory cell infiltration [30]. Thus, these multiple NO functions have been implicated in the pathogenesis of chronic inflammatory diseases of the airways [31].
NO is produced by a family of nitric oxide synthases (NOS), which metabolize L-arginine through the N-hydroxy intermediate L-arginine (NOHA) to form NO and L-citrulline using molecular oxygen and NADPH as cofactor. Three NOS isoenzymes have been identified in mammals, with variable distributions. Neuronal NOS (nNOS or NOS I) and endothelial NOS (eNOS or NOS III) are the constitutively expressed forms in the airway epithelium, nonadrenergic noncholinergic neurons (iNANC) and in endothelial cells of the airway vessels. Its activity is regulated by intracellular calcium, with rapid onset of activity and production of small amounts of NO in the order of magnitude of picomoles, that play an important role in maintaining respiratory homeostasis. Otherwise, the inducible NOS (iNOS or NOS II) is transcriptionally regulated by proinflammatory stimuli, with the ability to produce large amounts (nanomolar concentrations) of NO for a long period of time [30, 32]. About iNOS, its positive regulation is observed in the lungs of asthmatics, and the increased levels of exhaled NO are well described in patients with asthma, being a marker of the severity of the disease [33].
In the early phase of the asthmatic response, NO plays an important role in reducing airway hyperresponsiveness (AHR), and the hyperreactive response is related to NO deficiency, either due to the greater activity of the enzyme arginase, which prevents the conversion to NO and L-citrulline by NOS, or due to the lower activity of NOS constitutive isoforms. In this sense, the decrease in eNOS or nNOS expression has already been observed in guinea pigs exposed to repeated allergenic challenge and in asthmatic patients, respectively [34, 35]. In contrast, in the late phase of the asthmatic response, the increased activity of iNOS, which produces large amounts of NO, is responsible for AHR [36, 37].
This increased activity (or increased expression) of iNOS occurs due to the action of inflammatory cytokines [29, 33]. Corroborating these propositions, it was observed in models of acute asthma in guinea pigs that, in the rapid phase of the asthmatic response, the levels of exhaled NO decrease, and the iNOS expression is not altered as well; this is in contrast to the late phase, in which exhaled NO levels are high, as well as iNOS expression [37–39].
Inflammation and AHR in asthma are not the result of increased production of NO itself, but are due to the formation of the free radical, strong oxidizing agent, peroxynitrite, resulting from the reaction of NO with the superoxide anion in the airways [40, 41]. Peroxynitrite activates eosinophils, increases mucin 5AC (MUC5AC) expression, increases microvascular permeability, induces epithelial damage, and increases contraction of smooth muscle in the airways [42–44]. In this context, studies have shown that airway epithelial cells and bronchial biopsy inflammatory cells from asthmatic patients, as well as from sensitized guinea pigs, show an increase in nitrotyrosine labeling (a marker of tyrosine residue nitrosylation in proteins), which is also correlated with the increase of exhaled NO, expression of iNOS and AHR, and eosinophilic inflammation, having as an intermediary the action of free radicals and oxidative stress [41, 43, 45].
Recent studies have described a new source of superoxide anion, the decoupled endothelial nitric oxide (eNOS) synthase, a process that occurs when eNOS is not associated with cofactor or substrate [46]. This process can occur due to the oxidative action of peroxynitrite on the tetrahydrobiopterine cofactor [47] or by a reduction in the availability of L-arginine, common in asthma due to the increased expression of the enzyme arginase [31].
A summary of the multiple NO functions in the airways are presented in Figure 2.
Figure 2.
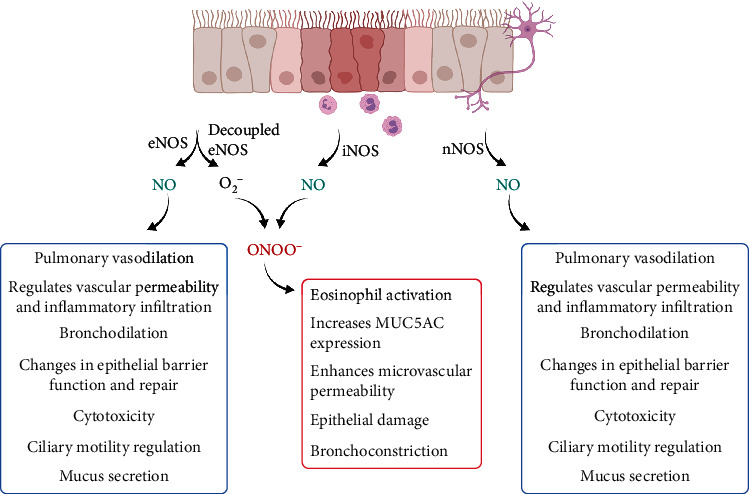
Summarized schema of the multiple NO functions in the airways. Created with BioRender.com.
The formation of the peroxynitrite radical, by itself, already produces harmful effects on the respiratory system that contribute to inflammatory damage, as well as reducing the availability of NO that has a role in regulating airway smooth muscle tone. A key molecule in increasing oxidative stress and decreasing NO availability is asymmetric dimethylarginine (ADMA). It is a product of posttranslational methylation of L-arginine, synthesized by the protein arginine methyltransferase (PRMT) and degraded by dimethylamino hydrolase (DDAH). ADMA binds to different NOS isoforms, inhibiting them and competing with NO for their binding site. Asthmatic subjects have high levels of ADMA, which may be one of the factors responsible for the asthmatic worsening [47].
4. Assessment of Oxidative Stress in the Respiratory System
The increase in ROS production enhances lipid peroxidation, as well as damage to proteins, aggravating airway inflammation through multiple mechanisms, including the release of inflammatory mediators and effects on mucus production and smooth muscle [48]. On the other hand, impaired antioxidant defense mechanisms contribute to oxidative damage in asthma [49]. Among the markers of oxidative stress are those specific to the lungs [50–53]. In this context, while increased levels of these markers in the plasma indicate systemic oxidative damage, which may or may not have originated from the respiratory system, the measurement of these markers in lung samples more accurately reflects the oxidative stress that occurs in the respiratory system [54].
The markers of pulmonary oxidative stress, determined in rodents and human subjects, are aldehyde products from the oxidative decomposition of polyunsaturated fatty acids in the membrane, such as malondialdehyde (MDA), hexanal, heptanal, nonanal, acrolein, 4-hydroxyhexanal (4-HHE), and 4-hydroxinonenal (4-HNE) [55], in addition to isoprostane, a product of the peroxidation of arachidonic acid by ROS, responsible for producing prostanoids in a pathway independent of cyclooxygenase, for example the 8-iso-PGF2α [56–58].
In this context, an increase in MDA levels in a model of chronic allergic asthma in mice (an indicator of elevated oxidative damage [59, 60]) and the rise in MDA levels have also been reported in other studies as a characteristic of oxidative damage caused by asthma, both in mice and rats [61–63]. Additionally, the increased levels of 8-iso-PGF2α have been described in a guinea pig model of chronic allergic lung inflammation [64]. Furthermore, Pinkerton et al. [65] evidenced increased levels of 3-nitrotyrosine and 8-isoprostane markers in a murine model of Chlamydia-induced steroid-resistant asthma.
5. Actions of Reactive Species in the Respiratory System
Several studies have already reported the effects of reactive species on the functioning of airway smooth muscle, involving numerous cells and signalling pathways being activated and/or inhibited by it. These effects are related to exacerbated production of ROS and RNS caused by the chronic inflammatory condition [66, 67], deficiency of intrinsic (for example, glutathione) or extrinsic (for example, natural vitamins and antioxidants in the diet) antioxidants [68–70], decreased activity or dysfunction of antioxidant enzymes [71], or excessive activity of prooxidative enzymes [72].
Hydrogen peroxide and increased oxygen levels have been shown to induce contraction of the guinea pig trachea [73] and to stimulate MAPKs involved in regulating the proliferation of tracheal myocytes [74]. In addition to its direct effects, studies show that ROS also influences airway reactivity to contractile and relaxing agonists; among them, the increased contractile response to acetylcholine and methacholine [75], histamine [42], serotonin [76], and the substance P [77], and the decrease in the number and function of β2 adrenergic receptors can be cited [78, 79].
Isoprostanes, such as 8-iso-PGF2α [80], are considered markers of oxidative stress in asthmatic patients and can induce contraction of airway smooth muscle [58, 59]. In this sense, the participation of 8-iso-PGF2α in the increase of airway resistance associated with oxidative stress has already been evidenced in previous studies [81, 82].
In an ovalbumin-induced asthma model, Vasconcelos et al. [64] demonstrated that the airway smooth muscle hyperreactivity was due to an increase in the formation of superoxide anion and hydrogen peroxide, in addition to a greater expression of iNOS, which culminated in a greater production of peroxynitrite radical and, as a consequence, 8-iso-PGF2α. In addition, it was shown that the antioxidant defenses in the asthmatic animals were impaired, contributing to oxidative stress.
Another marker of oxidative stress is 3-nitrotyrosine (3-NT), which is formed from the nitration of the amino acid tyrosine and has already been found in the airway epithelium, lung parenchyma, and inflammatory cells of asthmatic individuals at high levels [41, 83]. Tyrosine nitration can inhibit protein kinase phosphorylation, thereby interfering with the signal transduction mechanism [84], in addition to this role in increasing eosinophil chemotaxis by RANTES and IL-5 [85].
In vitro studies also demonstrated that the nitration of a specific tyrosine residue inactivated superoxide dismutase [86, 87], causing a reduction in its activity and generating an increase in the concentration of ROS and RNS, with consequent tissue damage [88]. Sugiura and Ichinose [89] also described a positive correlation between 3-nitrotyrosine, iNOS, and xanthine oxidase (XO) activity, indicating that together, iNOS and XO may be associated with the generation of RNS in the airways of patients with asthma.
The targets of ROS include catalytic receptors, phosphatases, and phospholipids, in addition to proteins from the MAPK pathway [90]. ROS comprise a type of damage-associated molecular pattern molecules (DAMP), which can activate dendritic cells, stimulating nuclear factor-κB (NF-κB) and triggering the inflammatory response [91]. In addition, the production of ROS by NADPH oxidase is related to the proliferation of airway smooth muscle in response to growth factors, followed by the activation of NF-κB, culminating in tissue remodeling and narrowing of the air lumen [92].
Neutrophils also play an important role in the production of ROS. Stimulated by various respiratory inflammatory diseases, such as asthma and chronic obstructive pulmonary disease (COPD), after activation, they release proinflammatory ROS and serine proteases, including neutrophil elastase and proteinase-3. These proteins degrade pulmonary elastin fibers, induce mucus production, and stimulate the secretion of metalloproteinase (MMP) subtypes 8 or 9 that break down elastin and collagen, worsening respiratory symptoms. Through positive feedback, neutrophils themselves also release factors, such as leukotriene B4 (LTB4) acting as a bronchoconstrictor, and IL-8, in which both attract other neutrophils and perpetuate chronic inflammation [93, 94].
Another important cellular signalling covers the activity of the nuclear factor erythroid 2-related factor (Nrf2), which is a transcription factor that regulates the expression of genes involved in the protection against oxidative damage [95, 96]. Physiologically, Nrf2 is bound and inhibited by Kelch-like ECH-associated protein 1 (Keap1), which prevents its binding to the antioxidant response element and is continuously degraded by the proteasome pathway. However, in the presence of ROS, increased during asthma, Keap1 inhibition occurs and Nrf2 migrates to the nucleus, increasing the synthesis of antioxidant enzymes that include GSH- and thyrodoxin- (TXN-) dependent systems [97, 98].
Changes and increased activity of Nrf2 are associated with several respiratory diseases. In asthma, there has been an increase in susceptibility, severity, and inflammation caused by it in mice [99], as well as an increase in the proliferation of smooth muscle cells in the airways in case of alteration [100]. Similarly, in COPD, a reduction in Nrf2 expression in pulmonary macrophages is reported [101], as well as a relationship between genetic alterations of this protein and the induction of disease onset, as well as emphysema [102].
Furthermore, activation of toll-like receptors (TLRs) was also reported in pulmonary inflammation in mice [103]. These receptors are expressed in several cells in the airways, including epithelial cells, macrophages, mast cells, and dendritic cells (DCs). The stimulation of TLRs by infectious agents activates antigen-presenting cells (APC) and controls the differentiation of T helper immune cells (Th1, Th2, and Th17) in a context-dependent manner, together with the activation of mast cells for the production of cytokines [104], which increase the production of ROS. In addition to the inflammatory component, bronchial hyperresponsiveness has also been associated with activity especially of TLR2 and TLR4 [105].
A summary of the mechanisms of cell damage in the respiratory system by reactive species is presented in Table 1.
Table 1.
Summary of main cells or effector systems altered in the airways due to increase of reactive species.
| Cell or effector system | Effect |
|---|---|
| MAPK | Cell proliferation |
| Hydrogen peroxide | Smooth muscle contraction |
| 8-Iso-PGF2α | Increase of airway resistance |
| 3-Nitrotyrosine | Increase of airway inflammation |
| Neutrophil | Increase of airway inflammation |
| Nrf2/Keap1 | Decrease of antioxidant activity |
6. Conclusions and Perspectives
Oxidative stress is a fundamental physiological cellular process related to several adaptations; however, when deregulated, it can lead to exacerbated cellular damage, culminating in the development of chronic diseases. The respiratory system is an environment susceptible to the action of reactive species by its close contact with air particles, allergens, pollutants, and pathogens capable of triggering inflammatory diseases, such as asthma. In this sense, the compiled data collected demonstrated a possible key role of ROS and RNS in the genesis of allergic inflammatory diseases of the respiratory system, especially the superoxide anion, hydrogen peroxide, and peroxynitrite, and how altered epithelial and pulmonary antioxidant systems play an important role in this process. However, additional studies, especially in humans, are necessary in order to make it possible to extrapolate these results obtained from animal models for asthmatic patients.
It has been shown that adult asthma is associated with a low dietary intake of fruits rich in antioxidant nutrients and low plasma vitamin C levels, suggesting that diet may be a potentially modifiable risk factor for the development of asthma [106]. The use of thiol compounds such as antioxidants in antiasthmatic therapy should be explored, due to the possible ability to mimic the endogenous effects promoted by GSH, as well as the use of plant-derived polyphenolic compounds, such as flavonoids. Furthermore, in this perspective, modulating the oxidative balance appears as a new perspective of therapeutics for the treatment of chronic respiratory diseases, such as asthma, and new research is needed to better explore it.
Acknowledgments
The authors thank PPgPNSB and UFPB for institutional support. This article was funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Grant number 426964/2016-0).
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Lambrecht B. N., Hammad H. The immunology of asthma. Nature Immunology. 2015;16(1):45–56. doi: 10.1038/ni.3049. [DOI] [PubMed] [Google Scholar]
- 2.Sies H., Berndt C., Jones D. P. Oxidative stress. Annual Review of Biochemistry. 2017;86(1):715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 3.Powers S. K., Jackson M. J. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiological Reviews. 2008;88(4):1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahal A., Kumar A., Singh V., et al. Oxidative stress, prooxidants, and antioxidants: the interplay. BioMed Research International. 2014;2014:19. doi: 10.1155/2014/761264.761264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finkel T. Oxidant signals and oxidative stress. Current Opinion in Cell Biology. 2003;15(2):247–254. doi: 10.1016/S0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y., Branicky R., Noë A., HEKIMI S. Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. Journal of Cell Biology. 2018;217(6):1915–1928. doi: 10.1083/jcb.201708007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalinina E. V., Chernov N. N., Novichkova M. D. Role of glutathione, glutathione transferase, and glutaredoxin in regulation of redox-dependent processes. Biochemistry. 2014;79(13):1562–1583. doi: 10.1134/S0006297914130082. [DOI] [PubMed] [Google Scholar]
- 8.Rahman I. Pharmacological antioxidant strategies as therapeutic interventions for COPD. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2012;1822(5):714–728. doi: 10.1016/j.bbadis.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki S., Matsukura S., Takeuchi H., et al. Increase in reactive oxygen metabolite level in acute exacerbations of asthma. International Archives of Allergy and Immunology. 2008;146(1):67–72. doi: 10.1159/000126064. [DOI] [PubMed] [Google Scholar]
- 10.Sugiura H., Komaki Y., Koarai A., Ichinose M. Nitrative stress in refractory asthma. Journal of Allergy and Clinical Immunology. 2008;121(2):355–360. doi: 10.1016/j.jaci.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Mishra V., Banga J., Silveyra P. Oxidative stress and cellular pathways of asthma and inflammation: therapeutic strategies and pharmacological targets. Pharmacology & Therapeutics. 2018;181:169–182. doi: 10.1016/j.pharmthera.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowler R., Crapo J. Oxidative stress in Airways. American Journal of Respiratory and Critical Care Medicine. 2002;166(supplement_1):S38–S43. doi: 10.1164/rccm.2206014. [DOI] [PubMed] [Google Scholar]
- 13.Bishopp A., Sathyamurthy R., Manney S. Biomarkers of oxidative stress and antioxidants in severe asthma: a prospective case-control study. Annals of Allergy, Asthma & Immunology. 2017;118(4):445–451. doi: 10.1016/j.anai.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Fridovich I. [5] Overview: Biological sources of O2−. Methods in Enzymology. 1984;105:59–61. doi: 10.1016/S0076-6879(84)05008-4. [DOI] [PubMed] [Google Scholar]
- 15.Vallyathan V., Shi X. The role of oxygen free radicals in occupational and environmental lung diseases. Environmental Health Perspectives. 1997;105(suppl 1):165–177. doi: 10.1289/ehp.97105s1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y., Zhu H., Kuppusamy P., Zweier J. L., Trush M. A. Mitochondrial electron transport chain-derived superoxide exits macrophages: implications for mononuclear cell-mediated pathophysiological processes. Reactive Oxygen Species. 2016;1(1):81–98. doi: 10.20455/ros.2016.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Babior B. M. NADPH oxidase: an update. Blood. 1999;93(5):1464–1476. doi: 10.1182/blood.V93.5.1464. [DOI] [PubMed] [Google Scholar]
- 18.Brunori M., Rotilio G. [2] Biochemistry of oxygen radical species. Methods in Enzymology. 1984;105:22–35. doi: 10.1016/S0076-6879(84)05005-9. [DOI] [PubMed] [Google Scholar]
- 19.Babior B. M. Oxygen-dependent microbial killing by phagocytes. New England Journal of Medicine. 1978;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- 20.Sanders S. P., Zweier J. L., Harrison S. J., Trush M. A., Rembish S. J., Liu M. C. Spontaneous oxygen radical production at sites of antigen challenge in allergic subjects. American Journal of Respiratory and Critical Care Medicine. 1995;151(6):1725–1733. doi: 10.1164/ajrccm.151.6.7767513. [DOI] [PubMed] [Google Scholar]
- 21.Nadeem A., Siddiqui N., Alharbi N. O., Alharbi M. M. Airway and systemic oxidant-antioxidant dysregulation in asthma: a possible scenario of oxidants spill over from lung into blood. Pulmonary Pharmacology & Therapeutics. 2014;29(1):31–40. doi: 10.1016/j.pupt.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Xiao C. Defective epithelial barrier function in asthma. Journal of Allergy and Clinical Immunology. 2011;128(3):549–556.e12. doi: 10.1016/j.jaci.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 23.Kinnula V. L., Yankaskas J. R., Chang L., et al. Primary and immortalized (BEAS 2B) human bronchial epithelial cells have significant antioxidative capacity in vitro. American Journal of Respiratory Cell and Molecular Biology. 1994;11(5):568–576. doi: 10.1165/ajrcmb.11.5.7946385. [DOI] [PubMed] [Google Scholar]
- 24.Repine J. E., Bast A. A. L. T., Lankhorst I. D. A. Oxidative stress in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 1997;156(2):341–357. doi: 10.1164/ajrccm.156.2.9611013. [DOI] [PubMed] [Google Scholar]
- 25.Pietarinen-Runtti P., Lakari E., Raivio K. O., Kinnula V. L. Expression of antioxidant enzymes in human inflammatory cells. American Journal of Physiology-Cell Physiology. 2000;278(1):C118–C125. doi: 10.1152/ajpcell.2000.278.1.C118. [DOI] [PubMed] [Google Scholar]
- 26.Loxham M., Davies D. E., Blume C. Epithelial function and dysfunction in asthma. Clinical and Experimental Allergy. 2014;44(11):1299–1313. doi: 10.1111/cea.12309. [DOI] [PubMed] [Google Scholar]
- 27.Su W. Y., Folz R., Chen J. S., Crapo J. D., Chang L. Y. Extracellular superoxide dismutase mRNA expressions in the human lung by in situ hybridization. American Journal of Respiratory Cell and Molecular Biology. 1997;16(2):162–170. doi: 10.1165/ajrcmb.16.2.9032123. [DOI] [PubMed] [Google Scholar]
- 28.Morwood K., Gillis D., Smith W., Kette F. Aspirin-sensitive asthma. Internal Medicine Journal. 2005;35(4):240–246. doi: 10.1111/j.1445-5994.2004.00801.x. [DOI] [PubMed] [Google Scholar]
- 29.Ricciardolo F. L., Sterk P. J., Gaston B., Folkerts G. Nitric oxide in health and disease of the respiratory system. Physiological Reviews. 2004;84(3):731–765. doi: 10.1152/physrev.00034.2003. [DOI] [PubMed] [Google Scholar]
- 30.Redington A. E. Modulation of nitric oxide pathways: therapeutic potential in asthma and chronic obstructive pulmonary disease. European Journal of Pharmacology. 2006;533(1–3):263–276. doi: 10.1016/j.ejphar.2005.12.069. [DOI] [PubMed] [Google Scholar]
- 31.Benson R. C., Hardy K. A., Morris C. R. Arginase and arginine dysregulation in asthma. Journal of Allergy. 2011;2011:12. doi: 10.1155/2011/736319.736319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Racke K., Warnken M. L-arginine metabolic Pathways~!2009-11-28~!2010-03-03~!2010-05-04~! The Open Nitric Oxide Journal. 2010;2(2):9–19. doi: 10.2174/1875042701002020009. [DOI] [Google Scholar]
- 33.Hamid Q., Springall D. R., Polak J. M., et al. Induction of nitric oxide synthase in asthma. Lancet. 1993;342(8886-8887):1510–1513. doi: 10.1016/S0140-6736(05)80083-2. [DOI] [PubMed] [Google Scholar]
- 34.Ricciardolo F. L. Multiple roles of nitric oxide in the airways. Thorax. 2003;58(2):175–182. doi: 10.1136/thorax.58.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samb A., Pretolani M., Dinh-Xuan A. T., et al. Decreased pulmonary and tracheal smooth muscle expression and activity of type 1 nitric oxide synthase (nNOS) after ovalbumin immunization and multiple aerosol challenge in guinea pigs. American Journal of Respiratory and Critical Care Medicine. 2001;164(1):149–154. doi: 10.1164/ajrccm.164.1.2004030. [DOI] [PubMed] [Google Scholar]
- 36.Kharitonov S. A., O’connor B. J., Evans D. J., Barnes P. J. Allergen induced late asthmatic reactions are associated with elevation of exhaled nitric oxide. American Journal of Respiratory and Critical Care Medicine. 1995;151(6):1894–1899. doi: 10.1164/ajrccm.151.6.7767537. [DOI] [PubMed] [Google Scholar]
- 37.Yan Z. Q., Hansson G. K., Skoogh B. E., Lotvall J. O. Induction of nitric oxide synthase in a model of allergic occupational asthma. Allergy. 1995;50(9):760–764. doi: 10.1111/j.1398-9995.1995.tb01221.x. [DOI] [PubMed] [Google Scholar]
- 38.Persson M. G., Gustafsson L. E. Allergen-induced airway obstruction in guinea-pigs is associated with changes in nitric oxide levels in exhaled air. Acta Physiologica Scandinavica. 1993;149(4):461–466. doi: 10.1111/j.1748-1716.1993.tb09643.x. [DOI] [PubMed] [Google Scholar]
- 39.De Boer J., Meurs H., Coers W., et al. Deficiency of nitric oxide in allergen-induced airway hyperreactivity to contractile agonists after the early asthmatic reaction: an ex vivo study. British Journal of Pharmacology. 1996;119(6):1109–1116. doi: 10.1111/j.1476-5381.1996.tb16011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sadeghi-Hashjin G., Folkerts G., Henricks P. A., Muijsers R. B., Nijkamp F. P. Peroxynitrite in airway diseases. Clinical and Experimental Allergy. 1998;28(12):1464–1473. doi: 10.1046/j.1365-2222.1998.00428.x. [DOI] [PubMed] [Google Scholar]
- 41.Saleh D., Ernst P., Lim S., Barnes P. J., Giaid A. Increased formation of the potent oxidant peroxynitrite in the airways of asthmatic patients is associated with induction of nitric oxide synthase: effect of inhaled glucocorticoid. The FASEB Journal. 1998;12(11):929–937. doi: 10.1096/fasebj.12.11.929. [DOI] [PubMed] [Google Scholar]
- 42.Sadeghi-Hashjin G., Folkerts G., Henricks P. A., et al. Peroxynitrite induces airway hyperresponsiveness in guinea pigs in vitro and in vivo. American Journal of Respiratory and Critical Care Medicine. 1996;153(5):1697–1701. doi: 10.1164/ajrccm.153.5.8630623. [DOI] [PubMed] [Google Scholar]
- 43.SUGIURA H., ICHINOSE M., OYAKE T., et al. Role of peroxynitrite in airway microvascular hyperpermeability during late allergic phase in guinea pigs. American Journal of Respiratory and Critical Care Medicine. 1999;160(2):663–671. doi: 10.1164/ajrccm.160.2.9807160. [DOI] [PubMed] [Google Scholar]
- 44.Hanazawa T., Kharitonov S. A., Barnes P. J. Increased nitrotyrosine in exhaled breath condensate of patients with asthma. American Journal of Respiratory and Critical Care Medicine. 2000;162(4):1273–1276. doi: 10.1164/ajrccm.162.4.9912064. [DOI] [PubMed] [Google Scholar]
- 45.Luo S., Lei H., Qin H., Xia Y. Molecular mechanisms of endothelial NO synthase uncoupling. Current Pharmaceutical Design. 2014;20(22):3548–3553. doi: 10.2174/13816128113196660746. [DOI] [PubMed] [Google Scholar]
- 46.Förstermann U., Sessa W. C. Nitric oxide synthases: regulation and function. European Heart Journal. 2012;33(7):829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhatraju N. K., Agrawal A. Mitochondrial dysfunction linking obesity and asthma. Annals of the American Thoracic Society. 2017;14(Supplement_5):S368–S373. doi: 10.1513/AnnalsATS.201701-042AW. [DOI] [PubMed] [Google Scholar]
- 48.Holguin F. Oxidative stress in airway diseases. Annals of the American Thoracic Society. 2013;10(Supplement):S150–S157. doi: 10.1513/AnnalsATS.201305-116AW. [DOI] [PubMed] [Google Scholar]
- 49.Galli F., Battistoni A., Gambari R., et al. Oxidative stress and antioxidant therapy in cystic fibrosis. Biochimica et Biophysica Acta. 2012;1822(5):690–713. doi: 10.1016/j.bbadis.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 50.Louhelainen N., Myllarniemi M., Rahman I., Kinnula V. L. Airway biomarkers of the oxidant burden in asthma and chronic obstructive pulmonary disease: current and future perspectives. International Journal of Chronic Obstructive Pulmonary Disease. 2008;3(4):585–603. doi: 10.2147/copd.s3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koutsokera A., Kostikas K., Nicod L. P., Fitting J. W. Pulmonary biomarkers in COPD exacerbations: a systematic review. Respiratory Research. 2013;14(1):p. 111. doi: 10.1186/1465-9921-14-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shoki A. H., Mayer-Hamblett N., Wilcox P. G., Sin D. D., Quon B. S. Systematic review of blood biomarkers in cystic fibrosis pulmonary exacerbations. Chest Journal. 2013;144(5):1659–1670. doi: 10.1378/chest.13-0693. [DOI] [PubMed] [Google Scholar]
- 53.Agusti A., Sin D. D. Biomarkers in COPD. Clinics in Chest Medicine. 2014;35(1):131–141. doi: 10.1016/j.ccm.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 54.Antus B. Oxidative stress markers in sputum. Oxidative Medicine and Cellular Longevity. 2016;2016:12. doi: 10.1155/2016/2930434.2930434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Del Rio D., Stewart A. J., Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutrition, Metabolism and Cardiovascular Diseases. 2005;15(4):316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 56.Morrow J. D., Roberts L. J. The isoprostanes: current knowledge and directions for future research. Biochemical Pharmacology. 1996;51(1):1–9. doi: 10.1016/0006-2952(95)02072-1. [DOI] [PubMed] [Google Scholar]
- 57.Janssen L. J. Isoprostanes: generation, pharmacology, and roles in free-radical-mediated effects in the lung. Pulmonary Pharmacology & Therapeutics. 2000;13(4):149–155. doi: 10.1006/pupt.2000.0244. [DOI] [PubMed] [Google Scholar]
- 58.Janssen L. J. Isoprostanes: an overview and putative roles in pulmonary pathophysiology. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2001;280(6):L1067–L1082. doi: 10.1152/ajplung.2001.280.6.L1067. [DOI] [PubMed] [Google Scholar]
- 59.Huang W. C., Fang L. W., Liou C. J. Phloretin attenuates allergic airway inflammation and oxidative stress in asthmatic mice. Frontiers in Immunology. 2017;8:1–13. doi: 10.3389/fimmu.2017.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nesi R. T., Kennedy-Feitosa E., Lanzetti M., et al. Inflammatory and oxidative stress markers in experimental allergic asthma. Inflammation. 2017;40(4):1166–1176. doi: 10.1007/s10753-017-0560-2. [DOI] [PubMed] [Google Scholar]
- 61.Bao H. R., Liu X. J., Li Y. L., Men X., Zeng X. L. Sinomenine attenuates airway inflammation and remodeling in a mouse model of asthma. Molecular Medicine Reports. 2016;13(3):2415–2422. doi: 10.3892/mmr.2016.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shakeri F., Soukhtanloo M., Boskabady M. H. The effect of hydro-ethanolic extract of <i>Curcuma longa</i> rhizome and curcumin on total and differential WBC and serum oxidant, antioxidant biomarkers in rat model of asthma. Iranian Journal of Basic Medical Sciences. 2017;20(2):155–165. doi: 10.22038/ijbms.2017.8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chekchaki N., Khaldi T., Rouibah Z., et al. Anti-inflammatory and antioxidant effects of two extracts from Pistacia lentiscus in liver and erythrocytes, in an experimental model of asthma. Methods. 2017;18:p. 19. [Google Scholar]
- 64.Vasconcelos L. H. C., Silva M. D. C. C., Costa A. C., et al. Virgin coconut oil supplementation prevents airway hyperreactivity of guinea pigs with chronic allergic lung inflammation by antioxidant mechanism. Oxidative Medicine and Cellular Longevity. 2020;2020:16. doi: 10.1155/2020/5148503.5148503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pinkerton J., Kim R., Essilfie A., et al. Investigating Antioxidant Therapy for Steroid-Resistant Asthma. European Respiratory Journal. 2016;48:p. PA570. [Google Scholar]
- 66.Calhoun W. J., Reed H. E., Moest D. R., Stevens C. A. Enhanced superoxide production by alveolar macrophages and air-space cells, airway inflammation, and alveolar macrophage density changes after segmental antigen bronchoprovocation in allergic subjects. The American Review of Respiratory Disease. 1992;145(2_part_1):317–325. doi: 10.1164/ajrccm/145.2_Pt_1.317. [DOI] [PubMed] [Google Scholar]
- 67.Iyer D., Mishra N., Agrawal A. Mitochondrial function in allergic disease. Current Allergy and Asthma Reports. 2017;17(5):p. 29. doi: 10.1007/s11882-017-0695-0. [DOI] [PubMed] [Google Scholar]
- 68.Rahman I. Oxidative stress and gene transcription in asthma and chronic obstructive pulmonary disease: antioxidant therapeutic targets. Current Drug Target -Inflammation & Allergy. 2002;1(3):291–315. doi: 10.2174/1568010023344607. [DOI] [PubMed] [Google Scholar]
- 69.Harik-Khan R. I., Muller D. C., Wise R. A. Serum vitamin levels and the risk of asthma in children. American Journal of Epidemiology. 2004;159(4):351–357. doi: 10.1093/aje/kwh053. [DOI] [PubMed] [Google Scholar]
- 70.Jiang L., Diaz P. T., Best T. M., Stimpfl J. N., He F., Zuo L. Molecular characterization of redox mechanisms in allergic asthma. Annals of Allergy, Asthma & Immunology. 2014;113(2):137–142. doi: 10.1016/j.anai.2014.05.030. [DOI] [PubMed] [Google Scholar]
- 71.Smith L. J., Shamsuddin M., Sporn P. H., Denenberg M., Anderson J. Reduced superoxide dismutase in lung cells of patients with asthma. Free Radical Biology and Medicine. 1997;22(7):1301–1307. doi: 10.1016/S0891-5849(96)00550-3. [DOI] [PubMed] [Google Scholar]
- 72.Li Y., Li G. P. Oxidative stress in asthma: a distinct clinical and pathologic feature? Journal of Biological Regulators and Homeostatic Agents. 2016;30(4):1053–1057. [PubMed] [Google Scholar]
- 73.Rhoden K. J., Barnes P. J. Effect of hydrogen peroxide on guinea-pig tracheal smooth muscle in vitro: role of cyclo-oxygenase and airway epithelium. British Journal of Pharmacology. 1989;98(1):325–330. doi: 10.1111/j.1476-5381.1989.tb16898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abe M. K., Chao T. S., Solway J., Rosner M. R., Hershenson M. B. Hydrogen peroxide stimulates mitogen-activated protein kinase in bovine tracheal myocytes: implications for human airway disease. American Journal of Respiratory Cell and Molecular Biology. 1994;11(5):577–585. doi: 10.1165/ajrcmb.11.5.7946386. [DOI] [PubMed] [Google Scholar]
- 75.Henricks P. A. J., Nijkamp F. P. Reactive oxygen species as mediators in asthma. Pulmonary Pharmacology & Therapeutics. 2001;14(6):409–421. doi: 10.1006/pupt.2001.0319. [DOI] [PubMed] [Google Scholar]
- 76.Szarek J. L., Schmidt N. L. Hydrogen peroxide-induced potentiation of contractile responses in isolated rat airways. American Journal of Physiology-Lung Cellular and Molecular Physiology. 1990;258(4):L232–L237. doi: 10.1152/ajplung.1990.258.4.l232. [DOI] [PubMed] [Google Scholar]
- 77.Murlas C. G., Murphy T. P., Lang Z. HOCl causes airway substance P hyperresponsiveness and neutral endopeptidase hypoactivity. American Journal of Physiology-Lung Cellular and Molecular Physiology. 1990;258(6):L361–L368. doi: 10.1152/ajplung.1990.258.6.l361. [DOI] [PubMed] [Google Scholar]
- 78.Nijkamp F. P., Henricks P. A. J. Beta-adrenoceptors in lung inflammation. American Review of Respiratory Disease. 1990;141(3_part_2):S145–S150. doi: 10.1164/ajrccm/141.3_Pt_2.S145. [DOI] [PubMed] [Google Scholar]
- 79.Ikuta N., Sugiyama S., Takagi K., Satake T., Ozawa T. Implication of oxygen radicals on airway hyperresponsiveness after ovalbumin challenge in guinea pigs. American Review of Respiratory Disease. 1992;145(3):561–565. doi: 10.1164/ajrccm/145.3.561. [DOI] [PubMed] [Google Scholar]
- 80.Hazbun M. E., Hamilton R., Holian A., Eschenbacher W. L. Ozone-induced increases in substance P and 8-Epi-Prostaglandin F2αin the airways of human subjects. American Journal of Respiratory Cell and Molecular Biology. 1993;9(5):568–572. doi: 10.1165/ajrcmb/9.5.568. [DOI] [PubMed] [Google Scholar]
- 81.Possa S. S., Charafeddine H. T., Righetti R. F., et al. Rho-kinase inhibition attenuates airway responsiveness, inflammation, matrix remodeling, and oxidative stress activation induced by chronic inflammation. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2012;303(11):L939–L952. doi: 10.1152/ajplung.00034.2012. [DOI] [PubMed] [Google Scholar]
- 82.Pigati P. A., Righetti R. F., Possa S. S., et al. Y-27632 is associated with corticosteroid-potentiated control of pulmonary remodeling and inflammation in guinea pigs with chronic allergic inflammation. BMC Pulmonary Medicine. 2015;15(1):85–100. doi: 10.1186/s12890-015-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaminsky D. A., Mitchell J., Carroll N., James A., Soultanakis R., Janssen Y. Nitrotyrosine formation in the airways and lung parenchyma of patients with asthma. Journal of Allergy and Clinical Immunology. 1999;104(4):747–754. doi: 10.1016/S0091-6749(99)70283-6. [DOI] [PubMed] [Google Scholar]
- 84.Van Der Vliet A., Eiserich J. P., Shigenaga M. K., Cross C. E. Reactive nitrogen species and tyrosine nitration in the respiratory tract: epiphenomena or a pathobiologic mechanism of disease? American Journal of Respiratory and Critical Care Medicine. 1999;160(1):1–9. doi: 10.1164/ajrccm.160.1.9807044. [DOI] [PubMed] [Google Scholar]
- 85.Sato E., Simpson K. L., Grisham M. B., Koyama S., Robbins R. A. Effects of Reactive Oxygen and Nitrogen Metabolites on RANTES. and IL-5-Induced Eosinophil Chemotactic Activity _in vitro_. The American Journal of Pathology. 1999;155(2):591–598. doi: 10.1016/S0002-9440(10)65154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yamakura F., Taka H., Fujimura T., Murayama K. Inactivation of Human Manganese-superoxide Dismutase by Peroxynitrite Is Caused by Exclusive Nitration of Tyrosine 34 to 3-Nitrotyrosine∗. Journal of Biological Chemistry. 1998;273(23):14085–14089. doi: 10.1074/jbc.273.23.14085. [DOI] [PubMed] [Google Scholar]
- 87.Mac Millan-Crow L. A., Thompson J. A. Tyrosine modifications and inactivation of active site manganese superoxide dismutase mutant (Y34F) by peroxynitrite. Archives of Biochemistry and Biophysics. 1999;366(1):82–88. doi: 10.1006/abbi.1999.1202. [DOI] [PubMed] [Google Scholar]
- 88.Andreadis A. A., Hazen S. L., Comhair S. A., Erzurum S. C. Oxidative and nitrosative events in asthma. Free Radical Biology and Medicine. 2003;35(3):213–225. doi: 10.1016/S0891-5849(03)00278-8. [DOI] [PubMed] [Google Scholar]
- 89.Sugiura H., Ichinose M. Oxidative and nitrative stress in bronchial asthma. Antioxidants & Redox Signaling. 2008;10(4):785–798. doi: 10.1089/ars.2007.1937. [DOI] [PubMed] [Google Scholar]
- 90.Bowler R. P. Oxidative stress in the pathogenesis of asthma. Current Allergy and Asthma Reports. 2004;4(2):116–122. doi: 10.1007/s11882-004-0056-7. [DOI] [PubMed] [Google Scholar]
- 91.Ckless K., Hodgkins S. R., Ather J. L., Martin R., Poynter M. E. Epithelial, dendritic, and CD4+ T cell regulation of and by reactive oxygen and nitrogen species in allergic sensitization. Biochimica et Biophysica Acta (BBA) - General Subjects. 2011;1810(11):1025–1034. doi: 10.1016/j.bbagen.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brar S. S., Kennedy T. P., Sturrock A. B., et al. NADPH oxidase promotes NF-κB activation and proliferation in human airway smooth muscle. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2002;282(4):L782–L795. doi: 10.1152/ajplung.00206.2001. [DOI] [PubMed] [Google Scholar]
- 93.Barnes P. J. New molecular targets for the treatment of neutrophilic diseases. Journal of Allergy and Clinical Immunology. 2007;119(5):1055–1062. doi: 10.1016/j.jaci.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 94.Santus P., Corsico A., Solidoro P., Braido F., di Marco F., Scichilone N. Oxidative stress and respiratory system: pharmacological and clinical reappraisal of N-acetylcysteine. COPD: Journal of Chronic Obstructive Pulmonary Disease. 2014;11(6):705–717. doi: 10.3109/15412555.2014.898040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Venugopal R., Jaiswal A. K. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD (P) H: quinone oxidoreductase1 gene. Proceedings of the National Academy of Sciences. 1996;93(25):14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Itoh K., Chiba T., Takahashi S. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochemical and Biophysical Research Communications. 1997;236(2):313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 97.Gong P., Stewart D., Hu B. Activation of the mouse heme oxygenase-1 gene by 15-Deoxy-Δ12,14-Prostaglandin J2Is mediated by the stress response elements and transcription factor Nrf2. Antioxidants & Redox Signaling. 2002;4(2):249–257. doi: 10.1089/152308602753666307. [DOI] [PubMed] [Google Scholar]
- 98.Hayes J. D., Dinkova-Kostova A. T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends in Biochemical Sciences. 2014;39(4):199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 99.Rangasamy T., Guo J., Mitzner W. A. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. Journal of Experimental Medicine. 2005;202(1):47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Michaeloudes C., Chang P. J., Petrou M., Chung K. F. Transforming growth Factor-β and nuclear factor E2–related factor 2 regulate antioxidant responses in airway smooth muscle Cells. American Journal of Respiratory and Critical Care Medicine. 2011;184(8):894–903. doi: 10.1164/rccm.201011-1780OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Suzuki M., Betsuyaku T., Ito Y., et al. Down-regulated NF-E2-related factor 2 in pulmonary macrophages of aged smokers and patients with chronic obstructive pulmonary disease. American Journal of Respiratory Cell and Molecular Biology. 2008;39(6):673–682. doi: 10.1165/rcmb.2007-0424OC. [DOI] [PubMed] [Google Scholar]
- 102.Rangasamy T., Blake D. J., Malhotra D. Targeting Nrf2 with the triterpenoid CDDO-imidazolide attenuates cigarette smoke-induced emphysema and cardiac dysfunction in mice. Proceedings of the National Academy of Sciences USA. 2009;106:250–255. doi: 10.1073/pnas.0804333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kleeberger S. R., Reddy S., Zhang L. Y., Jedlicka A. E. Genetic susceptibility to ozone-induced lung hyperpermeability: role of toll-like receptor 4. American Journal of Respiratory Cell and Molecular Biology. 2000;22(5):620–627. doi: 10.1165/ajrcmb.22.5.3912. [DOI] [PubMed] [Google Scholar]
- 104.Pulendran B., Kumar P., Cutler C. W., Mohamadzadeh M., van Dyke T., Banchereau J. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. The Journal of Immunology. 2001;167(9):5067–5076. doi: 10.4049/jimmunol.167.9.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Williams A. S., Leung S. Y., Nath P., et al. Role of TLR2, TLR4, and MyD88 in murine ozone-induced airway hyperresponsiveness and neutrophilia. Journal of Applied Physiology. 2007;103(4):1189–1195. doi: 10.1152/japplphysiol.00172.2007. [DOI] [PubMed] [Google Scholar]
- 106.Patel B. D., Welch A. A., Bingham S. A., et al. Dietary antioxidants and asthma in adults. Thorax. 2006;61(5):388–393. doi: 10.1136/thx.2004.024935. [DOI] [PMC free article] [PubMed] [Google Scholar]


