Abstract
Objectives
To determine SARS-CoV-2-antibody prevalence in pediatric healthcare workers (pHCWs).
Design
Baseline prevalence of anti-SARS-CoV-2-IgG was assessed in a prospective cohort study from a large pediatric healthcare facility. Prior SARS-CoV-2 testing history, potential risk factors and anxiety level about COVID-19 were determined. Prevalence difference between emergency department (ED)-based and non-ED-pHCWs was modeled controlling for those covariates. Chi-square test-for-trend was used to examine prevalence by month of enrollment.
Results
Most of 642 pHCWs enrolled were 31-40years, female and had no comorbidities. Half had children in their home, 49% had traveled, 42% reported an illness since January, 31% had a known COVID-19 exposure, and 8% had SARS-CoV-2 PCR testing. High COVID-19 pandemic anxiety was reported by 71%. Anti-SARS-CoV-2-IgG prevalence was 4.1%; 8.4% among ED versus 2.0% among non-ED pHCWs (p < 0.001). ED-work location and known COVID-19 exposure were independent risk factors. 31% of antibody-positive pHCWs reported no symptoms. Prevalence significantly (p < 0.001) increased from 3.0% in April–June to 12.7% in July–August.
Conclusions
Anti-SARS-CoV-2-IgG prevalence was low in pHCWs but increased rapidly over time. Both working in the ED and exposure to a COVID-19-positive contact were associated with antibody-seropositivity. Ongoing universal PPE utilization is essential. These data may guide vaccination policies to protect front-line workers.
Keywords: SARS-CoV-2, healthcare workers, COVID-19, Emergency Department, Personal Protective Equipment (PPE), Anti-SARS-CoV-2-IgG Antibodies
Introduction
Serologic studies examining prevalence of antibodies to SARS-CoV-2 vary widely by the group evaluated. Healthcare workers (HCW) are a population of considerable interest due to their essential roles in the pandemic and increased contact with known positive cases. The Center for Disease Control and Prevention (CDC) reported over 414,339 HCWs in the United States (US) afflicted with COVID-19 by February 2021, and at least 1,380 deaths reported (CDC Tracker, accessed February 27, 2021). Reports of positivity in HCW cohorts early in the pandemic range widely from 37% in a highly affected area in Spain (Pérez-García et al., 2020) to just 1% positivity in a California community with low COVID-19 levels (Brant-Zawadzki et al., 2020). Few studies have evaluated the prevalence of SARS-CoV-2 infection in HCWs working primarily in facilities dedicated to pediatrics (pHCW) (Insúa et al., 2020, Tokareva et al., 2021).
The exposure risk of pHCWs may be disparate from HCWs in adult-focused facilities for several reasons. Most children present with mild illness or are asymptomatic and children are less likely to be hospitalized compared to adults with COVID-19 (Li et al., 2020). Because of this, children with COVID-19 symptoms are more frequently seen and treated in the outpatient setting such as the emergency departments (ED), urgent cares and outpatient clinics. Particularly during the early months of the pandemic, the milder nature of COVID-19 infection in children led some to believe that children do not spread SARS-CoV-2 or are not affected. Data released from the CDC in April 2020 demonstrated that children do acquire the disease, although with fewer symptoms and the report cautioned that children may be a source of transmission of SARS-CoV-2 (CDC Morb Mortal Wkly Rep, 2020). Prior to these data being released, utilization of PPE in outpatient pediatric settings was inconsistent, which may have increased workplace exposure in the outpatient pediatric setting. The aim of this study was to determine the prevalence of IgG antibodies to SARS-CoV-2 in pHCWs at a large pediatric healthcare system that is comprised of three children’s hospitals, and to identify what characteristics may be associated with increased prevalence. In particular, we hypothesized that ED-based pHCWs were at higher risk of infection compared to those working in other areas of the hospital.
Methods
Study design
pHCWs were enrolled into a prospective, longitudinal cohort to determine the prevalence of IgG antibodies to SARS-CoV-2 in HCWs over time at a large pediatric healthcare system in Atlanta, Georgia that cares for children and adolescents up until their 21st birthday. There are no adult patient services within this healthcare system. Cross-sectional data presented here are from baseline visits for the cohort participants, which took place from April to August, 2020. The study received Institutional Review Board approval from Emory University and Children’s Healthcare of Atlanta.
Participants
Healthcare employees and support staff of the Department of Pediatrics at Emory University School of Medicine, Children’s Healthcare of Atlanta (Children’s) and private pediatric practitioners or contractors who regularly work at Children’s facilities were eligible for study participation. pHCWs were recruited by email, by postings in the facilities, and by personal communication. Staff and providers working in the ED were targeted with additional emails and communications to increase participation levels. A healthcare worker who self-identified as regularly working at one of the institutions above were eligible for study inclusion. For staff safety, all participants were required to be asymptomatic at the time of in person visits. If a participant was symptomatic near the time of participation, he/she was rescheduled to a later date to ensure the visit was scheduled at least 14 days post-symptoms.
Procedures
At baseline, verbal or electronic informed consent was obtained and each participant completed a brief online survey that included basic demographics (age range by decade, sex, history of comorbidity), job role, primary location within the pediatric facility, number of hours worked per week, perception of SARS-CoV-2 exposure, illnesses since January 2020, travel history, documented infection with SARS-CoV-2, current symptoms, belief as to whether they had anti-SARSCoV-2 antibodies, and level of interest in participating in donating blood for convalescent serum if positive (yes/no). pHCW participants were also asked their level of anxiety experienced due to the pandemic on a Likert scale of 1–5. All participants provided up to 30 ml of blood through venipuncture; blood specimens were processed within 1 h, and serum and plasma were stored by the Children's Healthcare of Atlanta and Emory University's Children’s Clinical and Translational Discovery Core for future analysis.
Laboratory methods
Venous blood for plasma and serum was collected, processed and stored at −20 °C until analysis. Qualitative positivity and quantitative SARS-CoV-2 antibodies were determined by measuring the IgG antibody responses to the receptor binding domain (RBD) of the spike (S) protein using an enzyme-linked immunosorbent assay (ELISA) as previously described (Suthar et al., 2020).
Outcomes
The dependent measure was prevalence of past SARS-CoV-2 infection as reflected by IgG antibody seropositivity. The primary job location was used to define the main independent measure as pediatric ED-based HCWs (ED or urgent care) or non-ED based pHCWs (pediatric intensive care [PICU], general wards, specialty wards, clinical and administrative services, and operating room). Other independent measures for the analyses included month of enrollment, HCW role, average number of clinical hours worked per week in 2020, potential risks for SARS-CoV-2 infection (children in the home, domestic and international travel), prior illness or known COVID-19 exposures since January 2020, and receipt of SARS-CoV-2 PCR testing. HCWs from urgent care settings were considered to be ED-based pHCWs. Pediatric ED-based HCWs, including respiratory therapists, worked primarily in the ED, and did not work in multiple units. The level of anxiety related to COVID-19 was quantified by dichotomizing the responses to the question “The COVID-19 pandemic has increased my anxiety.” Scores of 4 and 5 were defined as “high anxiety”, while scores of 1 and 2 were considered “low anxiety.”
Hospital COVID-19 policies and patient cohorting
In this large single-healthcare system, policies around response to the SARS-CoV-2 pandemic evolved over time. The first case of COVID-19 reported in Georgia was diagnosed on March 2, 2020. COVID-19 cohorting units at Children’s for patients under investigation (PUI) with respiratory symptoms and those with confirmed COVID-19 were established during the first few months of the pandemic between March 9–June 10, 2020. These were all private rooms. Staff followed Enhanced Contact Droplet precautions that included: procedure mask (N-95 mask if aerosol-generating procedure; once more N-95s available, staff could use as their default mask for all care of these patients), eye protection, gloves, and gown. Since turn-around time for COVID-19 testing in March/April was over 72 h, the COVID-19 status was often unknown for days. The PICU did not cohort PUI, however patients with respiratory symptoms were placed in isolation, and staff utilized Enhanced Contract Droplets precautions. Within the pediatric EDs at Children’s, a universal PPE policy was implemented that included use of surgical masks and goggles for all patient-facing activities on March 21, 2020, with N95 masks utilized for traumas, resuscitations and intubations. Prior to this time, masks were not routinely worn in the pediatric ED or at triage unless a patient was placed on respiratory isolation.
Patients seen in the pediatric EDs or admitted to the hospital were not routinely screened for COVID-19; testing was performed when the outcome could potentially influence clinical decision-making, and rapid testing was not available.
Statistical analysis
Prevalence of antibodies for SARS-CoV-2 was assessed using summary statistics, and reported overall and by HCW roles, and for pediatric ED-based vs. non-ED-based HCWs. Univariate tests for differences in proportions between groups were conducted using chi-square tests. Associations between participant characteristics and SARS-CoV-2 seropositivity were assessed by calculating prevalence ratios (PR) and 95% confidence intervals (CI) from modified Poisson regression models (Barros and Hirakata, 2003). Variables that were significant in bivariate analyses were included in the final multivariable model, and adjusted PR (aPR) and 95% CI were calculated for each exposure. Analyses were carried out in SAS v.9.4 (Cary, NC) and CRAN R v.3.3 (Vienna, Austria), and statistical significance was evaluated at the 0.05 threshold.
Results
Pediatric HCW cohort demographics, job and location, potential COVID-19 risk factors and anxiety
A total of 642 pHCWs (202 ED, 440 non-ED) were enrolled, the majority of whom were 31-40 years of age, female, and physicians or nurses. Overall and stratum-specific prevalence of antibodies are summarized in Table 1 . Thirty-one percent of participants were pediatric ED-based, 42% reported a viral illness between January 2020 and date of participation and 8% underwent SARS-CoV-2 PCR testing during an illness. The majority of pHCWs (84%) believed they were at high risk of COVID-19 due to their occupation in healthcare, 99% of the cohort believed it was helpful to know their SARS-CoV-2 IgG status, and 91% would be interested in donating convalescent plasma if seropositive. In response to the survey question “The COVID-19 pandemic has increased my anxiety”, 71% reported high anxiety (Table 2 ).
Table 1.
Characteristics and risk factors of pediatric healthcare workers enrolled in a prospective cohort study of SARS-CoV-2 prevalence, Atlanta, Georgia, April–August 2020.
| Characteristic | All (n = 642) | Pediatric ED (n = 202) | Non-ED (n = 440) | p-value |
|---|---|---|---|---|
| Median Age Range (years) | 31–40 | 31–40 | 31–40 | 0.05 |
| Gender- Female | 85% | 83% | 86% | 0.41 |
| Comorbidity-Yes | 23% | 26% | 21% | 0.23 |
| IgG Results | ||||
| SeroPositive (%) | 4% | 8% | 2% | <0.001 |
| SeroNegative (%) | 96% | 92% | 98% | <0.001 |
| Job Position | ||||
| Medical doctor | 23% | 39% | 16% | <0.001 |
| NP/APP | 10% | 8% | 11% | 0.26 |
| Nurse | 33% | 37% | 31% | 0.15 |
| Respiratory Therapist | 6% | 2% | 7% | 0.02 |
| Administration | 7% | 1% | 10% | <0.001 |
| Other | 21% | 13% | 25% | <0.001 |
| Hours per week worked | ||||
| Mean ± SC | 35.0 ± 10.0 | 30.6 ± 8.1 | 37.1 ± 10.2 | <0.001 |
| Median | 36 (32–40) | 32 (26.3–36) | 40 (36–40) | |
| Children in home | 49% | 47% | 49% | 0.61 |
| 0-2yrs | 16% | 18% | 15% | 0.35 |
| 3-5yrs | 16% | 15% | 16% | 1.00 |
| 6-12yrs | 21% | 20% | 21% | 1.00 |
| 13-17yrs | 15% | 19% | 14% | 0.10 |
| 18-21yrs | 8% | 6% | 9% | 0.28 |
| Senior or high-risk in home | 17% | 17% | 17% | 0.91 |
| Travel since January 2020 | 50% | 46% | 52% | 0.17 |
| USA | 44% | 42% | 45% | 0.49 |
| International | 8% | 8% | 8% | 0.88 |
| History of viral-like symptomsa | 42% | 54% | 36% | < 0.001 |
| Known COVID-19 exposure | 31% | 40% | 27% | 0.002 |
| Believes higher risk as HCW | 84% | 94% | 80% | < 0.001 |
| SARS-CoV-2 PCR test | 8% | 13% | 6% | 0.002 |
| SARS-CoV-2 PCR positive | 1% | 2% | 0.9% | 0.15 |
| Mean (SD) COVID-19 anxiety | 3.7 ± 0.99 | 3.7 ± 1.0 | 3.7 ± 0.97 | 0.64 |
Bold for p-values highlighted significant observations.
Viral symptoms included fever, URI symptoms, sore throat, myalgias, new cough, shortness of breath, vomiting or diarrhea. ED = Emergency Department.
Table 2.
Prevalence and correlates of anti-SARS-CoV-2-IgG among pediatric healthcare workers enrolled in a prospective cohort study, Atlanta, Georgia, April-August 2020.
| ALL HCWs (N) | IgG Positive n (%) | PR (95% CI) | aPR (95% CI)a | |
|---|---|---|---|---|
| Overall | 642 | 26 (4.0) | ||
| Age (years) | ||||
| 18–30 | 132 | 7 (5.3) | Ref | |
| 31–40 | 237 | 10 (4.2) | 0.80 (0.31, 2.04) | |
| 41–50 | 144 | 6 (4.2) | 0.79 (0.27, 2.28) | |
| 51+ | 129 | 3 (2.3) | 0.44 (0.12, 1.66) | |
| Sex | ||||
| Male | 97 | 6 (6.2) | 1.69 (0.69, 4.09) | |
| Female | 545 | 20 (3.7) | Ref | |
| Work location | ||||
| Pediatric ED only | 202 | 17 (8.4) | 4.11 (1.87, 9.07) | 2.90 (1.19, 7.03) |
| Pediatric Non-ED | 440 | 9 (2.0) | Ref | Ref |
| Job Position | ||||
| Medical Doctor | 148 | 11 (7.4) | 2.45 (1.15, 5.21) | 1.72 (0.71, 4.15) |
| Other | 494 | 15 (3.0) | Ref | Ref |
| Hours worked per week | ||||
| <35 | 199 | 13 (6.5) | 2.23 (1.05, 4.71) | 1.42 (0.62, 3.24) |
| 35+ | 443 | 13 (2.9) | Ref | Ref |
| Children in home | 1.23 (0.58, 2.63) | |||
| Yes | 312 | 14 (4.5) | Ref | |
| No | 330 | 12 (3.6) | ||
| Senior/high-risk in home | 0.90 (0.31, 2.57) | |||
| Yes | 110 | 4 (3.6) | Ref | |
| No | 519 | 21 (4.0) | ||
| Travel | 0.76 (0.18, 3.22) | |||
| Any international | 53 | 2 (3.8) | 0.61 (0.26, 1.40) | |
| Domestic only | 266 | 8 (3.0) | Ref | |
| None | 323 | 16 (5.0) | 1.23 (0.58, 2.63) | |
| Exposed to known COVID+ | ||||
| Yes | 200 | 15 (7.5) | 3.01 (1.41, 6.44) | 2.72 (1.26, 5.88) |
| No | 442 | 11 (2.5) | Ref | Ref |
Bold for p-values highlighted significant observations.
Adjusted prevalence ratio (aPR) from modified Poisson regression model including the following variables: Work Location (Pediatric ED vs. Non-ED), Job Position (MD vs. other), Hours Worked and Exposure to Known COVID + Individual. ED = Emergency Department.
SARS-CoV-2 IgG-positive vs. IgG-negative pHCW cohort
The prevalence of SARS-CoV-2 seropositivity was 26 out of 642 participants (4.1%, Figure 1 ). Most of the IgG positive pHCW were physicians and nurses (73%). Only 31% of the entire cohort reported an exposure to a known COVID-19 contact. The majority of IgG-positive HCWs were pediatric ED-based with a prevalence of 8.4% vs. 2.0% in ED vs. non-ED pHCWs (p < 0.001). No PICU-based HCWs tested positive for SARS-CoV-2 antibodies. One out of 36 (2.6%) respiratory therapist in our cohort was seropositive.
Figure 1.
Anti-SARS-CoV-2-IgG endpoint titers in 26 seropositive pediatric healthcare workers enrolled in a prospective cohort study, Atlanta, Georgia, April–August 2020.
A total of 642 participants enrolled. Seroprevalence is 4.1%, with 26 pediatric healthcare workers (HCWs) testing positive for anti-SARS-CoV-2 IgG antibodies. Endpoint titers <200 are considered negative (n=616).
Thirty-five percent of IgG-positive pHCWs were tested for SARS-CoV-2 by PCR during an acute illness; 8/9 were PCR-positive. Seropositive pHCWs were more likely to report a history of viral illness since January 2020 compared to those who were IgG-negative (69% vs. 41%, p = 0.004), and had a higher exposure to a known COVID-19 positive case (58% vs. 30%, p = 0.004). There was no difference in travel history or presence of children in the household. Of the 26 IgG-positive participants, 31% reported no viral illness or symptoms since January 2020, suggesting asymptomatic COVID-19 disease. No seropositive HCW reported hospitalization, but 19% sought medical care for the acute illness, 31% described their illness as moderate and impacting daily activities in addition to staying home from work, while 2 (8%) reported severe symptoms including being bedbound for at least 1 day and/or requiring an ED visit. There was no difference in self-reported anxiety level between pHCWs in the ED vs. those outside the pediatric ED, nor was there a difference in those who were seropositive vs. negative.
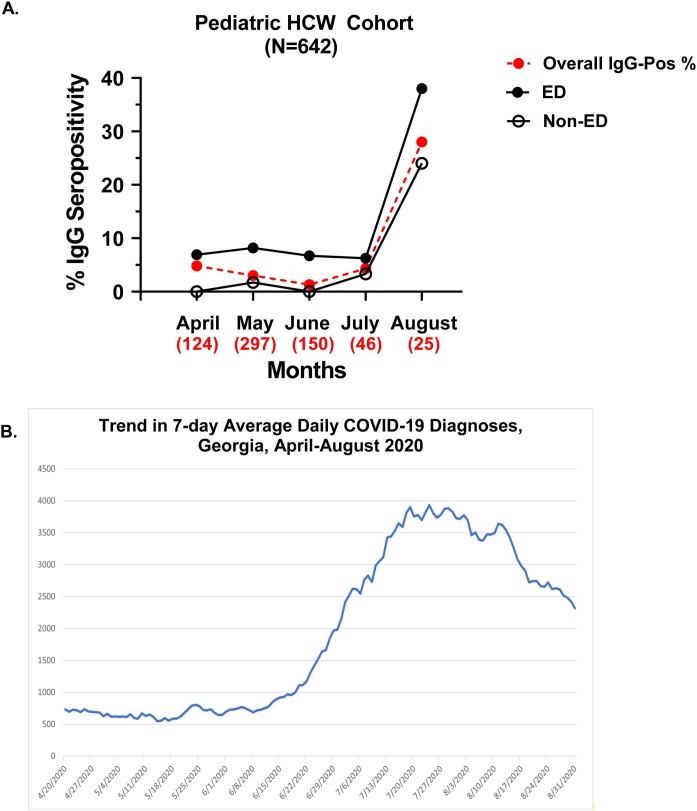
Prevalence of antibody seropositivity by month of enrollment
Seropositivity by month of pHCW participant enrollment between April–August 2020 is illustrated in Figure 2 A. Overall prevalence by month of enrollment decreased from 4.8% among those enrolled in April to 1.3% among those enrolled in June. Seropositivity among the pediatric ED participants remained stable in those enrolled in the months April through July, while an increase in IgG positivity was detected in participants enrolled in August regardless of job location. Reported COVID-19 cases in Georgia between April–August 2020 are illustrated in Figure 2B in order to demonstrate activity in the community during this same timeframe (CDC Tracker, accessed January 31, 2021).
Figure 2.
(A) Percent anti-SARS-CoV-2-IgG seropositive by month for A. 642 Children’s Healthcare of Atlanta pediatric healthcare workers enrolled in a prospective cohort study in Atlanta, Georgia and B. COVID-19 infections reported by month in Georgia, April–August 2020, and (B) Trend in 7-day average daily COVID-19 diagnoses in Georgia from April–August 2020 (Data source: Centers for Disease Control and Prevention, COVID-19 Response. COVID-19 Case Surveillance Public Data Access, Summary, and Limitations; version date: January 31, 2021) In 2A, the filled black circles represent emergency department (ED)-based pediatric healthcare workers (pHCWs) while non-ED based pHCWs are represented by opened black circles, and red filled circles represent the overall % seropositive enrolled each month. Numbers beneath each month represent the number of pHCWs enrolled that month. IgG positivity in this cohort is driven primarily by the pediatric ED-based HCWs. The first case of COVID-19 reported in Georgia was diagnosed on March 2, 2020. A Georgia statewide shelter-in-place order took effect on April 3, which was lifted May 1, 2020 for some businesses and restaurants, and lifted for medically fragile Georgians and those over 65 years old on June 12, 2020. Enforcement of the shelter-in-place order was limited, and there was no federal mask mandate in Georgia during the time of this study. Public social distancing practices liberalized over the summer, associated with a spike in community cases in July-August 2020 in Georgia. A universal PPE policy was implemented within the pediatric EDs at Children’s Healthcare of Atlanta that included use of surgical masks and goggles for all patient-facing activities on March 21, 2020, with N95 masks utilized for traumas, resuscitations and intubations.
Discussion
In this prospective study of SARS-CoV-2 IgG antibodies in pHCWs, the overall prevalence of positivity was 4.1%. Factors associated with seropositive status included working in the pediatric ED, fewer work hours, being a physician and known exposure to a COVID-19 positive person; ED-based location and a known COVID-19 exposure remained independent variables associated with IgG antibody positivity in multivariate regression modeling.
Our observed prevalence of antibodies in pHCWs fell within ranges observed in other studies of HCWs assessed at a similar point in time. For example, a study at the University Hospital Essen, a tertiary care hospital in Germany, reported 1.6% seroprevalence among 316 HCWs (Korth et al., 2020). Seroprevalence was 7.4% among a small cohort of 202 HCWs in an Italian hospital in Milan (Sotgiu et al., 2020), and just 1% in a HCW cohort in California (Brant-Zawadzki et al., 2020). A very small study of physicians working in a children’s hospital in Argentina reported just 1% seropositivity (Insúa et al., 2020). A larger study of 530 pHCWs at an urban children’s hospital in the Seattle, Washington also detected a low anti-SARS-CoV-2 IgG prevalence of only 0.9% (May 4–June 2, 2020) early in the pandemic, which is more than 4-fold lower than that identified in the Atlanta cohort. However, 2.3% of participants working in the Seattle pediatric ED tested positive, whereas only 0.01% of non-ED personnel tested positive (Tokareva et al., 2021), supporting the premise of the pediatric ED as a likely higher-risk clinical location. Given that the first case of SARS-CoV-2 infection in the US was reported on January 21, 2020 just outside Seattle, Washington, Seattle Children’s Hospital activated a hospital incident command structure on January 22 in response to the first US case occurring regionally. The pediatric ED operations committee began pandemic preparation on February 12, 2020 (Hartford et al., 2020). We speculate that implementation of an early response to the pandemic that rapidly incorporated new ED and hospital based processes that included extra PPE for staff may be a factor that contributed to a lower seroprevalence among HCWs in Seattle compared to Atlanta.
Few reports break down HCW work location within the hospital or clinical specialty. One small study looking at 152 symptomatic HCWs and their household contacts in the United Kingdom found 25% of the HCWs were positive for SARS-CoV-2 by PCR assay. Of those identified with COVID-19, respiratory staff nurses were most frequently positive (35%) (Bird et al., 2020). Information on access to and utilization of PPE was not included in the report. Jeremias et al. identified a 9.8% positivity rate for SARS-CoV-2 IgG antibodies among 1699 HCWs tested in a large tertiary community hospital in NY, which was a lower prevalence of infection compared to the general public in the surrounding community (16.7% for Long Island and 21.2% for New York City). They also found no meaningful difference in prevalence with respect to job title or work area, including the ED (Jeremias et al., 2020).
In contrast, the seropositivity of approximately 4% in our pHCW cohort was higher than a general population surveillance of the Atlanta region (Biggs et al., 2020), and was predominantly driven by ED-based HCWs. In a household-based surveillance survey conducted by the CDC during April 28-May 3, 2.7% of 696 participants were seropositive (weighted seroprevalence 2.5% (CI 95%: 1.4 – 4.5) (Biggs et al., 2020). Although the first case of COVID-19 reported in Georgia was diagnosed on March 2, 2020, 7% of the ED-based pHCWs tested were already seropositive by the end of April, compared to zero cases outside the ED in our cohort. Development of SARS-CoV-2 IgG antibodies in pediatric ED-based HCWs so early in the pandemic also suggests that the virus was likely circulating for some time before the pandemic risk was appreciated, during which stage pediatric ED-based HCWs may have been exposed to infectious children with unrecognized COVID-19, or their infectious parent, family member or caretaker. Use of universal PPE was not standard practice in our pediatric EDs before the Georgia statewide shelter-in-place order took effect on April 3, 2020. High volumes of children with mild symptoms are commonly seen in pediatric facilities. Low acuity “fast track” patients make up 40–60% patient volume across the three Children’s EDs included in this analysis and represent pediatric facilities, that combined, evaluate over 240,000 ill or injured children annually. With over a 4-fold increased risk of seropositivity compared to their non-ED based colleagues, these data show that pediatric ED-based HCWs are at increased risk of viral infection with SARS-CoV-2 compared to other pHCWs. These data add to growing evidence that children likely spread the novel coronavirus, and HCWs in the ED may be at higher risk for exposure and infection. These data also have implications for frontline pHCWs during future epidemics regarding workplace safety.
PPE may contribute to the variation in HCW positivity seen across various studies. Chen et al. from Nanjing, China reported a high IgG antibody positivity rate of 17% in 105 HCWs known to be exposed to patients with COVID-19. Not all HCWs utilized PPE in this study. The authors identified a lower risk of seroconversion among HCWs that was closely related to wearing a facemask (Chen et al., 2020). In our study, the upward trend in seropositivity noted in July among non-ED pHCWs and in August among all pHCWs in our study likely reflects escalation in community spread as local businesses began to re-open and social activities increased, correlating with an increase in acute cases reported in Georgia following the 4th of July weekend (CDC Tracker, accessed December 10, 2020) We hypothesize that exposure risk in the workplace early in the pandemic mitigated by universal PPE utilization transitioned to greater risk of exposure in the community as the prevalence of COVID-19 rose briskly in Atlanta (CDC Tracker, accessed February 27, 2021).
By professional classification, physicians were found to be at highest risk, however this association disappeared in multivariate analysis, possibly driven by the higher number of physicians in the pediatric ED-based participant group. Respiratory therapy could be considered a high-risk profession given the infectious exposures resulting from aerosolizing procedures. A report of high infection rates among respiratory nurses in the United Kingdom supports this notion (Bird et al., 2020). However, only 1/36 (2.6%) respiratory therapist in our cohort was seropositive, similar to the prevalence among non-ED based pHCWs. In contrast to pediatric ED providers at our facilities, most respiratory therapists at our facilities typically wore PPE as standard practice when working with children in the hospital even before the pandemic, which may have provided increased protection (Ferioli et al., 2020, Sommerstein et al., 2020). It is interesting to note that no staff from the PICU, another closed unit with potentially high COVID-19 exposure risk, were found to be seropositive even though they made up 10% of our pHCW cohort. There are several possible explanations for the difference including differences in typical pre-pandemic PPE utilization and differences in patient exposure levels between inpatient and outpatient settings. Although recent studies suggest that children are just as likely as adults to become infected with the virus, they have fewer symptoms and less severe disease (Zimmermann and Curtis, 2020), so they may present to acute care settings with COVID-19 symptoms, while only a small portion will require hospitalization (Sinha et al., 2020, Zimmermann and Curtis, 2020). COVID-19 testing of pediatric patients at our facilities was limited at the time of this study, so most HCWs caring for a child infected with SARS-CoV-2 were unaware of their diagnosis.
Working less than 35 hours per week was another univariate risk factor that fell out of the multivariate model, leaving only ED-based work location and known exposure to COVID-19 as significant risk factors. The association with working fewer hours was likely driven by the ED-based physician seropositivity as this group commonly works less than 30 clinical hours per week. Travel and presence of children in the household were not predictors of seropositivity, although these factors are often assumed to be risks.
Testing for SARS-CoV-2 by PCR during acute illness was infrequent among our participants, with only 8% of the cohort tested for SARS-CoV-2, despite 42% reporting viral symptoms and flu-like illness since January 2020. Even among those who were identified as seropositive through this surveillance study, only about a third of staff were PCR tested during an acute illness, with 8 out of 9 tested found to be PCR-positive. Interestingly, 31% of seropositive HCWs reported no history of illness in 2020, representing an asymptomatic group similar to what has been documented in the literature (Oran and Topol, 2020).
HCWs who are exposed to SARS-CoV-2 in the workplace may be concerned about inadvertently bring the virus home to their families, adversely impacting household members. This could potentially generate fear and anxiety among HCWs not typically experienced previously in the US, although the psychosocial impact of past epidemic/pandemic outbreaks on HCWs is well described in the literature (Preti et al., 2020). Our study confirms this suspicion, with 71% of our pHCW cohort reporting high anxiety during the current pandemic. Anxiety was similarly high between ED-based and non-ED HCWs, although 94% of pediatric ED-based HCWs reported a belief that they were at high risk of COVID-19 compared to 80% of HCWs located outside the ED. In a large cohort of over 1200 HCWs from 34 hospitals in China, 72% of HCWs reported distress and 34–50% reported symptoms manifesting as stress, anxiety, insomnia and/or depression.(Lai et al., 2020) Evidence is emerging that the psychological toll of the COVID-19 pandemic is being felt by HCWs worldwide (Chew et al., 2020). HCWs are known for their stamina and emotional resilience in the workplace, however COVID-19 has changed the playing field. Pressures of the pandemic may add to the long-recognized problem of physician burnout (Yates, 2020), and emergency medicine physicians in particular are already at high-risk (Lim et al., 2020). Additional resources may be needed to address this growing issue among HCWs in order to preserve mental health and resilience, particularly among front-line workers (Santarone et al., 2020).
This study has several limitations. First, this was a convenience sample, and the antibody prevalence of pHCWs who chose to participate may be systematically different from those who chose not to participate. Second, this study was not designed to identify the source of infection for the pHCWs. Adult family members, parents and/or caregivers of the children seeking care were also a potential source of infection. Additionally, it is possible that pHCWs were exposed to SARS-CoV-2 by household contacts, through domestic or international travel, or infected via community spread rather than work exposure. However, neither children in the home nor travel between January-March 2020 were associated with seropositivity. The significant difference in seropositivity between ED-based pHCWs vs. those outside the ED supports the hypothesis that spread by children evaluated in the ED occurred early in the pandemic. Alternatively, it is possible that the high prevalence of SARS-CoV-2 IgG antibodies in ED-based pHCWs is because of spread among staff working closely together in an environment with a limited ability to socially distance within the ED (Cave, 2020). However, the consistently high prevalence in ED-based pHCWs across three separate pediatric hospitals within the Children’s network makes this less likely. Given this study takes place in a region that acquired high SARS-CoV-2 activity, the conclusions may not be generalizable to all healthcare settings, particularly in communities with lower contagion prevalence. Also, although pHCWs were recruited from a large pediatric healthcare system, a small percentage of pHCWs in the network was tested, which could lead to selection bias and an overestimate or underestimate of the true prevalence. Finally, false positive or false negative SARS-CoV-2 IgG antibody test results could impact the integrity of the data, however the method used in this study has demonstrated a >96% sensitivity and specificity (Suthar et al., 2020).
The study has several strengths. This is a large, prospective cohort and represents many departments and position types among the pHCWs in our institutions. To our knowledge, it is the largest report on pediatric-specific HCWs to date. The participant enrollment began near the beginning of the epidemic, thus allowing us to characterize both the early seropositivity prevalence and the change over time as the level of COVID-19 has fluctuated within our region. These data also highlight increased risk to pediatric frontline workers, and the psychological toll of this pandemic on HCWs that is likely not unique to our institution.
In conclusion, we found that the seropositivity prevalence for antibodies to SARS-CoV-2 in pHCWs was higher compared to reported levels in our community early in the pandemic, while the percent of positive participants increased in those pHCWs enrolled subsequent to a time of increased community spread (July 2020, CDC Tracker, accessed January 31, 2021). Both known exposure to COVID-19 and working in the pediatric ED were independently associated with seropositivity, while travel and having children at home were not. In the months after the pandemic began, most healthcare facilities switched to universal PPE, including ours. Ongoing access to PPE should be a top priority for all HCWs (Ferioli et al., 2020, Sommerstein et al., 2020, Huang et al., 2020) including those working in pediatric facilities. These results have implications for ongoing public health recommendations for ensuring workplace safety.
Contributions
CRM and MBV designed the research questions, co-wrote the study protocol, obtained funding, insured compliance and regulatory oversight, analyzed and interpreted the data and wrote the first draft of the manuscript. PS, WKL, MS, JW helped designed research questions, assisted with data analysis, and assisted with writing the manuscript. GM, MZ helped with data analysis, and critically reviewed the manuscript. TS, SH, AC-G, JF helped designed research question, assisted data analysis, and critically reviewed the manuscript. BH, SI assisted with the study protocol design, obtained and processed biological samples, and reviewed the manuscript. SM, DK, PB, TH, CC, RM and RK obtained informed consent/assent, insured compliance and regulatory oversight, analyzed data, and reviewed the manuscript. RC assisted with design of the research question, helped write the protocol, assisted with obtaining funding, insured compliance and regulatory oversight, and reviewed the manuscript.
Funding source
This work was supported in part by the Wilbur and Hilda Glenn Family Foundation, a donation by Michael and Natalia Beinenson, by the E mory University Woodruff Health Sciences Center Synergy Award, an Emory COVID-19 CUREaward made possible by philanthropic support from the O. Wayne Rollins Foundation and the William Randolph Hearst Foundation, and by a generous donation by the Scott Hudgens Family Foundation. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Conflicts of interests
We declare no conflicts of interests. Claudia R. Morris, MD, is the inventor or co-inventor of several UCSF-Benioff Children’s Hospital Oakland patents/patent-pending applications that include nutritional supplements, and biomarkers of cardiovascular disease related to arginine bioavailability, is an inventor of several Emory University School of Medicine patent applications, and is a consultant for Pfizer, Hoffmann-La Roche Ltd. and CSL Behring. Miriam B. Vos, MD, MSPH is a consultant for Boehringer Ingelheim, Bristol Myers Squibb, Intercept, Eli Lilly, Novo Nordisk, Target Pharmasolutions and has research funding from Bristol Myers Squibb and Target Pharmasolutions.
Ethical approval
The study received Institutional Review Board approval from Emory University and Children’s Healthcare of Atlanta; verbal or electronic informed consent was obtained from all participants.
Acknowledgements
The authors acknowledge the clinical staff, nurses and physicians working at the Emory University and Children’s Healthcare of Atlanta facilities and in the Emory Children’s Center Research Unit, who contributed to the success of this study. The authors also recognize the significant contributions of the participants, without whom this study would not have been possible. This study was funded in part by the Wilbur and Hilda Glenn Family Foundation, a generous donation by Michael and Natalia Beinenson, by the Woodruff Health Sciences Center Synergy Award, an Emory COVID-19 CURE award made possible by philanthropic support from the O. Wayne Rollins Foundation and the William Randolph Hearst Foundation, and by a generous donation by the Scott Hudgens Family Foundation. Sample processing for the HCW cohort was supported by the Children's Healthcare of Atlanta and Emory University's Children’s Clinical and Translational Discovery Core. The authors would like to acknowledge the support and contributions from Children's Healthcare of Atlanta and Emory University Pediatric Biostatistics Core.
References
- Barros A.J., Hirakata V.N. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs H., Harris J., Breakwell L., Dahlgren S., Abedi G.R., Szablewski C.M. Estimated community seroprevalence of SARS-CoV-2 antibodies — two Georgia counties, April 28–May 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(29):965–970. doi: 10.15585/mmwr.mm6929e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird P., Badhwar V., Fallon K., Kwok K.O., Tang J.W. High SARS-CoV-2 infection rates in respiratory staff nurses and correlation of COVID-19 symptom patterns with PCR positivity and relative viral loads. J Infect. 2020;81(3):452–482. doi: 10.1016/j.jinf.2020.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brant-Zawadzki M., Fridman D., Robinson P.A., Zahn M., Chau C., German R. SARS-CoV-2 antibody prevalence in health care workers: Preliminary report of a single center study. PLoS One. 2020;15(11) doi: 10.1371/journal.pone.0240006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave E. COVID-19 super-spreaders: definitional quandaries and implications. Asian Bioeth Rev. 2020:1–8. doi: 10.1007/s41649-020-00118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CCD Tracker. Trends in number of COVID-19 cases and deaths in the US reported to CDC, by state/territory; Available from: https://covid.cdc.gov/covid-data-tracker/#trends_dailytrendscases. [Accessed 31 January 2021].
- CDC Tracker. Cases & deaths among healthcare personnel; Available from: https://covid.cdc.gov/covid-data-tracker/#health-care-personnel. [Accessed 27 February2021].
- CDC Morb Mortal Wkly Rep Coronavirus disease 2019 in children — United States, February 12–April 2, 2020. Morb Mortal Wkly Rep (MMWR) 2020;69(14):422–426. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Tong X., Wang J., Huang W., Yin S., Huang R. High SARS-CoV-2 antibody prevalence among healthcare workers exposed to COVID-19 patients. J Infect. 2020;81(3):420–426. doi: 10.1016/j.jinf.2020.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew N.W.S., Lee G.K.H., Tan B.Y.Q., Jing M., Goh Y., Ngiam N.J.H. A multinational, multicentre study on the psychological outcomes and associated physical symptoms amongst healthcare workers during COVID-19 outbreak. Brain Behav Immun. 2020;88:559–565. doi: 10.1016/j.bbi.2020.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferioli M., Cisternino C., Leo V., Pisani L., Palange P., Nava S. Protecting healthcare workers from SARS-CoV-2 infection: practical indications. Eur Respir Rev. 2020;29(155) doi: 10.1183/16000617.0068-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartford E.A., Keilman A., Yoshida H., Migita R., Chang T., Enrequez B. Pediatric emergency department responses to COVID-19: transitioning from surge preparation to regional support. Disaster Med Public Health Prep. 2020 doi: 10.1017/dmp.2020.197. online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Liu F., Teng Z., Chen J., Zhao J., Wang X. Care for the psychological status of frontline medical staff fighting against COVID-19. Clin Infect Dis. 2020;71(12):3268–3269. doi: 10.1093/cid/ciaa385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insúa C., Stedile G., Figueroa V., Hernández C., Svartz A., Ferrero F. Seroprevalence of SARS-CoV-2 antibodies among physicians from a children’s hospital. Arch Argent Pediatr. 2020;118(6):381–385. doi: 10.5546/aap.2020.eng.381. [DOI] [PubMed] [Google Scholar]
- Jeremias A., Nguyen J., Levine J., Pollack S., Engellenner W., Thakore A. Prevalence of SARS-CoV-2 infection among health care workers in a tertiary community hospital. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.4214. online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korth J., Wilde B., Dolff S., Anastasiou O.E., Krawczyk A., Jahn M. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol. 2020;128:104437. doi: 10.1016/j.jcv.2020.104437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J., Ma S., Wang Y., Cai Z., Hu J., Wei N. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA network open. 2020;3(3) doi: 10.1001/jamanetworkopen.2020.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Zhang S., Zhang R., Chen X., Wang Y., Zhu C. Epidemiological and clinical characteristics of COVID-19 in children: a systematic review and meta-analysis. Front Pediatr. 2020;8:591132. doi: 10.3389/fped.2020.591132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R., Van Aarsen K., Gray S., Rang L., Frizpatrick J., Fischer L. Emergency medicine physician burnout and wellness in Canada before COVID19: a national survey. CJEM. 2020;22(5):603–607. doi: 10.1017/cem.2020.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oran D.P., Topol E.J. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med. 2020;173(5):362–367. doi: 10.7326/M20-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-García F., Pérez-Zapata A., Arcos N., De la Mata M., Ortiz M., Simón E. SARS-CoV-2 infection among hospital workers of one of the most severely affected institutions in Madrid, Spain: a surveillance cross-sectional study. Infect Control Hosp Epidemiol. 2020:1–28. doi: 10.1017/ice.2020.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preti E., Di Mattei V., Perego G., Ferrari F., Mazzetti M., Taranto P. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence. Curr Psychiatry Rep. 2020;22(8):43. doi: 10.1007/s11920-020-01166-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarone K., McKenney M., Elkbuli A. Preserving mental health and resilience in frontline healthcare workers during COVID-19. Am J Emerg Med. 2020;38(7):1530–1531. doi: 10.1016/j.ajem.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha I.P., Harwood R., Semple M.G., Hawcutt D.B., Thursfield R., Narayan O. COVID-19 infection in children. Lancet Respir Med. 2020;8(5):446–447. doi: 10.1016/S2213-2600(20)30152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerstein R., Fux C.A., Vuichard-Gysin D., Abbas M., Marschall J., Balmelli C. Risk of SARS-CoV-2 transmission by aerosols, the rational use of masks, and protection of healthcare workers from COVID-19. Antimicrob Resist Infect Control. 2020;9(1):100. doi: 10.1186/s13756-020-00763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotgiu G., Barassi A., Miozzo M., Saderi L., Piana A., Orfeo N. SARS-CoV-2 specific serological pattern in healthcare workers of an Italian COVID-19 forefront hospital. BMC Pulm Med. 2020;20(1):203. doi: 10.1186/s12890-020-01237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthar M.S., Zimmerman M.G., Kauffman R.C., Mantus G., Linderman S.L., Hudson W.H. Rapid generation of neutralizing antibody responses in COVID-19 patients. Cell Rep Med. 2020;1(3):100040. doi: 10.1016/j.xcrm.2020.100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokareva Y., Englund J.A., Dickerson J.A., Brown J.C., Zerr D.M., Walter E. Prevalence of healthcare and hospital worker SARS-CoV-2 IgG antibody in a pediatric hospital. Hosp Pediatr. 2021;11(3):e48–e53. doi: 10.1542/hpeds.2020-003517. [Epub 2020 Dec 23] [DOI] [PubMed] [Google Scholar]
- Yates S.W. Physician stress and burnout. Am J Med. 2020;133(2):160–164. doi: 10.1016/j.amjmed.2019.08.034. [DOI] [PubMed] [Google Scholar]
- Zimmermann P., Curtis N. COVID-19 in children, pregnancy and neonates: a review of epidemiologic and clinical features. Pediatr Infect Dis J. 2020;39(6):469–477. doi: 10.1097/INF.0000000000002700. [DOI] [PMC free article] [PubMed] [Google Scholar]