Abstract
Tissue engineering combines principles of engineering and biology to generate living tissue equivalents for drug testing, disease modeling, and regenerative medicine. As techniques for reprogramming human somatic cells into induced pluripotent stem cells (iPSCs) and subsequently differentiating them into cardiomyocytes and other cardiac cells have become increasingly efficient, progress toward the development of engineered human cardiac muscle patch (hCMP) and heart tissue analogs has accelerated. A few pilot clinical studies in patients with post-infarction LV remodeling have been already approved. Conventional methods for hCMP fabrication include suspending cells within scaffolds, consisting of biocompatible materials, or growing two-dimensional sheets that can be stacked to form multilayered constructs. More recently, advanced technologies, such as micropatterning and three-dimensional bioprinting, have enabled fabrication of hCMP architectures at unprecedented spatiotemporal resolution. However, the studies working on various hCMP-based strategies for in vivo tissue repair face several major obstacles, including the inadequate scalability for clinical applications, poor integration and engraftment rate, and the lack of functional vasculature. Here, we review many of the recent advancements and key concerns in cardiac tissue engineering, focusing primarily on the production of hCMPs at clinical/industrial scales that are suitable for administration to patients with myocardial disease. The wide variety of cardiac cell types and sources that are applicable to hCMP biomanufacturing are elaborated. Finally, some of the key challenges remaining in the field and potential future directions to address these obstacles are discussed.
Keywords: tissue engineering, cardiac patch, myocardium, heart failure, myocardial infarction, regenerative medicine, cardiac regeneration and remodeling
Introduction
Despite advancements in preventive medicine, cardiovascular disease (CVD) remains a leading cause of morbidity and mortality worldwide (1, 2) with estimated 17.9 million people died of cardiovascular disease in 2016, accounting for 31% of all deaths globally (3). The molecular and cellular basis for progressive heart failure is the result of the inability of damaged and apoptotic myocytes to be replaced. The regenerative capacity of mammalian hearts declines rapidly after birth, and <1% of cardiomyocytes (CMs) in the hearts of adult humans are replaced each year (4); thus, myocardial injury leads to adverse cardiac remodeling and fibrosis as the injured myocardium is replaced by fibrotic scar tissue (5, 6). The only available procedure for end-stage patients is whole-heart transplantation which is restricted by an inadequate supply of donors. Therefore, alternative strategies for limiting post-injury cardiac remodeling and remuscularizing the damaged myocardium are urgently needed (7, 8). Administration of exogenous stem cells was initially considered a promising approach (9). The cells were expected to differentiate into CMs after transplantation (10, 11); however, the results from subsequent preclinical and clinical studies indicated that the benefits were only marginal and likely attributable to the cells' paracrine activity, because the proportion of cells that were retained and survived at the site of injury (i.e., the engraftment rate) was drastically low (12, 13).
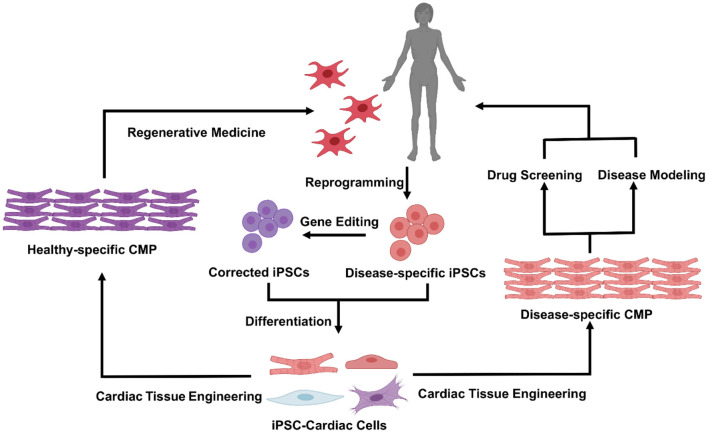
Tissue engineering combines the principles of engineering and life sciences to better understand the structure-function relationships in normal and pathological tissues and to generate living tissue equivalents for drug testing (14, 15), disease modeling (16–18), and regenerative medicine [(6, 19, 20); Figure 1]. In the cardiovascular field, the first proof-of-concept study was conducted in 1997, when embryonic chick CMs were suspended in collagen solution, and the mixture subsequently solidified and contracted coherently between two glass tubes (21). With the emergence of techniques for reprogramming human somatic cells into induced pluripotent stem cells (iPSCs) and differentiating them into CMs (22) and other cardiac cells, the field has progressed to the development of engineered human cardiac muscle patch (hCMP) constructs (6). These engineered patch constructs are often associated with higher rates of engraftment and appear to support the injured myocardium more effectively than transplanted cells (2, 23). Nevertheless, vascularization of the hCMP (either during manufacture or via the infiltration of native vessels after transplantation) is not extensive and efficient enough to support the high metabolic demand of the heart (13, 24). Consequently, the thickness/dimensions of most hCMPs are limited to just a few hundreds of micrometers (13, 25). In this review, we discuss many of the most recent advancements in cardiac tissue engineering, with a primary focus on techniques for generating thicker and more integrative hCMP systems.
Figure 1.
Cardiac tissue engineering and its applications. Patient specific iPSC can be derived by reprogramming of somatic cells from the patient, in healthy vs. diseased states, and used to generate a variety of functional cardiovascular cells. Incorporation of cells within specifically tuned 3D biomaterial systems will enable fabrication of the human cardiac muscle patch (hCMP) that could be used either in a variety of in vitro applications [drug screening and disease modeling (Right)], or as cardiac patch for in vivo regenerative therapies (Left).
Cell Types and Sources for hCMP Fabrication
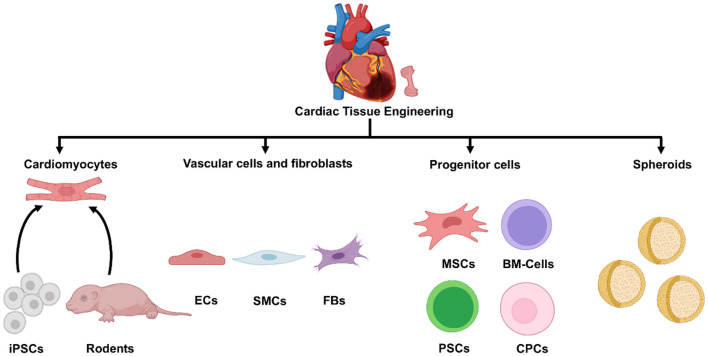
CMs are the fundamental contractile units of the myocardium and occupy 70–85% of myocardial volume in adult mammals (26); thus, many investigations of cell therapy have been conducted with CMs alone, either as dissociated cells or contiguous sheets (27, 28). However, hCMPs are designed to comprehensively recapitulate the physical structure and signaling pathways present in native heart tissue (13, 29) and, therefore, are typically composed of multiple cardiac cell types, including CMs, endothelial cells (ECs), smooth muscle cells (SMCs), and cardiac fibroblasts (6, 26, 30). Other cell types or clusters of multiple cell types (e.g., progenitor cells and spheroids) have also been incorporated into cardiac patches and evaluated in preclinical models of myocardial injury (Figure 2).
Figure 2.
Cell sources for cardiac tissue engineering. A variety of cell types can be used in tissue engineered cardiac constructs, including cardiomyocytes derived from iPSCs or isolated from rodent hearts, cardiac vascular cells and fibroblasts, different progenitor and stem cells, and various spheroids. iPSC, induced pluripotent cells; ECs, endothelial cells; SMCs, smooth muscle cells; FBs, fibroblasts; MSCs, mesenchymal stem cells; BM-cells, bone marrow-cells; PSCs, pluripotent stem cells; CPCs, cardiac progenitor cells.
Cardiomyocytes (CMs)
Healthy adult human CMs were largely unavailable for early hCMP studies due to scarcity of healthy heart donors and their non-proliferative phenotype. Thus, most of what we have explored and learned about the structural and functional properties of engineered cardiac tissues was initially investigated in experiments using primary rodent CMs (31). Pioneering work with fetal CMs from 15-day-old mouse embryos demonstrated that the cells were engrafted and survived after administration into the mouse hearts (32). Rhythmically contracting hydrogels were generated by plating neonatal rat heart cells on collagen, which enabled researchers to study how factors, such as cell density and collagen concentration, influenced contractile activity (33) and to demonstrate that these cellular constructs generated electrocardiography (ECG)-like potentials (34). Subsequent studies confirmed that patches composed of fetal rat ventricular cells and gelatin could survive and continue to contract when implanted subcutaneously in the adult rat legs. The engineered graft formed junctions with native heart cells when delivered to the scarred region of cryoinjured hearts, but whether the treatment could improve cardiac function remained uncertain (35). Patches consisting of fetal rat cardiac cells suspended in an alginate scaffold were among the first to preserve measures of cardiac function and impede adverse cardiac remodeling when administered to infarcted rat hearts (36).
Embryonic stem cells (ESCs) and iPSCs are the most readily available sources of human-lineage CMs, because they can proliferate indefinitely and be differentiated into cells of different lineages (37). The first human vascularized, contracting hCMP was generated by combining CMs and ECs derived from human ESCs with mouse embryonic fibroblasts in porous sponges composed of 50% poly-l-lactic acid (PLLA) and 50% polylactic-glycolic acid (PLGA) (38). Experiments in both rodent (19, 39, 40) and swine (23) models of myocardial injury suggest that iPSC-based hCMPs are associated with higher levels of cell survival and engraftment than those associated with iPSC-based cell injection, and that the cells' paracrine activity can be modestly beneficial. Both ESC- and iPSC-derived CMs are, however, structurally and functionally more similar to fetal or neonatal cells than to the adult mature CMs. This could hinder downstream applications of hCMPs composed of immature PSC-CMs. The electrical immaturity of PSC-CMs may induce ventricular arrhythmias as demonstrated in non-human primate models, raising a safety concern for clinical translation (41, 42). Therefore, various methods for improving stem cell-derived CM maturity before transplantation, including electrical stimulation, and treatment with neurohormonal factors, have been frequent topics of research (43–46). hCMP maturation can be influenced by the purity of the initial CM population, as evidenced by increases in conduction velocity and contractile force (44). Further, mechanical conditioning of cardiac patches has been shown effective in maturation and functional improvement of encapsulated CMs. For instance, hCMPs consisting of a collagen matrix and CMs differentiated from mouse ESCs, more closely resembled native myocardial tissue after undergoing 7 days of in vitro cyclic stretching (47).
Cardiac Vascular Cells and Fibroblasts
Cardiac ECs and SMCs not only promote the vascularization and survival of transplanted hCMPs but are also key mediators of the signaling mechanisms that regulate CM activity (23, 48, 49). Cardiac fibroblasts can further improve CM survival by remodeling the extracellular matrix (ECM) and releasing cytoprotective paracrine factors (6, 9, 50–52). Except for providing a reliable source of CMs, hiPSCs demonstrate great potential in offering vascular cells and fibroblasts. Robust protocols for differentiating hiPSCs to ECs (53), SMCs (50), and cardiac fibroblasts (54, 55) have been established. The inclusion of ECs and stromal cells, combined with uniaxial mechanical stimulation during manufacturing, has been shown to improve the hCMP maturity (56). However, early protocols for hiPSC-EC differentiation are not efficient and the phenotype of generated hiPSC-ECs remain rather unstable. Refined EC differentiation protocols, utilizing spatiotemporal 3D environments, have prolonged the maintenance of EC phenotype to up to 4 weeks (57). With deeper insights into the signaling pathways affecting cardiac cell differentiation, novel protocols for EC and SMC differentiation have yielded efficiencies exceeding 80% within 6-day time periods (58). Great efforts have also been devoted to deriving arterial EC from hiPSC, which demonstrated arterial-specific functional characteristics unlike generic ECs, thus offering reliable cell sources for hCMP (59). Cardiac fibroblasts have been successfully differentiated from hPSCs, which resembled native cardiac fibroblasts in morphology, gene expression, and proliferation. Notably, hPSC-derived cardiac fibroblasts could generate three-dimensional (3D) ECM scaffolds and their co-culture with hPSC-CMs could increase action potential propagation rate compared to co-culture with dermal fibroblasts (60). Despite development of refined protocols for generation of vascular cells and fibroblasts of arterial lineage, the optimal combination and proportion of cell types for recapitulating the complex 3D environment of native heart tissue continues to be an active area of research (52, 61–64).
Pluripotent Stem Cells
Pluripotent Stem Cells (PSCs), including ESCs and iPSCs, have revolutionized the field of cardiac tissue engineering by providing a platform of unlimited numbers and types of cells due to their ability to self-renew indefinitely and the plasticity to differentiate into any type of cells (6, 45). ESCs were initially derived from inner cell mass of developing blastocyst (65), thus raising ethical issues due to destruction of fertilized human embryos. ESCs could also be collected via parthenogenesis which alleviates the ethical issues (66). In contrast, iPSCs were initially reprogrammed from somatic cells via overexpression of four transcription factors, Oct4 (octamer-binding transcription factor 4), Sox2 (sex determining region Y-box 2), Klf4 (KLF family of transcription factor 4), and Myc (67). Different combinations of transcription factors, such as Oct4, Sox2, Nanog, and Lin28 (68) or only Oct4 and Sox2 (69) can be also used to generate iPSCs. Despite the controversy on immunogenicity of autologous iPSCs (70–73), devoid of ethical issues qualifies them as one of the most promising cell sources for cardiac tissue engineering.
PSCs have revolutionized biomedicine by providing robust platforms for regenerative medicine, drug screening, and disease modeling, all requiring reproducible and efficient protocols for differentiating PSCs to CMs. Initially, contractile CMs were spontaneously differentiated from hESCs in 3D embryoid bodies (EBs) with low efficiencies (74–76). These early protocols suffered from line-to-line variability, inclusion of undefined components, and heterogeneous EB sizes (77, 78). To advance the yield and purity of CM differentiation protocols, in-depth knowledge of the pathways involved in embryonic heart developments was utilized to optimize the process. CM differentiation stages typically involve early mesendoderm priming, cardiac progenitor specification, and differentiation into CM subtypes such as ventricular and atrial like phenotypes (79–81). Modulation of TGFβ signaling superfamily via serial application of activin A and bone morphogenetic protein 4 (BMP4) yielded >30% CMs (82); manipulation of Activin/Nodal and BMP4 signaling pathways could efficiently induce cardiac mesoderm, resulting >60% CMs in mouse PSCs and >50% in human PSCs (83). Robust CM differentiation protocols have been developed by mimicking biphasic pattern of WNT pathway with chemical compounds. Temporal modulating Wnt pathway via GSK inhibitor CHIR99021 and Wnt inhibitor IWP2 generated 80–98% CMs (84). Suspension culture in stirred tank bioreactors was utilized to upscale hPSC expansion and lineage differentiation (85, 86). Efforts have been made to develop suspension culture based hPSC-CM production with good manufacturing practice standards (79, 87). Pioneering works of hCMP with PSC-CMs have laid foundation for optimization of manufacturing methods and demonstrated beneficial efficacy after transplantation into animal models with higher engraftment and survival rate than those associated with iPSC-based cell injection (38, 47, 56). Notably, hCMP fabrication typically incorporates PSC-derived cardiac cells with refined protocols to differentiate PSC into ECs, SMCs, and fibroblasts which benefit hCMPs via pre-vascularization and secretion of ECM proteins (50, 52).
Progenitor Cells
The results from early clinical trials suggested that the modest benefits associated with transplanted bone marrow-derived cells and cardiac progenitor cells (CPCs) occurred via the cells' paracrine activity, rather than by directly repairing the damaged heart tissue (30). Nevertheless, bone marrow mesenchymal stem cells (MSCs) have been suspended in 3D hydrogels and tested in a rat model of myocardial infarction (MI) (88). The development of a program for generating human ESC-derived CPCs under Good Manufacturing Practice (GMP) conditions (89) led to the first clinical trial of hESC-CPCs, in which the cells were administered by suspending them in fibrin and suturing the patch to the surface of the infarcted heart. Heart function improved symptomatically and no safety concerns were observed 3 months after transplantation (90). Further research was conducted to investigate the efficacy of ESC-derived cardiovascular progenitors administered in a fibrin patch in six patients with severe ischemic left ventricular dysfunction. Results demonstrated uneventful recoveries with no complications such as tumor formation, arrhythmias or alloimmunization (91). These trials demonstrated feasibility and safety of clinical translation of hESC-derived cardiovascular progenitors, paving the way for further efforts of efficacy studies. Recently, CPCs have shown promise as a highly reproductive cell source in additive cardiac tissue manufacturing, as an alternative to the non-proliferative, mature CMs, to create highly cellularized function hCMPs (92–94).
Spheroids
During the iPSC differentiation process, the cells tend to aggregate into spheroids containing multiple cell types, which can be used for high-throughput screening of heterocellular interactions and drug testing (6, 9). Spheroids could also enhance differentiation and promote maturation, with CMs comprising 80–100% of the cells in microtissues, while refined CM differentiation protocols in monolayer cultures could yield 80–98% CMs (84, 95). Furthermore, spheroids containing proportions of iPSC-derived CMs, ECs, and fibroblasts somewhat recapitulate the morphological and physiological properties of human myocardium, as well as the response to treatment with pharmacological agents, which suggests that spheroids could provide a valuable platform for in vitro disease modeling (96, 97). Measurements of engraftment and survival also appear to be greater when cells are administered in the form of spheroids, rather than disaggregated cellular suspensions (98, 99). In another study, engrafted spheroids composed of CPCs expressing the ISL1-LIM-homeodomain transcription factor differentiated into CMs and ECs and contributed to the formation of new blood vessels in infarcted mouse hearts (100). The maturation of iPSC-CM spheroids can be increased via mechanical and electrical stimulation (101, 102), but measurements of isometric force and electrical conduction are hindered by the lack of a functional architecture (9). The use of cardiac spheroids in advanced biomanufacturing procedures, such as bioprinting, has recently attracted increasing attention, as they can provide improved printed cell viability, fusion, and function to fabricate large-scale cardiovascular constructs (103, 104).
Advanced Cardiac Tissue Manufacturing Strategies
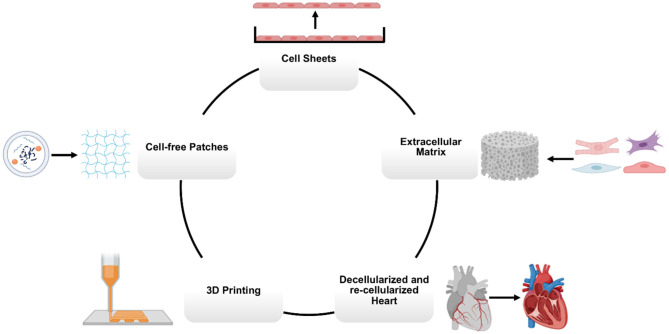
Conventional methods for manufacturing hCMPs include generating contiguous sheets of cardiac cells (mainly CMs) or suspending cells of a variety of types in scaffolds of biocompatible material (94, 105–108). More recently, the emergence of 3D printing technologies, combined with cell-containing “bioinks” and computer-aided design (CAD), has enabled researchers to define the architecture of hCMPs with previously unattainable precision (Figure 3).
Figure 3.
Main manufacturing methods for cardiovascular tissue engineering. Human cardiac muscle patch (hCMP) constructs can be fabricated using a variety of bioengineering methods, including cell sheets, scaffolds, decellularized heart tissues, 3D (bio)printing, and cell-free patches.
Cell Sheet Approaches to Fabricate hCMPs
Cell sheets are typically produced by culturing cells on dishes coated with a temperature-sensitive polymer, such as poly(N-isopropyl acrylamide) (PIPAAm), which releases the attached cells when the temperature is reduced from 37 to 32°C, thereby maintaining the ECM and intercellular connections produced during the culture period (109). Since cell sheets lack the exogenous/synthetic scaffold, concerns regarding the potential immunogenicity of the scaffold material are abolished. The sheets can be stacked to generate 3D hCMPs that contract spontaneously (110). Furthermore, the cells of adjacent layers form connections that facilitate communication between layers, including gap junctions, which are required for electrical coupling (111). Although, constructs composed of more than four cellular layers are typically resistant to vascularization, the vascularity of fabricated cell sheets can be improved by the inclusion of stromal cells or omentum during manufacture (112, 113). Further, CM survival and contractile function were significantly improved when iPSC-CM sheets were covered with an omentum flap after transplantation into infarcted pig hearts (114–116). However, transplanted cell sheets tend to remain electromechanically isolated from the native myocardium, which suggests that the observed benefits likely occur primarily through the activation of paracrine mechanisms (117). Clinical trials utilizing cell sheets of skeletal myoblast demonstrated safety and feasibility with no complications, paving the way for further therapeutic efficacy studies (118, 119). Notably, the Japanese health ministry recently approved the use of iPSC-derived CM sheets in a small study of patients with heart disease, which represents only the second clinical application of iPSC-derived cells (115, 120).
Extracellular Matrix
The biomaterials used in scaffold-based hCMPs (121) are designed to mimic the native cardiac ECM which consists of a highly specialized, 3D network of structural (e.g., collagen) and non-structural (e.g., glycoproteins, proteoglycans, and glycosaminoglycans) proteins that support cardiac function (122, 123). The patch biomaterial is aimed to provide the architecture for cell attachment and cell-cell or cell-matrix interactions (122, 124) and facilitate the coordinated transmission of electrical and mechanical signals (9). Therefore, several design criteria for scaffold biomaterials have been established. Biocompatibility of materials is critical to minimize immunogenicity and cytotoxicity, while promoting cell attachment, differentiation and proliferation (30, 125). Biodegradability is another important aspect of scaffolding biomaterials, allowing the material to degrade at an appropriate rate while new ECM proteins are being synthesized (106, 126). Biomechanical and biophysical properties of materials such as stiffness, elasticity, and porosity enable effective electromechanical coupling of cardiac scaffolds and facilitate mass transport within the constructs (9, 94, 127, 128). Toward that goal, strategies utilizing natural hydrogels (e.g., collagen, fibrin, and Matrigel), synthetic polymers (e.g., polyglycolic acid, polycaprolactone, polylactic acid, and poly-D, L-lactic-co-glycolic acid), or nanofibrous materials as scaffolds have been attempted (9, 129–135). Natural materials have the advantages of promoting cell attachment and viability without cytotoxicity. Nevertheless, physical properties of natural materials could vary from batch to batch and source to source. Immunogenicity is also a concern for xeno-transplantation. Synthetic materials could be designed to precisely control mechanical properties and degradation rate, thus inducing minimal immunogenicity. However, the novel composition of synthetic materials often struggles to fully support cell adhesion and survival. Various scaffold architectures and geometries that have been tested include cylindrical constructs, which typically contain only small numbers of cells, and therefore, are usually scaled to much larger hCMPs at clinically relevant dimensions (2 cm × 4 cm × 1.25 mm) (23). Other structures used are various rodent-sized ventricular organoids (9).
Decellularized Heart Tissues
The role of the ECM in cardiac differentiation, organization, and vascularization is well-established by many studies with decellularized heart tissues (136). The ECM from decellularized human ventricles is shown to induce CM gene expression in human CPCs and MSCs. Further, human umbilical vein ECs formed endocardial and vascular linings in these constructs, and fully differentiated human CMs aggregated into muscular bundles with mature calcium dynamics and electrical coupling (137). Whole rat hearts have been also decellularized, seeded with cardiac and endothelial cells, and perfused in bioreactors, demonstrating macroscopic contractions after only 4 days of culture. By day 8 in culture, the recellularized hearts displayed pumping function in response to electrical stimulation, equivalent to about 2% of adult rat hearts (138). Even in the absence of seeded cells, application of a decellularized porcine myocardial tissue resulted in significant improvements in cardiac functional parameters when evaluated in a rat model of MI, and the patch was vascularized by cells that had migrated from the native tissues (139).
3D Bioprinting—Additive Biomanufacturing
3D bioprinting technologies utilize CAD modeling to guide the assembly of living cells and other biological materials into large-scale tissue and organ analogs with precisely controlled, native-like architectures (92, 140–143). In an early work, tissue printing technology was utilized to generate a construct composed of human cardiac-derived CPCs and alginate. The printed construct remained committed to cardiac lineage with high viability after culturing for 7 days (144). In subsequent studies, human CM progenitor cells were bioprinted into a matrix of hyaluronic acid and gelatin to form a patch with six perpendicularly printed layers and a total surface area of 4 cm2. When evaluated in a murine MI model, the patch improved measures of cardiac function and prevented remodeling. The expression of cardiac and vascular differentiation markers increased during the 4-week follow-up period (145). Scaffold-free bioprinted hCMPs have been also generated by loading spheroids, one-by-one, onto an array of needles, allowing them to fuse, and then removing the hCMP and culturing it until the needle holes were filled in with surrounding tissue. The construct remained engrafted and displayed evidence of vascularization 1 week after implantation into the infarcted rat hearts (104). A more recent, customized device has been developed that can load an entire layer of spheroids onto the needle array simultaneously, which will substantially reduce the time required to print larger engineered constructs (146).
The resolution of traditional bioprinting techniques cannot accommodate the structural details that facilitate interactions with individual cells. To address this limitation, a more advanced technique, multiphoton-excited (MPE) 3D bioprinting, has emerged, allowing the control of the architecture of photoactive polymers to resolutions of <1 μm and, consequently, reproducing the structural features of the ECM with high fidelity (147). MPE 3D-printed hCMPs composed of iPSC-derived CMs, ECs, and SMCs in a photoactive gelatin scaffold began generating calcium transients and beating synchronously within 1 day post-manufacturing. The printed patches were associated with significant improvements in cardiac function (left ventricular ejection fraction and fractional shortening), infarct size, apoptosis, vascularity, and cell proliferation when tested in mice with surgically induced MI (62). Photoactivated 3D bioprinting has also been conducted with a bioink containing both ECM proteins and hiPSCs to generate two-chambered structures with both inlet and outlet vessels. The hiPSCs proliferated and differentiated into mature cardiac cells in situ to form a living pump with contiguous walls of human cardiac muscle that mimicked the chambers and large vessels of a native heart (148).
Cell-Free Cardiac Patches
Cell- and tissue-based treatments will likely require specialized methods of storage and transportation to maintain cell viability (149). Since most of the benefits associated with transplanted cells and engineered tissues appear to evolve from the paracrine factors produced by the cells, rather than the cells themselves (150), strategies that exploit the regenerative activity of these paracrine factors may be more easily translated to the clinics. The three primary categories of secreted, biologically active cellular products are growth factors (typically proteins that function as signaling molecules), non-coding RNA (short, single-stranded oligonucleotides that regulate gene expression), and extracellular vesicles of endosomal origin (151). Artificial cardiac patches have been manufactured by encapsulating the factors secreted by cardiac stromal cells in PLGA and then embedding the capsules in decellularized porcine myocardium. Applying these factor-laden patches to the infarcted hearts of both immunocompetent rats and pigs resulted in reduced scarring and improved cardiac function, without inducing an immune response (152). In other works, the controlled release of miRNAs from injected hydrogels has shown improvement in cardiac function in infarcted mouse hearts (153). Further, hydrogel patches containing the extracellular vesicles produced by human iPSC-CMs reduced infarct size and cardiac hypertrophy in a rat MI model (154). Collectively, these results suggest that the controlled release of paracrine factors from cell-free patches or injected biomaterials may be a feasible alternative approach to transplanted hCMPs for the treatment of myocardial disease.
Increasing hCMP Thickness/Dimensions
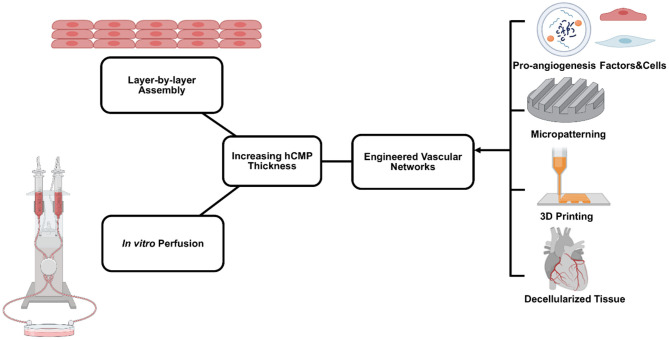
Despite substantial improvements in the components and protocols used for hCMP manufacturing, few studies have been conducted with constructs of clinically relevant size. Even patches with comparatively large surface areas (e.g., 8 cm2) are relatively thin (1.25 mm) (23), with inability of direct perfusion limiting thickness of hCMP to 1–2 mm (155, 156). Thus, production methods continue to be optimized and enhanced for the development of larger and thicker hCMP constructs. The inability to engineer and maintain thick and viable hCMP jeopardizes the clinical applications due to their inability to mimic characteristics of native myocardium, such as generating adult-like forces and action potentials (157, 158). In practice, hCMP thickness is often limited by the diffusion of oxygen and nutrients from the vasculature after transplantation, which requires CMs to be within 100–200 μm distance from the capillaries (159). Diffusion can be facilitated by including molecular crystals (e.g., sucrose) in the matrix solution and leaching them out after the matrix solidifies to increase the porosity of the scaffold (160). However, hCMPs of clinically relevant thicknesses require formation of a dense internal vascular network that couples with the native circulation after transplantation (9, 157). Vascularization can be increased by including combinations of vascular and other cell types (ECs, SMCs, and/or fibroblasts) (23, 62, 161) during manufacturing and/or via the nanoparticle-mediated (151) extended release of pro-angiogenic factors [e.g., vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and the Wnt activator CHIR99021; (162, 163)], which can promote infiltration of the native circulatory system. Further, the spatial orientation of the vascular network can be controlled with more technologically advanced fabrication methods (e.g., micropatterning and 3D bioprinting) to enhance the mass transport and perfusion [(164); Figure 4].
Figure 4.
Primary bioengineering techniques to manufacture and maintain relatively thick human cardiac muscle patch (hCMP) constructs. A variety of methods are used, including layer-by-layer assembly, in vitro perfusion using various bioreactor systems, and engineered vascular networks.
Layer-By-Layer Assembly
In theory, the layer-by-layer assembly technique enables hCMPs of any desired thickness to be generated simply by stacking the required number of individual CM sheets. However, since the individual layers of multilayered hCMPs are grown in isolation before assembly, intercellular connectivity is likely to be greater between cells within the same layer than between the cells of adjacent layers. Techniques for enhancing the formation of physical and electromechanical connections between layers include the use of graphene oxide (GO)-based thin films, which improved not only adhesion but also electrical coupling, maturation and cell organization (165), as well as the Tissue-Velcro platform, in which cardiac cells are cultured on 2D cell meshes that incorporate a microfabricated hook-and-loop system. After layering, the hooks and loops of adjacent layers interlocked and the hCMP contracted in response to electrical stimulation (166). The vascularization of multilayered hCMPs can be promoted by sandwiching vascular cells between the CM layers (167, 168). Coherent vascular networks have been generated in stacked hCMPs by using resected tissue as a vascular bed. When the resected vasculature was overlaid with sheets of cardiac cells (including ECs), connected to a bioreactor, and perfused with culture medium, ECs in the cardiac-cell layers connected to capillaries in the vascular bed and formed tubular structures. The vascularized hCMPs could survive after transplantation into the necks of rats via blood vessel anastomosis (169).
In vitro Perfusion
Techniques for maintaining adequate oxygen and nutrient availability during the manufacturing process include the development of cartridges that reproduce the convective-diffusive properties of oxygen transportation present in native myocardial tissue (170). Another approach is culturing cells on a simulated capillary network with medium containing an oxygen carrier (perfluorocarbon) that mimics hemoglobin (171, 172). The hemoglobin mimic significantly increased the expression of cardiac markers and improved the contractile performance of constructs composed of primary neonatal CMs and fibroblasts. Small intestinal submucosa could be utilized to enhance perfusion with rat primary endothelial cells forming a network in pre-existing vessel structures (173). AngioChip technology (174) incorporates a perfusable, 3D microchannel network that recapitulates the native vascular interface and, because it is compatible with current practices in both laboratories and industry, can be adapted to produce a variety of tissue types. Perfusion can also be improved by culturing cardiac tissue constructs under dynamic conditions [e.g., on a rocking platform; (158)]. This approach was used during the manufacture of large (2 cm × 4 cm) patches for subsequent testing in a swine MI model and was associated with improvements in not only cell viability, but also in measures of hCMP maturation (23). Notably, some evidence suggests that the contractile properties of hCMPs can be enhanced by perfusing them in rhythmic pulses, rather than a continuous static flow (175).
Engineered Vascular Networks
Rather than relying solely on infiltration from the native circulatory system, thicker hCMPs will likely require at least some amount of engineered vascularity before transplantation. Vascularization can be induced during the manufacturing process by encapsulating a sacrificial gelatin mesh in scaffold material and then melting the gelatin mesh away, leaving behind a network of interconnected microfluidic channels. When seeded with human microvascular ECs, the sacrificial scaffolds produced a rudimentary endothelial network (176). An alternative strategy mimics the endogenous angiogenic process by using a sustained-release preparation of the angiogenic factor thymosin β to promote and guide the outgrowth of vessels from explanted arteries and veins to form a capillary bed within a hydrogel scaffold (177). Vessel growth can also be directed with micropatterned polyglycerol sebacate scaffolds. After transplantation, host blood cells infiltrated into the microvessels as the scaffold degraded (178). Micropatterning has also been used to organize ECs into “cords,” which guide the formation of capillaries that integrate with the host tissue after transplantation (179).
One of the novel reports of 3D printed vessels used a thermal inkjet printer to print mixtures of human microvascular ECs and fibrin, forming micro-sized fibrin channels lined with confluent cells (180). Vascular networks have also been bioprinted with an advanced extrusion system that produced a sheath of photoactive, cell-laden bioink around an alginate core. After UV crosslinking, the alginate was dissolved with a Ca2+-chelating agent, allowing the cells to proliferate, spread, and form a perfusable biomimetic vascular network (140). However, the penetration of UV radiation is limited. Polymerization can be induced at greater depths via enzymatic reactions, such as the thrombin-induced cleavage of fibrinogen into fibrin. This strategy has been combined with the co-printing of vascular and cellular inks in cast ECM material to generate markedly thick (>1 cm) engineered osteogenic tissues (181).
Transplantation of hCMP Constructs
hCMP Delivery Methods
Delivery methods for cells and tissues could be categorized into invasive and non-invasive approaches, both often featuring low engraftment rates (12). Transplantation of hCMPs mainly falls into invasive delivery, requiring open-chest surgery, and suturing/attaching the patch onto the epicardium (23, 62). This method typically offers an enhanced engraftment and survival rate compared with cell injection into the injured myocardium. The widespread application of intra-myocardial cell injections has demonstrated a poor cell retention rate with induced damages to both exogenous and host cells due to a dramatic increase in flow velocity and shear stress (182). Studies have shown that repeated cell injections increase the therapeutic effects (183). However, invasive delivery methods are not optimal for repeated applications due to requirements of dedicated facilities and highly trained staff to perform open-chest surgery. As a result, invasive delivery of cells and hCMPs has challenged clinical translation of these therapies (184).
Alternatively, non-invasive delivery methods, such as intravascular (or intravenous) delivery and injectable hydrogel approaches, have been tested. Intravascular delivery has shown recirculation and redistribution of injected cells to other organs besides the target site (6). These methods have demonstrated improved cardiac function potentially due to the paracrine signaling mechanism as previously discussed (185). This method has the advantages of being non-invasive and possibility of repeated administrations, qualifying them a good choice from a clinical viewpoint (184). Recently, a novel minimally invasive method demonstrated epicardial delivery of hydrogels through the pericardial space (186). A pericardial device (catheter) delivers the hydrogel components through separate (coaxial) lumens and combines them after exiting the device, forming a stable hydrogel construct between the pericardium and epicardium layers. This method could help minimize the risks of extensive myocardial injury, thrombotic occlusion, and arrhythmia. Injectable hydrogels have been also increasingly tested as a minimally (or non) invasive delivery approach for cardiac patch systems (187, 188). In a clinical study, acellular alginate-based hydrogels were injected in MI patients, demonstrating the preservation of the LV indices and ejection fraction (189). However, a more recent large clinical trial, investigating the effect of injectable alginate hydrogels in patients with advanced HF, reported about 9% death within 30 days post-injection, while the control group had no fatalities (190). Therefore, while significant progress has been made in developing injectable cardiac patch systems, more efforts are required for their further improvement for efficient clinical use. These include further enhancement in biomechanical and biochemical properties of the hydrogel constructs to mimic the native tissue, improving cell viability and biomolecules activity, controlled degradation and immune response, and enhancement in the in vivo tracking of the patch (187, 191, 192).
Animal Models to Test hCMPs
Preclinical studies have utilized different animal models, including mice, rats, guinea pigs, swine, and non-human primates (Table 1). Choice of a suitable animal model with high predictive validity for safety profile and therapeutic outcomes is crucial for the clinical translational purposes. Initial studies of therapeutic efficacy of transplanted human cells or cardiac tissues were performed in immunocompromised rodents, including athymic rats and severe combined immunodeficiency mice, due to genetic manipulations and easy handling. However, the disparity of anatomy and physiology between rodents and human dampens the reliability of therapeutic outcomes (197). Subsequent preclinical studies utilize large animal models, including non-human primates and swine, demonstrating remuscularization and therapeutic efficacy with safety concerns of ventricular arrhythmias (42, 196, 198). Large animal models are more reliable and relevant for preclinical studies compared with rodent models. However, the high cost associated with large animal studies would limit their applications (41).
Table 1.
Summary of various hCMP studies conducted in animal models of heart injuries.
| Animal model | Cell type and number | Delivery method/Material | Vascularization | Observations | Follow up |
|---|---|---|---|---|---|
| NOD/SCID gamma mice with MI (101) | Five Spheroids composed of hiPSC-CMs (2 × 105/spheroid) | Engineered fibrin cardiac patch | N/A | Improved LVEF and FS; enhanced engraftment | 4 weeks |
| NOD/SCID gamma mice with MI (163) | 1 × 106 hiPSC-CM | Engineered fibrin cardiac patch containing nanoparticles releasing CHIR99021 and FGF1 | N/A | Enhanced cardiac function; reduced infarct size; increased engraftment with enhanced cell cycle activity of hiPSC-CMs | 4 weeks |
| Guinea pigs with cryoinjury (193) | 1 × 108 hESC-CMs | IM | N/A | Improved mechanical function; reduced incidence of arrhythmias | 4 weeks |
| Guinea pigs with MI (52) | 5 × 106 hiPSC-CM and 2 × 106 hiPSC-ECs | Engineered fibrin cardiac patch | Inclusion of iPSC-ECs | Improve left ventricular function by 31%; remuscularization; vascularization; electrical coupling with host myocardium | 28 days |
| Rats with MI (35) | 4 × 107 Fetal rat ventricular muscle | Engineered gelatin cardiac patch | N/A | No significant change in cardiac function; surviving grafts enhanced angiogenesis | 5 weeks |
| Rats with MI (36) | 3 × 105 Fatal rat CMs | Engineered alginate cardiac patch | N/A | No significant change; Engraftment with intensive neovascularization; integration; attenuate LV dilatation | 9 weeks |
| Normal athymic rats Sprague Dawley (56) | ESC-CMs and iPSC-CMs; HUVEC; MSCs; MEFs | Engineered collagen cardiac patch | Inclusion of stromal supporting cells | Form grafts containing microvessels; Enhanced formation of vessel-like structures | 1 week |
| Rats with MI (88) | 1 × 106 Bone marrow mesenchymal stem cells | Engineered silk fibroin/hyaluronic acid cardiac patch | N/A | Therapeutic efficacy; improved LV wall thickness; high viability; neovascularization | 8 weeks |
| Nude athymic rats with MI (39, 194) | 2.5 × 106 hESC-CMs | Engineered collagen cardiac patch | N/A | No significant changes of LVEF; Preserved heart function revealed by tagged magnetic resonance imaging; high engraftment | 4 weeks |
| Athymic rats with MI (40) | 2.2 × 106 iPSC-CMs; 3.4 × 105 human pericytes | Engineered fibrin cardiac patch | Inclusion of human pericytes | Improved cardiac function; reduced infarct size; engraft onto host heart | 4 weeks |
| Rats with acute MI (139) | Acellular | Decellularized porcine myocardial slice | Utilization of decellularized porcine myocardium mimicking native ECM | Improved cardiac function; attach to host myocardium; prevent LV wall thinning; vascularization | 4 weeks |
| Rats and porcine with MI (152) | Acellular; synthetic cardiac stromal cells | Cardiac patch composed of a decellularized porcine myocardium | Enhanced cardiac recovery with reduced scar and promoted angiogenesis in rat model; therapeutic efficacy in porcine model | 3 weeks | |
| Porcine with MI (113) | 1.5 × 107 Skeletal myoblasts from mini-pigs | Cell sheets covered with omentum flap | Utilization of omentum flap for revasularization | Improved cardiac function; Reduced infarct size; increased angiogenesis | 8 weeks |
| Porcine minipigs with MI (114) | iPSC-CMs | Cell sheets | N/A | Improved cardiac performance and attenuated left ventricular remodeling; poor engraftment | 8 weeks |
| Normal porcine mini-pigs (115) | iPSC-CMs | Cell sheets with an omentum flap | Inclusion of an omentum flap | Enhanced survival and engraftment; rich vasculature | 8 weeks |
| Immunosuppressed Yorkshire pigs with MI (51) | 4 × 106 hiPSC-VC (ECs and SMCs) | Engineered fibrin cardiac patch | Inclusion of iPSC-VCs (ECs and SMCs) | Increased BZ contractile function and ATP turnover rate with attenuated regional wall stress, neovascularization and improved BZ perfusion | 4 weeks |
| Porcine with acute MI (50) | 6 × 106 total iPSC-cardiovascular cells (CMs, ECs, and SMCs; each 2 × 106) | IM + fibrin patch containing IGF | Inclusion of iPSC-ECs, SMCs | Improved left ventricular function; reduced infarct size; Integration with host myocardium; vascularization; | 4 weeks |
| Porcine with ICM (116) | 5 × 106 iPSC-CMs | Cell sheets with omental flap | Utilization of omentum flap | Enhanced therapeutic effects and survival; | 3 months |
| Porcine with MI (23) | iPSC-Cardiac cells (4 × 106 CMs, 2 × 106 ECs, and 2 × 106 SMCs) | Engineered fibrin cardiac patch | Inclusion of iPSC-ECs, -SMCs | Improved left ventricular function; reduced infarct size; reduced LV wall stress; no significant changes in arrhythmogenicity | 4 weeks |
| Porcine with MI (195) | Acellular; exosomes derived from iPSC-Cardiac cells (iPSC-CMs, -ECs, -SMCs) | IM | N/A | Improve myocardial recovery without increasing the frequency of arrhythmogenic | 4 weeks |
| Non-human primate Macaca nemestrina with MI (41) | 1 × 109 (1 billion) hESC-CMs | IM | N/A | Remuscularization; vascularization; electromechanical coupling; arrhythmias | 3 months |
| Non-human primate Macaca fascicularis with MI (196) | 1 × 109 iPSC-CMs | IM | N/A | Improved cardiac function; Survive for 12 weeks; electrical coupling; ventricular tachycardia (transient) | 12 weeks |
| Non-human primate macaque monkeys with MI (42) | ~750 million hESC-CMs | IM | N/A | Improved cardiac function; formation of electromechanical junctions; ventricular arrhythmias | 3 months |
| One patient with severe heart failure (90) | 4 × 106 hESC-derived Isl-1+ SSEA-1+ cells | Engineered fibrin cardiac patch | N/A | Symptomatically improved LVEF; new-onset contractility; no complications | 3 months |
| Seven patients with ischemic cardiomyopathy (UMIN000008013) (118) | 3 × 108 autologous skeletal muscle | Cell sheet | N/A | Improved LVEF; improved patient status | 26 weeks |
| Six patients with severe ischemic left ventricular dysfunction (NCT02057900) (91) | 8.2 × 106 hESC-derived cardiovascular progenitors | Engineered fibrin cardiac patch | N/A | Uneventful recoveries; no safety concerns, such as tumor, arrhythmias, alloimmunization | 1 year |
| Four patients with dilated cardiomyopathy (UMIN000000660) (119) | 4.5–7.5 × 108 autologous skeletal muscle | Cell sheet | N/A | Improved LVEF in two patients; reduced cardiac hypertrophy | 3 months |
FGF1, fibroblast growth factor 1; LVEF, left ventricular ejection fraction; FS, fractional shortening; IM, intra-myocardial delivery; HUVECs, human umbilical vein endothelial cells; MSCs, human marrow stromal cells; MEFs, mouse embryonic fibroblasts; IGF, insulin growth factor; ICM, ischemic cardiomyopathy.
Mechanisms of Action of hCMPs
The ultimate goal of cardiac patch transplantation is to replace the injured myocardium with exogenously functional cardiac muscle. Preclinical studies from mice (199, 200), rats (201), guinea pigs (52, 193), porcine (50), and non-human primates (41) have demonstrated some degree of myocardial remuscularization of the fibrotic scar tissue with transplanted cardiac tissues. The clinical translation of this approach has met some challenges, first of which is the requirement of large quantities of exogenous CMs (and/or other cardiac cells) used to replenish lost tissue. Robust protocols for CM differentiation and scalability of CM production, together with cryopreservation and retrieval procedures following current good manufacturing practice (cGMP), have enabled generation of well-characterized PSC-CMs as an off-the-shelf therapy (202). Furthermore, it remains a critical issue to ensure long-term graft retention for maximal therapeutic efficacy based on the hypothesis that contractile force is correlated to the electromechanically integrated CMs (202). Gene manipulation has been adopted to increase cell retention. Overexpression of CCND2 (cyclin D2), a cell cycle activator, increased cell cycle activity and proliferation rate in hiPSC-CMs, thus improving engraftment rate from average 10 to 25% with a significant remuscularization of injured myocardium in mice (203). CM retention could also be enhanced via co-administration of pro-survival factors, such as Matrigel, cyclosporine A, pinacidil, ZVAD-fmk, insulin-like growth factor-1 (IGF-1), CHIR99021, and fibroblast growth factor 1(FGF1) (50, 82, 163).
The lack of electromechanical coupling between hCMPs and host tissue is another challenge on the way of clinical translation of patch-based therapies. Super-aligned carbon nanotubes were utilized during the fabrication of cardiac tissues to enhance electrophysiological homogeneity due to the anisotropic conductivity of their aligned structure (204). In another study, electrospun nanofibrous scaffolds with enhanced conductivity were demonstrated to promote electrical coupling of the patch, showing potential for fabrication of clinically relevant hCMP products (205). One major aspect that must be carefully studied, when working on electromechanical integration of exogenous CMs with host myocardium is the likely potential to trigger arrhythmias, as discussed in the previous sections (41).
Low CM survival rates, as little as 10%, have been usually associated with functional benefits from hCMP transplantations (39, 51, 194). Patch constructs composed of non-CMs have also shown improved cardiac function (51). These data collectively suggest an alternative mechanism of action, based on paracrine signaling, where cell secreted signals, including extracellular vesicles, mediate cardiac repair via increasing CM survival and neovascularization and decreasing apoptosis and inflammation (206). Paracrine signaling was further evidenced by a study where exosomes secreted from combination of iPSC-derived CMs, ECs, and SMCs yielded cardioprotective effects similar to that obtained from direct cells injection in a porcine model of MI (195). Further, acellular hCMPs composed of non-viable/irradiated cells (39) or decellularized porcine myocardial tissues (139) have shown therapeutic benefits after transplantation, through the proposed mechanism of mechanical stabilization. Thus, the therapeutic benefits associated with hCMP transplantation could be a combination of any or all of mechanisms including remuscularization, paracrine signaling, and mechanical stabilization (202, 207).
Safety Concerns
Occurrence of arrhythmias is a critical safety concern for clinical applicability of cardiac patch therapies. Sine large animal models more closely approximate the human heart's physiology, function, and anatomy, they have been increasingly used to assess arrhythmias in cardiac patch studies (41). Preclinical studies of non-human primates demonstrated transient non-fatal arrhythmias during 2 weeks after transplantation, followed by a subsequent decrease in those irregularities (41, 196). Ventricular arrhythmias occurred in porcine models have been more frequent and lethal than those in non-human primate models (2 out of every 7 pigs) (198). It is currently viewed that disorder of impulse generation at the interface between engrafted CMs and host myocardium could induce ventricular arrhythmias (42, 198). Further investigation of arrhythmic complications will be necessary before translation into patients.
Autologous cell transplantation offers an advantageous option based on the hypothesis that autologous cell transplantation could induce no immunological response due to the acceptable human leukocyte antigens (HLA) match. However, accumulating evidence prove that the clinical translation of autologous cells could become complicated by limitations, such as variability from batch to batch, requiring a detailed characterization and quality control of each batch. The initial hypothesis that transplantation of autologous iPSC derivatives would induce no immunogenicity has been challenged by some recent studies (70, 71).
For a long time in clinical practice, inhibition of the immunological responses has been mainly through the use of immunosuppressive drugs, such as cyclosporine, dexamethasone, and FK-506. With side effects induced by such immunosuppressants, there is a trade-off between efficacy and toxicity. High-dose immunosuppression would result in toxicity and associated complications, while low-dose immunosuppression would lead to allograft rejection. The choice of transient or chronic exposure of immunosuppressants would be based on the presumed mechanism of action, as discussed previously (184, 208). In addition, immunological tolerance could be induced via host conditioning by activation/adoptive transfer of regulatory T cells (209). Short-term administration of nanobiologics targeting macrophages could benefit long-term allograft survival (210). Other strategies have been adopted to reduce immunogenicity. Modern genome-editing tools [e.g., zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), or the clustered regulatory interspaced short palindromic repeat (CRISPR)/Cas-9 system; (37, 211, 212)] have enabled the development of minimally immunogenic iPSC lines by knocking out key components of major histocompatibility complexes (MHCs) I and II [Beta2 microglobulin (B2M) and MHC II transactivator (CIITA)] (213). This is done by combining MHC gene inactivation with the overexpression of CD47, a ubiquitous membrane protein that directly regulates T-cell immunity (214), and by disrupting the genes for HLA (215). These genetically modified hiPSCs could be used to generate a stockpile of “off-the-shelf” cardiac cells and hCMPs for administration to patients in emergency situations without the need for concomitant immunosuppressive therapies.
Summary—Current Challenges and Future Perspectives
The potential benefits of hCMPs for the treatment of myocardial injury and disease are readily observable in preclinical studies, and at least one small study in patients is currently underway (120). Conventional methods for manufacturing hCMPs include suspending cells in scaffolds of biocompatible material or growing 2D sheets in culture and stacking them to form multilayered constructs. More advanced technologies, such as micropatterning and CAD-guided 3D printing with bioinks have given researchers the tools to control the architecture of hCMPs at resolutions that match the scale of interactions between individual cells or between cells and the ECM. Most studies, however, have been conducted with hCMPs at relatively small scales that are not suitable for administration to patients. The size and (especially) thickness of hCMPs are often restricted by the diffusion limitations of oxygen, nutrients, and other biologically active molecules, which requires CMs to be within 100–200 μm distance from the capillaries. Thus, the clinical translation of hCMP technology will require the development of protocols for manufacturing larger and thicker constructs that are adequately vascularized and fully couple with the circulatory and electromechanical systems of the native myocardium.
Author Contributions
LW, VS, and JZ contributed to the overall design of the manuscript, collecting the data, and writing the article. JZ supervised the entire manuscript preparation process, figures design and creation, obtaining the necessary permits, etc. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
References
- 1.Laslett LJ, Alagona P, Jr., Clark BA, III, Drozda JP, Saldivar F, Wilson SR, et al. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol. (2012) 60(Suppl. 25):S1–49. 10.1016/j.jacc.2012.11.002 [DOI] [PubMed] [Google Scholar]
- 2.Tzatzalos E, Abilez OJ, Shukla P, Wu JC. Engineered heart tissues and induced pluripotent stem cells: macro- and microstructures for disease modeling, drug screening, and translational studies. Adv Drug Deliv Rev. (2016) 96:234–44. 10.1016/j.addr.2015.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans MA, Sano S, Walsh K. Cardiovascular disease, aging, and clonal hematopoiesis. Annu Rev Pathol. (2020) 15:419–38. 10.1146/annurev-pathmechdis-012419-032544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, et al. Dynamics of cell generation and turnover in the human heart. Cell. (2015) 161:1566–75. 10.1016/j.cell.2015.05.026 [DOI] [PubMed] [Google Scholar]
- 5.Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC. Cardiac fibrosis: the fibroblast awakens. Circ Res. (2016) 118:1021–40. 10.1161/CIRCRESAHA.115.306565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J, Zhu W, Radisic M, Vunjak-Novakovic G. Can we engineer a human cardiac patch for therapy? Circ Res. (2018) 123:244–65. 10.1161/CIRCRESAHA.118.311213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parsa H, Ronaldson K, Vunjak-Novakovic G. Bioengineering methods for myocardial regeneration. Adv Drug Deliv Rev. (2016) 96:195–202. 10.1016/j.addr.2015.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujita B, Zimmermann WH. Myocardial tissue engineering for regenerative applications. Curr Cardiol Rep. (2017) 19:78. 10.1007/s11886-017-0892-4 [DOI] [PubMed] [Google Scholar]
- 9.Pomeroy JE, Helfer A, Bursac N. Biomaterializing the promise of cardiac tissue engineering. Biotechnol Adv. (2020) 42:107353. 10.1016/j.biotechadv.2019.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. (2001) 410:701–5. 10.1038/35070587 [DOI] [PubMed] [Google Scholar]
- 11.Nygren JM, Jovinge S, Breitbach M, Säwén P, Röll W, Hescheler J, et al. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. (2004) 10:494–501. 10.1038/nm1040 [DOI] [PubMed] [Google Scholar]
- 12.Nguyen PK, Rhee JW, Wu JC. Adult stem cell therapy and heart failure, 2000 to 2016: a systematic review. JAMA Cardiol. (2016) 1:831–41. 10.1001/jamacardio.2016.2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogle BM, Bursac N, Domian I, Huang NF, Menasché P, Murry CE, et al. Distilling complexity to advance cardiac tissue engineering. Sci Transl Med. (2016) 8:342ps13. 10.1126/scitranslmed.aad2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldfracht I, Efraim Y, Shinnawi R, Kovalev E, Huber I, Gepstein A, et al. Engineered heart tissue models from hiPSC-derived cardiomyocytes and cardiac ECM for disease modeling and drug testing applications. Acta Biomater. (2019) 92:145–59. 10.1016/j.actbio.2019.05.016 [DOI] [PubMed] [Google Scholar]
- 15.Meyer T, Tiburcy M, Zimmermann WH. Cardiac macrotissues-on-a-plate models for phenotypic drug screens. Adv Drug Deliv Rev. (2019) 140:93–100. 10.1016/j.addr.2019.03.002 [DOI] [PubMed] [Google Scholar]
- 16.Turnbull IC, Mayourian J, Murphy JF, Stillitano F, Ceholski DK, Costa KD. Cardiac tissue engineering models of inherited and acquired cardiomyopathies. Methods Mol Biol. (2018) 1816:145–59. 10.1007/978-1-4939-8597-5_11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Y, Rafatian N, Feric NT, Cox BJ, Aschar-Sobbi R, Wang EY, et al. A platform for generation of chamber-specific cardiac tissues and disease modeling. Cell. (2019) 176:913–27.e18. 10.1016/j.cell.2018.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giacomelli E, Meraviglia V, Campostrini G, Cochrane A, Cao X, van Helden RWJ, et al. Human-iPSC-Derived cardiac stromal cells enhance maturation in 3D cardiac microtissues and reveal non-cardiomyocyte contributions to heart disease. Cell Stem Cell. (2020) 26:862–79.e11. 10.1016/j.stem.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shadrin IY, Allen BW, Qian Y, Jackman CP, Carlson AL, Juhas ME, et al. Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues. Nat Commun. (2017) 8:1825. 10.1038/s41467-017-01946-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinberger F, Mannhardt I, Eschenhagen T. Engineering cardiac muscle tissue: a maturating field of research. Circ Res. (2017) 120:1487–500. 10.1161/CIRCRESAHA.117.310738 [DOI] [PubMed] [Google Scholar]
- 21.Eschenhagen T, Fink C, Remmers U, Scholz H, Wattchow J, Weil J, et al. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system. Faseb J. (1997) 11:683–94. 10.1096/fasebj.11.8.9240969 [DOI] [PubMed] [Google Scholar]
- 22.Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci USA. (2012) 109:E1848–57. 10.1073/pnas.1200250109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao L, Gregorich ZR, Zhu W, Mattapally S, Oduk Y, Lou X, et al. Large cardiac muscle patches engineered from human induced-pluripotent stem cell–derived cardiac cells improve recovery from myocardial infarction in swine. Circulation. (2018) 137:1712–30. 10.1161/CIRCULATIONAHA.117.030785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimizu I, Minamino T. Physiological and pathological cardiac hypertrophy. J Mol Cell Cardiol. (2016) 97:245–62. 10.1016/j.yjmcc.2016.06.001 [DOI] [PubMed] [Google Scholar]
- 25.Cimetta E, Godier-Furnémont A, Vunjak-Novakovic G. Bioengineering heart tissue for in vitro testing. Curr Opin Biotechnol. (2013) 24:926–32. 10.1016/j.copbio.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou P, Pu WT. Recounting cardiac cellular composition. Circ Res. (2016) 118:368–70. 10.1161/CIRCRESAHA.116.308139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimizu T, Yamato M, Kikuchi A, Okano T. Cell sheet engineering for myocardial tissue reconstruction. Biomaterials. (2003) 24:2309–16. 10.1016/S0142-9612(03)00110-8 [DOI] [PubMed] [Google Scholar]
- 28.Bursac N, Loo Y, Leong K, Tung L. Novel anisotropic engineered cardiac tissues: studies of electrical propagation. Biochem Biophys Res Commun. (2007) 361:847–53. 10.1016/j.bbrc.2007.07.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol Heart Circ Physiol. (2007) 293:H1883–91. 10.1152/ajpheart.00514.2007 [DOI] [PubMed] [Google Scholar]
- 30.Fleischer S, Vunjak-Novakovic G. Cardiac tissue engineering: from repairing to modeling the human heart. In: Reis RL. editor. Encyclopedia of Tissue Engineering and Regenerative Medicine. Oxford: Academic Press; (2019). p. 131–44. 10.1016/B978-0-12-801238-3.65543-5 [DOI] [Google Scholar]
- 31.Wobma H, Vunjak-Novakovic G. Tissue engineering and regenerative medicine 2015: a year in review. Tissue Eng Part B Rev. (2016) 22:101–13. 10.1089/ten.teb.2015.0535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soonpaa MH, Koh GY, Klug MG, Field LJ. Formation of nascent intercalated disks between grafted fetal cardiomyocytes and host myocardium. Science. (1994) 264:98–101. 10.1126/science.8140423 [DOI] [PubMed] [Google Scholar]
- 33.Souren JE, Schneijdenberg C, Verkleij AJ, Van Wijk R. Factors controlling the rhythmic contraction of collagen gels by neonatal heart cells. In Vitro Cell Dev Biol. (1992) 28a(3 Pt 1):199–204. 10.1007/BF02631092 [DOI] [PubMed] [Google Scholar]
- 34.Souren JE, Peters RC, Van Wijk R. Collagen gels populated with rat neonatal heart cells can be used for optical recording of rhythmic contractions which also show ECG-like potentials. Experientia. (1994) 50:712–6. 10.1007/BF01919368 [DOI] [PubMed] [Google Scholar]
- 35.Li RK, Jia ZQ, Weisel RD, Mickle DA, Choi A, Yau TM. Survival and function of bioengineered cardiac grafts. Circulation. (1999) 100(Suppl. 19):Ii63–9. 10.1161/01.CIR.100.suppl_2.II-63 [DOI] [PubMed] [Google Scholar]
- 36.Leor J, Aboulafia-Etzion S, Dar A, Shapiro L, Barbash IM, Battler A, et al. Bioengineered cardiac grafts: a new approach to repair the infarcted myocardium? Circulation. (2000) 102(19 Suppl. 3):Iii56–61. 10.1161/01.CIR.102.suppl_3.III-56 [DOI] [PubMed] [Google Scholar]
- 37.Chen IY, Matsa E, Wu JC. Induced pluripotent stem cells: at the heart of cardiovascular precision medicine. Nat Rev Cardiol. (2016) 13:333–49. 10.1038/nrcardio.2016.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caspi O, Lesman A, Basevitch Y, Gepstein A, Arbel G, Habib IH, et al. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ Res. (2007) 100:263–72. 10.1161/01.RES.0000257776.05673.ff [DOI] [PubMed] [Google Scholar]
- 39.Riegler J, Tiburcy M, Ebert A, Tzatzalos E, Raaz U, Abilez OJ, et al. Human engineered heart muscles engraft and survive long term in a rodent myocardial infarction model. Circ Res. (2015) 117:720–30. 10.1161/CIRCRESAHA.115.306985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wendel JS, Ye L, Tao R, Zhang J, Zhang J, Kamp TJ, et al. Functional effects of a tissue-engineered cardiac patch from human induced pluripotent stem cell-derived cardiomyocytes in a rat infarct model. Stem Cells Transl Med. (2015) 4:1324–32. 10.5966/sctm.2015-0044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. (2014) 510:273–7. 10.1038/nature13233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu YW, Chen B, Yang X, Fugate JA, Kalucki FA, Futakuchi-Tsuchida A, et al. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat Biotechnol. (2018) 36:597–605. 10.1038/nbt.4162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nunes SS, Miklas JW, Liu J, Aschar-Sobbi R, Xiao Y, Zhang B, et al. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat Methods. (2013) 10:781–7. 10.1038/nmeth.2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang D, Shadrin IY, Lam J, Xian HQ, Snodgrass HR, Bursac N. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials. (2013) 34:5813–20. 10.1016/j.biomaterials.2013.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang X, Pabon L, Murry CE. Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circ Res. (2014) 114:511–23. 10.1161/CIRCRESAHA.114.300558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruan JL, Tulloch NL, Razumova MV, Saiget M, Muskheli V, Pabon L, et al. Mechanical stress conditioning and electrical stimulation promote contractility and force maturation of induced pluripotent stem cell-derived human cardiac tissue. Circulation. (2016) 134:1557–67. 10.1161/CIRCULATIONAHA.114.014998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo XM, Zhao YS, Chang HX, Wang CY, E LL, Zhang XA, et al. Creation of engineered cardiac tissue in vitro from mouse embryonic stem cells. Circulation. (2006) 113:2229–37. 10.1161/CIRCULATIONAHA.105.583039 [DOI] [PubMed] [Google Scholar]
- 48.Talman V, Kivelä R. Cardiomyocyte-endothelial cell interactions in cardiac remodeling and regeneration. Front Cardiovasc Med. (2018) 5:101. 10.3389/fcvm.2018.00101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zakharova IS, Zhiven MK, Saaya SB, Shevchenko AI, Smirnova AM, Strunov A, et al. Endothelial and smooth muscle cells derived from human cardiac explants demonstrate angiogenic potential and suitable for design of cell-containing vascular grafts. J Transl Med. (2017) 15:54. 10.1186/s12967-017-1156-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ye L, Y.-Chang H, Xiong Q, Zhang P, Zhang L, Somasundaram P, et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell. (2014) 15:750–61. 10.1016/j.stem.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiong Q, Ye L, Zhang P, Lepley M, Tian J, Li J, et al. Functional consequences of human induced pluripotent stem cell therapy: myocardial ATP turnover rate in the in vivo swine heart with postinfarction remodeling. Circulation. (2013) 127:997–1008. 10.1161/CIRCULATIONAHA.112.000641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weinberger F, Breckwoldt K, Pecha S, Kelly A, Geertz B, Starbatty J, et al. Cardiac repair in guinea pigs with human engineered heart tissue from induced pluripotent stem cells. Sci Transl Med. (2016) 8:363ra148. 10.1126/scitranslmed.aaf8781 [DOI] [PubMed] [Google Scholar]
- 53.Choi KD, Yu J, Smuga-Otto K, Salvagiotto G, Rehrauer W, Vodyanik M, et al. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells. (2009) 27:559–67. 10.1002/stem.20080922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shu B, Xie JL, Xu YB, Yu JX, Shi Y, Liu J, et al. Directed differentiation of skin-derived precursors into fibroblast-like cells. Int J Clin Exp Pathol. (2014) 7:1478–86. [PMC free article] [PubMed] [Google Scholar]
- 55.Gao L, Yang L, Wang L, Geng Z, Wei Y, Gourley G, et al. Relationship between the efficacy of cardiac cell therapy and the inhibition of differentiation of human iPSC-derived nonmyocyte cardiac cells into myofibroblast-like cells. Circ Res. (2018) 123:1313–25. 10.1161/CIRCRESAHA.118.313094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tulloch NL, Muskheli V, Razumova MV, Korte FS, Regnier M, Hauch KD, et al. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ Res. (2011) 109:47–59. 10.1161/CIRCRESAHA.110.237206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang S, Dutton JR, Su L, Zhang J, Ye L. The influence of a spatiotemporal 3D environment on endothelial cell differentiation of human induced pluripotent stem cells. Biomaterials. (2014) 35:3786–93. 10.1016/j.biomaterials.2014.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patsch C, Challet-Meylan L, Thoma EC, Urich E, Heckel T, O'Sullivan JF, et al. Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nat Cell Biol. (2015) 17:994–1003. 10.1038/ncb3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang J, Chu LF, Hou Z, Schwartz MP, Hacker T, Vickerman V, et al. Functional characterization of human pluripotent stem cell-derived arterial endothelial cells. Proc Natl Acad Sci USA. (2017) 114:E6072–8. 10.1073/pnas.1702295114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang J, Tao R, Campbell KF, Carvalho JL, Ruiz EC, Kim GC, et al. Functional cardiac fibroblasts derived from human pluripotent stem cells via second heart field progenitors. Nat Commun. (2019) 10:2238. 10.1038/s41467-019-09831-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giacomelli E, Bellin M, Orlova VV, Mummery CL. Co-differentiation of human pluripotent stem cells-derived cardiomyocytes and endothelial cells from cardiac mesoderm provides a three-dimensional model of cardiac microtissue. Curr Protoc Hum Genet. (2017) 95:21.9.1–21.9.22. 10.1002/cphg.46 [DOI] [PubMed] [Google Scholar]
- 62.Gao L, Kupfer ME, Jung JP, Yang L, Zhang P, Da Sie Y, et al. Myocardial tissue engineering with cells derived from human-induced pluripotent stem cells and a native-like, high-resolution, 3-dimensionally printed scaffold. Circ Res. (2017) 120:1318–25. 10.1161/CIRCRESAHA.116.310277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liau B, Jackman CP, Li Y, Bursac N. Developmental stage-dependent effects of cardiac fibroblasts on function of stem cell-derived engineered cardiac tissues. Sci Rep. (2017) 7:42290. 10.1038/srep42290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, Sirabella D, et al. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature. (2018) 556:239–43. 10.1038/s41586-018-0016-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. (1981) 292:154–6. 10.1038/292154a0 [DOI] [PubMed] [Google Scholar]
- 66.Kim K, Lerou P, Yabuuchi A, Lengerke C, Ng K, West J, et al. Histocompatible embryonic stem cells by parthenogenesis. Science. (2007) 315:482–6. 10.1126/science.1133542 [DOI] [PubMed] [Google Scholar]
- 67.Sayed N, Liu C, Wu JC. Translation of human-induced pluripotent stem cells: from clinical trial in a dish to precision medicine. J Am Coll Cardiol. (2016) 67:2161–76. 10.1016/j.jacc.2016.01.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. (2007) 318:1917–20. 10.1126/science.1151526 [DOI] [PubMed] [Google Scholar]
- 69.Huangfu D, Osafune K, Maehr R, Guo W, Eijkelenboom A, Chen S, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. (2008) 26:1269–75. 10.1038/nbt.1502 [DOI] [PubMed] [Google Scholar]
- 70.Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. (2011) 474:212–5. 10.1038/nature10135 [DOI] [PubMed] [Google Scholar]
- 71.Kaneko S, Yamanaka S. To be immunogenic, or not to be: that's the iPSC question. Cell Stem Cell. (2013) 12:385–86. 10.1016/j.stem.2013.03.008 [DOI] [PubMed] [Google Scholar]
- 72.Kim EM, Manzar G, Zavazava N. Induced pluripotent stem cell-derived gamete-associated proteins incite rejection of induced pluripotent stem cells in syngeneic mice. Immunology. (2017) 151:191–7. 10.1111/imm.12722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen Z, Zhao T, Xu Y. The genomic stability of induced pluripotent stem cells. Protein Cell. (2012) 3:271–7. 10.1007/s13238-012-2922-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, et al. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med. (2000) 6:88–95. 10.1007/BF03401776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. (2001) 108:407–14. 10.1172/JCI200112131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res. (2003) 93:32–9. 10.1161/01.RES.0000080317.92718.99 [DOI] [PubMed] [Google Scholar]
- 77.Osafune K, Caron L, Borowiak M, Martinez RJ, Fitz-Gerald CS, Sato Y, et al. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat Biotechnol. (2008) 26:313–5. 10.1038/nbt1383 [DOI] [PubMed] [Google Scholar]
- 78.Mummery CL, Zhang J, Ng ES, Elliott DA, Elefanty AG, Kamp TJ. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ Res. (2012) 111:344–58. 10.1161/CIRCRESAHA.110.227512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Halloin C, Schwanke K, Löbel W, Franke A, Szepes M, Biswanath S, et al. Continuous WNT control enables advanced hPSC cardiac processing and prognostic surface marker identification in chemically defined suspension culture. Stem Cell Rep. (2019) 13:366–79. 10.1016/j.stemcr.2019.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Devalla HD, Schwach V, Ford JW, Milnes JT, El-Haou S, Jackson C, et al. Atrial-like cardiomyocytes from human pluripotent stem cells are a robust preclinical model for assessing atrial-selective pharmacology. EMBO Mol Med. (2015) 7:394–410. 10.15252/emmm.201404757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee JH, Protze SI, Laksman Z, Backx PH, Keller GM. Human pluripotent stem cell-derived atrial and ventricular cardiomyocytes develop from distinct mesoderm populations. Cell Stem Cell. (2017) 21:179–94.e4. 10.1016/j.stem.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 82.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. (2007) 25:1015–24. 10.1038/nbt1327 [DOI] [PubMed] [Google Scholar]
- 83.Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. (2011) 8:228–40. 10.1016/j.stem.2010.12.008 [DOI] [PubMed] [Google Scholar]
- 84.Lian X, Zhang J, Azarin SM, Zhu K, Hazeltine LB, Bao X, et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat Protoc. (2013) 8:162–75. 10.1038/nprot.2012.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kropp C, Kempf H, Halloin C, Robles-Diaz D, Franke A, Scheper T, et al. Impact of feeding strategies on the scalable expansion of human pluripotent stem cells in single-use stirred tank bioreactors. Stem Cells Transl Med. (2016) 5:1289–301. 10.5966/sctm.2015-0253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ackermann M, Kempf H, Hetzel M, Hesse C, Hashtchin AR, Brinkert K, et al. Bioreactor-based mass production of human iPSC-derived macrophages enables immunotherapies against bacterial airway infections. Nat Commun. (2018) 9:5088. 10.1038/s41467-018-07570-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen VC, Ye J, Shukla P, Hua G, Chen D, Lin Z, et al. Development of a scalable suspension culture for cardiac differentiation from human pluripotent stem cells. Stem Cell Res. (2015) 15:365–75. 10.1016/j.scr.2015.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chi NH, Yang MC, Chung TW, Chen JY, Chou NK, Wang SS. Cardiac repair achieved by bone marrow mesenchymal stem cells/silk fibroin/hyaluronic acid patches in a rat of myocardial infarction model. Biomaterials. (2012) 33:5541–51. 10.1016/j.biomaterials.2012.04.030 [DOI] [PubMed] [Google Scholar]
- 89.Menasché P, Vanneaux V, Fabreguettes JR, Bel A, Tosca L, Garcia S, et al. Towards a clinical use of human embryonic stem cell-derived cardiac progenitors: a translational experience. Eur Heart J. (2015) 36:743–50. 10.1093/eurheartj/ehu192 [DOI] [PubMed] [Google Scholar]
- 90.Menasché P, Vanneaux V, Hagège A, Bel A, Cholley B, Cacciapuoti I, et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur Heart J. (2015) 36:2011–7. 10.1093/eurheartj/ehv189 [DOI] [PubMed] [Google Scholar]
- 91.Menasché P, Vanneaux V, Hagège A, Bel A, Cholley B, Parouchev A, et al. Transplantation of human embryonic stem cell-derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J Am Coll Cardiol. (2018) 71:429–38. 10.1016/j.jacc.2017.11.047 [DOI] [PubMed] [Google Scholar]
- 92.Qasim M, Haq F, Kang MH, Kim JH. 3D printing approaches for cardiac tissue engineering and role of immune modulation in tissue regeneration. Int J Nanomedicine. (2019). 14:1311–33. 10.2147/IJN.S189587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tomov ML, Theus A, Sarasani R, Chen H, Serpooshan V. 3D bioprinting of cardiovascular tissue constructs: cardiac bioinks. In: Serpooshan V, Wu SM. editors. Cardiovascular Regenerative Medicine: Tissue Engineering Clinical Applications. Cham: Springer International Publishing; (2019). p. 63–77. 10.1007/978-3-030-20047-3_4 [DOI] [Google Scholar]
- 94.Tomov ML, Gil CJ, Cetnar A, Theus AS, Lima BJ, Nish JE, et al. Engineering functional cardiac tissues for regenerative medicine applications. Curr Cardiol Rep. (2019) 21:105. 10.1007/s11886-019-1178-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nguyen DC, Hookway TA, Wu Q, Jha R, Preininger MK, Chen X, et al. Microscale generation of cardiospheres promotes robust enrichment of cardiomyocytes derived from human pluripotent stem cells. Stem Cell Rep. (2014) 3:260–8. 10.1016/j.stemcr.2014.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Polonchuk L, Chabria M, Badi L, Hoflack JC, Figtree G, Davies MJ, et al. Cardiac spheroids as promising in vitro models to study the human heart microenvironment. Sci Rep. (2017) 7:7005. 10.1038/s41598-017-06385-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Campbell M, Chabria M, Figtree GA, Polonchuk L, Gentile C. Stem cell-derived cardiac spheroids as 3D in vitro models of the human heart microenvironment. Methods Mol Biol. (2019) 2002:51–9. 10.1007/7651_2018_187 [DOI] [PubMed] [Google Scholar]
- 98.Oltolina F, Zamperone A, Colangelo D, Gregoletto L, Reano S, Pietronave S, et al. Human cardiac progenitor spheroids exhibit enhanced engraftment potential. PLoS ONE. (2015) 10:e0137999. 10.1371/journal.pone.0137999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao S, Xu Z, Wang H, Reese BE, Gushchina LV, Jiang M, et al. Bioengineering of injectable encapsulated aggregates of pluripotent stem cells for therapy of myocardial infarction. Nat Commun. (2016) 7:13306. 10.1038/ncomms13306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bartulos O, Zhuang ZW, Huang Y, Mikush N, Suh C, Bregasi A, et al. ISL1 cardiovascular progenitor cells for cardiac repair after myocardial infarction. JCI Insight. (2016) 1:e80920. 10.1172/jci.insight.80920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mattapally S, Zhu W, Fast VG, Gao L, Worley C, Kannappan R, et al. Spheroids of cardiomyocytes derived from human-induced pluripotent stem cells improve recovery from myocardial injury in mice. Am J Physiol Heart Circ Physiol. (2018) 315:H327–39. 10.1152/ajpheart.00688.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.LaBarge W, Mattappally S, Kannappan R, Fast VG, Pretorius D, Berry JL, et al. Maturation of three-dimensional, hiPSC-derived cardiomyocyte spheroids utilizing cyclic, uniaxial stretch and electrical stimulation. PLoS ONE. (2019) 14:e0219442. 10.1371/journal.pone.0219442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ong CS, Fukunishi T, Nashed A, Blazeski A, Zhang H, Hardy S, et al. Creation of cardiac tissue exhibiting mechanical integration of spheroids using 3D bioprinting. J Vis Exp. (2017). 10.3791/55438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ong CS, Fukunishi T, Zhang H, Huang CY, Nashed A, Blazeski A, et al. Biomaterial-free three-dimensional bioprinting of cardiac tissue using human induced pluripotent stem cell derived cardiomyocytes. Sci Rep. (2017) 7:4566. 10.1038/s41598-017-05018-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhu Y, Serpooshan V, Wu S, Demirci U, Chen P, Güven S. Tissue engineering of 3D organotypic microtissues by acoustic assembly. Methods Mol Biol. (2019) 1576:301–12. 10.1007/7651_2017_68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee S, Serpooshan V, Tong X, Venkatraman S, Lee M, Lee J, et al. Contractile force generation by 3D hiPSC-derived cardiac tissues is enhanced by rapid establishment of cellular interconnection in matrix with muscle-mimicking stiffness. Biomaterials. (2017) 131:111–20. 10.1016/j.biomaterials.2017.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Serpooshan V, Chen P, Wu H, Lee S, Sharma A, Hu DA, et al. Bioacoustic-enabled patterning of human iPSC-derived cardiomyocytes into 3D cardiac tissue. Biomaterials. (2017) 131:47–57. 10.1016/j.biomaterials.2017.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Haraguchi Y, Shimizu T, Yamato M, Okano T. Regenerative therapies using cell sheet-based tissue engineering for cardiac disease. Cardiol Res Pract. (2011) 2011:845170. 10.4061/2011/845170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Okano T, Yamada N, Sakai H, Sakurai Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide). J Biomed Mater Res. (1993) 27:1243–51. 10.1002/jbm.820271005 [DOI] [PubMed] [Google Scholar]
- 110.Shimizu T, Yamato M, Isoi Y, Akutsu T, Setomaru T, Abe K, et al. Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. Circ Res. (2002) 90:e40. 10.1161/hh0302.105722 [DOI] [PubMed] [Google Scholar]
- 111.Haraguchi Y, Shimizu T, Yamato M, Kikuchi A, Okano T. Electrical coupling of cardiomyocyte sheets occurs rapidly via functional gap junction formation. Biomaterials. (2006) 27:4765–74. 10.1016/j.biomaterials.2006.04.034 [DOI] [PubMed] [Google Scholar]
- 112.Kreutziger KL, Muskheli V, Johnson P, Braun K, Wight TN, Murry CE. Developing vasculature and stroma in engineered human myocardium. Tissue Eng Part A. (2011) 17:1219–28. 10.1089/ten.tea.2010.0557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shudo Y, Miyagawa S, Fukushima S, Saito A, Shimizu T, Okano T, et al. Novel regenerative therapy using cell-sheet covered with omentum flap delivers a huge number of cells in a porcine myocardial infarction model. J Thorac Cardiovasc Surg. (2011) 142:1188–96. 10.1016/j.jtcvs.2011.07.002 [DOI] [PubMed] [Google Scholar]
- 114.Kawamura M, Miyagawa S, Miki K, Saito A, Fukushima S, Higuchi T, et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation. (2012) 126(11 Suppl. 1):S29–37. 10.1161/CIRCULATIONAHA.111.084343 [DOI] [PubMed] [Google Scholar]
- 115.Kawamura M, Miyagawa S, Fukushima S, Saito A, Miki K, Ito E, et al. Enhanced survival of transplanted human induced pluripotent stem cell-derived cardiomyocytes by the combination of cell sheets with the pedicled omental flap technique in a porcine heart. Circulation. (2013) 128(11 Suppl. 1):S87–94. 10.1161/CIRCULATIONAHA.112.000366 [DOI] [PubMed] [Google Scholar]
- 116.Kawamura M, Miyagawa S, Fukushima S, Saito A, Miki K, Funakoshi S, et al. Enhanced therapeutic effects of human iPS cell derived-cardiomyocyte by combined cell-sheets with omental flap technique in porcine ischemic cardiomyopathy model. Sci Rep. (2017) 7:8824. 10.1038/s41598-017-08869-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Masumoto H, Matsuo T, Yamamizu K, Uosaki H, Narazaki G, Katayama S, et al. Pluripotent stem cell-engineered cell sheets reassembled with defined cardiovascular populations ameliorate reduction in infarct heart function through cardiomyocyte-mediated neovascularization. Stem Cells. (2012) 30:1196–205. 10.1002/stem.1089 [DOI] [PubMed] [Google Scholar]
- 118.Sawa Y, Yoshikawa Y, Toda K, Fukushima S, Yamazaki K, Ono M, et al. Safety and efficacy of autologous skeletal myoblast sheets (TCD-51073) for the treatment of severe chronic heart failure due to ischemic heart disease. Circ J. (2015) 79:991–9. 10.1253/circj.CJ-15-0243 [DOI] [PubMed] [Google Scholar]
- 119.Yoshikawa Y, Miyagawa S, Toda K, Saito A, Sakata Y, Sawa Y. Myocardial regenerative therapy using a scaffold-free skeletal-muscle-derived cell sheet in patients with dilated cardiomyopathy even under a left ventricular assist device: a safety and feasibility study. Surg Today. (2018) 48:200–10. 10.1007/s00595-017-1571-1 [DOI] [PubMed] [Google Scholar]
- 120.Cyranoski D. ‘Reprogrammed’ stem cells approved to mend human hearts for the first time. Nature. (2018). 557:619–620. 10.1038/d41586-018-05278-8 [DOI] [PubMed] [Google Scholar]
- 121.Zhang J. Engineered tissue patch for cardiac cell therapy. Curr Treat Options Cardiovasc Med. (2015). 17:399. 10.1007/s11936-015-0399-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rienks M, Papageorgiou AP, Frangogiannis NG, Heymans S. Myocardial extracellular matrix: an ever-changing and diverse entity. Circ Res. (2014) 114:872–88. 10.1161/CIRCRESAHA.114.302533 [DOI] [PubMed] [Google Scholar]
- 123.Nielsen SH, Mouton AJ, DeLeon-Pennell KY, Genovese F, Karsdal M, Lindsey ML. Understanding cardiac extracellular matrix remodeling to develop biomarkers of myocardial infarction outcomes. Matrix Biol. (2019) 75-76:43–57. 10.1016/j.matbio.2017.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Adv Drug Deliv Rev. (2016) 97:4–27. 10.1016/j.addr.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 125.Leor J, Landa N, Cohen S. Renovation of the injured heart with myocardial tissue engineering. Expert Rev Cardiovasc Ther. (2006) 4:239–52. 10.1586/14779072.4.2.239 [DOI] [PubMed] [Google Scholar]
- 126.Reis LA, Chiu LL, Feric N, Fu L, Radisic M. Biomaterials in myocardial tissue engineering. J Tissue Eng Regen Med. (2016) 10:11–28. 10.1002/term.1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen QZ, Harding SE, Ali NN, Lyon AR, Boccaccini AR. Biomaterials in cardiac tissue engineering: ten years of research survey. Mater Sci Eng R Rep. (2008) 59:1–37. 10.1016/j.mser.2007.08.001 [DOI] [Google Scholar]
- 128.Serpooshan V, Mahmoudi M, Hu DA, Hu JB, Wu SM. Bioengineering cardiac constructs using 3D printing. J 3D Print Med. (2017) 1:123–39. 10.2217/3dp-2016-0009 [DOI] [Google Scholar]
- 129.Fukuhara S, Tomita S, Nakatani T, Fujisato T, Ohtsu Y, Ishida M, et al. Bone marrow cell-seeded biodegradable polymeric scaffold enhances angiogenesis and improves function of the infarcted heart. Circ J. (2005) 69:850–7. 10.1253/circj.69.850 [DOI] [PubMed] [Google Scholar]
- 130.Engelmayr GC, Jr., Cheng M, Bettinger CJ, Borenstein JT, Langer R, et al. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat Mater. (2008) 7:1003–10. 10.1038/nmat2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Prabhakaran MP, Kai D, Ghasemi-Mobarakeh L, Ramakrishna S. Electrospun biocomposite nanofibrous patch for cardiac tissue engineering. Biomed Mater. (2011) 6:055001. 10.1088/1748-6041/6/5/055001 [DOI] [PubMed] [Google Scholar]
- 132.Liu BH, Yeh HY, Lin YC, Wang MH, Chen DC, Lee BH, et al. Spheroid formation and enhanced cardiomyogenic potential of adipose-derived stem cells grown on chitosan. Biores Open Access. (2013) 2:28–39. 10.1089/biores.2012.0285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu Q, Tian S, Zhao C, Chen X, Lei I, Wang Z, et al. Porous nanofibrous poly(L-lactic acid) scaffolds supporting cardiovascular progenitor cells for cardiac tissue engineering. Acta Biomater. (2015) 26:105–14. 10.1016/j.actbio.2015.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sidorov VY, Samson PC, Sidorova TN, Davidson JM, Lim CC, Wikswo JP. I-wire heart-on-a-Chip I: three-dimensional cardiac tissue constructs for physiology and pharmacology. Acta Biomater. (2017) 48:68–78. 10.1016/j.actbio.2016.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hajipour MJ, Mehrani M, Abbasi SH, Amin A, Kassaian SE, Garbern JC, et al. Nanoscale technologies for prevention and treatment of heart failure: challenges and opportunities. Chem Rev. (2019) 119:11352–90. 10.1021/acs.chemrev.8b00323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Khan OF, Sefton MV. Endothelialized biomaterials for tissue engineering applications in vivo. Trends Biotechnol. (2011) 29:379–87. 10.1016/j.tibtech.2011.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sánchez PL, Fernández-Santos ME, Costanza S, Climent AM, Moscoso I, Gonzalez-Nicolas MA, et al. Acellular human heart matrix: a critical step toward whole heart grafts. Biomaterials. (2015) 61:279–89. 10.1016/j.biomaterials.2015.04.056 [DOI] [PubMed] [Google Scholar]
- 138.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. (2008) 14:213–21. 10.1038/nm1684 [DOI] [PubMed] [Google Scholar]
- 139.Shah M, Kc P, Zhang G. In vivo assessment of decellularized porcine myocardial slice as an acellular cardiac patch. ACS Appl Mater Interfaces. (2019) 11:23893–900. 10.1021/acsami.9b06453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jia W, Gungor-Ozkerim PS, Zhang YS, Yue K, Zhu K, Liu W, et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials. (2016) 106:58–68. 10.1016/j.biomaterials.2016.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Datta P, Ayan B, Ozbolat IT. Bioprinting for vascular and vascularized tissue biofabrication. Acta Biomater. (2017) 51:1–20. 10.1016/j.actbio.2017.01.035 [DOI] [PubMed] [Google Scholar]
- 142.Hu JB, Hu DA, Buikema JW, Chirikian O, Venkatraman S, Serpooshan V, et al. Bioengineering of vascular myocardial tissue; a 3D bioprinting approach. Tissue Engineering Part A. (2017) 23:S158–9. [Google Scholar]
- 143.Serpooshan V, Hu JB, Chirikian O, Hu DA, Mahmoudi M, Wu SM, et al. Chapter 8-4D printing of actuating cardiac tissue. In:. Min J, Mosadegh B, Dunham S, Al'Aref S. editors. 3D Printing Applications in Cardiovascular Medicine. (2018) Boston, MA: (2018). p. 153–62. 10.1016/B978-0-12-803917-5.00008-0 [DOI] [Google Scholar]
- 144.Gaetani R, Doevendans PA, Metz CH, Alblas J, Messina E, Giacomello A, et al. Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials. (2012) 33:1782–90. 10.1016/j.biomaterials.2011.11.003 [DOI] [PubMed] [Google Scholar]
- 145.Gaetani R, Feyen DA, Verhage V, Slaats R, Messina E, Christman KL, et al. Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials. (2015) 61:339–48. 10.1016/j.biomaterials.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 146.LaBarge W, Morales A, Pretorius D, Kahn-Krell AM, Kannappan R, Zhang J. Scaffold-Free bioprinter utilizing layer-by-layer printing of cellular spheroids. Micromachines. (2019) 10:570. 10.3390/mi10090570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ajeti V, Lien CH, Chen SJ, Su PJ, Squirrell JM, Molinarolo KH, et al. Image-inspired 3D multiphoton excited fabrication of extracellular matrix structures by modulated raster scanning. Opt Express. (2013) 21:25346–5. 10.1364/OE.21.025346 [DOI] [PubMed] [Google Scholar]
- 148.Kupfer ME, Lin WH, Ravikumar V, Qiu K, Wang L, Gao L, et al. In situ expansion, differentiation and electromechanical coupling of human cardiac muscle in a 3D bioprinted, chambered organoid. Circ Res. (2020). 10.1161/CIRCRESAHA.119.316155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Marbán E. The secret life of exosomes: what bees can teach Us about next-generation therapeutics. J Am Coll Cardiol. (2018). 71:193–200. 10.1016/j.jacc.2017.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Merino-González C, Zuñiga FA, Escudero C, Ormazabal V, Reyes C, Nova-Lamperti E, et al. Mesenchymal stem cell-derived extracellular vesicles promote angiogenesis: potencial clinical application. Front Physiol. (2016) 7:24. 10.3389/fphys.2016.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bar A, Cohen S. Inducing endogenous cardiac regeneration: can biomaterials connect the dots? Front Bioeng Biotechnol. (2020) 8:126. 10.3389/fbioe.2020.00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Huang K, Ozpinar EW, Su T, Tang J, Shen D, Qiao L, et al. An off-the-shelf artificial cardiac patch improves cardiac repair after myocardial infarction in rats and pigs. Sci Transl Med. (2020) 12:eaat9683. 10.1126/scitranslmed.aat9683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang LL, Liu Y, Chung JJ, Wang T, Gaffey AC, Lu M, et al. Local and sustained miRNA delivery from an injectable hydrogel promotes cardiomyocyte proliferation and functional regeneration after ischemic injury. Nat Biomed Eng. (2017) 1:983–92. 10.1038/s41551-017-0157-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu B, Lee BW, Nakanishi K, Villasante A, Williamson R, Metz J, et al. Cardiac recovery via extended cell-free delivery of extracellular vesicles secreted by cardiomyocytes derived from induced pluripotent stem cells. Nat Biomed Eng. (2018) 2:293–303. 10.1038/s41551-018-0229-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA, Lewis JA. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater. (2014). 26:3124–30. 10.1002/adma.201305506 [DOI] [PubMed] [Google Scholar]
- 156.Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen DH, Cohen DM, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater. (2012) 11:768–4. 10.1038/nmat3357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fleischer S, Shapira A, Feiner R, Dvir T. Modular assembly of thick multifunctional cardiac patches. Proc Natl Acad Sci USA. (2017) 114:1898–903. 10.1073/pnas.1615728114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jackman CP, Carlson AL, Bursac N. Dynamic culture yields engineered myocardium with near-adult functional output. Biomaterials. (2016) 111:66–79. 10.1016/j.biomaterials.2016.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kannan RY, Salacinski HJ, Sales K, Butler P, Seifalian AM. The roles of tissue engineering and vascularisation in the development of micro-vascular networks: a review. Biomaterials. (2005) 26:1857–75. 10.1016/j.biomaterials.2004.07.006 [DOI] [PubMed] [Google Scholar]
- 160.Park JH, Chung BG, Lee WG, Kim J, Brigham MD, Shim J, et al. Microporous cell-laden hydrogels for engineered tissue constructs. Biotechnol Bioeng. (2010) 106:138–48. 10.1002/bit.22667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC, et al. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. (2005) 23:879–84. 10.1038/nbt1109 [DOI] [PubMed] [Google Scholar]
- 162.Oduk Y, Zhu W, Kannappan R, Zhao M, Borovjagin AV, Oparil S, et al. VEGF nanoparticles repair the heart after myocardial infarction. Am J Physiol Heart Circ Physiol. (2018) 314:H278–84. 10.1152/ajpheart.00471.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fan C, Tang Y, Zhao M, Lou X, Pretorius D, Menasche P, et al. CHIR99021 and fibroblast growth factor 1 enhance the regenerative potency of human cardiac muscle patch after myocardial infarction in mice. J Mol Cell Cardiol. (2020) 141:1–0. 10.1016/j.yjmcc.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kim JJ, Hou L, Huang NF. Vascularization of three-dimensional engineered tissues for regenerative medicine applications. Acta Biomater. (2016) 41:17–26. 10.1016/j.actbio.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shin SR, Aghaei-Ghareh-Bolagh B, Gao X, Nikkhah M, Jung SM, Dolatshahi-Pirouz A. Layer-by-layer assembly of 3D tissue constructs with functionalized graphene. Advanced functional materials. (2014) 24:6136–44. 10.1002/adfm.201401300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang B, Montgomery M, Davenport-Huyer L, Korolj A, Radisic M. Platform technology for scalable assembly of instantaneously functional mosaic tissues. Sci Adv. (2015) 1:e1500423. 10.1126/sciadv.1500423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yang J, Yamato M, Kohno C, Nishimoto A, Sekine H, Fukai F, et al. Cell sheet engineering: recreating tissues without biodegradable scaffolds. Biomaterials. (2005) 26:6415–22. 10.1016/j.biomaterials.2005.04.061 [DOI] [PubMed] [Google Scholar]
- 168.Haraguchi Y, Shimizu T, Sasagawa T, Sekine H, Sakaguchi K, Kikuchi T, et al. Fabrication of functional three-dimensional tissues by stacking cell sheets in vitro. Nat Protoc. (2012) 7:850–8. 10.1038/nprot.2012.027 [DOI] [PubMed] [Google Scholar]
- 169.Sekine H, Shimizu T, Sakaguchi K, Dobashi I, Wada M, Yamato M, et al. In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat Commun. (2013) 4:1399. 10.1038/ncomms2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Radisic M, Yang L, Boublik J, Cohen RJ, Langer R, Freed LE, et al. Medium perfusion enables engineering of compact and contractile cardiac tissue. Am J Physiol Heart Circ Physiol. (2004) 286:H507–16. 10.1152/ajpheart.00171.2003 [DOI] [PubMed] [Google Scholar]
- 171.Radisic M, Deen W, Langer R, Vunjak-Novakovic G. Mathematical model of oxygen distribution in engineered cardiac tissue with parallel channel array perfused with culture medium containing oxygen carriers. Am J Physiol Heart Circ Physiol. (2005) 288:H1278–89. 10.1152/ajpheart.00787.2004 [DOI] [PubMed] [Google Scholar]
- 172.Radisic M, Park H, Chen F, Salazar-Lazzaro JE, Wang Y, Dennis R, et al. Biomimetic approach to cardiac tissue engineering: oxygen carriers and channeled scaffolds. Tissue Eng. (2006) 12:2077–91. 10.1089/ten.2006.12.2077 [DOI] [PubMed] [Google Scholar]
- 173.Vukadinovic-Nikolic Z, Andrée B, Dorfman SE, Pflaum M, Horvath T, Lux M, et al. Generation of bioartificial heart tissue by combining a three-dimensional gel-based cardiac construct with decellularized small intestinal submucosa. Tissue Eng Part A. (2014) 20:799–809. 10.1089/ten.tea.2013.0184 [DOI] [PubMed] [Google Scholar]
- 174.Zhang B, Montgomery M, Chamberlain MD, Ogawa S, Korolj A, Pahnke A, et al. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nat Mater. (2016) 15:669–78. 10.1038/nmat4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Brown MA, Iyer RK, Radisic M. Pulsatile perfusion bioreactor for cardiac tissue engineering. Biotechnol Prog. (2008) 24:907–20. 10.1002/btpr.11 [DOI] [PubMed] [Google Scholar]
- 176.Golden AP, Tien J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip. (2007) 7:720–5. 10.1039/b618409j [DOI] [PubMed] [Google Scholar]
- 177.Chiu LL, Montgomery M, Liang Y, Liu H, Radisic M. Perfusable branching microvessel bed for vascularization of engineered tissues. Proc Natl Acad Sci USA. (2012) 109:E3414–23. 10.1073/pnas.1210580109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ye X, Lu L, Kolewe ME, Park H, Larson BL, Kim ES, et al. A biodegradable microvessel scaffold as a framework to enable vascular support of engineered tissues. Biomaterials. (2013) 34:10007–15. 10.1016/j.biomaterials.2013.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chaturvedi RR, Stevens KR, Solorzano RD, Schwartz RE, Eyckmans J, Baranski JD, et al. Patterning vascular networks in vivo for tissue engineering applications. Tissue Eng Part C Methods. (2015) 21:509–17. 10.1089/ten.tec.2014.0258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cui X, Boland T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials. (2009) 30:6221–7. 10.1016/j.biomaterials.2009.07.056 [DOI] [PubMed] [Google Scholar]
- 181.Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci U S A. (2016) 113:3179–84. 10.1073/pnas.1521342113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Aguado BA, Mulyasasmita W, Su J, Lampe KJ, Heilshorn SC. Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng Part A. (2012) 18:806–15. 10.1089/ten.tea.2011.0391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tang XL, Nakamura S, Li Q, Wysoczynski M, Gumpert AM, Wu WJ, et al. Repeated Administrations of Cardiac Progenitor Cells Are Superior to a Single Administration of an Equivalent Cumulative Dose. J Am Heart Assoc. (2018) 7(4). 10.1161/JAHA.117.007400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Desgres M, Menasché P. Clinical Translation of Pluripotent Stem Cell Therapies: Challenges and Considerations. Cell Stem Cell. (2019) 25:594–606. 10.1016/j.stem.2019.10.001 [DOI] [PubMed] [Google Scholar]
- 185.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Reisman MA, Schaer GL, Sherman W, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. (2009) 54:2277–86. 10.1016/j.jacc.2009.06.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Garcia JR, Campbell PF, Kumar G, Langberg JJ, Cesar L, Wang L, et al. A Minimally Invasive, Translational Method to Deliver Hydrogels to the Heart Through the Pericardial Space. JACC Basic Transl Sci. (2017) 2:601–609. 10.1016/j.jacbts.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pena B, Laughter M, Jett S, Rowland TJ, M.Taylor RG, Mestroni L, et al. Injectable Hydrogels for Cardiac Tissue Engineering. Macromol Biosci. (2018) 18:e1800079. 10.1002/mabi.201800079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wu T, Cui C, Huang Y, Liu Y, Fan C, Han X, et al. Coadministration of an Adhesive Conductive Hydrogel Patch and an Injectable Hydrogel to Treat Myocardial Infarction. ACS Appl Mater Interfaces. (2020) 12:2039–2048. 10.1021/acsami.9b17907 [DOI] [PubMed] [Google Scholar]
- 189.Frey N, Linke A, Suselbeck T, Muller-Ehmsen J, Vermeersch P, Schoors D, et al. Intracoronary delivery of injectable bioabsorbable scaffold (IK-5001) to treat left ventricular remodeling after ST-elevation myocardial infarction: a first-in-man study. Circ Cardiovasc Interv. (2014) 7:806–12. 10.1161/CIRCINTERVENTIONS.114.001478 [DOI] [PubMed] [Google Scholar]
- 190.Anker SD, Coats AJ, Cristian G, Dragomir D, Pusineri E, Piredda M, et al. A prospective comparison of alginate-hydrogel with standard medical therapy to determine impact on functional capacity and clinical outcomes in patients with advanced heart failure (AUGMENT-HF trial). Eur Heart J. (2015) 36:2297–309. 10.1093/eurheartj/ehv259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gil CJ, Tomov ML, Theus AS, Cetnar A, Mahmoudi M, Serpooshan V. In vivo tracking of tissue engineered constructs. Micromachines. (2019) 10:474. 10.3390/mi10070474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mahmoudi M, Zhao M, Matsuura Y, Laurent S, Yang PC, Bernstein D, et al. Infection-resistant MRI-visible scaffolds for tissue engineering applications. Bioimpacts. (2016) 6:111–5. 10.15171/bi.2016.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J, et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. (2012) 489:322–5. 10.1038/nature11317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Qin X, Riegler J, Tiburcy M, Zhao X, Chour T, Ndoye B, et al. Magnetic resonance imaging of cardiac strain pattern following transplantation of human tissue engineered heart muscles. Circ Cardiovasc Imaging. (2016) 9:e004731. 10.1161/CIRCIMAGING.116.004731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gao L, Wang L, Wei Y, Krishnamurthy P, Walcott GP, Menasché P, et al. Exosomes secreted by hiPSC-derived cardiac cells improve recovery from myocardial infarction in swine. Sci Transl Med. (2020) 12:eaay1318. 10.1126/scitranslmed.aay1318 [DOI] [PubMed] [Google Scholar]
- 196.Shiba Y, Gomibuchi T, Seto T, Wada Y, Ichimura H, Tanaka Y, et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature. (2016) 538:388–91. 10.1038/nature19815 [DOI] [PubMed] [Google Scholar]
- 197.Rai V, Sharma P, Agrawal S, Agrawal DK. Relevance of mouse models of cardiac fibrosis and hypertrophy in cardiac research. Mol Cell Biochem. (2017) 424:123–45. 10.1007/s11010-016-2849-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Romagnuolo R, Masoudpour H, Porta-Sánchez A, Qiang B, Barry J, Laskary A, et al. Human embryonic stem cell-derived cardiomyocytes regenerate the infarcted pig heart but induce ventricular tachyarrhythmias. Stem Cell Rep. (2019) 12:967–81. 10.1016/j.stemcr.2019.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wei K, Serpooshan V, Hurtado C, Diez-Cunado M, Zhao M, Maruyama S, et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature. (2015) 525:479–85. 10.1038/nature15372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Serpooshan V, Zhao M, Metzler SA, Wei K, Shah PB, Wang A, et al. The effect of bioengineered acellular collagen patch on cardiac remodeling and ventricular function post myocardial infarction. Biomaterials. (2013) 34:9048–55. 10.1016/j.biomaterials.2013.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Masumoto H, Nakane T, Tinney JP, Yuan F, Ye F, Kowalski WJ, et al. The myocardial regenerative potential of three-dimensional engineered cardiac tissues composed of multiple human iPS cell-derived cardiovascular cell lineages. Sci Rep. (2016) 6:29933. 10.1038/srep29933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zimmermann WH. Translating myocardial remuscularization. Circ Res. (2017). 120:278–81. 10.1161/CIRCRESAHA.116.310194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhu W, Zhao M, Mattapally S, Chen S, Zhang J. CCND2 overexpression enhances the regenerative potency of human induced pluripotent stem cell–derived cardiomyocytes: remuscularization of injured ventricle. Circ Res. (2018) 122:88–96. 10.1161/CIRCRESAHA.117.311504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ren J, Xu Q, Chen X, Li W, Guo K, Zhao Y, et al. Superaligned carbon nanotubes guide oriented cell growth and promote electrophysiological homogeneity for synthetic cardiac tissues. Adv Mater. (2017) 29:1702713. 10.1002/adma.201702713 [DOI] [PubMed] [Google Scholar]
- 205.Wang L, Wu Y, Hu T, Guo B, Ma PX. Electrospun conductive nanofibrous scaffolds for engineering cardiac tissue and 3D bioactuators. Acta Biomater. (2017) 59:68–81. 10.1016/j.actbio.2017.06.036 [DOI] [PubMed] [Google Scholar]
- 206.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. (2008) 103:1204–19. 10.1161/CIRCRESAHA.108.176826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zimmermann WH. Remuscularization of the failing heart. J Physiol. (2017). 595:3685–90. 10.1113/JP273098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Malat G, Culkin C. The ABCs of immunosuppression: a primer for primary care physicians. Med Clin North Am. (2016) 100:505–18. 10.1016/j.mcna.2016.01.003 [DOI] [PubMed] [Google Scholar]
- 209.Pan Y, Leveson-Gower DB, de Almeida PE, Pierini A, Baker J, Florek M, et al. Engraftment of embryonic stem cells and differentiated progeny by host conditioning with total lymphoid irradiation and regulatory T cells. Cell Rep. (2015) 10:1793–802. 10.1016/j.celrep.2015.02.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Braza MS, M.van Leent MT, Lameijer M, Sanchez-Gaytan BL, Arts RJW, Pérez-Medina C, et al. Inhibiting inflammation with myeloid cell-specific nanobiologics promotes organ transplant acceptance. Immunity. (2018) 49:819–28.e6. 10.1016/j.immuni.2018.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. (2014) 157:1262–78. 10.1016/j.cell.2014.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kim H, Kim JS. A guide to genome engineering with programmable nucleases. Nat Rev Genet. (2014) 15:321–4. 10.1038/nrg3686 [DOI] [PubMed] [Google Scholar]
- 213.Mattapally S, Pawlik KM, Fast VG, Zumaquero E, Lund FE, Randall TD, et al. Human leukocyte antigen class I and II knockout human induced pluripotent stem cell–derived cells: universal donor for cell therapy. J Am Heart Assoc. (2018) 7:e010239. 10.1161/JAHA.118.010239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Deuse T, Hu X, Gravina A, Wang D, Tediashvili G, De C, et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat Biotechnol. (2019) 37:252. 10.1038/s41587-019-0016-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Xu H, Wang B, Ono M, Kagita A, Fujii K, Sasakawa N, et al. Targeted disruption of HLA genes via CRISPR-Cas9 generates iPSCs with enhanced immune compatibility. Cell Stem Cell. (2019) 24:566–78. e7. 10.1016/j.stem.2019.02.005 [DOI] [PubMed] [Google Scholar]