Abstract
Background
SDZ-ADL (GP2017; Sandoz GmbH, Austria) is an EMA-/FDA-approved adalimumab biosimilar. The effect of SDZ-ADL on quality of life (QoL) and patient-reported outcomes (PROs) was assessed as part of two phase III studies, one in patients with moderate‐to‐severe chronic plaque psoriasis (PsO; ADACCESS) and the other in patients with rheumatoid arthritis (RA; ADMYRA). Additionally, ADACCESS included patients with psoriatic arthritis (PsA).
Methods
ADACCESS included 465 patients with PsO, whereas ADMYRA included 353 patients with RA. Both studies evaluated and confirmed equivalent efficacy, similar safety, and immunogenicity of SDZ-ADL with reference adalimumab (ref-ADL). A third of patients underwent multiple (four) treatment switches between study treatments starting at Week 17 (ADACCESS); all patients switched from ref-ADL to SDZ-ADL at Week 24 (ADMYRA). Assessed PROs included Dermatology Life Quality Index (DLQI) and EuroQol five-dimension health status questionnaire (EQ-5D-5L) in ADACCESS, Functional Assessment of Chronic Illness Therapy–Fatigue Scale (FACIT-Fatigue) score in ADMYRA, and Health Assessment Questionnaire–Disability Index (HAQ-DI) in both studies.
Results
In both studies, baseline scores for all PRO assessments were comparable between the two treatment groups. In ADACCESS, mean DLQI decreased from baseline in both groups, and the mean (standard deviation [SD]) percent reductions from baseline in DLQI were comparable between groups at Week 17 (SDZ-ADL, − 64.5 [80.3]; ref-ADL, − 70.6 [41.7]), which were sustained after the switch at Week 51 (‘continued SDZ-ADL,’ − 79.7 [36.2]; ‘continued ref-ADL,’ − 80.8 [44.6]; ‘switched to SDZ-ADL,’ − 70.7 [32.2]; ‘switched to ref-ADL,’ − 69.3 [49.6]). In ADACCESS, the proportion of patients with an EQ-5D-5L score of 1 (no problems) increased from baseline for all five dimensions in all treatment groups and was comparable between treatment groups at Week 51. In ADACCESS, in patients with PsA at baseline, mean (SD) HAQ-DI scores decreased from baseline in both treatment groups, and scores were comparable between groups at Week 17 (SDZ-ADL, 0.5 [0.6]; ref-ADL, 0.5 [0.6]) and after switching at Week 51 (‘continued SDZ-ADL,’ 0.4 [0.5]; ‘continued ref-ADL,’ 0.4 [0.6]; ‘switched to SDZ-ADL,’ 0.5 [0.8]; ‘switched to ref-ADL,’ 0.7 [0.6]). In ADMYRA, proportion of patients achieving HAQ-DI in the normal range (≤ 0.5) was comparable between treatment groups at Week 24 (SDZ-ADL, 37.8%; ref-ADL, 36.3%) and after switching at Week 48 (‘SDZ-ADL,’ 41.6%; ‘ref-ADL/switched to SDZ-ADL,’ 40.0%). In ADMYRA, mean FACIT-Fatigue scores increased from baseline in both treatment groups. At Week 24, mean (SD) percent change from baseline in the FACIT-Fatigue scores was 75.4 (135.5) in SDZ-ADL and 73.0 (96.3) in ref-ADL groups; the scores were sustained after switching at Week 48.
Conclusion
Treatment with SDZ-ADL and ref-ADL resulted in comparable improvements in PROs as well as QoL scores across the three diseases, PsO, PsA, and RA. Switching between SDZ-ADL and ref-ADL had no negative impact on PROs across the reported period.
Clinical trials.gov identifier
NCT02744755, NCT02016105.
Supplementary Information
The online version of this article (10.1007/s40259-021-00470-1) contains supplementary material, which is available to authorized users.
Key Points
| Meaningful improvements in patient-reported outcomes (PROs) and quality of life scores were observed in both SDZ-ADL and reference adalimumab (ref-ADL) treatment groups. These data, together with evidence of comparable efficacy and safety findings, further support the biosimilarity of SDZ-ADL and ref-ADL. |
| To date, this is the first report describing PROs with an adalimumab biosimilar and reference medicine across three diseases, psoriasis, psoriatic arthritis, and rheumatoid arthritis. |
Introduction
Immune-mediated inflammatory diseases (IMIDs), such as rheumatoid arthritis (RA), psoriasis (PsO), and psoriatic arthritis (PsA), are often associated with impaired physical functioning, work productivity, and quality of life (QoL) [1–3]. Biologics, including anti-TNF (tumor necrosis factor) medications, represent excellent therapeutic options in the management of these IMIDs [4–6]. The advent of biosimilars provides the potential to increase patient access to treatment at lower prices without compromising patient outcomes [7, 8].
Patient-reported outcomes (PROs) are standardized measures that provide a critical understanding of the benefits of the drug as well as reflect patient perspectives related to the disease and treatment [9]. The importance of PRO assessment has gained increasing recognition by regulatory authorities, clinicians, and patients [10–12]. PRO data from clinical trials may provide valuable evidence to labeling claims, clinical guidelines, and health policies [11–13]. In addition, incorporation of patient perspectives helps gather patient acceptability information, which in turn could assist clinicians and patients in selecting the best treatment by providing a clearer picture of the benefits that lead to improved quality of care [14, 15].
Although the risk of any negative clinical consequences of switching a patient from a reference biologic medicinal product to a biosimilar is not substantiated by clinical evidence, this perception still exists among physicians and patients [16, 17]. Patients switching from reference biologic medicinal products to biosimilars sometimes perceive disease worsening and/or occurrence of adverse events following the switch, which may be due to negative patient expectations concerning treatment, that is, the nocebo effect [18, 19]. This could lead to nonadherence to a medicine, which in turn can affect disease and QoL.
SDZ-ADL (Sandoz adalimumab; GP2017; Hyrimoz®; Sandoz GmbH, Austria) is an adalimumab biosimilar [20–22]. The equivalent efficacy and comparable safety of SDZ-ADL to reference adalimumab (ref-ADL; Humira®; AbbVie Ltd., Maidenhead, United Kingdom) in patients with moderate‐to‐severe chronic plaque PsO was demonstrated in the phase III ADACCESS study. The primary endpoint of the Psoriasis Area and Severity Index (PASI)-75 response at Week 16 was met; the 95% confidence interval (CI) for the difference in PASI-75 between the SDZ-ADL and ref-ADL treatment groups was contained within a prespecified margin of ±18%. In addition, switching between SDZ-ADL and ref-ADL up to four times had no impact on efficacy, safety, or immunogenicity [23]. Another study (ADMYRA) in patients with moderate-to-severe RA also demonstrated equivalent efficacy of SDZ-ADL and ref-ADL, with a sustained and comparable efficacy after switching patients from ref-ADL to SDZ-ADL [24]. One of the objectives of these two studies was to compare QoL improvements in patients treated with SDZ-ADL versus ref-ADL. The effect of treatment switch between SDZ-ADL and ref-ADL on PROs was also evaluated. Furthermore, the influence of the treatment on patients’ level of functional ability and activity was assessed in patients with concurrent PsA in the ADACCESS study. Here, we present the PRO assessment results across PsO, PsA, and RA indications from the two studies.
Methods
Study Design
The study design and patient populations of the ADACCESS and ADMYRA studies have been reported previously [23, 24]. ADACCESS was a randomized, double-blinded, multicenter, phase III confirmatory study in patients with plaque PsO. Eligible patients were randomized 1:1 to receive 80 mg SDZ-ADL or ref-ADL by subcutaneous (SC) injection, followed by 40 mg every other week starting 1 week after the initial dose until Week 15 (treatment period 1 [TP1]). Patients who had achieved at least 50% improvement in PASI at Week 16 were re-randomized (2:1) at Week 17 to either continue the initial treatment (defined as the ‘continued SDZ-ADL’ and ‘continued ref-ADL’ groups) or undergo a sequence of three treatment switches between SDZ-ADL and ref-ADL until Week 35 (defined as the ‘switched to SDZ-ADL’ and ‘switched to ref-ADL’ groups) (treatment period 2 [TP2]). From Weeks 35 to 51, all patients received the treatment originally assigned at randomization.
ADMYRA was a randomized, double-blinded, parallel-group, multicenter study in patients with moderate-to-severe RA. Eligible patients were randomized 1:1 to receive 40 mg SC SDZ-ADL or ref-ADL every other week for 24 weeks (study period 1 [SP1]). At Week 24, all patients with at least a moderate response by disease activity score, including the 28-joint count Disease Activity Score–C-reactive protein (DAS28-CRP), according to the European League Against Rheumatism (EULAR) response criteria [25, 26] continued SDZ-ADL (defined as the ‘continued SDZ-ADL’ group) in the SDZ-ADL group and were switched to SDZ-ADL (defined as the ‘ref-ADL to SDZ-ADL’ group) in the ref-ADL group for up to 46 weeks (study period 2 [SP2]), with an end-of-study visit at Week 48. The 48-week results are reported for the treatment groups that continued SDZ-ADL (‘SDZ-ADL’) and groups that switched from ref-ADL to SDZ-ADL (‘ref-ADL/switched to SDZ-ADL’).
Both studies were conducted in accordance with the ethical principles derived from the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practices and in compliance with local regulatory requirements. The study protocols were approved by the Independent Ethics Committee or Institutional Review Board for each center. All patients provided written informed consent before entering the study.
Patient-Reported Outcome (PRO) Assessments
ADACCESS Study: Patients with Moderate‐to‐Severe Plaque Psoriasis (PsO)
The PRO assessments included (1) mean actual scores and percent change from baseline in Dermatology Life Quality Index (DLQI) and the proportion of patients achieving a DLQI of 0 or 1 (indicating no impairment in health-related QoL [HRQoL]) up to Weeks 17 and 51; (2) mean EuroQol five-dimension health status questionnaire v2 (EQ-5D-5L) visual analog scale (VAS) scores by visit and proportion of patients achieving an EQ-5D-5L score of 1 (‘no problems’) up to Weeks 17 and 51; and (3) mean actual scores and percent change from baseline in Health Assessment Questionnaire Disability Index (HAQ-DI) scores up to Weeks 17 and 51 in the subset of patients with concurrent PsA (detailed methods in Electronic Supplementary Material [ESM]).
ADMYRA Study: Patients with Moderate-to-Severe Rheumatoid Arthritis (RA)
The endpoints assessed included (1) mean actual scores and percent change from baseline in the HAQ-DI score and the proportion of patients achieving the HAQ index in the normal range (≤ 0.5) and a score improvement > 0.3 at Weeks 4, 12, 24, and 48; (2) mean actual scores and percent change from baseline in the Functional Assessment of Chronic Illness Therapy–Fatigue Scale (FACIT-Fatigue©) Scale scores at Weeks 4, 12, 24, and 48; (3) mean actual scores and percent change from baseline in Patient Global Assessment (PtGA) of disease activity and patient assessment of RA pain at Weeks 2, 4, 12, 24, and 48 (detailed methods in ESM).
Statistical Analysis
Demographics, baseline characteristics, and continuous PRO variables were summarized using descriptive statistics. Categorical PRO variables were summarized by treatment group and visit using frequency and proportion. Continuous PRO variables included the absolute values and percent change from baseline for DLQI, EQ-5D-5L VAS scores, HAQ-DI scores, FACIT-Fatigue Scale, pain, and PtGA VAS scores. Categorical variables included the proportion of patients achieving an HAQ-DI score ≤ 0.5 and a score improvement > 0.3, and EQ-5D-5L dimension scores.
In both studies, PROs were analyzed in the full analysis set (FAS). During TP1, FAS consisted of all randomized patients in whom the study treatment had been administered (FAS for ADACCESS or SP1 FAS for ADMYRA). During TP2 and the extension phase (EP) of the ADACCESS study, FAS consisted of all patients who were re-randomized into TP2 (TP2 + EP FAS). During SP2 of the ADMYRA study, FAS included all patients entering SP2 (SP2 FAS).
Results
Patient Disposition and Baseline Characteristics
ADACCESS Study: Patients with Moderate-to-Severe Plaque PsO
Of the 465 patients randomized in the study, 402 (86.5%) (SDZ-ADL, n = 201 [87.0%]; ref-ADL, n = 201 [85.9%]) completed TP1 (ESM, Fig. S1a). A total of 379 (81.5%) patients were re-randomized to TP2; 323 (69.5%) patients continued to EP; and 301 (64.7%) patients completed the study. TP1 FAS and TP2 + EP FAS included 465 and 379 patients, respectively. Baseline patient demographics and disease characteristics were well balanced between the treatment groups in TP1 FAS and TP2 + EP FAS [23]. Similarly, the PRO instrument scores were balanced at baseline between groups in TP1 and TP2 + EP FAS (ESM Table S1). Overall, 52 (22.5%) patients in the SDZ-ADL and 46 (19.7%) patients in the ref-ADL groups had PsA at baseline. In these patients, mean (standard deviation [SD]) HAQ-DI total scores at baseline were comparable between treatment groups (ESM Table S1).
ADMYRA Study: Patients with Moderate-to-Severe RA
Of the 353 patients randomized in the study, 331 (93.8%) (SDZ-ADL, n = 163 [92.1%]; ref-ADL, n = 168 [95.5%]) completed SP1 (ESM, Fig. S1b). A total of 325 (92.1%) patients entered SP2 and 303 (85.8%) completed the study [24]. The SP1 and SP2 sets included 353 and 325 patients, respectively. Baseline demographics and disease characteristics were well balanced and comparable between the treatment groups in SP1 and SP2 FAS; a majority of the patients were female in both groups. At baseline, the PRO instrument scores were also balanced between the groups for SP1 and SP2 FAS (ESM Table S2).
PROs
ADACCESS Study: Patients with Moderate-to-Severe Plaque PsO
DLQI Scores at Week 17 and at Week 51
The mean (SD) DLQI scores were comparable in the SDZ-ADL and ref-ADL groups at baseline and at Week 17 (Table 1). After switching, mean (SD) DLQI continued to decrease and was comparable between the continued and switched treatment groups at Week 51.
Table 1.
Mean scores of DLQI and EQ-5D-5L in patients with moderate-to-severe plaque psoriasis and HAQ-DI scores in patients with psoriatic arthritis (ADACCESS study; TP1 FAS, TP2 + EP FAS)
| Patient-reported outcomes Mean (SD) |
TP1 | TP2 | ||||
|---|---|---|---|---|---|---|
| SDZ-ADL N = 231 |
ref-ADL N = 234 |
Switched to SDZ-ADL N = 63 |
Continued ref-ADL N = 127 |
Switched to ref-ADL N = 63 |
Continued SDZ-ADL N = 126 |
|
| DLQI | ||||||
| Baseline | 14.1 (7.8) | 13.5 (7.6) | 13.1 (7.4) | 13.7 (7.7) | 12.3 (7.7) | 14.5 (7.7) |
| Week 17 | 4.2 (5.7) | 3.9 (5.3) | 3.8 (5.0) | 3.6 (4.9) | 3.3 (4.2) | 3.6 (4.8) |
| Week 51 | NA | NA | 4.1 (5.3) | 2.3 (3.2) | 3.1 (5.0) | 2.4 (4.1) |
| EQ-5D-5L score | ||||||
| Baseline | 69.7 (22.5) | 69.2 (23.0) | 70.7 (24.1) | 68.3 (23.1) | 72.7 (20.6) | 68.2 (23.3) |
| Week 17 | 80.6 (17.6) | 80.9 (19.4) | 82.0 (18.4) | 80.9 (19.7) | 82.6 (15.0) | 80.5 (18.1) |
| Week 51 | NA | NA | 85.0 (16.5) | 88.3 (10.9) | 85.5 (12.4) | 87.4 (10.2) |
| HAQ-DI scorea | n = 52 | n = 46 | n = 13 | n = 25 | n = 15 | n = 24 |
| Baseline | 0.6 (0.6) | 0.7 (0.6) | 0.9 (0.6) | 0.6 (0.5) | 0.6 (0.7) | 0.7 (0.6) |
| Week 17 | 0.5 (0.6) | 0.5 (0.6) | 0.6 (0.7) | 0.4 (0.6) | 0.6 (0.6) | 0.4 (0.5) |
| Week 51 | NA | NA | 0.5 (0.8) | 0.4 (0.6) | 0.7 (0.6) | 0.4 (0.5) |
DLQI Dermatology Life Quality Index, EP extension phase, EQ-5D-5L EuroQol five-dimension health status questionnaire, FAS full analysis set, HAQ-DI Health Assessment Questionnaire Disability Index, NA not available, ref-ADL reference adalimumab, SD standard deviation, SDZ-ADL Sandoz biosimilar adalimumab, TP1 treatment period 1, TP2 treatment period 2
aPerformed only in patients with psoriatic arthritis.
TP1 FAS consisted of all randomized patients in whom the study treatment had been administered during TP1. TP2 + EP FAS consisted of all patients who were re-randomized into TP2
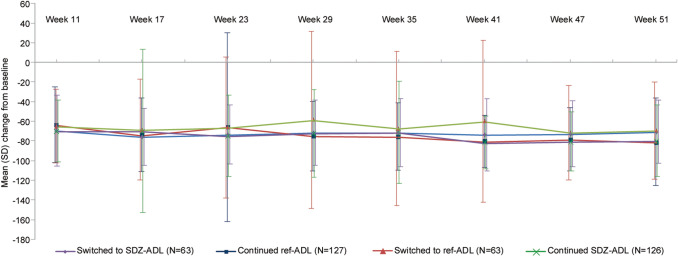
Mean (SD) percent change from baseline in total DLQI was comparable between groups during TP1; the scores continued to decrease after the switch during TP2 and were comparable between the continued and switched treatment groups at Week 51 (Fig. 1). However, a high variability in mean (SD) percent change in total DLQI scores from baseline was observed. Mean (SD) percent change from baseline at Weeks 17 and 51 was also comparable between treatment groups for each of the six DLQI subscales (ESM Table S3).
Fig. 1.
Percent change in DLQI from baseline to Week 51 in patients with moderate‐to‐severe plaque PsO (ADACCESS study; TP2 + EP FAS). The vertical bars represent SD. TP2 + EP FAS consists of all patients who were re-randomized into TP2. Patients were analyzed according to the treatment assigned at re-randomization. DLQI scores range from 0 to 30, with higher scores indicating greater impairment in the health-related quality of life. DLQI Dermatology Life Quality Index, EP extension phase, FAS full analysis set, PsO psoriasis, ref-ADL reference adalimumab, SD standard deviation, SDZ-ADL Sandoz biosimilar adalimumab, TP2 treatment period 2
The proportion of patients achieving a DLQI of 0 or 1 was comparable between treatment groups at Week 17 (SDZ ADL, 104/201 [51.7%]; ref-ADL, 99/200 [49.5%]) and after the switch at Week 51 (‘continued SDZ-ADL,’ 61/100 [61.0%]; ‘continued ref-ADL,’ 59/104 [56.7%]; ‘switched to SDZ-ADL,’ 24/47 [51.1%]; ‘switched to ref ADL,’ 28/50 [56.0%]).
EQ-5D-5L Scores at Week 17 and at Week 51
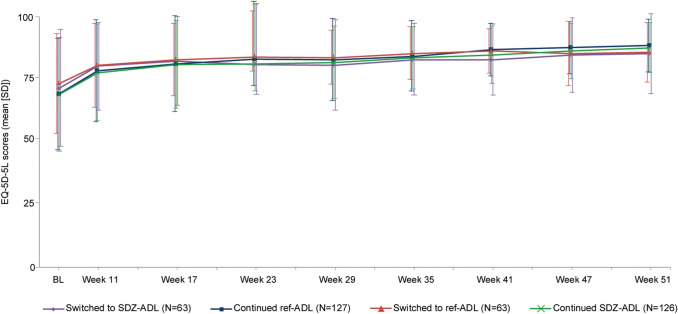
The mean (SD) EQ-5D-5L VAS scores were comparable between treatment groups at baseline; there was a ten-point increase from baseline at Week 17. Mean (SD) EQ-5D-5L scores continued to increase and were comparable across the continued and switched groups after switching (Table 1, Fig. 2).
Fig. 2.
Mean EQ-5D-5L scores up to Week 51 in patients with moderate‐to‐severe plaque PsO (ADACCESS study; TP2 + EP FAS). TP2 + EP FAS consists of all patients who were re-randomized into TP2. Following the intent-to-treat principle, patients were analyzed according to the treatment assigned at re-randomization. EQ-5D-5L visual analog scores range from 0 to 100, with lower scores indicating greater impairment in the health-related quality of life. BL baseline, EP extension phase, EQ-5D-5L EuroQol five-dimension health status questionnaire, FAS full analysis set, PsO psoriasis, ref-ADL reference adalimumab, SD standard deviation, SDZ-ADL Sandoz biosimilar adalimumab, TP2 treatment period 2
The proportion of patients with EQ-5D-5L scores of 1 (no problems) increased from baseline to Week 51 for all five dimensions of the scale and was comparable between treatment groups (ESM Fig. S2). Improvements in all categories of EQ-5D dimensions observed during TP1 continued after the switch during TP2 and were comparable across the continued and switched groups.
HAQ-DI Scores at Week 17 and at Week 51 in Patients with PsA at Baseline
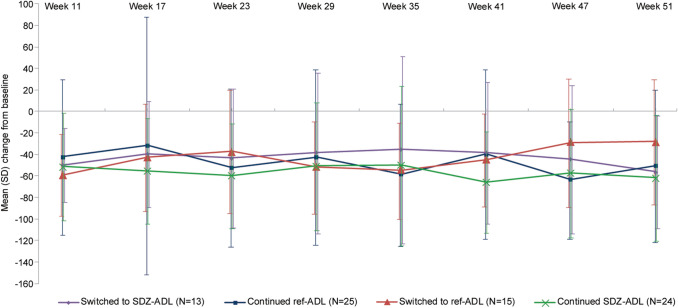
In the subset of patients with concurrent PsA at baseline, mean (SD) HAQ-DI scores were comparable in the SDZ-ADL and ref-ADL groups at baseline and at Week 17 (Table 1). There was an overall decrease in mean (SD) HAQ-DI total scores across the treatment groups at Week 51. The mean percent change from baseline in HAQ-DI total scores was similar between the continued and switched treatment groups at all time points (Fig. 3). However, as the treatment groups were small, a high variability in mean (SD) percent change in HAQ-DI total scores from baseline was observed.
Fig. 3.
Mean percent change from baseline in HAQ-DI total scores up to Week 51 in patients with PsA (ADACCESS study; TP2 + EP FAS). TP2 + EP FAS consists of all patients who were re-randomized into TP2. Patients were analyzed according to the treatment assigned at re-randomization. Each item in HAQ-DI is scored on a four-point scale from 0 to 3, representing ‘without any difficulty’ (0), ‘with some difficulty’ (1), ‘with much difficulty’ (2), and ‘unable to do’ (3). EP extension phase, FAS full analysis set, HAQ-DI Health Assessment Questionnaire Disability Index, PsA psoriatic arthritis, ref-ADL reference adalimumab, SD standard deviation, SDZ-ADL Sandoz biosimilar adalimumab, TP2 treatment period 2
ADMYRA Study: Patients with Moderate-to-Severe RA
HAQ-DI scores at Week 24 and at Week 48
Mean HAQ-DI scores decreased in the SDZ-ADL and ref-ADL treatment groups at Week 24, indicating an improvement in HRQoL (Table 2). Mean (SD) percent change from baseline in HAQ-DI scores was also comparable between both treatment groups at Week 24. After switching, mean HAQ-DI scores remained stable and mean (SD) percent change from baseline at Week 48 was sustained, with no clinically meaningful differences between the treatment groups (ESM, Fig. S3a).
Table 2.
Mean scores of HAQ-DI and FACIT-Fatigue in patients with moderate-to-severe rheumatoid arthritis (ADMYRA study; SP1, SP2 FAS)
| Patient-reported outcomes Mean (SD) |
SP1 | SP2 | ||
|---|---|---|---|---|
| SDZ-ADL N = 177 |
ref-ADL N = 176 |
Continued SDZ-ADL N = 159 |
ref-ADL/switched to SDZ-ADL N = 166 |
|
| HAQ-DI© score | ||||
| Baseline | 1.49 (0.6) | 1.46 (0.6) | 1.48 (0.7) | 1.44 (0.6) |
| Week 24 | 0.84 (0.6) | 0.85 (0.6) | 0.81 (0.6) | 0.85 (0.6) |
| Week 48 | NA | NA | 0.85 (0.7) | 0.82 (0.7) |
| FACIT-Fatigue score | ||||
| Baseline | 24.71 (8.8) | 25.33 (10.1) | 24.86 (8.9) | 25.47 (10.1) |
| Week 24 | 37.08 (9.2) | 38.08 (9.3) | 37.31 (9.0) | 38.06 (9.4) |
| Week 48 | NA | NA | 36.22 (9.8) | 37.01 (9.7) |
SP1 FAS consisted of all randomized patients in whom the study drug was administered. SP2 FAS consisted of all SP1 FAS patients who entered SP2
FACIT Functional Assessment of Chronic Illness Therapy, FAS full analysis set, HAQ-DI Health Assessment Questionnaire Disability Index, NA not available, ref-ADL reference adalimumab, SD standard deviation, SDZ-ADL Sandoz biosimilar adalimumab, SP1 study period 1, SP2 study period 2, VAS visual analog scale
The proportion of patients achieving HAQ-DI in the normal range (≤ 0.5) was comparable between treatment groups at Week 24 (SDZ-ADL, 62/164 [37.8%]; ref-ADL, 61/168 [36.3%]) and after switching at Week 48 (‘SDZ-ADL,’ 69/166 [41.6%]; ‘ref-ADL/switched to SDZ-ADL,’ 66/165 [40.0%]). Similarly, the proportion of patients who achieved HAQ-DI score improvement > 0.3 was comparable in both treatment groups at Week 24 (SDZ-ADL, 116/164 [70.7%]; ref-ADL, 128/168 [76.2%]) and after switching at Week 48 (‘SDZ-ADL,’ 115/166 [69.3%]; ‘ref-ADL/switched to SDZ-ADL,’ 116/165 [70.3%]).
FACIT-Fatigue Scale Scores at Week 24 and at Week 48
The mean FACIT-Fatigue scale scores were comparable in the SDZ-ADL and ref-ADL treatment groups at Week 24 (Table 2). The mean (SD) percent change from baseline indicated a similar improvement in the FACIT-Fatigue Scale for both treatment groups at Week 24. The improvement was sustained further up to Week 48 (ESM, Fig. S3b).
PtGA of Disease Activity Scores at Week 24 and at Week 48
At baseline, the mean (SD) PtGA VAS scores were comparable between SDZ-ADL (64.4 [17.4]) and ref-ADL (65.2 [18.5]) treatment groups. Mean (SD) change from baseline in PtGA VAS scores improved and was comparable in the SDZ-ADL versus ref-ADL groups at Week 24 (− 38.2 [24.1] vs − 41.5 [23.9]) and in the ‘SDZ-ADL’ versus ‘ref-ADL/switched to SDZ-ADL’ groups at Week 48 (− 35.1 [25.7] vs − 39.4 [25.7]).
Patient Assessment of Pain Scores at Week 24 and at Week 48
The mean (SD) pain scores were comparable in the SDZ-ADL versus ref-ADL treatment groups (64.1 [18.7] vs 64.3 [18.4]). Mean (SD) reduction from baseline in pain scores was comparable in the SDZ-ADL versus ref-ADL (− 38.5 [25.7] vs − 41.4 [24.2]) treatment groups at Week 24 and in the ‘SDZ-ADL’ versus ‘ref-ADL/switched to SDZ-ADL’ treatment groups at Week 48 (− 36.0 [27.7] vs − 39.2 [26.9]).
Discussion
HRQoL is reportedly impaired in IMIDs, including RA, PsA, and PsO [1–3]; PROs are therefore gaining considerable interest as they provide valuable evidence to inform clinical decision–making, pharmaceutical labeling claims, and health policies [10–13]. The primary results of the ADACCESS and ADMYRA studies demonstrated equivalent efficacy between SDZ-ADL and ref-ADL in patients with PsO [23] and RA [24], respectively. Results from the PROs assessed in the two studies confirm that both treatments lead to comparable improvements in HRQoL across the three diseases, PsO, PsA, and RA. Further, switching between treatments did not affect HRQoL between the continued and switched treatment groups, as seen by similar outcomes for all PRO assessment tools, including DLQI, EQ-5D-5L, HAQ-DI, and FACIT-Fatigue Scale.
In comparison with DLQI data for reference biologic medicinal product in PsO [27], baseline data and improvement up to Week 16 were comparable, showing mean improvements of 8.4 in the historical data in over 800 patients compared with 9.7/9.1 for SDZ-ADL/ref-ADL in the ADACCESS study; these values are well above the minimal clinically important difference (MCID) of 4 [28]. The results from this study are also consistent with data from other trials, such as the AURIEL-PsO study, wherein treatment with a reference biologic medicinal product versus an adalimumab biosimilar in patients with moderate-to-severe plaque-type PsO resulted in comparable improvements in QoL measures until Week 16. A single switch from the reference biologic medicinal product to biosimilar adalimumab at Week 16 had no impact on patient QoL, indicated by sustained QoL improvements observed until Week 52 [29]. In the AURIEL-RA study, no clinically meaningful differences in QoL measures were observed until Week 52 after a single switch from the reference biologic medicinal product to biosimilar adalimumab at Week 24 [30].
In the subset of patients with PsA in the ADACCESS study, mean improvements of 0.1 (SDZ-ADL) and 0.2 (ref-ADL) in HAQ-DI scores up to Week 17 were seen. These results are in line with previous studies, such as ADEPT, wherein treatment with a reference biologic medicinal product resulted in a mean improvement of 0.3 in the HAQ-DI score at Week 24 [31]. Improvements in HAQ-DI scores observed in patients with RA in the ADMYRA study suggest that SDZ-ADL improves functional capacity and reduces disability in patients with RA, and the switch does not affect QoL. In the ARMADA study wherein patients with RA were treated with reference adalimumab [32], the mean baseline HAQ-DI score was 1.55, with a mean improvement of 0.62 at Week 24. Similar trends were observed in the ADMYRA study; mean improvements in the HAQ-DI total score at Week 24 were 0.65 and 0.58 in the SDZ-ADL and ref-ADL groups, respectively, exceeding an MCID of 0.22 [33]. Limitations of these analyses are their descriptive nature and the high variability observed, as seen in the large standard deviations for all outcome measures, particularly in the small group of PsA patients.
The strength of the analyses is that this is the first report describing PROs of an adalimumab biosimilar and reference biologic medicinal product across the three diseases: PsO, PsA, and RA. To date, limited QoL data are available on biosimilars. Given the chronic nature of IMIDs, PROs providing QoL data could assist physicians in decision–making, leading to improved quality of care [14, 15]. PRO data, together with evidence of comparable clinical and safety findings [23, 24] from the ADACCESS and ADMYRA studies, further support the biosimilarity of SDZ-ADL and ref-ADL. In addition, these data provide reassurance to patients and physicians that SDZ-ADL provides good and sustained improvements in QoL, similar to ref-ADL. Patient registry studies that monitor the real-world clinical use of approved biosimilars would help to further validate the effects of switching from reference biologic medicinal products to biosimilars on effectiveness, safety, and QoL.
Conclusions
Treatment with SDZ-ADL and ref-ADL resulted in comparable improvements in PROs as well as QoL scores across the three diseases, PsO, PsA, and RA. These improvements were sustained after switching. Switching between SDZ-ADL and ref-ADL had no negative impact on PROs across the reported period.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank all investigators (Clinicaltrials.gov: NCT02744755, NCT02016105) and participating patients who contributed to the successful conduct of this study, and Divya Chandrasekhar (Product Lifecycle Services-NBS, Novartis Healthcare Pvt. Ltd., Hyderabad, India) for medical writing and editorial assistance.
Declarations
Funding
This study was funded by Hexal AG, a Sandoz company.
Conflict of interest
Andrew Blauvelt: Received honoraria from Sandoz for scientific consulting. His company, Oregon Medical Research Center, received funds to conduct the clinical study reported herein. Also, he has served as a scientific adviser and/or clinical study investigator for Sandoz, AbbVie, Almirall, Arena, Athenex, Boehringer Ingelheim, Bristol-Myers Squibb, Dermavant, Eli Lilly and Company, Evommune, Forte, Galderma, Incyte, Janssen, Leo, Novartis, Pfizer, Rapt, Regeneron, Sanofi Genzyme, Sun Pharma, and UCB Pharma. Craig L. Leonardi: Consultant and/or advisory board member for AbbVie, Amgen, Boehringer-Ingelheim, Dermira, Eli Lilly, Janssen, Leo, Pfizer, Sandoz, UCB and Vitae; Speaker bureau for AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Novartis, Ortho Dermatologies, Sun Pharmaceuticals, and UCB; Investigator for Actavis, AbbVie, Allergan, Amgen, Boehringer-Ingelheim, Celgene, Coherus, Cellceutix, Corrona, Dermira, Eli Lilly, Galderma, Glenmark, Janssen, Leo Pharma, Merck, Novartis, Novella, Pfizer, Sandoz, Sienna, Stiefel, UCB and Wyeth; writing assistance received from Sandoz. Norman Gaylis: None declared. Julia Jauch Lembach: Employee of Sandoz. Alison Balfour: Employee of Sandoz. Lena Lemke: Employee of Sandoz. Sohaib Hachaichi: Employee of Sandoz. Ines Brueckmann: Employee of Sandoz. Teodora Festini: Employee of Sandoz; stock/stock options as Sandoz employee, not related to publication. Piotr Wiland: Participation in clinical trial ‘ADMYRA’, received the fee as principal investigator in his center.
Ethical approval
The two studies (ADACCESS and ADMYRA) were conducted in accordance with the ethical principles derived from the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practices and in compliance with local regulatory requirements. The study protocols were approved by the Independent Ethics Committee or Institutional Review Board for each center.
Consent to participate
All patients provided written informed consent before entering the study.
Consent for publication
All patients provided their consent for their data to be published.
Availability of data and material
All data generated or analyzed in relation to PROs are included in this article and the supplementary information files. Anonymized datasets and related documents, such as the statistical analysis plan, protocol, and amendments, can be shared upon reasonable request through a data sharing agreement.
Author contributions
JJL and LL contributed to the design of the studies. AB, CLL, NG, and PW contributed to the conduct of the clinical studies. JJL, LL, and AB contributed to the analysis and interpretation of the results. SH, IB, and TF discussed the results and contributed to the manuscript. All authors discussed the results and contributed to the manuscript.
References
- 1.Sokka T, Kautiainen H, Möttönen T, Hannonen P. Work disability in rheumatoid arthritis 10 years after the diagnosis. J Rheumatol. 1999;26(8):1681–1685. [PubMed] [Google Scholar]
- 2.Krueger G, Koo J, Lebwohl M, Menter A, Stern RS, Rolstad T. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280–284. [PubMed] [Google Scholar]
- 3.Taylor WJ. Impact of psoriatic arthritis on the patient: through the lens of the WHO international classification of functioning, health, and disability. Curr Rheumatol Rep. 2012;14:369–374. doi: 10.1007/s11926-012-0263-5. [DOI] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration. Remicade® (infliximab) [prescribing information] Horsham, PA: Janssen Biotech, Inc. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/103772s5359lbl.pdf. Accessed 3 Dec 2019.
- 5.US Food and Drug Administration. Enbrel® (etanercept) [prescribing information] Thousand Oaks, CA: Immunex Corporation. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/103795s5503lbl.pdf. Accessed 03 Dec 2019.
- 6.US Food and Drug Administration. Humira® (adalimumab) [Prescribing information] North Chicago, IL: AbbVie Inc. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/125057s0110lbl.pdf. Accessed 28 Nov 2019.
- 7.Hirsch BR, Lyman GH. Biosimilars: a cure to the U.S. health care cost conundrum? Blood Rev. 2014;28:263–8. [DOI] [PubMed]
- 8.Dorner T, Strand V, Castaneda-Hernandez G, Ferraccioli G, Isaacs JD, Kvien TK, et al. The role of biosimilars in the treatment of rheumatic diseases. Ann Rheum Dis. 2013;72:322–328. doi: 10.1136/annrheumdis-2012-202715. [DOI] [PubMed] [Google Scholar]
- 9.Gossec L, Dougados M, Dixon W. Patient-reported outcomes as end points in clinical trials in rheumatoid arthritis. RMD Open. 2015;1:e000019. doi: 10.1136/rmdopen-2014-000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doward LC, Gnanasakthy A, Baker MG. Patient reported outcomes: looking beyond the label claim. Health Qual Life Outcomes. 2010;8(1):89. doi: 10.1186/1477-7525-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US Food and Drug Administration. Guidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. Silver Spring, MD: US Food and Drug Administration; 2009. https://www.fda.gov/media/77832/download. Accessed 28 Nov 2019.
- 12.European Medicines Agency Committee for Medicinal Products for Human Use. Appendix 2 to the Guideline on the Evaluation of Anticancer Medicinal Products in Man: The Use of Patient-Reported Outcome (PRO) Measures in Oncology Studies EMA/CHMP/292464/2014. London, England: European Medicines Agency; 2016. https://www.ema.europa.eu/en/documents/other/appendix-2-guideline-evaluation-anticancer-medicinal-products-man_en.pdf. Accessed 28 Nov 2019.
- 13.Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA. 2003;290(12):1624–1632. doi: 10.1001/jama.290.12.1624. [DOI] [PubMed] [Google Scholar]
- 14.Sokka T. Morning stiffness and other patient-reported outcomes of rheumatoid arthritis in clinical practice. Scand J Rheumatol Suppl. 2011;125:23–27. doi: 10.3109/03009742.2011.566437. [DOI] [PubMed] [Google Scholar]
- 15.Smolen JS, Landewe R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs update. Ann Rheum Dis. 2016;2017:1–18. doi: 10.1136/annrheumdis-2016-210715. [DOI] [PubMed] [Google Scholar]
- 16.Inotai A, Prins CPJ, Csanádi M, Vitezic D, Codreanu C, Kaló Z. Is there a reason for concern or is it just hype? A systematic literature review of the clinical consequences of switching from originator biologics to biosimilars. Expert Opin Biol Ther. 2017;17:915–926. doi: 10.1080/14712598.2017.1341486. [DOI] [PubMed] [Google Scholar]
- 17.Moots R, Azevedo V, Coindreau JL, Dörner T, Mahgoub E, Mysler E, et al. Switching between reference biologics and biosimilars for the treatment of rheumatology, gastroenterology, and dermatology inflammatory conditions: considerations for the clinician. Curr Rheumatol Rep. 2017;19:37. doi: 10.1007/s11926-017-0658-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikiphorou E, Kautiainen H, Hannonen P, Asikainen J, Kokko A, Rannio T, et al. Clinical effectiveness of CT-P13 (infliximab biosimilar) used as a switch from Remicade (infliximab) in patients with established rheumatic disease. Report of clinical experience based on prospective observational data. Expert Opin Biol Ther. 2015;15:1677–83. [DOI] [PubMed]
- 19.Glintborg B, Sørensen IJ, Loft AG, Lindegaard H, Linauskas A, Hendricks O, et al. A nationwide non-medical switch from originator infliximab to biosimilar CT-P13 in 802 patients with inflammatory arthritis: 1-year clinical outcomes from the DANBIO registry. Ann Rheum Dis. 2017;76:1426–1431. doi: 10.1136/annrheumdis-2016-210742. [DOI] [PubMed] [Google Scholar]
- 20.US Food and Drug Administration. Hyrimoz. Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761071lbl.pdf. Accessed 31 Oct 2018.
- 21.European Medicines Agency. Hyrimoz. https://www.ema.europa.eu/en/documents/assessment-report/hyrimoz-epar-public-assessment-report_en.pdf. Accessed 31 Oct 2018.
- 22.von Richter O, Lemke L, Haliduola H, Fuhr R, Koernicke T, Schuck E, et al. GP2017, an adalimumab biosimilar: pharmacokinetic similarity to its reference medicine and pharmacokinetics comparison of different administration methods. Expert Opin Biol Ther. 2019;30:1–9. doi: 10.1080/14712598.2019.1571580. [DOI] [PubMed] [Google Scholar]
- 23.Blauvelt A, Lacour JP, Fowler JF, Jr, Weinberg JM, Gospodinov D, Schuck E, et al. Phase III randomized study of the proposed adalimumab biosimilar GP2017 in psoriasis: impact of multiple switches. Br J Dermatol. 2018;179(3):623–631. doi: 10.1111/bjd.16890. [DOI] [PubMed] [Google Scholar]
- 24.Wiland P, Slawomir J, Dokoupilová E, Brandt-Jürgens J, Miranda-Limón JM, Cantalejo Moreira M, et al. Switching to biosimilar SDZ-ADL in patients with moderate-to-severe active rheumatoid arthritis: 48-week efficacy, safety and immunogenicity results from the phase III, randomized, double-blind ADMYRA study. Biodrugs. 2020;34(6):809–823. doi: 10.1007/s40259-020-00447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fransen J, van Riel PL. The disease activity score and the EULAR response criteria. Rheum Dis Clin N Am. 2009;35(4):745–757. doi: 10.1016/j.rdc.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 26.van Gestel AM, Prevoo ML, van 't Hof MA, van Rijswijk MH, van de Putte LB, van Riel PL. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum. 1996;39(1):34–40. [DOI] [PubMed]
- 27.Revicki DA, Willian MK, Menter A, Gordon KB, Kimball AB, Leonardi CL, et al. Impact of adalimumab treatment on patient-reported outcomes: results from a phase III clinical trial in patients with moderate to severe plaque psoriasis. J Dermatol Treat. 2007;18(6):341–350. doi: 10.1080/09546630701646172. [DOI] [PubMed] [Google Scholar]
- 28.Basra MK, Salek MS, Camilleri L, Sturkey R, Finlay AY. Determining the minimal clinically important difference and responsiveness of the dermatology life quality index (DLQI): further data. Dermatology. 2015;230(1):27–33. doi: 10.1159/000365390. [DOI] [PubMed] [Google Scholar]
- 29.Hercogová J, Papp KA, Chyrok V, et al. Quality-of-life outcomes comparing the proposed biosimilar MSB11022 to reference adalimumab in patients with moderate-to-severe chronic plaque-type psoriasis. Presented at: International Society for Pharmacoeconomics and Outcomes Research 24th Annual International Meeting; May 18-22, 2019; New Orleans, Louisiana. Abstract PBI35. https://www.centerforbiosimilars.com/conferences/ispor-2019/fresenius-kabis-adalimumab-biosimilar-produces-comparable-qol-improvements-to-humira. Accessed 28 Nov 2019.
- 30.Edwards CJ, Monnet J, Ullmann M, Vlachos P, Chyrok V, Ghori V. Safety of adalimumab biosimilar MSB11022 (acetate-buffered formulation) in patients with moderately-to-severely active rheumatoid arthritis. Clin Rheumatol. 2019;38:3381–3390. doi: 10.1007/s10067-019-04679-y. [DOI] [PubMed] [Google Scholar]
- 31.Mease PJ, Ory P, Sharp JT, Ritchlin CT, Van den Bosch F, Wellborne F, et al. Adalimumab for long-term treatment of psoriatic arthritis: 2-year data from the Adalimumab Effectiveness in Psoriatic Arthritis Trial (ADEPT) Ann Rheum Dis. 2009;68(5):702–709. doi: 10.1136/ard.2008.092767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, a fully human anti–tumor necrosis factor α monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48(1):35–45. doi: 10.1002/art.10697. [DOI] [PubMed] [Google Scholar]
- 33.Wells GA, Tugwell P, Kraag GR, Baker PR, Groh J, Redelmeier DA. Minimum important difference between patients with rheumatoid arthritis: the patient’s perspective. J Rheumatol. 1993;20:557–560. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.