Abstract
Objective
Our objective was to update the understanding of the development of paradoxical immune-mediated glomerular disorders (IGDs) in patients with rheumatic diseases treated with biologics and targeted synthetic drugs (ts-drugs).
Methods
A systematic literature review was performed by searching PubMed for articles published between 1 January 2014 and 1 January 2020 reporting on the development of IGD in adult patients with rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis or systemic lupus erythematosus (SLE) who were receiving biologics or ts-drugs. IGDs were classified on the basis of clinical, laboratory and histopathological data as (1) glomerulonephritis associated with systemic vasculitis (GNSV), (2) isolated autoimmune renal disorder (IARD) or (3) glomerulonephritis in SLE and in lupus-like syndrome (GNLS). The World Health Organization-Uppsala Monitoring Centre (WHO-UMC) system for standardized case causality assessment was applied to evaluate the causal relationship between IGD and specific drugs. The classification was based on a six-category scale, where the “certain” and “probable” categories were deemed clinically relevant relationships.
Results
The literature search retrieved 875 articles. Of these, 16 articles reported IGD data, for a total of 25 cases. According to the WHO-UMC assessment, the strength of the causal relationship between IGDs and investigated drugs was higher for anti-tumor necrosis factor-α agents (a clinically relevant relationship was found in four of six cases), abatacept (one of two cases), tocilizumab (two cases), ustekinumab (one case) and tofacitinib (one case) than for rituximab (nine cases), belimumab (three cases) or secukinumab (one case), which showed a weak causal relationship with these paradoxical events. No cases associated with apremilast or baricitinib were found. The retrieved cases were classified as 11 GNLS, seven IARD and seven GNSV.
Conclusions
Biologics and ts-drugs can cause IGDs. These events are rare, and the causative effect of a specific drug is hard to establish. When a patient is suspected of having an IGD, the drug should be discontinued, and treatment for the new-onset renal disorder should be promptly started.
Key Points
| Biologics and targeted synthetic drugs may induce rare paradoxical immune-mediated glomerular disorders. |
| Drug-induced immune-mediated glomerular disorders are rare but could be irreversible, leading to dialysis or death. |
| The immune-mediated mechanisms underlying biologics and targeted synthetic drug-induced immune-mediated glomerular disorders have not yet been fully identified. |
Introduction
Over the past 20 years, many treatment options have become available for people with rheumatic diseases [1] thanks to a better understanding of disease pathogenesis. New biotechnological or synthetic drugs have been developed to better control the course of immune-mediated chronic diseases, including rheumatoid arthritis (RA) [2], psoriatic arthritis (PsA) [3], ankylosing spondylitis (AS) [4] and systemic lupus erythematosus (SLE) [5], through different mechanisms of action. Biotechnological products, or biologics, used in rheumatic diseases are drugs that inhibit the effects of specific cytokines (e.g., interleukin [IL]-1, IL-6, IL-17, IL-12, IL-23, B-cell activating factor [BAFF], or tumor necrosis factor [TNF]-α) or selectively target cluster of differentiation (CD)-20-positive B cells or prevent antigen-presenting cells from delivering the costimulatory signal to T lymphocytes by binding to CD80 and CD86, thereby blocking interaction with CD28 [6–8]. Targeted synthetic drugs (ts-drugs) are recently developed small molecules that suppress inflammation by interfering with intracellular signaling pathways; the so-called Janus kinase inhibitors (JAKi) inhibit the activity of one or more of the JAK family of enzymes (JAK1, JAK2, JAK3) [9], whereas other ts-drugs inhibit the activity of phosphodiesterase-4, the enzyme responsible for breaking down cyclic adenosine monophosphate.
The use of these agents has significantly improved patients’ symptoms by controlling disease activity, inhibiting the progression of structural damage and reducing the risk of comorbidities [10]. Nevertheless, all of these drugs have a range of shared adverse effects [11, 12].
Notably, biologics can cause the paradoxical development of autoimmune processes [13], whereas the nonproteinic structure of ts-drugs makes them apparently unlikely to induce immunogenicity. In a recent systematic literature review (SLR) [14], we reported that anti-TNFα can lead to the development of autoimmune renal disorders that could be life threatening. Stopping the treatment can reverse the adverse events and normalize renal function, so physicians need to be aware of drug-induced immune-mediated glomerular disorders (IGDs). The underlying pathogenetic mechanisms range from self-limited reactions against the drug, resulting in immunocomplex deposition and kidney damage, to imbalance of cytokine production and lymphocyte functions [15]. Since our first review was published, new drugs have become routinely used in rheumatology practice, so an update describing their risk of inducing IGD was necessary.
The purpose of this study was to provide an updated survey of the reports on biologic- and ts-drug-induced IGD, to assess the causality relationship between the drug and the adverse event and to describe IGD features in adult patients with rheumatic diseases through an SLR.
Methods
Systematic Review
Two investigators performed an SLR in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [16] by searching for articles published between 1 January 2014 and 1 January 2020 reporting on the development of IGD (outcome) in adult patients with the rheumatic diseases RA, PsA, AS or SLE (population) receiving biologics or ts-drugs (intervention). The following search strategy through MEDLINE via PubMed was designed using the following combination of medical subject heading (MeSH) terms: (“arthritis, rheumatoid”[MeSH]) OR “arthritis, psoriatic”[MeSH]) OR “spondylitis, ankylosing”[MeSH]) OR “lupus erythematosus, systemic”[MeSH]) AND “infliximab”[MeSH]) OR “etanercept”[MeSH]) OR “adalimumab”[MeSH]) OR “golimumab” [supplementary concept]) OR “certolizumab pegol”[MeSH]) OR “rituximab”[MeSH]) OR “belimumab” [supplementary concept]) OR “tocilizumab” [supplementary concept]) OR “abatacept”[MeSH]) OR “tofacitinib” [supplementary concept]) OR “baricitinib” [supplementary concept]) OR “apremilast”[supplementary concept]) OR “Janus kinase inhibitors”[MeSH]) AND “glomerulonephritis”[MeSH]) OR “nephrotic syndrome”[MeSH]) OR “nephrosis, lipoid”[MeSH].
Additional papers were obtained by checking the reference lists of the selected studies, review articles and other sources known to the authors. All types of studies were allowed, but only full publications reporting on adult patients and written in English were included in the literature search. The investigators independently selected the articles initially on the basis of titles and abstracts and then, if necessary, on the basis of the full texts. Then, eligibility assessment was performed independently in a blinded and standardized manner. Whenever papers reported duplicate data, the most recent article was selected. To be included in the review, the retrieved papers had to provide descriptive features of each reported case of induced IGD. In particular, demographic, clinical, histopathological (if performed), treatment and outcome data were required.
Case Classification
According to clinical manifestations and kidney histology, the identified cases were classified as (1) glomerulonephritis associated with systemic vasculitis (GNSV), (2) glomerulonephritis in SLE and lupus-like syndrome (GNLS) or (3) isolated autoimmune renal disorder (IARD), i.e., autoimmune glomerular disorders not classifiable in the context of a specific systemic disease. Clinical outcomes were defined as (1) complete resolution (i.e., inactive urinary sediment, absent proteinuria and normal or stable renal function), (2) partial resolution (i.e., significant improvement of proteinuria, urinary sediment and renal function that did not return to normal values) or (3) worsening of clinical conditions (i.e., absence of improvement or worsening of proteinuria and/or urinary sediment, deterioration of renal function until end-stage renal disease or death).
Case Causality Assessment
In an attempt to clarify whether IGDs are specific adverse reactions to biologics and ts-drugs, the World Health Organization-Uppsala Monitoring Centre (WHO-UMC) system for standardized case causality assessment [45] was applied, and the reported adverse drug reactions were classified on a six-category scale: “certain,” “probable/likely,” “possible,” “unlikely,” “conditional/unclassified” and “unassessable/unclassifiable” [17]. The “certain” and “probable” categories were deemed clinically relevant relationships. Case reports classified as “conditional/unclassified” and “unassessable/unclassifiable” were excluded from the analysis. Causality assessment was performed independently in a blinded and standardized manner by the two reviewers. Disagreements between reviewers were solved by consensus.
Results
Literature Search
The literature search identified 875 articles; 11 were initially considered relevant for the present study. The manual search retrieved 12 additional articles. Finally, 16 articles accounting for 25 case reports were included in the study (Fig. 1).
Fig. 1.
Flow chart illustrating the literature search and study selection. IGD immune-mediated glomerular disorders, IRD inflammatory rheumatic diseases, ts-drugs targeted synthetic drugs
Demographic Features
The updated search identified nine IGD cases associated with rituximab, three with belimumab, two with etanercept, abatacept and tocilizumab and one each with secukinumab, ustekinumab, tofacitinib, adalimumab, infliximab, certolizumab pegol and golimumab. No cases of IGD associated with apremilast or baricitinib were identified. Of 25 cases of IGD, ten were reported in patients affected by RA, nine in patients affected by SLE, three in patients affected by cryoglobulinemic vasculitis and one each in patients affected by AS, PsA or RA overlapping with SLE.
IGDs developed within a median of 3 months (interquartile range 1–6.5) from the beginning of treatment. In seven cases, all in patients treated with rituximab, IGD appeared within the first month of treatment, whereas in four cases (16.0%) IGDs had a long latency onset, and renal disorders developed after 2 years of treatment. Seven of the 25 patients were classified as affected by GNSV (Table 1) [18–22], seven as affected by IARD (Table 2) [23–28] and 11 as affected by GNLS (Table 3) [29–33].
Table 1.
Demographic and clinical features of patients developing glomerulonephritis in systemic vasculitis following treatment with a biologic or targeted synthetic drug
| Drug | Age, sex | IRD (duration) | Latency | Associated features | Renal abnormalities | Kidney biopsy | Treatment | Outcome | WHO-UMC assessment | References |
|---|---|---|---|---|---|---|---|---|---|---|
| ETN | 48, F | RA (24 years) | 72 months | Purpura, RF | u-RBC, u-Prot (16.2 g/gCr) | IgA mGN (HSP) | ETN withdrawal, IV MPRE, PRE | Complete resolution | Possible | [18] |
| ETN | 30, F | RA (16 years) | 2 months | Polyarthritis, lower limb edema, hypertension, MPO-ANCA, RF, pre-existing u-RBC, u-Prot | u-RBC, casts, u-Prot | NCGN, mesangial IgA deposits | ETN withdrawal, CYC, PRE | Complete resolution | Possible | [19] |
| SEC | 55, M | RA (28 years) | 6 months | Arthritis, ACPA, ↓C3 and C4, MPO-ANCA, RF, pre-existing u-RBC, u-Prot | u-RBC, ↑s-Cr, u-Prot (2.6 g/day) | NCGN | SEC withdrawal, IV MPRE, RTX | Partial resolution | Possible | [20] |
| TOF | 67, F | RA (16 years) | 6 months | Purpura, arthralgia, lower limbs edema, RF | u-RBC, casts, u-Prot (8 g/day) | IgA mGN (HSP) | TOF withdrawal, IV MPRE, PRE | Complete resolution | Certain | [21] |
| RTX | 49, M | CV (NA) | 8 days | Fever, purpura, intestinal vasculitis ↓C4, pre-existing HCV, lymphoproliferation, nephritis | ARI, u-Prot (0.3 g/day), u-RBC | MP-GN | PEX, RTX, IV CYC, IV MPRE | Worsened (dialysis and death) | Possible | [22] |
| RTX | 78, M | CV (NA) | 12 days | ↓C4, pre-existing HCV, marginal zone lymphoma, nephritis | ARI, u-Prot (1.37 g/day), u-RBC | MP-GN | RTX withdrawal, PEX, IV MPRE | Worsened (dialysis) | Possible | [22] |
| RTX | 34, M | CV (NA) | 13 days | Purpura, ↓C4; pre-existing HCV, nephritis | ARI, u-Prot (7 g/L), u-RBC | MP-GN | RTX withdrawal, PEX, IV MPRE | Partial resolution | Possible | [22] |
↑ indicates increased, ACPA anti-citrullinated protein antibodies, ARI acute renal injury, CV cryoglobulinemic vasculitis, CYC Cyclophosphamide:, ETN etanercept, F female, HCV hepatitis C virus infection, HSP Henoch-Schönlein purpura, IgA immunoglobulin A, IRD inflammatory rheumatic disease, IV intravenous, M male, mGN mesangial glomerulonephritis, MP-GN membranoproliferative glomerulonephritis, MPO-ANCA myeloperoxidase anti-neutrophil cytoplasmic antibodies, MPRE methylprednisolone, NA not available, NCGN necrotizing crescentic glomerulonephritis, PEX plasma exchange, PRE prednisone, RA rheumatoid arthritis, RF rheumatoid factor, RTX rituximab, s-Cr serum creatinine, SEC secukinumab, TOF tofacitinib, u-Prot proteinuria, u-RBC urinary red blood cells (hematuria), WHO-UMC World Health Organization Uppsala Monitoring Centre
Table 2.
Demographic and clinical features of patients developing glomerulonephritis in isolated autoimmune renal disorders following treatment with a biologic or targeted synthetic drug
| Drug | Age, sex | IRD (duration) | Latency (months) | Associated features | Renal abnormalities | Kidney biopsy | Treatment | Outcome | WHO-UMC assessment | References |
|---|---|---|---|---|---|---|---|---|---|---|
| IFX | 40, F | AS (1 year) | 6 | Previous treatment with NSAIDs, SSZ | u-RBC, u-Prot (3.7 g/day) | FSGS | IFX, NSAIDs, SSZ withdrawal, IV MPRE, PRE, ACEi | Partial resolution | Probable | [23] |
| CTZ | 63, F | RA (15 years) | 6 | Arthritis, lower limbs edema, ↓alb, RF, ACPA, ANA | u-Prot (14 g/day) | MGN with glomerular sclerosis | CTZ withdrawal, diuretics and ACEi | Partial resolution | Certain | [24] |
| ABA | 60, F | RA, sSS (11 years) | 7 |
Edema, fatigue, lymphopenia, ↓alb, ↓complement, RF, ANA, ↑anti-dsDNA, Pre-existing PBC, T2DM Previous treatment with bucillamine, SSZ |
Casts, u-Prot (12.6 g/day) | MGN | ABA withdrawal, IV MPRE, PRE | Partial resolution | Possible | [25] |
| ADA | 62, M | RA (10 years) | 2 | Lower limb edema, ↑WBC, ↑CRP, ANA 1:80 homo, aCL IgG, MPO-ANCA, ↓C3, RF | u-RBC, u-Prot (5.41 g/day), ↓GFR (23 ml/min) | IgA mGN | ADA withdrawal, IV MPRE, PRE | Worsened (dialysis) | Probable | [26] |
| ABA | 76, F | RA (16 years) | NA | Polyarthritis, lower limbs edema | u-RBC, casts, ↑s-Cr, u-Prot (2.60 g/day) | IgA mGN, amyloidosis | ABA withdrawal, TOF | Complete resolution | Probable | [27] |
| TCZ | 48, F | RA (13 years) | 36 | Lower limbs edema, ANA 1:40 homo, Sm weakly +, ↓complement | u-RBC, u-Prot (3.5 g/day) | MP-GN | TCZ withdrawal, PRE, diuretics and ACEi | Complete resolution | Certain | [28] |
| TCZ | 74, M | RA (25 years) | 24 | Lower limbs edema, ↓complement | u-RBC, u-Prot (2.67 g/gCr), ↑s-Cr, ↓GFR (34 ml/min) | MP-GN | TCZ withdrawal, PRE | Worsened (death: severe infection) | Probable | [28] |
↓ indicates decreased, ↓alb hypoalbuminemia, ↑ indicates increased, ABA abatacept, ACEi angiotensin-converting enzyme inhibitor, aCL anti-cardiolipin, ACPA anti-citrullinated protein antibodies, ADA adalimumab, ANA antinuclear antibodies, AS ankylosing spondylitis, CRP C-reactive protein, CTZ certolizumab pegol, CYC cyclophosphamide, dsDNA double-stranded DNA, F female, FSGS focal segmental glomerular sclerosis, GFR glomerular filtration rate, IFX infliximab, IgA immunoglobulin A, IgG immunoglobulin G, IRD inflammatory rheumatic disease, IV intravenous, M male, MGN membranous glomerulonephritis, mGN mesangial glomerulonephritis, MP-GN membranoproliferative glomerulonephritis, MPO-ANCA myeloperoxidase anti-neutrophil cytoplasmic antibodies, MPRE methylprednisolone, NSAID nonsteroidal anti-inflammatory drug, PBC primary biliary cirrhosis, PRE prednisone, RA rheumatoid arthritis, RF rheumatoid factor, s-Cr serum creatinine, Sm anti-Smith autoantibody, sSS secondary Sjogren syndrome, SSZ sulfasalazine, T2DM type 2 diabetes mellitus, TCZ tocilizumab, TOF tofacitinib, u-Prot increased proteinuria, u-RBC urinary red blood cells (hematuria), WBC white blood cells, WHO-UMC World Health Organization Uppsala Monitoring Centre
Table 3.
Demographic and clinical features of patients developing glomerulonephritis in systemic lupus erythematosus and lupus-like syndromes following treatment with a biologic or targeted synthetic drug
| Drug | Age, sex | IRD (duration) | Latency | Associated features | Renal abnormalities | Kidney biopsy | Treatment | Outcome | WHO-UMC assessment | References |
|---|---|---|---|---|---|---|---|---|---|---|
| BLM | 31, F | SLE (13 years) | 3 months | Rash, ANA, ↑anti-DNA, previous anti-RNP, anti-Sm, ↓serum complement | u-RBC, u-Prot (1.6 g/day) | Class III | BLM withdrawal, AZA, ACEi | Complete resolution | Possible | [29] |
| BLM | 38, F | SLE (9 years) | 3 months | Fever, arthritis, lower limb edema, serositis, adenopathy, previous ANA, anti-SSA, ↓serum complement | u-Prot (6.0 g/day) | Class V | BLM withdrawal, MMF | Partial resolution | Possible | [29] |
| GOL | 62, F | RA + SLE (21 years) | 1 month | Fatigue, appetite loss, previous ACPA, ANA, pre-existing LN class IV | u-RBC, u-Prot (16.6 g/gCr) | Class IV | GOL withdrawal, PRE | Worsened (dialysis) | Possible | [30] |
| RTX | 38, F | SLE (10 years) | 1 week | Rash, edema, ↓alb, nephrotic syndrome, pre-existing LN class V | ↑s-Cr, u-Prot | NR | II infusion RTX interrupted, IV MPRE, ↓MMF | Complete resolution | Unlikely | [31] |
| RTX | 26, F | SLE (1 year) | 2 weeks | Hypertension, pre-existing proliferative LN, sickle cell disease | ↑s-Cr | Class IV | RTX withdrawal, hemodialysis, IV MPRE, CYC, antihypertensive | Partial resolution | Unlikely | [31] |
| RTX | 30, F | SLE (11 years) | 6 months | Arthralgia, edema, weight gain (13 kg), dyspnea, ↓alb, nephrotic syndrome, pre-existing LN class III–V | ↑s-Cr, u-Prot | NR | RTX withdrawal, PRE, diuretics, ↓MMF | Complete resolution | Unlikely | [31] |
| RTX | 33, F | SLE (5 years) | 1 month | Edema, weight gain (15 kg), pre-existing LN class IV | ↑s-Cr, u-Prot | Class IV | RTX withdrawal, MMF, diuretics | Partial resolution | Possible | [31] |
| RTX | 19, F | SLE (2 years) | 3 months | Fever, ↓WBC, ↓alb, synovitis, ↓Hb, pleural effusion, pre-existing LN class III–V | u-Prot | Class IV–V | RTX withdrawal, MMF | Worsened | Unlikely | [31] |
| RTX | 18, F | SLE (6 years) | 3 weeks | ↓alb, nephrotic syndrome, pre-existing LN class IV | ↑s-Cr, u-Prot | NR | RTX withdrawal, PRE, MMF | Partial resolution | Unlikely | [31] |
| BLM | 62, F | SLE (9 years) | 10 months | Arthritis, VT, serositis, ↑anti-DNA, ↓serum complement, previous Coombs, LAC, aCL IgG, carcinoma, family history of RA and ESRD | u-RBC, casts, u-Prot | Class III | BLM withdrawal, PRE, CYC | Complete resolution | Possible | [32] |
| UST | 40, M | PsA (15 years) | 24 months | Purpura, ANA, ↓C3 | ↓GFR | Class V and proliferative aspects | UST withdrawal, CYC | Worsened (persistent renal failure) | Probable | [33] |
↓ indicates decreased, ↑ indicates increased, ↓alb hypoalbuminemia, ACEi angiotensin-converting enzyme inhibitor , aCL anti-cardiolipin, ACPA anti-citrullinated protein antibodies, ANA antinuclear antibodies, AZA azathioprine, BLM belimumab, CYC cyclophosphamide, ESRD end-stage renal disease, F female, GFR glomerular filtration rate, GOL golimumab, Hb hemoglobin, IgG immunoglobulin G, IRD inflammatory rheumatic disease, IV intravenous, LAC lupus anticoagulant, LN lupus nephritis, M male, MMF mycophenolate mofetil, MPRE methylprednisolone, NR not repeated, PRE prednisone, PsA psoriatic arthritis, RA rheumatoid arthritis, RNP ribonucleoprotein, RTX rituximab, s-Cr serum creatinine, SLE systemic lupus erythematosus, SSA Sjögren’s syndrome-related antigen A autoantibody, u-Prot proteinuria, u-RBC urinary red blood cells (hematuria), UST ustekinumab, VT venous thrombosis, WBC white blood cells, WHO-UMC World Health Organization Uppsala Monitoring Centre
Large differences in the age of IGD onset were found, with the youngest patients in the GNLS group (mean age 36.1 years; range 18–62), followed by the GNSV (mean age 51.6 years; range 30–78) and IARD groups (mean age 60.4 years; range 40–76). At the time of IGD development, all three groups had a long primary disease duration: GNLS had a mean of 9.3 years (range 1–21), IARD had a mean of 13 years (range 1–25), and the longest disease duration was found in the GNSV group, which had a mean of 21 years (range 16–28).
In the GNLS group, 10 of the 11 patients had a previous diagnosis of SLE before the biologic treatment (one overlapping with RA), seven of whom already had documented nephritis; the eleventh patient had PsA. In the IARD group, one of the seven patients had AS. Three of the seven patients in the GNSV group had cryoglobulinemic vasculitis with renal involvement. All remaining patients in the IARD and GNSV groups had RA.
Clinical, Serological and Histopathological Features
The most typical clinical presentation was peripheral edema, which was more frequent in patients with IARD (six cases, 85.7%), followed by patients with GNLS (four cases, 36.4%) and patients with GNSV (two cases, 28.6%). Patients with GNSV had frequent cutaneous (four cases, 57.1%) and joint (three cases, 42.9%) involvement (Table 1). Among all patients, only one (4.5%) in the IARD group had no other associated clinical manifestations (Table 2).
Of the 25 patients, 23 presented proteinuria (92.0%), which was in the nephrotic range (> 3 g/24 h or > 3.5 g/gCr) in five patients with IARD (71.4%), six with GNLS (54.5%) and three with GNSV (42.9%). Hematuria was the most frequent sediment abnormality and was present in all GNSV cases, five IARD cases (57.1%) and three GNLS cases (27.3%). Impaired renal function with increased serum creatinine was reported in six patients with GNLS (54.5%), two patients with IARD (28.6%) and one patient with GNSV (14.3%). Casts (frequently granular) were the second urinary abnormality, accounting for two cases each in the GNSV and IARD groups (28.6%) and one case in the GNLS group (9.1%). Pyuria was never reported.
At the time of IGD development, renal biopsy was performed for the first time in 15 of the 25 cases; seven of the ten remaining cases underwent repeat biopsy after deterioration of renal function, whereas biopsy was not repeated in three cases. Patients with GNLS had been treated with golimumab, belimumab, rituximab and ustekinumab and showed proliferative aspects of lupus nephritis in nine cases (class IV, five cases, 45.5%; class III, three cases, 27.3%; class not specified in one case); five cases were class V (45.5%) (Table 3). Seven of the ten patients with SLE had previous lupus nephritis; it was active in six patients for whom treatment with rituximab was started.
Patients treated with etanercept [18, 19], secukinumab [20] and tofacitinib [21], and three patients treated with rituximab [22], developed GNSV, showing crescentic mesangial immunoglobulin A (IgA) deposits in the context of Schönlein–Henoch vasculitis (two cases, 28.6%) [18, 21] or necrotizing crescentic glomerulonephritis with clinical and serological pictures of myeloperoxidase anti-neutrophil cytoplasmic antibodies (MPO-ANCA) vasculitis (two cases [28.6%]) [19, 20], one of whom also presented mesangial IgA deposits [19]. Three patients affected by hepatitis C virus-related cryoglobulinemic vasculitis had a renal flare after rituximab treatment and presented deterioration of the known membrane-proliferative glomerulonephritis.
IARD developed after treatment with anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA4), anti-TNFα and anti-IL-6R: two cases, treated with tocilizumab, were classified as membrane-proliferative glomerulonephritis (28.6%); two cases, treated with certolizumab pegol and abatacept, presented membranous glomerulonephritis (28.6%); two cases, treated with adalimumab and abatacept, showed mesangial proliferative crescentic IgA nephritis (28.6%); and one case, treated with infliximab, showed a focal segmental glomerulosclerosis (14.3%).
Of the two case reports of membranous glomerulonephritis, one was in the context of a new diagnosis of SLE (antinuclear antibodies [ANA] 1:1280, low complement, anti-DNA positive and lymphopenia). Seven patients (31.8%) out of the total developed autoantibodies after biologic treatment: MPO-ANCA in three cases [19, 20, 26], ANA >1:80 in three cases [25, 26, 33] and anti-DNA in three cases [25, 29, 32]. Nevertheless, a systematic search for autoantibodies before the start of biological therapy was performed only in a few cases among those identified in the literature.
The search for possible predisposing or precipitating factors revealed the presence of an underlying nephropathy (40.0%) or urinary abnormality (microhematuria and trace of proteinuria) (4.0%) and the assumption of potential nephrotoxic drugs (bucillamine or nonsteroid anti-inflammatory drugs in two patients). Description of the presence of infection prior to the onset of IGD was found in one case (cytomegalovirus infection, noticed 4 years before the nephritic flare) [32], whereas, in one case, the presence of infection was suggested by the detection of leukocytosis accompanied by increased C-reactive protein [26]. In total, 18 of the 25 cases had no other comorbidities (see Tables 1, 2, 3). Three patients underwent cutaneous biopsy showing leukocytoclastic vasculitis [18, 21, 33].
Treatment and Outcomes
In all but one described case, biologics and JAKi were discontinued at the time of IGD clinical presentation; in one patient, the biologic (rituximab) was continued [22], which led to the patient’s death. In one patient, the resolution of renal manifestations was secondary to withdrawal of the biologic drug and the administration of antihypertensive and angiotensin-receptor blockers [24]. Rituximab was used as a rescue treatment in one case [20], and tofacitinib [27] was used in another case.
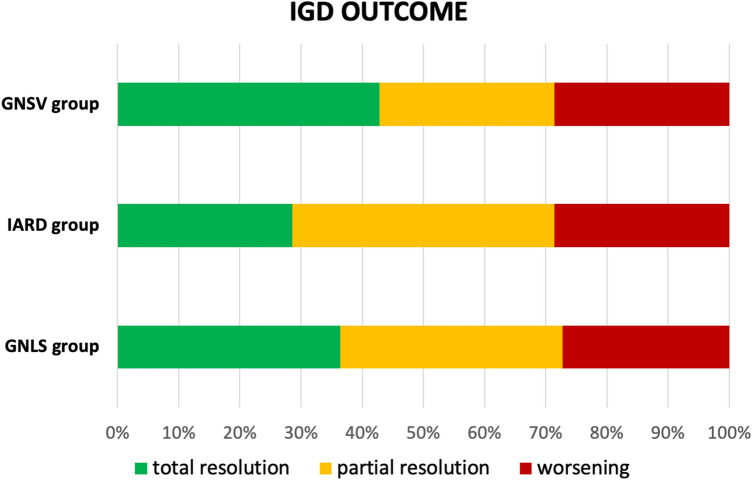
In all groups, corticosteroids were the most commonly adopted treatment (GNSV 100.0%; IARD 71.4%; GNLS 63.6%), whereas immunosuppressants were used most commonly in the GNLS group (90.9%; GNSV 42.9%; IARD 14.3%). In five cases, hemodialysis was required [22, 26, 30, 31]. Six of the 25 patients experienced a deterioration of renal function that led to end-stage renal disease (n = 3) or death (n = 1); one patient died of severe infection. The clinical outcomes are summarized in Fig. 2.
Fig. 2.
Bar chart illustrating the outcomes following drug discontinuation and intervention in three different groups. GNLS glomerulonephritis in SLE and in lupus-like syndrome, GNSV glomerulonephritis associated with systemic vasculitis, IARD isolated autoimmune renal disorders, IGD immune-mediated glomerular disorder, SLE systemic lupus erythematosus
Causality Assessment
The case causality assessment for IGD identified three cases classified in the “certain” category (one with tofacitinib in GNSV and one each with certolizumab and tocilizumab in IARD) [21, 24, 28] and five cases classified as “probable” (one each with infliximab, adalimumab, abatacept, tocilizumab in IARD; one with ustekinumab in GNLS), with evidence for an etiologic role for biologics and JAKi in inducing IGD [23, 26–28, 33] (see Tables 1, 2, 3). The majority of cases showed weaker evidence of causality between biologic treatment and IGD development (see Tables 1, 2, 3). The major reason for classifying cases into the “possible” and “unlikely” categories was the presence of another equally likely explanation for IGD development, namely, pre-existing or co-occurring causes of kidney disease and, in one case, the very long latency (72 months) between biologic intake and IGD development.
Discussion
It is well-established that some drugs used in the treatment of autoimmune diseases can themselves induce paradoxical immune-mediated processes. Biologics and ts-drugs target cytokines or lymphocytes involved in normal immune homeostasis, and blocking these cells might result in adverse events [34], with an estimated frequency of eight cases of biologic-induced autoimmune disease per 1000 patients [35, 36]. We previously reported that IGD induced by biologics is a rare but not exceptional event, being reported in 0.9 cases per 1000 patient-years [14]. In the last 2 decades, an increasing number of agents for the management of rheumatic diseases have been developed, and we have witnessed new unexpected paradoxical immune-mediated adverse events.
Through this SLR, updated data on biologic- and ts-drug-induced IGD were found in 25 new case reports published since 2014. The present SLR confirmed the role of anti-TNFα (six cases), anti-CTLA4 (two cases) and anti-IL-6R agents (two cases) in inducing renal disease but also highlighted the potential relationship between the development of IGD and other drugs, such as rituximab (nine cases), belimumab (three cases), ustekinumab (one case), secukinumab (one case) and tofacitinib (one case). To date, no cases involving apremilast or baricitinib have been found in the literature. According to clinical manifestations and kidney histology, IGD was classified into three groups: nephropathy developed as part of induced autoimmune systemic disease, such as systemic vasculitis (GNSV 28.0%) or SLE (GNLS 44.0%), or as an induced autoimmune process limited to the kidney and not classifiable in the context of a specific systemic disease (IARD 28.0%). Overall, IGD showed a better prognosis than previously reported, probably because clinicians have become more aware of this adverse event [14].
The pathogenetic mechanisms underlying biologic- and ts-drug-induced IGD have not yet been identified [37]. Although still debated, different pathways may conceivably act to induce IGD depending on individual drug molecules [11], and a predisposing genetic background may play a key role [36]. The literature especially focused on the role of anti-TNFα agents: a review conducted on the BIOGEAS Spanish registry [36] analyzed 12,731 cases of autoimmune diseases induced by biologics and found that, in most cases, the responsible agents were anti-TNFα agents (n = 9133 cases), whereas rituximab (n = 678), tocilizumab (n = 224), ustekinumab (n = 17) and abatacept (n = 14) were less frequently responsible, and no data were shown on belimumab, secukinumab or JAKi. Additionally, anti-TNFα agents are those most frequently reported as suspected drug inducers of lupus symptoms [38]. Nevertheless, it must be taken into consideration that they also represent the most commonly used biologic agents. Since biologics are large protein molecules, they can be intrinsically immunogenic and can lead to immunologic side effects that might impact both treatment efficacy and safety [13]. Different mechanisms by which anti-TNFα agents may provoke autoantibody production have been proposed. Anti-TNFα agents might bind to immune cell products, determining the formation of immunocomplexes or inducing inflammatory cell apoptosis, which causes the release of immunogenic nucleosome antigens [39, 40]. Moreover, infections, which are a well-known side effect of anti-TNFα treatment, might act as an immunostimulatory trigger for autoimmune disorders [32, 41]. TNFα inhibition also exerts a direct effect on lymphocyte function and cytokine production, switching the cytokine response from T-helper type 1 to type 2 [34] or inducing the production of type I interferon by activating plasmacytoid dendritic cells [42]. These considerations are probably applicable to other drugs with proteinic structures.
As monoclonal antibodies, belimumab and rituximab are immunogenic and could cause paradoxical inflammatory or autoimmune adverse events [34]. Nevertheless, all belimumab- and rituximab-related IGD cases were reported in patients diagnosed with active vasculitis [22], SLE [29, 32] or active lupus nephritis [30, 31], thus reducing the strength of our observation. In this setting, in fact, distinguishing between a worsening of the disease because of the lack of drug effect and a paradoxical adverse reaction could be extremely difficult, as demonstrated by the low grade of causality obtained using the WHO-UMC assessment. In a longitudinal cohort study published after our literature search was completed [43], the use of belimumab was associated with an increased frequency of de novo lupus nephritis. The authors concluded that studies of whether the effects of BAFF inhibition on lymphocyte subsets contribute to lupus nephritis susceptibility are warranted. Moreover, it is already known that viral or bacterial infections might promote autoimmune reactions by different mechanisms, such as molecular mimicry, bystander activation or epitope spreading [44]. Therefore, particular attention should be given to patients with SLE who develop signs of infection during biologic treatment, which may potentially trigger a renal flare.
Another novel finding of this SLR is that single IGD cases involving ustekinumab, secukinumab and tofacitinib have been reported [20, 21, 32]. For the first two, a mechanism linked to their proteinic structure has been hypothesized, whereas, for tofacitinib, which is a ts-drug, a different pathogenesis needs to be identified. Notably, the small number of IGD cases associated with ustekinumab, secukinumab and tofacitinib could be linked to their limited use in clinical practice compared with that of other drugs.
Applying the WHO-UMC causality assessment, the high likelihood of causality associated with the grades “certain” [21, 23, 27] and “probable” [22, 25–27, 32] was especially supported by the absence of other possible causes and good outcomes following drug withdrawal. In one case, discontinuing the biologic was sufficient to achieve complete resolution of renal function [23].
Our review has some limitations. First, searching a single database did not allow us to detect all possible reports on adverse renal events. However, we were able to retrieve 12 of 25 publications from other sources, providing a comprehensive overview of currently available data. Second, the results are based on case reports and retrospective data and not on a pharmacovigilance registry designed to systematically collect adverse events. Third, interstitial nephritis was not included in our search. In fact, we thought this topic deserved a separate discussion because of the differences in its pathogenetic mechanisms, clinical manifestations and outcomes compared with those of IGD.
Conclusions
Biologics and ts-drugs can be responsible for IGD. Clinicians should be aware of this rare event when administering such drugs because of their potential negative outcomes. A close evaluation of kidney parameters at baseline and in a quarterly follow-up is recommended to reveal renal alterations early and avoid irreversible manifestations. For the same reason, nonspecific symptoms, such as asthenia, fever, cutaneous rashes, arthralgia and/or myalgia, must always be considered suggestive of a systemic drug-induced paradoxical autoimmune reaction. A baseline laboratory work-up to exclude underlying and active infections is recommended not only to avoid reactivation of chronic viral infections but also to identify potential autoimmunity triggers. The management of IGD with systemic involvement (GNSV and GNLS) usually needs treatment with high doses of glucocorticoids and immunosuppressants, whereas the heterogeneity of IARD is mirrored by the variety in therapeutic approaches for IARD. For all IGDs, the discontinuation of the implicated drug is mandatory because of the potential severity of renal involvement. A rechallenge test of the drug should be avoided, whereas switching to a different class of biologic treatment or ts-drug is a reasonable option.
Declarations
Funding
Open access funding provided by Università degli Studi di Cagliari within the CRUI-CARE Agreement.
Conflict of Interest
AC has received consulting fees or honorarium for lectures or travel grants from Pfizer, BMS, Lilly, Janssen, Celgene, AbbVie, Novartis and MSD. EC, MP, AF, MC, IC and AM have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author Contributions
MP, EC and AM contributed to the study conception and design. Material preparation, data collection and analysis were performed by MP and EC. AAC, AF, MC, IC and AM commented on previous versions of the manuscript. All authors read and approved the final manuscript.
References
- 1.Sepriano A, Kerschbaumer A, Smolen JS, van der Heijde D, Dougados M, van Vollenhoven R, et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2019 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2020;79:760–770. doi: 10.1136/annrheumdis-2019-216653. [DOI] [PubMed] [Google Scholar]
- 2.Smolen JS, Landewé RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79:685–699. doi: 10.1136/annrheumdis-2019-216655. [DOI] [PubMed] [Google Scholar]
- 3.Gossec L, Baraliakos X, Kerschbaumer A, de Wit M, McInnes I, Dougados M, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis. 2020;79:700–712. doi: 10.1136/annrheumdis-2020-217159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Heijde D, Ramiro S, Landewé R, Baraliakos X, den Bosch FV, Sepriano A, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76:978–991. doi: 10.1136/annrheumdis-2016-210770. [DOI] [PubMed] [Google Scholar]
- 5.Fanouriakis A, Kostopoulou M, Alunno A, Aringer M, Bajema I, Boletis JN, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78:736–745. doi: 10.1136/annrheumdis-2019-215089. [DOI] [PubMed] [Google Scholar]
- 6.Aletaha D, Smolen JS. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA. 2018;320:1360–1372. doi: 10.1001/jama.2018.13103. [DOI] [PubMed] [Google Scholar]
- 7.Toussirot E. Pharmacological management of axial spondyloarthritis in adults. Expert Opin Pharmacother. 2019;20:1483–1491. doi: 10.1080/14656566.2019.1617853. [DOI] [PubMed] [Google Scholar]
- 8.Dörner T, Furie R. Novel paradigms in systemic lupus erythematosus. Lancet. 2019;393:2344–2358. doi: 10.1016/S0140-6736(19)30546-X. [DOI] [PubMed] [Google Scholar]
- 9.Tektonidou MG. JAK inhibitors: promising for a wider spectrum of autoimmune diseases? Lancet. 2019;394:2047–2048. doi: 10.1016/S0140-6736(19)32681-9. [DOI] [PubMed] [Google Scholar]
- 10.Joensuu JT, Huoponen S, Aaltonen KJ, Konttinen YT, Nordström D, Blom M. The cost-effectiveness of biologics for the treatment of rheumatoid arthritis: a systematic review. PLoS ONE. 2015;10:e0119683. doi: 10.1371/journal.pone.0119683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radhakrishnan J, Perazella MA. Drug-induced glomerular disease: attention required! Clin J Am Soc Nephrol. 2015;10:1287–1290. doi: 10.2215/CJN.01010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferner R, Aronson J. Susceptibility to adverse drug reactions. Br J Clin Pharmacol. 2019;85:2205–2212. doi: 10.1111/bcp.14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jani M, Dixon WG, Chinoy H. Drug safety and immunogenicity of tumour necrosis factor inhibitors: the story so far. Rheumatol (Oxf) 2018;57:1896–1907. doi: 10.1093/rheumatology/kex434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piga M, Chessa E, Ibba V, Mura V, Floris A, Cauli A, et al. Biologics-induced autoimmune renal disorders in chronic inflammatory rheumatic diseases: systematic literature review and analysis of a monocentric cohort. Autoimmun Rev. 2014;13:873–879. doi: 10.1016/j.autrev.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Hogan JJ, Markowitz GS, Radhakrishnan J. Drug-induced glomerular disease: immune-mediated injury. Clin J Am Soc Nephrol. 2015;10:1300–1310. doi: 10.2215/CJN.01910215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 17.Rehan HS, Chopra D, Kakkar AK. Physician’s guide to pharmacovigilance: terminology and causality assessment. Eur J Intern Med. 2009;20:3–8. doi: 10.1016/j.ejim.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 18.Karasawa K, Iwabuchi Y, Kyoda M, Akihisa T, Yamaguchi E, Suzuki S, et al. Primary IgA vasculitis with nephritis in a patient with rheumatoid arthritis diagnosed by anti-galactose-deficient IgA1 immunostaining. Intern Med. 2019;58:2551–2554. doi: 10.2169/internalmedicine.2640-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alkhunaizi AM, Dawamneh MF. ANCA-positive crescentic glomerulonephritis in a patient with rheumatoid arthritis treated with anti-tumor necrosis factor alpha. Int J Rheum Dis. 2017;20:1843–1847. doi: 10.1111/1756-185X.12612. [DOI] [PubMed] [Google Scholar]
- 20.Góis M, Messias A, Carvalho D, Carvalho F, Sousa H, Sousa J, et al. MPO-ANCA-associated necrotizing glomerulonephritis in rheumatoid arthritis; a case report and review of literature. J Nephropathol. 2017;6:58–62. doi: 10.15171/jnp.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Itoh I, Kasuno K, Yamamoto C, Takahashi N, Shimizu H, Ojima T, et al. IgA vasculitis developed as an adverse effect of tofacitinib taken for rheumatoid arthritis. Intern Med. 2020;59:817–821. doi: 10.2169/internalmedicine.3668-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desbois AC, Biard L, Sène D, Brocheriou I, Rouvier P, Lioger B, et al. Rituximab-associated vasculitis flare: incidence, predictors, and outcome. J Rheumatol. 2020;47(6):896–902. doi: 10.3899/jrheum.190076. [DOI] [PubMed] [Google Scholar]
- 23.Yarkan Tuğsal H, Zengin B, Kenar G, Can G, Ünlü M, Önen F, et al. Infliximab-associated focal segmental glomerulosclerosis in a patient with ankylosing spondylitis. Rheumatol Int. 2019;39:561–567. doi: 10.1007/s00296-019-04241-8. [DOI] [PubMed] [Google Scholar]
- 24.Butendieck RR, Bhattacharya T, Geiger X. Development of nephrotic syndrome in a patient with rheumatoid arthritis treated with certolizumab. J Rheumatol. 2016;43:1770–1772. doi: 10.3899/jrheum.160147. [DOI] [PubMed] [Google Scholar]
- 25.Asami Y, Ishiguro H, Ueda A, Nakajima H. First report of membranous nephropathy and systemic lupus erythematosus associated with abatacept in rheumatoid arthritis. Clin Exp Rheumatol. 2016;34:1122. [PubMed] [Google Scholar]
- 26.Li X, Ma J, Zhao Y, Wang H-Y, Li X-M. Development of crescentic immunoglobulin A nephritis and multiple autoantibodies in a patient during adalimumab treatment for rheumatoid arthritis. Chin Med J (Engl) 2015;128:2555–2556. doi: 10.4103/0366-6999.164992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe T, Hattori T, Ogawa Y, Jodo S. Successful treatment with tofacitinib for renal disorder due to amyloid A amyloidosis and immunoglobulin A nephropathy in a patient with rheumatoid arthritis. Clin Exp Rheumatol. 2018;36:683–684. [PubMed] [Google Scholar]
- 28.Fukaya D, Inoue T, Kogure Y, Kajiyama H, Ishizawa K, Seto T, et al. Tocilizumab-induced immunocomplex glomerulonephritis: a report of two cases. CEN Case Rep. 2020 doi: 10.1007/s13730-020-00478-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Staveri C, Karokis D, Liossis SNC. New onset of lupus nephritis in two patients with SLE shortly after initiation of treatment with belimumab. Semin Arthritis Rheum. 2017;46:788–790. doi: 10.1016/j.semarthrit.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Saka Y, Taniguchi Y, Nagahara Y, Yamashita R, Karasawa M, Naruse T, et al. Rapidly progressive lupus nephritis associated with golimumab in a patient with systemic lupus erythematosus and rheumatoid arthritis. Lupus. 2017;26:447–448. doi: 10.1177/0961203316662724. [DOI] [PubMed] [Google Scholar]
- 31.Manou-Stathopoulou S, Robson MG. Risk of clinical deterioration in patients with lupus nephritis receiving rituximab. Lupus. 2016;25:1299–1306. doi: 10.1177/0961203316641768. [DOI] [PubMed] [Google Scholar]
- 32.Sjöwall C, Cöster L. Belimumab may not prevent lupus nephritis in serologically active patients with ongoing non-renal disease activity. Scand J Rheumatol. 2014;43:428–430. doi: 10.3109/03009742.2014.887769. [DOI] [PubMed] [Google Scholar]
- 33.Al Khalili A, Scott L, Dutz JP. New-onset autoantibody-mediated nephritis during ustekinumab therapy for psoriasis in patients with and without prior systemic lupus erythematosus. JAAD Case Rep. 2019;5:682–685. doi: 10.1016/j.jdcr.2019.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Her M, Kavanaugh A. Alterations in immune function with biologic therapies for autoimmune disease. J Allergy Clin Immunol. 2016;137:19–27. doi: 10.1016/j.jaci.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 35.Perez-Alvarez R, Pérez-de-Lis M, Ramos-Casals M. BIOGEAS study group. Biologics-induced autoimmune diseases. Curr Opin Rheumatol. 2013;25:56–64. doi: 10.1097/BOR.0b013e32835b1366. [DOI] [PubMed] [Google Scholar]
- 36.Pérez-De-Lis M, Retamozo S, Flores-Chávez A, Kostov B, Perez-Alvarez R, Brito-Zerón P, et al. Autoimmune diseases induced by biological agents. A review of 12,731 cases (BIOGEAS Registry) Expert Opin Drug Saf. 2017;16:1255–1271. doi: 10.1080/14740338.2017.1372421. [DOI] [PubMed] [Google Scholar]
- 37.Kim S-K, Choe J-Y. Gender is a risk factor for annual decline in estimated glomerular filtration rate in patients treated with biological DMARDs in rheumatoid arthritis and ankylosing spondylitis: a retrospective observational study. J Korean Med Sci. 2018;33:e188. doi: 10.3346/jkms.2018.33.e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arnaud L, Mertz P, Gavand P-E, Martin T, Chasset F, Tebacher-Alt M, et al. Drug-induced systemic lupus: revisiting the ever-changing spectrum of the disease using the WHO pharmacovigilance database. Ann Rheum Dis. 2019;78:504–508. doi: 10.1136/annrheumdis-2018-214598. [DOI] [PubMed] [Google Scholar]
- 39.Boehncke W-H, Brembilla NC. Immunogenicity of biologic therapies: causes and consequences. Expert Rev Clin Immunol. 2018;14:513–523. doi: 10.1080/1744666X.2018.1468753. [DOI] [PubMed] [Google Scholar]
- 40.Heineke MH, Ballering AV, Jamin A, et al. New insights in the pathogenesis of immunoglobulin A vasculitis (Henoch-Schönlein purpura) Autoimmun Rev. 2017;16(12):1246–1253. doi: 10.1016/j.autrev.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 41.Prinz JC. Autoimmune-like syndromes during TNF blockade: does infection have a role? Nat Rev Rheumatol. 2011;7:429–434. doi: 10.1038/nrrheum.2011.35. [DOI] [PubMed] [Google Scholar]
- 42.Michel M, Henri P, Vincent FB, Leon N, Marcelli C. Mesangial immunoglobulin (Ig)A glomerulonephritis in a patient with rheumatoid arthritis treated with abatacept. Joint Bone Spine. 2013;80:660–663. doi: 10.1016/j.jbspin.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 43.Parodis I, Vital EM, Hassan SU, Jönsen A, Bengtsson AA, Eriksson P, et al. De novo lupus nephritis during treatment with belimumab. Rheumatol (Oxf) 2020;20:keaa796. doi: 10.1093/rheumatology/keaa796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arango M-T, Shoenfeld Y, Cervera R, Anaya J-M. Infection and autoimmune diseases [Internet]. autoimmunity: from bench to bedside [Internet]. El Rosario University Press. 2013. https://www.ncbi.nlm.nih.gov/books/NBK459437/. Accessed 21 Jun 2020. [PubMed]
- 45.The Uppsala Monitoring Centre. The use of the WHO-UMC system for standardised case causality assessment. 2019. https://www.who.int/medicines/areas/quality_safety/safety_efficacy/WHOcausality_assessment.pdf. Accessed 30 May 2020.