Abstract
Mitigating the risk of acquiring coronaviruses including SARS-CoV-2 requires awareness of the survival of virus on high-touch environmental surfaces (HITES) and skin, and frequent use of targeted microbicides with demonstrated efficacy. The data on stability of infectious SARS-CoV-2 on surfaces and in suspension have been put into perspective, as these inform the need for hygiene. We evaluated the efficacies of formulated microbicidal actives against alpha- and beta-coronaviruses, including SARS-CoV-2. The coronaviruses SARS-CoV, SARS-CoV-2, human coronavirus 229E, murine hepatitis virus-1, or MERS-CoV were deposited on prototypic HITES or spiked into liquid matrices along with organic soil loads. Alcohol-, quaternary ammonium compound-, hydrochloric acid-, organic acid-, p-chloro-m-xylenol-, and sodium hypochlorite-based microbicidal formulations were evaluated per ASTM International and EN standard methodologies. All evaluated formulated microbicides inactivated SARS-CoV-2 and other coronaviruses in suspension or on prototypic HITES. Virucidal efficacies (≥ 3 to ≥ 6 log10 reduction) were displayed within 30 s to 5 min. The virucidal efficacy of a variety of commercially available formulated microbicides against SARS-CoV-2 and other coronaviruses was confirmed. These microbicides should be useful for targeted surface and hand hygiene and disinfection of liquids, as part of infection prevention and control for SARS-CoV-2 and emerging mutational variants, and other emerging enveloped viruses.
Subject terms: Microbiology, Diseases
Introduction
The guidance provided by the U.S. Centers for Disease Control and Prevention (CDC)1, the World Health Organization (WHO)2,3, and other regional centers for disease prevention and control discuss the infection prevention and control (IPAC) strategies of most utility in dealing with the COVID-19 pandemic. These would appear to be social distancing, the wearing of face masks, and the use of microbicides for hand hygiene and for sanitization of high-touch environmental surfaces (HITES)4. The latter include, but are not limited to, the toilet, bathroom and kitchen sinks, food preparation surfaces, door knobs, toys, desk tops, coins and paper currency, cell phones and other small electronic devices, automatic teller machines, and shopping carts, etc.)5. The use of surface- and hand-hygiene agents should be informed by knowledge of the likelihood of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) contamination of such HITES. Other important factors include the persistence (survival or stability) of infectious virus released within bodily secretions/excretions from infected individuals on HITES, the likelihood of transfer of virus from HITES to hands, the persistence (survival) of virus on the skin once transferred, and the hierarchy of susceptibility of virus to microbicides. The hygiene agents should be targeted to HITES and to skin, and should be applied with appropriate frequency. The microbicides should be used as instructed, and applied using contact times that have been demonstrated empirically to have adequate virucidal efficacy. Reports of improper use of cleaning agents have surfaced6. As a result, scientists at the U.S. CDC have stressed that “Public messaging should continue to emphasize evidence-based, safe cleaning and disinfection practices to prevent SARS-CoV-2 transmission in households, including hand hygiene and cleaning and disinfection of high-touch surfaces”6.
On the basis of the known susceptibility of lipid-enveloped viruses, such as the coronaviruses, to microbicides7–9, reduction of the burden of infectious SARS-CoV-2 and other emerging coronaviruses remaining on HITES should readily be achieved through use of a variety of commonly-employed formulated microbicides. This paper is intended to complement and expand on a previous report on the virucidal efficacy of a number of commercially available formulated microbicides10. We have now included antiseptic liquids, disinfectant wipes, disinfectant liquids, disinfectant sprays, and sodium hypochlorite against SARS-CoV-2 and other coronaviruses tested on inanimate non-porous surfaces per ASTM E1053-2011. In addition, we have also tested a bar soap, an antiseptic liquid, a surface cleanser, two hand sanitizing gels, a liquid handwash, two foaming handwashes, and a toilet bowl cleanser for virucidal efficacy against SARS-CoV-2 and human coronavirus 229E in suspension studies conducted per ASTM E1052-2012 or EN 14,476:2013 + A2:201913. We have expanded the evaluation to include additional beta-coronaviruses, including murine hepatitis virus-1 (MHV-1), severe acute respiratory syndrome coronavirus (SARS-CoV), and Middle East respiratory syndrome coronavirus (MERS-CoV), and the alpha-coronavirus human coronavirus 229E (HCoV-229E). In addition, we have developed the theme of persistence (survival) of infectious SARS-CoV-2, once deposited or spilled and then dried on HITES or on skin. This persistence information informs the need for targeted surface- and hand-hygiene applied at an appropriate frequency. In addition, we discuss the risk associated with incomplete inactivation of coronaviruses that subsequently might be released to the environment. This information informs the need for properly formulated microbicidal actives that may be used to decontaminate SARS-CoV-2 and other coronaviruses suspended in liquid matrices, such as pathophysiological secretions/excretions, residual virus in pre-soaked wipes following use for sanitizing surfaces, and waste handwash rinse water.
Results
Survival of SARS-CoV-2 on inanimate surfaces (prototypic HITES) and animate surfaces (swine skin)
Several studies of the survival (persistence of infectivity) of SARS-CoV-2 experimentally dried on prototypic HITES, or added to human secretions/excretions or skin have been reported in the recent literature14–22. These studies have evaluated the recovery of infectious SARS-CoV-2 from hard non-porous surfaces (such as steel and glass), from relatively porous surfaces (such as wood and cardboard), or from skin or within bodily secretions/excretions. The data sets have included the determined infectious SARS-CoV-2 titer at various times following deposition and drying on the prototypic HITES or after being added to skin or bodily secretions. The survival half-life values (t½, time required to reduce the SARS-CoV-2 titer by one-half) have been provided in the cited literature or were, in some cases16,18, calculated here from reported raw data to reflect biphasic or monophasic decay values, as appropriate to the reported data sets.
These viral persistence data (Table 1) indicate that, once deposited on prototypic HITES or swine skin, or when added to human secretions/excretions, infectious SARS-CoV-2 is recoverable from the surfaces/suspensions for hours to weeks. Survival half-life on surfaces was found to depend a number of factors. These include: (1) the type and porosity of the surface (including skin), (2) the presence and type of organic matrix in which the virus is suspended at the time of deposition onto the surface, (3) time, and (4) environmental factors such as temperature and relative humidity (RH). In suspension inactivation studies, relatively short half-lives (1.9 to 3.7 h) were observed in human sputum, mucus, or fecal suspensions17,20. A longer half-life (16 h) was determined in human urine20 for SARS-CoV-2. While some of the studies14,17,19,21 examined the impact of temperature or RH on viral persistence, for the most part, the studies evaluated virus survival at ambient temperature and RH, and we have reported only these data in Table 1.
Table 1.
Literature values for terminal survival half-life (t½) of SARS-CoV-2 on prototypic HITES, on skin, or in suspension.
| Prototypic fomite/suspension | Organic load | Temperature (RH) | Survival t½ (h) | Time needed for 1 log10 reduction in titer (h) | Time needed to decrease viral burden below MID (h)a | References |
|---|---|---|---|---|---|---|
| Plastic | None | 21–23 °C (40%) | 6.8 | 23 | 91 | 15 |
| None | 22 °C (65%) | 11 | 37 | 147 | 14 | |
| None | 25–27 °C (35%) | 16 | 53 | 213 | 20 | |
| None | 19–21 °C (45–55%) | 35 | 115 | 460 | 16 | |
| 10 mg/mL BSA | 19–21 °C (45–55%) | 24 | 79 | 316 | 16 | |
| Human sputum | 21 °C (40%) | 3.1 | 10 | 40 | 17 | |
| Human mucus | 21 °C (40%) | 3.1 | 10 | 40 | 17 | |
| Tripartite soil | 20 °C (35–40%) | 38 | 130 | 520 | 18 | |
| Stainless steel | None | 21–23 °C (40%) | 5.6 | 19 | 75 | 15 |
| None | 22 °C (65%) | 15 | 50 | 200 | 14 | |
| None | 25–27 °C (35%) | 23 | 77 | 306 | 20 | |
| Tripartite soil | 20 °C (35–40%) | 29 | 95 | 380 | 18 | |
| Tripartite soil | 20 °C (50%) | 43 | 143 | 573 | 21 | |
| Aluminum | None | 19–21 °C (45–55%) | 0.33 | 1.1 | 4.4 | 16 |
| 10 mg/mL BSA | 19–21 °C (45–55%) | 15 | 51 | 204 | 16 | |
| Glass | None | 22 °C (65%) | 4.8 | 16 | 64 | 14 |
| None | 25–27 °C (35%) | 22 | 73 | 293 | 20 | |
| None | 19–21 °C (45–55%) | 7.0 | 23 | 93 | 16 | |
| 10 mg/mL BSA | 19–21 °C (45–55%) | 25 | 83 | 333 | 16 | |
| Tripartite soil | 20 °C (50%) | 46 | 153 | 613 | 21 | |
| Wood | None | 22 °C (65%) | 0.71 | 2.4 | 9.5 | 14 |
| None | 25–27 °C (35%) | 21 | 70 | 280 | 20 | |
| Vinyl | Tripartite soil | 20 °C (50%) | 46 | 153 | 613 | 21 |
| Copper | None | 21–23 °C (40%) | 0.77 | 2.6 | 10 | 15 |
| Cardboard | None | 21–23 °C (40%) | 3.5 | 12 | 47 | 15 |
| Liquid sputum | N/A | 21 °C | 1.9 | 6.3 | 25 | 17 |
| Liquid mucus | N/A | 21 °C | 3.7 | 12 | 47 | 17 |
| Swine skin | None | 20–24 °C (40–50%) | 3.5 | 12 | 47 | 19 |
| Human feces (10% suspension) | None | 25–27 °C (35%) | 2.6 | 8.7 | 35 | 20 |
| Human urine | None | 25–27 °C (35%) | 16 | 53 | 212 | 20 |
Abbreviations used: BSA, bovine serum albumin; MID, human minimal infectious dose; RH, relative humidity; t½, half-life.
aCalculated assuming an initial deposited virus burden of 1.0 × 106 plaque-forming units (PFU) and an estimated human MID of 250 PFU (see “Methods” section).
Viral stability data, on their own, do not greatly inform the implications for virus transmission. In order to put these SARS-CoV-2 survival data into perspective, we have calculated and displayed in Table 1 the durations of time required to reduce an initial viral burden of 1.0 × 106 plaque-forming units (PFU) to a level beneath an estimate of the minimal human infectious dose of 250 PFU. These calculations were performed as described in Table 1 on the basis of the stability values reported in the literature. The SARS-CoV-2 may remain infectious on different types of fomites, or in suspensions of various types of body discharges, for hours to days.
Virucidal efficacy of an antiseptic liquid formulation for SARS-CoV-2 and other coronaviruses
We evaluated the virucidal efficacy of an antiseptic liquid, with p-chloro-m-xylenol (PCMX) as active ingredient tested at a final active concentration of ~ 0.12%, against various alpha- and beta-coronaviruses (MHV-123, HCoV-229E, SARS-CoV, MERS-CoV, and SARS-CoV-2). The results of virucidal efficacy of PCMX for inactivating viruses dried on glass (Table 2) demonstrate complete inactivation of each tested coronavirus within 0.5 to 10 min contact time at ambient temperature. Complete inactivation of the infectious virus within the limits of detection of the assays used was observed in the case of each virus. The difference in log10 reduction noted relate more to limit of virus-detection of the assay than to differences in virucidal-efficacy. The suspension inactivation testing against SARS-CoV-2 also demonstrate complete inactivation within 1 min.
Table 2.
Virucidal efficacy of an antiseptic liquid contining ~ 0.12% p-chloro-m-xylenol (PCMX) for inactivating a variety of coronaviruses in suspension or on a hard surface (glass).
| Coronavirus | Contact time | Temperature | Organic load | Log10 reduction | |
|---|---|---|---|---|---|
| Hard surface testing (glass) | |||||
| MHV-123 | Beta-coronavirus | 0.5 min | Ambient | None | ≥ 4.2, ≥ 4.5, ≥ 4.5, ≥ 4.5, ≥ 4.5a |
| HCoV-229E | Alpha-coronavirus | 10 min | 20 ± 2 °C | 10 FBS | ≥ 4.0 |
| SARS-CoV | Beta-coronavirus | 5 min | 20 ± 1 °C | 5% FBS | ≥ 6.0, ≥ 6.0b |
| MERS-CoV | Beta-coronavirus | 5 min | Ambient | 5% BSA | ≥ 5.0, ≥ 5.2a |
| Suspension testing | |||||
| SARS-CoV-2 | Beta-coronavirus | 1 min | 20 ± 1 °C | 5% FBS | ≥ 5.0 |
Abbreviations used: BSA, bovine serum albumin; FBS, fetal bovine serum; HCoV-229E, human coronavirus strain 229E; MERS-CoV, Middle Eastern respiratory syndrome virus; MHV-1, murine hepatitis virus 1; PCMX, p-chloro-m-xylenol; SARS-CoV, severe acute respiratory syndrome coronavirus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aThe values are for technical replicates.
bThe values are for independent lots.
Virucidal efficacy of formulated microbicidal actives for SARS-CoV-2 and other coronaviruses experimentally deposited on glass
The virucidal efficacy of a variety of formulated microbicidal actives was tested per ASTM E1053-20 Standard11 using infectious SARS-CoV-2 and other beta- and alpha-coronaviruses dried on a glass surface in the presence of an organic load at ambient temperature (20 ± 1 °C). The results are displayed in Table 3. Virucidal efficacy displayed by the microbicides against the two beta-coronaviruses and the alpha-coronavirus were similar, as expected on theoretical grounds7–9,24. Contact times of 0.5 to 10 min led to ≥ 3.0 to ≥ 6.0 log10 reduction in coronavirus titer in the case of each of the formulated microbicidal actives evaluated, including PCMX, QAC, organic acids, ethanol/QAC, and sodium hypochlorite. Lot-to-lot variability of virucidal efficacy of the formulated microbicidal actives was minimal.
Table 3.
Virucidal efficacy of formulated microbicides tested per ASTM E1053-20 Standard against HCoV-229E, SARS-CoV, or SARS-CoV-2 dried on a glass surface in the presence of an organic load.
| Product type | Active ingredient concentration | Temperature (°C) | Contact time (min) | Organic load | Log10 reduction in infectious titer achieveda | |||
|---|---|---|---|---|---|---|---|---|
| In product | Tested | Alpha-coronavirus | Beta-coronavirus | |||||
| HCoV-229E | SARS-CoV | SARS-CoV-2 | ||||||
| Antiseptic liquid | PCMX (4.7% w/v) | 0.125% w/v (tested at 1:40 of supplied) | 20 ± 1 | 5, 10e | 5, 10% FBSe | ≥ 4.0 | ≥ 6.0, ≥ 6.0 | NT |
| Disinfectant wipes | QACb (0.19% w/w) | 0.19% w/w (tested as supplied) | 20 ± 1 | 1.75 | 5% FBS | ≥ 6.0 | ≥ 5.8 | ≥ 3.5, ≥ 3.5, ≥ 3.5 |
| Citric acid (2.4% w/w) |
2.4% w/w (tested as supplied) |
20 ± 1 | 0.5 | 5% FBS | ≥ 4.3, ≥ 4.3 | ≥ 3.0, ≥ 3.0 | ≥ 3.0, ≥ 3.0, ≥ 3.0 | |
| Disinfectant spray | Ethanol (50% w/w)/ QACc (0.082% w/w) | 50% w/w ethanol, 0.082% w/w QACc (tested as supplied) | 20 ± 1 | 0.5, 1.75f | 5% FBS | ≥ 5.5, ≥ 5.5, | NT | ≥ 4.6, ≥ 4.7, ≥ 4.5 |
| Dilutable cleaner | QACb (2.9% w/w) | 0.0916% (tested at 1:32 of supplied) | 20 ± 1 | 5 | 5% FBS | ≥ 3.5, ≥ 3.5 | ≥ 4.8, ≥ 4.8 | NT |
| RTU cleaner | QACd (0.092% w/w) | 0.092% (tested as supplied) | 20 ± 1 | 2 | 5% FBS | ≥ 3.3, ≥ 3.3 | ≥ 3.8, ≥ 3.8 | ≥ 4.0, ≥ 4.0, ≥ 4.0 |
In all cases, one technical replicate was performed per data point.
Abbreviations used: FBS, fetal bovine serum; HCoV-229E, human coronavirus strain 229E; NT, not tested; PCMX, p-chloro-m-xylenol; QAC, quaternary ammonium compound; RTU, ready to use; SARS-CoV, severe acute respiratory syndrome coronavirus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; w/v, weight-to-volume; w/w, weight-to-weight.
aWhere multiple values are displayed, this reflects the testing of multiple independent lots of the formulated microbicide.
bAlkyl (50% C14, 40% C12, 10% C16) dimethyl benzyl ammonium chloride.
cAlkyl (50% C14, 40% C12, 10% C16) dimethyl benzyl ammonium saccharinate.
dAlkyl (67% C12, 25% C14, 7% C16, 1% C8-C10-C18) dimethyl benzyl ammonium chloride; Alkyl (50% C14, 40% C12, 10% C16) dimethyl benzyl ammonium chloride.
eThe 10-min contact time and 10% FBS load were used in the HCoV-229E study.
fThe 0.5-min contact time was used for the HCoV-229E study and the 1.75-min contact time was used for the SARS-CoV-2 study.
Virucidal efficacy of formulated microbicidal actives for SARS-CoV-2 and other coronaviruses evaluated in suspension
The virucidal efficacy of a variety of formulated microbicidal actives was tested per ASTM-E1052-2012 (handwash agents; Table 4) or EN 14,476:2013 + A2:201913 (hand sanitizers, antiseptic liquids and sprays, surface cleaners, toilet cleaners, etc.; Table 5), using infectious SARS-CoV-2 and HCoV-229E in suspension studies. Contact times of 0.5 to 1 min at ~ 37 °C led to ≥ 3.0 to ≥ 3.6 log10 reduction in coronavirus titer in the case of the handwash agents containing actives such as PCMX, salicylic acid, or benzalkonium chloride (Table 4). Contact times of 0.5 to 5 min at ambient temperature led to ≥ 4.0 to ≥ 5.5 log10 reduction in coronavirus titer in the case of each of the actives-based formulations evaluated in the EN 14,476 studies, including PCMX, benzalkonium chloride, organic and inorganic acids, ethanol, and sodium hypochlorite (Table 5).
Table 4.
Virucidal efficacy of formulated microbicidal actives tested per ASTM-E1052-20 Standard against HCoV-229E or SARS-CoV-2 in suspension inactivation studies.
| Product type | Active ingredient concentration | Temperature (°C) | Contact time (min) | Organic load | Log10 reduction in infectious titer achieved | ||
|---|---|---|---|---|---|---|---|
| In product | Tested | Alpha-coronavirus | Beta-coronavirus | ||||
| HCoV-229E | SARS-CoV-2 | ||||||
| Bar soap | PCMX (0.090% w/w) | 0.014% w/w (tested at 1:6.25 of supplied) | 37 ± 1 | 0.5, 1a | 5% FBS | ≥ 3.3 | ≥ 4.1 |
| Liquid gel handwash | Salicylic acid (0.10% w/w) | 0.025% w/w (tested at 1:4 of supplied) | 37 ± 1 | 0.5, 1a | 5% FBS | ≥ 3.6, ≥ 3.6, | ≥ 3.6 |
| Foaming handwash | Benzalkonium chloride (0.10% w/w) | 0.025% w/w (tested at 1:4 of supplied) | 37 ± 1 | 1 | 5% FBS | ≥ 3.3, ≥ 3.3 | ≥ 3.4 |
| Salicylic acid (0.09% w/w) | 0.023% w/w (tested at 1:4 of supplied) | 37 ± 1 | 0.5, 1a | 5% FBS | ≥ 3.5, ≥ 3.8 | ≥ 5.0 | |
Where multiple cvalues are shown, these represent different technical replicates.
Abbreviations used: FBS, fetal bovine serum; HCoV-229E, human coronavirus strain 229E; PCMX, p-chloro-m-xylenol; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2, w/w, weight-to-weight.
aA 1-min contact time was used for testing against HCoV-229E; an 0.5-min contact time was used for testing against SARS-CoV-2.
Table 5.
Virucidal efficacy of formulated microbicidal actives tested per EN 14,476:2013 + A2:2019 Standard against HCoV-229E or SARS-CoV-2 in suspension inactivation studies.
| Product type | Active ingredient concentration | Temperature (°C) | Contact time (min) | Organic load | Log10 reduction in infectious titer achieved | ||
|---|---|---|---|---|---|---|---|
| In product | Tested | Alpha-coronavirus | Beta-coronavirus | ||||
| HCoV-229E | SARS-CoV-2 | ||||||
| Hand hygiene agents | |||||||
| Antiseptic liquid | PCMX (4.7% w/v) | 0.021% w/v (tested at 1:200 of supplied) | 20 ± 1 | 5 | Dirtya | ≥ 5.2 | ≥ 4.7 |
| Hand sanitizer gel | Ethanol (67% w/w) | 53% w/w (tested at 1:1.25 of supplied) | 20 ± 1 | 1 | Dirty, cleanb | ≥ 5.4 | ≥ 4.2 |
| Citric acid (1.9% w/w), lactic acid (0.51% w/w) | 1.5% w/w citric acid, 0.41% w/w lactic acid (tested at 1:1.25 of supplied) | 20 ± 1 | 0.5, 1c | Cleanb | ≥ 5.2 | ≥ 4.7 | |
| Surface hygiene agents | |||||||
| Surface cleaner | QACd (0.096% w/w) | 0.077% w/w (tested at 1:1.25 of supplied) | 20 ± 1 | 5 | Dirty | NT | ≥ 4.1 |
| Lactic acid (2.4% w/w) | 1.9% (1:1.25 of supplied) | 20 ± 1 | 5 | Dirty | NT | ≥ 5.5 | |
| Toilet bowl cleaner | Hydrochloric acid (6.9% w/w) | 0.25% w/w (tested at 1:27 of supplied) | 20 ± 1 | 0.5 | Dirty | NT | ≥ 4.1 |
| Dilutable cleaner | Sodium hypochlorite (3.6% w/w) | 0.14% w/w (tested at 1:26 of supplied) | 20 ± 1 | 0.5 | Cleanb | NT | ≥ 5.1 |
| RTU cleaner | Benzalkonium chloride (0.56% w/w) | 0.45% w/w (tested at 1:1.25 of supplied) | 20 ± 1 | 5 | Dirty | NT | ≥ 4.5 |
| Disinfectant spray | Ethanol (55% w/w) | Ethanol (44% w/w) used as supplied | 20 ± 1 | 5 | Dirty | ≥ 4.0 | ≥ 4.1 |
| Bathroom cleaner | Sodium hypochlorite (0.40% w/w) | 0.32% w/w (tested at 1:1.25 of supplied) | 20 ± 1 | 5 | Dirty | NT | ≥ 5.1 |
In all cases, one technical replicate was performed per data point.
Abbreviations used: BSA, bovine serum albumin; HCoV-229E, human coronavirus strain 229E; NT, not tested; PCMX, p-chloro-m-xylenol; RTU, ready to use; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2, w/v, weight-to-volume, w/w, weight-to-weight.
aDirty means 3 g/L BSA + 3 mg/L erythrocyte suspension.
bClean means 0.3 g/L BSA, used for testing SARS-CoV-2.
cA 1-min contact time was used for testing against HCoV-229E; an 0.5-min contact time was used for testing against SARS-CoV-2.
dAlkyl dimethyl benzyl ammonium chloride (C12-16).
Discussion
In response to the current outbreak of SARS-CoV-2 and the urgency around establishing evidence-based IPAC approaches, we and others have hypothesized that the virucidal efficacy of commonly used microbicides against this emerging coronavirus should be predictable on the basis of the known susceptibility of enveloped viruses in general to microbicides7–9,24. In this paper, we confirm the virucidal efficacy of a variety of formulated microbicidal actives against SARS-CoV-2 and a number of members of the Coronaviridae family (HCoV-229E, MERS-CoV, SARS-CoV, and MHV-1), indicating similar virucidal efficacy across members of the Coronaviridae. On the basis of these results, we predict that any potential future emerging coronaviruses or other emerging enveloped viruses also readily would be inactivated by these microbicides. The necessity for use of microbicides in IPAC for emerging viruses is informed by the routes of transmission of the viruses, the likelihood that they will be deposited on HITES, the expected duration of survival of the viruses on such HITES, and the frequency of recontamination of the HITES by infected persons.
The primary route of person-to-person transmission of SARS-CoV-2 is thought to involve respiratory droplets and aerosols, as reviewed in25–27, leading predominantly to a respiratory tract infection. Secondary (indirect) transmission of SARS-CoV-2 through contamination of HITES by droplets and respiratory aerosols or other patient secretions/excretions (bronchoalveolar fluid, sputum, mucus, blood, lacrimal fluid, semen, urine, and feces) also is thought to occur25–28. The indirect transmission pathway may be envisioned as a patient’s bodily fluids-HITES-hands-mucous membrane nexus. The relevance of this pathway is supported by experimental transmission studies in animal models29 and by the results of investigations of the contamination of HITES with SARS-CoV-2 RNA in healthcare settings26,30–32. The detection of infectious SARS-CoV-2 in patient feces33,34 and urine35, together with the data on survival of SARS-CoV-2 in fecal and urine suspensions20,22, suggest that a fecal/oral or fecal/respiratory route of transmission is possible. Zang et al.36, upon being unable to recover infectious SARS-CoV-2 from RNA-positive human fecal samples, have argued that the virus is rapidly inactivated by simulated human colonic fluid. This conclusion is not consistent, however, with the findings of Xiao et al.33 or Zhang et al.34, who were able to recover infectious SARS-CoV-2 from human feces, as reviewed in37, or with the reports of Liu et al.20 and Chan et al.22 that SARS-CoV-2 remains infectious for hours in human urine and fecal suspensions. The conclusion is also not consistent with results obtained for other coronaviruses, such as SARS-CoV and MERS-CoV37. These routes of transmission could involve direct transmission or indirect transmission involving the patient’s bodily fluids-HITES-hands-mucous membrane nexus mentioned above. The U.S. CDC has stated that “transmission of novel coronavirus to persons from surfaces contaminated with the virus has not been documented”, but nevertheless has provided guidance on surface disinfection38. The finding of SARS-CoV-2 RNA in untreated wastewater39 and sewage40, is suggestive of, but certainly not proof of, the possibility for survival of infectious virus within these human waste streams, as reviewed in37,41,42. Unfortunately, there are, to our knowledge, no data on the detection or persistence of infectious SARS-CoV-2 in wastewater, and this topic, therefore, remains a knowledge gap37,41,42. For the moment, on the basis of the reported survival of SARS-CoV-2 in human fecal suspensions and urine20,22, we assume the possibility of the contamination of wastewater streams with infectious SARS-CoV-2, and associated risk of virus dissemination through this route.
In order to inform the necessity of effective and frequent HITES decontamination during a virus pandemic, such as that being experienced currently with SARS-CoV-2, we have summarized and put into perspective the recent data on the survival of infectious SARS-CoV-2 on such surfaces under ambient conditions. Infectivity half-life values obtained from virus survival studies can be used to calculate the burden of infectious SARS-CoV-2 expected to remain on a surface after varying durations of time following initial virus deposit. This assumes, of course, that the initial virus load on the surface is known. There, unfortunately, is a paucity of empirical data on infectious SARS-CoV-2 burden (loads) on HITES in the literature thus far. The existing data consist primarily of measurements of nucleic acid burden on HITES. Findings from Matson et al.17 suggest that caution should be taken when making inferences regarding the possible presence of infectious virus on a surface based solely on RT-PCR detection of viral RNA. We very much share this concern.
The data on the survival of SARS-CoV-2 on surfaces14–22,43, like previous data obtained for other coronaviruses43–51, demonstrate that viral persistence (survival) on HITES is dependent upon: (1) the type of surface, (2) the presence and type of organic matrix in which the virus is suspended at the time of deposition and drying upon the surface, and (3) time. The survival data for SARS-CoV-2 dried on surfaces (Table 1) indicate that the virus remains infectious longer on hard non-porous surfaces, such as plastic and stainless steel, than on wood or cardboard. The presence of an organic load during drying of the virus typically results in increased half-life of SARS-CoV-216,18,21. The result of Matson et al.17 that SARS-CoV-2 displayed a shorter half-life when dried on a surface in the presence of human sputum and mucus than when dried in a culture medium matrix was therefore unexpected, and requires confirmation. Temperature and relative humidity likely also play a role in the persistence of coronaviruses on HITES, although the data sets appearing in the literature specifically for SARS-CoV-214–22 have primarily evaluated survival under ambient conditions. For survival dependence on temperature, see references17,19,21,22,43.
The viral persistence data indicate that infectious virus may remain on non-porous HITES for one or two weeks. The risk of acquiring a SARS-CoV-2 infection indirectly, through transfer of virus from a contaminated HITES to a susceptible mucous membrane through the intermediacy of the hands, therefore may remain for weeks after the initial surface contamination event. The requirement for frequent sanitization of HITES is driven by the possibility for recontamination of these environmental surfaces by infected persons52. Infectivity data addressing the frequency of recontamination of HITES with SARS-CoV-2 have not yet appeared in the literature, to our knowledge. This represents another knowledge gap. In the case of human coronavirus 229E, Bonny et al.53 demonstrated that infectious virus could be recovered from HITES (desktops and door knobs) in a university classroom that was cleaned daily with a commercial cleaning solution consisting of non-ionic and anionic surfactants. This result suggested the possibility of the frequent recontamination of the HITES, although the possible inadequacy of the daily cleaning regimen was not ruled out by the authors53.
The stability of SARS-CoV-2 in suspensions and on skin has also been investigated. The survival of the virus in human sputum and mucus is similar to that on porous surfaces (half-lives of 1.9 to 3.5 h, respectively)17. Survival on skin (3.5 h)19 is similar (Table 1). These half-life values indicate that the virus may remain infectious for days on HITES or skin following a contamination event, in the absence of hygiene interventions.
From the foregoing, it is apparent that there is a risk of indirect transmission of SARS-CoV-2 from contaminated HITES, via the intermediacy of hands. This risk may be mitigated through targeted hygiene interventions, including frequent surface hygiene as well as hand hygiene. The required frequency of surface hygiene interventions is dependent on the expected rate of recontamination of HITES by patients. This suggests that greater vigilance with respect to targeted hygiene practices is required in intensive care units and other contamination hot spots, as emphasized by Zhang54 and by the results of Wu et al.55.
The required efficacy of targeted hygiene agents (formulated microbicidal actives) for reducing the infectious titer of SARS-CoV-2 and other coronaviruses depends, in large part, on the burden of infectious virus on the surface or in the suspension being sanitized56 and the human minimal infectious dose (MID). Expected virucidal efficacy usually is expressed in terms of a minimal log10 reduction in viral titer to be achieved in standardized testing. For instance, the U.S. Environmental Protection Agency (EPA) specified in its 2012 disinfectant product guidance57 that “The product should demonstrate complete inactivation of the virus at all dilutions. If cytotoxicity is present, the virus control titer should be increased to demonstrate a ≥ 3 log10 reduction in viral titer beyond the cytotoxic level.” On the other hand, in the case of disinfectants that do not cause cytotoxicity in the cell-based infectivity assays used in virucidal efficacy testing, a 4-log10 reduction in viral titer is considered to demonstrate effectiveness. These EPA requirements were revised in the 2018 revision58. In the 2018 guidance, a valid test requires the following: (1) ≥ 4.8 log10 of infectivity per carrier be recovered from the dried virus control film; (2) ≥ 3 log10 reduction in titer is observed in the presence or absence of cytotoxicity; (3) if cytotoxicity is present, ≥ 3 log10 reduction in titer is observed beyond the cytotoxic level; and (4) cell controls (cells not spiked with virus) be negative for evidence of infectivity (i.e., viral cytopathic effect or plaques). The revised guidance therefore does not require that complete inactivation be observed at all dilutions for a product to be deemed effective.
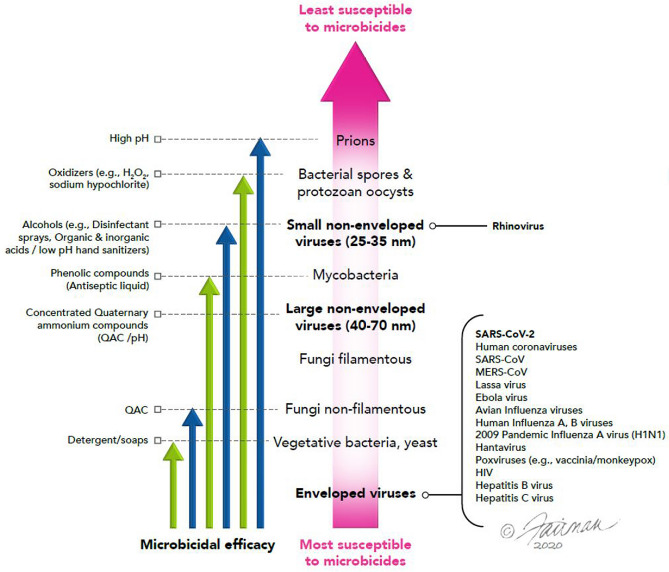
In our virucidal efficacy studies, a variety of formulated microbicidal actives displayed complete inactivation of the challenge coronaviruses (including SARS-CoV-2). The maximum log10 reduction values achieved depended on the limitations of the assays (namely, the maximum titer of virus applied to the test and the cytotoxicity associated with the formulated microbicidal active). In any event, log10 reduction values of ≥ 3 to ≥ 6 were obtained after relatively short contact times (i.e., 0.5 to 5 min.). These contact times are relevant for surface disinfection interventions and, notably, the contact times required for the hand hygiene agents evaluated (handwash agents and hand sanitizing gels) were ≤ 1 min. The active ingredients used in the formulated microbicidal agents evaluated in Tables 2, 3, 4, and 5 included agents with differing mechanisms of action59. These included lipid envelope-disrupting agents such as ethanol, QAC, detergents, and phenolics. Protein- and capsid-denaturing agents evaluated included ethanol, phenolics, sodium hypochlorite, inorganic and organic acids. The genome-degrading agents evaluated included ethanol, and sodium hypochlorite. Each of these types of microbicidal actives was expected, on the basis of the known susceptibility of pathogens to microbicides7–9,24,59 (Fig. 1), to display virucidal efficacy against lipid-enveloped viruses, including SARS-CoV-2 and other coronaviruses. This principle of the hierarchy of pathogen susceptibility has also been embraced by the U.S. EPA60. Our efficacy data presented herein confirm this, and indicate that the virucidal activities are approximately equivalent for a variety of alpha- and beta-coronaviruses. In addition, reviews and empirical reports of the efficacy of microbicides against SARS-CoV-210,22,48,49 and other coronaviruses23,44,46–49,61 have confirmed the expected virucidal efficacy of a variety of microbicides against these viruses in surface disinfection studies. Efficacy of microbicides tested in suspension studies has been discussed in recent reviews and empirical reports of the efficacy of microbicides against SARS-CoV-210,14,22,49 and other coronaviruses44,46,48,49,61,62. These also have confirmed the expected virucidal efficacy of a variety of microbicides against these viruses in suspension.
Figure 1.
Heirarchy of susceptibility of pathogens to microbicidal active ingredients. Certain formulated microbicides may include combinations of active ingredients, resulting in synergistic virucidal efficacy greater than that displayed by the individual active ingredients (modified from Sattar8).
Taken together, these results imply that similar virucidal efficacies should be displayed by such microbicides against future emerging coronaviruses, including mutational variants (isolates) of SARS-CoV-2 such as the recently emerging and highly transmissible 20I/501Y.V1, VOC 202,012/01, or B.1.1.7 variant63,64. The virucidal efficacies would be expected24 to apply also to other emerging enveloped viruses, such as Ebola virus65,66, Lassa virus, Nipah virus, and influenza viruses such as the recently emerging G4 genotype H1N1 swine influenza virus67 and the variant influenza viruses (H1N1v, H3N2v, H1N2v) in humans68. The latter expectation is supported by our own unpublished data on influenza strains and by a recent literature review61. These are important conclusions, given that there is a likelihood of emergence of novel coronaviruses and other enveloped viruses in the future.
A large diversity of alpha- and beta-coronaviruses currently circulate in bat reservoirs69. These include the alpha-coronavirus, swine acute diarrhea syndrome coronavirus, which caused large-scale pig die-offs in southern China, and is able to infect human cells in the laboratory70. They also include a substantial diversity of SARS-related coronaviruses that include the progenitor lineages of SARS-CoV and SARS-CoV-2, primarily carried by horseshoe bats (Rhinolophus spp.)71–76. SARS-CoV emerged in 2002 within urban live animal markets in Guangdong, where a range of animal species being held there, as well as animal vendors themselves, were infected77. While the exact route of SARS-CoV-2 spillover from bats to humans is uncertain, evidence strongly implicates a similar live animal market as a site where infections were amplified, and where SARS-CoV-2 was identified on contaminated surfaces71,78. Subsequent clusters of COVID-19 have been reported in a large seafood market in Beijing, perhaps as a direct result of contamination of cold surfaces used to prepare food79. Thus, the role of food animals, food preparation, and contaminated surfaces in the spillover of these bat coronaviruses suggests a key role for disinfecting surfaces to mitigate spillover or early spread of novel bat coronaviruses.
There is also evidence that bat coronaviruses are transmitted regularly to people in southeast Asia, without involvement of wildlife consumption. First, diverse behaviors that bring people into contact with wildlife have been reported in South China80,81. Secondly, 2.79% of people sampled from communities living close to a bat cave in Yunnan, China, where SARS coronaviruses have been reported, were serologically positive for bat coronavirus immunoglobulin G (IgG)82. Extrapolating to rural communities across Southeast Asia where similar bats exist, and given that SARS-CoV IgG had a half-life of 2–3 years in SARS survivors, it is likely that hundreds of thousands of people are infected by novel bat coronaviruses each year. Surface disinfection and personal hygiene using agents that are effective at inactivating coronaviruses may, therefore, be critical to the control of the current SARS-CoV-2 pandemic, and in reducing the risk of future coronavirus spillover events.
Assuming, for the purpose of argument, that SARS-CoV-2 is transmitted from person-to-person in part through the patient’s bodily fluids-HITES-hands-mucous membrane nexus, what evidence do we have that implementing surface and hand hygiene interventions will mitigate risk of disseminating SARS-CoV-2? It is clear that face touching is a frequent human behavior83, suggesting that the indirect route of transmission occurring through the intermediacy of the hands is relevant, and highlighting the need for strict implementation of hand hygiene. This especially is the case when coming in contact with patients’ bodily fluids and when touching potentially contaminated HITES. Evidence has now been reported that disinfection can lead to reduction in dissemination of SARS-CoV-2 from infected persons to uninfected family members. For instance, Wang et al.4 reported that the daily use of chlorine- or ethanol-based disinfectants for household cleaning was 77% effective in reducing transmission of SARS-CoV-2 within the families investigated. Diarrhea as a symptom of the primary infected household member was also found to be a risk factor for transmission within families, informing the importance of sanitizing the toilets and the bathroom itself4.
The relatively high risk of the bathroom for deposition of SARS-CoV-2 from patients onto HITES was also highlighted in the study of Ding et al.84 In that study, frequency of sanitization of HITES was twice daily using a chlorine-releasing agent. Out of 107 surface samples and 46 air samples taken from a COVID-19 hospital ward, only eight were found to be positive for SARS-CoV-2 RNA. These included seven surface samples (two door handles, one toilet seat, one toilet seat cover, one bathroom washbasin tap handle, one bathroom ceiling exhaust louver, and one bathroom door handle) and one air sample (a corridor air sample)84.
Since it is not known yet whether infectious SARS-CoV-2 persists in wastewater streams41,42, we cannot address the question of whether hygiene interventions can reduce the infectious viral burden of such waste streams. This remains a significant knowledge gap that has yet to be closed85. There are data on the persistence of infectious virus in water for other coronaviruses, such as transmissible gastroenteritis virus, mouse hepatitis virus-1, and SARS-CoV, as reviewed in42,43. For the moment, the use of wastewater/sewage SARS-CoV-2 RNA data is limited to a biomarker for monitoring of ongoing COVID-19 outbreak intensity39,40. It is evident from the foregoing discussion, however, that targeted surface/hand hygiene, appropriately practiced under healthcare, community and home settings, can help to ensure that infectious SARS-CoV-2 is not released into the environment via wastewater streams.
Conclusions
Indirect person-to-person transmission of SARS-CoV-2 from contaminated HITES through the intermediacy of the hand (i.e. through the patient’s bodily fluids-HITES-hands-mucous membrane nexus), is a relevant mechanism for dissemination of SARS-CoV-2 and the associated disease (COVID-19). Here, we have expanded on a previous report on the virucidal efficacy of a number of commercially available formulated microbicidal actives10 to now include antiseptic liquids, disinfectant wipes, disinfectant liquids, disinfectant sprays, and sodium hypochlorite for virucidal efficacy against SARS-CoV-2 and other coronaviruses on inanimate surfaces (prototypic HITES). In addition, we have also tested bar soap, antiseptic liquid, surface cleanser, hand sanitizing gels, liquid handwash, foaming handwash, and toilet bowl cleanser for efficacy against SARS-CoV-2 and human coronavirus 229E in suspensions intended to model animate surfaces/solutions (skin and bodily fluids). Each of these formulated microbicidal actives resulted in complete inactivation (≥ 3 to ≥ 6 log10 reduction in infectious titer within the limits of virus detection) of the challenge coronaviruses, including SARS-CoV-2. These surface- and personal-care hygiene agents should, therefore, be useful in IPAC for SARS-CoV-2, including newly emerging mutational variants63,64, future emerging coronaviruses, and other emerging enveloped viruses23 (such as Ebola virus, Lassa virus, Nipah virus, and new strains of influenza virus such as the recently emerging G4 genotype H1N1 swine influenza virus67 and the variant influenza viruses (H1N1v, H3N2v, H1N2v) in humans)68.
Methods
Challenge viruses, host cell lines, and reagents
Virucidal efficacy testing against alpha- and beta-coronaviruses was performed for a variety of formulated microbicidal active-containing products per standardized methods. Details on the challenge viruses and their sources and the detector (host) cell lines used for propagation of viral stocks and for cell-based infectivity (titration) assay are displayed in Supplementary Table S1. This table also indicates the culture media used in these assays and the organizations that performed the virucidal efficacy testing.
Standardized efficacy testing methodologies
Virucidal efficacy evaluations of formulated microbicidal actives against coronaviruses experimentally deposited on a non-porous surface (glass) were conducted per ASTM E1053-2011. The active ingredient concentrations, contact times, and exposure temperatures evaluated and the organic soil load are indicated in Table 3. Virucidal efficacy evaluations of formulated microbicidal actives against coronaviruses suspended in liquid matrices were conducted per ASTM E1052-2012 or EN 14,476:2013 + A2:201913, depending upon the geographical region in which the formulated microbicide was intended to be marketed. The challenge matrix in each case was cell culture medium containing various organic loads. The active ingredient concentrations in the formulations and the concentrations actually tested (if different), contact times, and exposure temperature evaluated, and the organic soil load, if applicable, are indicated in Tables 4 and 5.
A summary of the standardized methods is presented in Supplemental Materials.
Calculation of log10 reduction, survival half-lives, and time required to reach surface burdens below the MID
Virucidal efficacy data obtained from suspension inactivation and non-porous surface (glass) inactivation studies are presented in terms of log10 reduction in titer of the virus, with titers being calculated on the basis of viral cytopathic effect (CPE) (CPE for SARS-CoV-2 in Vero E6 cells is shown in Supplementary Figure S1) and expressed in units of log10 tissue culture infectious dose50 per mL (TCID50/mL).
Survival half-life (t½) of SARS-CoV-2 on experimentally contaminated prototypic HITES, skin, urine, and feces were calculated from data reported in the literature14–22. These data consisted of infectious viral titers (log10 TCID50/mL) measured at various time intervals following drying of the virus on prototypic HITES or skin. Biphasic linear regression plots (log10 titer vs. time) of the survival data were used to calculate the survival half-lives (t½), as t½ = 0.301/-m, where m = the slope of the log10 titer vs. time plots. The time required to reach viral burdens below the human minimal infectious dose (MID) was calculated assuming an initial viral burden of 1 × 106 plaque-forming units (PFU). The time required to reduce the initial viral burden by 1 log10 (D) was calculated by multiplying the t½ × 3.33 (one t½ = 0.301 log10 reduction in titer).
Assuming a human MID for SARS-CoV-2 of ~ 250 PFU (estimated on the basis of mouse infectious dose50 values obtained for MHV-186 and SARS-CoV87), the time required to bring the burden to 100 PFU was calculated as 4 log10 reduction × the time (D) required to achieve 1 log10 reduction in titer. This calculation was performed as an illustrative example only. It is acknowledged that the selection of an initial viral burden of 1 × 106 PFU is somewhat arbitrary. The latter was based, in part, on estimates of viral particle counts expected to be generated by SARS-CoV-2 by infected persons during loud speaking (> 1 × 103 virion-containing droplet nuclei per minute)87, and the assumption that once generated, the droplet nuclei will eventually settle and contaminate environmental surfaces. The use of ~ 250 PFU as the human MID is a very conservative approach based not on empirical human data, but only on animal (transgenic mouse) studies87–89.
Supplementary Information
Acknowledgements
This work was funded by Reckitt Benckiser LLC. We thank Dr. Chris Jones and Dr. Mark Ripley, both from Reckitt Benckiser R&D, for their critical review of the manuscript and feedback. The authors gratefully acknowledge Jennifer Musyoki of Reckitt Benckiser R&D for assistance with compilation of SARS-CoV-2 virucidal efficacy data of microbicides investigated, and Jennifer Fairman, CMI, FAMI (Fairman Studios, LLC), for illustrating Fig 1.
Author contributions
J. R. R., M. K. I. & J. M., designed and approved the project and experimental design; K. W. & V. S. coordinated the efficacy testing performed at the various contract testing organizations and aided in assembling the efficacy results and experimental conditions; S. S. Z., T. K. & S. F performed the SARS-CoV-2 efficacy experiments and contributed the images used in Figure S1; R. W. N. & M. K. I performed the data analysis, interpretation, and presentation, including the figures. J. H. E. & P. D. contributed the discussion section on emerging coronaviruses. All authors (M. K. I., R. W. N., S. S. Z., K. W., V. S., T. K., S. F., J. H. E., P. D., J. R. R. & J. M. participated in authoring, reviewing, and approving the manuscript.
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
Competing interests
R. W. N. received a fee from Reckitt Benckiser LLC for assistance in authoring the manuscript. No other authors have declared a competing interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-84842-1.
References
- 1.U.S. Centers for Disease Prevention and Control. Coronavirus 2019 (COVID-19) How to protect yourself and others. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention-H.pdf (2020).
- 2.World Health Organization.Coronavirus disease (COVID-19) advice for the public. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. (2020).
- 3.World Health Organization. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations. (2020).
- 4.Wang Y, et al. Reduction of secondary transmission of SARS-CoV-2 in households by face mask use, disinfection and social distancing: a cohort study in Beijing. China. BMJ Glob. Health. 2020;5:e002794. doi: 10.1136/bmjgh-2020-002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott EA, Bruning E, Nims RW, Rubino JR, Ijaz MK. A 21st century view of infection control in everyday settings: moving from the germ theory of disease to the microbial theory of health. Am. J. Infect. Control. 2020;48:1387–1392. doi: 10.1016/j.ajic.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gharpure R, et al. Knowledge and practices regarding safe household cleaning and disinfection for COVID-19 prevention—United States, May 2020. MMWR Morb. Mortal. Wkly. Rep. 2020 doi: 10.15585/mmwr.mm6923e2externalicon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein, M. & Deforest, A. Principles of viral inactivation. In: Block, S. S., editor. Disinfection, sterilization, and preservation, 3rd edition: Philadelphia: Lea and Febiger; pp. 422–434 (1983).
- 8.Sattar SA. Hierarchy of susceptibility of viruses to environmental surface disinfectants: a predictor of activity against new and emerging viral pathogens. J. AOAC Int. 2007;90(6):1655–1658. doi: 10.1093/jaoac/90.6.1655. [DOI] [PubMed] [Google Scholar]
- 9.Ijaz MK, Rubino JR. Should test methods for disinfectants use vertebrate virus dried on carriers to advance virucidal claims? Inf. Control Hosp. Epidemiol. 2008;29(2):192–194. doi: 10.1086/526441. [DOI] [PubMed] [Google Scholar]
- 10.Ijaz MK, et al. Microbicidal actives with virucidal efficacy against SARS-CoV-2. Am. J. Infect. Control. 2020;48:972–973. doi: 10.1016/j.ajic.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ASTM International. ASTM E1053–20. Standard practice to assess virucidal activity of chemicals intended for disinfection of inanimate, nonporous environmental surfaces. 10.1520/E1053-20. https://www.astm.org/Standards/E1053.htm (2020).
- 12.ASTM International. ASTM E1052–20. Standard practice to assess the activity of microbicides against viruses in suspension. 10.1520/E1052-20. https://www.astm.org/Standards/E1052.htm (2020).
- 13.British Standards Institute. BS EN 14476:2013+A2:2019. Chemical disinfectants and antiseptics. Quantitative suspension test for the evaluation of virucidal activity in the medical area. Test method and requirements (Phase 2/Step 1). https://infostore.saiglobal.com/en-us/Standards/BS-EN-14476-2013-A2-2019-238423_SAIG_BSI_BSI_2753744/ (2019).
- 14.Chin, A. W. H. et al. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 1(1).https://www.thelancet.com/journals/lanmic/article/PIIS2666-5247(20)30003-3/fulltext (2020) [DOI] [PMC free article] [PubMed]
- 15.van Doremalen N, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pastorino B, Touret F, Gilles M, de Lamballerie X, Charr RN. Prolonged infectivity of SARS-CoV-2 in fomites. Emerg. Infect. Dis. 2020;26(9):1. doi: 10.3201/eid2609.201788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matson MJ, et al. Effect of environmental conditions on SARS-CoV-2 stability in human nasal mucus and sputum. Emerg. Infect. Dis. 2020;26(9):2276–2278. doi: 10.3201/eid2609.202267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasloff SB, Strong JE, Funk D, Cutts TA. Stability of SARS-CoV-2 on critical personal protective equipment. Sci. Rep. 2021;11:984. doi: 10.1038/s41598-020-80098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harbourt DE, et al. Modeling the stability of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on skin, currency, and clothing. PLOS Negl. Trop. Dis. 2020;14(11):e0008831. doi: 10.1371/journal.pntd.0008831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, et al. Stability of SARS-CoV-2 on environmental surfaces and in human excreta. J. Hosp. Infect. 2021;107:105–107. doi: 10.1016/j.jhin.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riddell S, Goldie S, Hill A, Eagles D, Drew TW. The effect of temperature on persistence of SARS-CoV-2 on common surfaces. Virol. J. 2020;17:145. doi: 10.1186/s12985-020-01418-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan K-H, et al. Factors affecting stability and infectivity of SARS-CoV-2. J. Hosp. Infect. 2020;106(2):226–231. doi: 10.1016/j.jhin.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dellanno C, Vega Q, Boesenberg D. The antiviral action of common household disinfectants and antiseptics against murine hepatitis virus, a potential surrogate for SARS coronavirus. Am. J. Infect. Control. 2009;37:649–652. doi: 10.1016/j.ajic.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ijaz MK, Sattar SA, Rubino JR, Nims RW, Gerba CP. Combating SARS-CoV-2: Leveraging microbicidal experiences with other emerging/re-emerging viruses. PeerJ. 2020;8:e9914. doi: 10.7717/peerj.9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morawska L, et al. How can airborne transmission of COVID-19 indoors be minimised? Environ. Int. 2020;142:105832. doi: 10.1016/j.envint.2020.105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel KP, et al. Transmission of SARS-CoV-2: an update of current literature. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39(11):2005–2011. doi: 10.1007/s10096-020-03961-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. Transmission of SARS-CoV-2: implications for infection prevention precautions. https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions (2020).
- 28.Wang W, et al. Detection of SARS-CoV-2 in different types of clinical specimens. J. Am. Med. Assoc. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sia S-F, et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature. 2020;583:834–838. doi: 10.1038/s41586-020-2342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ong SWX, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. J. Am. Med. Assoc. 2020;323(16):1610–1611. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye G, et al. Environmental contamination of SARS-CoV-2 in healthcare premises. J. Infect. 2020;81(2):e1–e5. doi: 10.1016/j.jinf.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang F-C, et al. Detection of severe acute respiratory syndrome coronavirus 2 RNA on surfaces in quarantine rooms. Emerg. Infect. Dis. 2020;26(9):2162–2164. doi: 10.3201/eid2609.201435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao F, et al. Infectious SARS-CoV-2 in feces of patient with severe COVID-19. Emerg. Infect. Dis. 2020;26(8):1920–1922. doi: 10.3201/eid2608.200681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, et al. Isolation of 2019-nCoV from a stool specimen of a laboratory-confirmed case of the coronavirus disease 2019 (COVID-19) China CDC Weekly. 2020;2(8):123–124. doi: 10.46234/ccdcw2020.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun A, et al. Isolation of infectious SARS-CoV-2 from urine of a COVID-19 patient. Emerg. Microb. Infect. 2020;9:991–993. doi: 10.1080/22221751.2020.1760144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zang R, et al. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci. Immunol. 2020;5:eavc3582. doi: 10.1126/sciimmunol.abc3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jefferson, T., Spencer, E. A., Brassey, J. & Heneghan, C. SARS-CoV-2 and the role of orofecal transmission: Evidence brief. CEBM. https://www.cebm.net/covid-19/sars-cov-2-orofecal-transmission/ (2020). [DOI] [PMC free article] [PubMed]
- 38.U.S. Centers for Disease Prevention and Control. Cleaning and disinfection for households. Interim recommendations for U.S. households with suspected or confirmed coronavirus disease 2019 (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cleaning-disinfection.html (2020).
- 39.Ahmed W, et al. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728(138764):2020. doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peccia J, et al. SARS-CoV-2 RNA concentrations in primary municipal sewage sludge as a leading indicator of COVID-19 outbreak dynamics. Nat. Biotechnol. 2020;38:1164–1167. doi: 10.1038/s41587-020-0684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhowmick GD, et al. Coronavirus disease (COVID-19) outbreak: some serious consequences with urban and rural water cycle. NPJ Clean Water. 2019;3:32. doi: 10.1038/s41545-020-0079-1. [DOI] [Google Scholar]
- 42.La Rosa G, Bonadonna L, Lucentini L, Kenmoe S, Suffredini E. Coronavirus in water environments: occurrence, persistence and concentration methods—a scoping review. Water Res. 2020;179:115899. doi: 10.1016/j.watres.2020.115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aboubakr HA, Sharafeldin TA, Goyal SM. Stability of SARS-CoV-2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: a review. Dis. Transbound. Emerg. 2020 doi: 10.1111/tbed.13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolff, M. H., Sattar, S. A., Adegbunrin, O. & Tetro, J. Environmental survival and microbicide inactivation of coronaviruses. In Coronaviruses with special emphasis on first insights concerning SARS. Schmidt, A., Wolff, M. H. & Weber, O., editors, Birkhäuser Verlag, Basel Switzerland (2005).
- 45.Otter JA, et al. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J. Hosp. Infect. 2016;92(3):235–250. doi: 10.1016/j.jhin.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kampf, G., Todt, D., Pfaender, S. & Steinmann, E. Persistence of coronaviruses on inanimate surfaces and its inactivation with biocidal agents. J. Hosp. Infect. 104, 246–251 (2020) and corrigendum: J. Hosp. Infect. 105, 587 (2020). [DOI] [PMC free article] [PubMed]
- 47.Kumar GD, et al. Biocides and novel antimicrobial agents for mitigation of coronaviruses. Front. Microbiol. 2020;11:1351. doi: 10.3389/fmicb.2020.01351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castaño, N. et al. Fomite transmission and disinfection strategies for SARS-CoV-2 and related viruses. arXiv. 2005.11443 [q-bio.OT} (2020).
- 49.Cimolai N. Environmental and decontamination issues for human coronaviruses and their potential strategies. J. Med. Virol. 2020;92(11):2498–2510. doi: 10.1002/jmv.26170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ren S-Y, et al. Stability and infectivity of coronaviruses in inanimate environments. World J. Clin. Cases. 2020;8(8):1391–1399. doi: 10.12998/wjcc.v8.i8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duan S-M, et al. Stability of SARS coronavirus in human specimens and environment and its sensitivity to heating and UV irradiation. Biomed. Environ. Sci. 2003;16:246–255. [PubMed] [Google Scholar]
- 52.Ikner, L. A., Torrey, J. R., Gundy, P. M. & Gerba, C. P. A continuously active antimicrobial coating effective against human coronavirus 229E. medRxiv. (2020) DOI 10.1101/2020.05.10.20097329
- 53.Bonny TS, Yezli S, Lednicky JA. Isolation and identification of human coronavirus 229E from frequently touched environmental surfaces of a university classroom that is cleaned daily. Am. J. Infect. Control. 2018;46:105–107. doi: 10.1016/j.ajic.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang DX. SARS-CoV-2: air/aerosols and surfaces in laboratory and clinical settings. J. Hosp. Infect. 2020;105:577–579. doi: 10.1016/j.jhin.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu S, et al. Environmental contamination by SARS-CoV-2 in a designated hospital for coronavirus disease 2019. Am. J. Infect. Control. 2020;48:910–924. doi: 10.1016/j.ajic.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scott, E. A., Bruning, E. & Ijaz, M. K. Targeted decontamination of environmental surfaces in everyday settings. In: McDonnell, G. & Hansen, J., editors. Block’s disinfection, sterilization, and preservation, 6th edition: Philadelphia: Wolters Kluwer, (2020).
- 57.U.S. Environmental Protection Agency. Product Performance Test Guidelines OCSPP 810.2200: Disinfectants for Use on Hard Surfaces – Efficacy Data Recommendations. [EPA 712-C-07–074] (2012). https://www.regulations.gov/document?D=EPA-HQ-OPPT-2009-0150-0021
- 58.U.S. Environmental Protection Agency. Product Performance Test Guidelines OCSPP 810.2200: Disinfectants for Use on Environmental Surfaces – Guidance for Efficacy Testing. [EPA 712-C-17–004] (2018). https://www.regulations.gov/document?D=EPA-HQ-OPPT-2009-0150-0036
- 59.Maillard, J.-Y., Sattar, S. A. & Pinto, F. Virucidal activity of microbicides. in Russell, Hugo & Ayliffe's: Principles and Practice of Disinfection, Preservation and Sterilization, Fifth Edition. Fraise, A. P., Maillard, J.-Y. & Sattar, S. A., editors, Blackwell Publishing (2013).
- 60.U.S. Environmental Protection Agency. Guidance to Registrants: Process for Making Claims against Emerging Viral Pathogens not on EPA-Registered Disinfectant Labels. (2016). https://www.epa.gov/sites/production/files/2016-09/documents/emerging_viral_pathogen_program_guidance_final_8_19_16_001_0.pdf
- 61.Lin Q, et al. Sanitizing agents for virus inactivation and disinfection. View. 2020;1:e16. doi: 10.1002/viw2.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Golin AP, Choi D, Ghahary A. Hand sanitizers: a review of ingredients, mechanisms of action, modes of delivery, and efficacy against coronaviruses. Am. J. Infect. Control. 2020;48:P1062–1067. doi: 10.1016/j.ajic.2020.06.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.U.S. Centers for Disease Control and Prevention. Emerging SARS-CoV-2 Variants. https://www.cdc.gov/coronavirus/2019-ncov/more/science-and-research/scientific-brief-emerging-variants.html [PubMed]
- 64.Lauring AS, Hodcroft EB. Genetic Variants of SARS-CoV-2—What Do They Mean? J. Am. Med. Assoc. 2021 doi: 10.1001/jama.2020.27124. [DOI] [PubMed] [Google Scholar]
- 65.Cutts TA, Ijaz MK, Nims RW, Rubino JR, Theriault SS. Effectiveness of dettol antiseptic liquid for inactivation of ebola virus in suspension. Sci Rep. 2019;9:6590. doi: 10.1038/s41598-019-42386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cutts TA, et al. Assessing the contributions of inactivation, removal, and transfer of Ebola virus and vesicular stomatitis virus by disinfectant pre-soaked wipes. Front. Pub. Health. 2020;8:183. doi: 10.3389/fpubh.2020.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun H, et al. Prevalent Eurasian avian-like H1N1 swine influenza virus with 2009 pandemic viral genes facilitating human infection. Proc. Natl. Acad. Sci. USA. 2020;117(29):17204–17210. doi: 10.1073/pnas.1921186117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.U.S. Centers for Disease Control and Prevention. Variant Influenza Viruses in Humans. https://www.cdc.gov/flu/swineflu/variant-flu-in-humans.htm (2020).
- 69.Anthony SJ, et al. Global patterns in coronavirus diversity. Virus Evolution. 2017;3(1):vex012. doi: 10.1093/ve/vex012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou P, et al. Fatal swine acute diarrhoea syndrome caused by an HKU2-related coronavirus of bat origin. Nature. 2018;556:255–258. doi: 10.1038/s41586-018-0010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boni MF, et al. Evolutionary origins of the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19 pandemic. Nat. Microbiol. 2020;5:1408–1417. doi: 10.1038/s41564-020-0771-4. [DOI] [PubMed] [Google Scholar]
- 72.Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nature Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou P, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li W, et al. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2020;310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- 75.Yang X-L, et al. Isolation and characterization of a novel bat coronavirus closely related to the direct progenitor of severe acute respiratory syndrome coronavirus. J. Virol. 2020;90:3253–3256. doi: 10.1128/JVI.02582-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Latinne A, et al. Origin and cross-species transmission of bat coronaviruses in China. Nat. Commun. 2020;11:4235. doi: 10.1038/s41467-020-17687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guan Y, et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in Southern China. Science. 2020;302:276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- 78.Huang C, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan China. Lancet. 2020 doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhuang, P. Coronavirus: Beijing market outbreak investigation zeros in on seafood stalls. https://www.scmp.com/news/china/society/article/3089674/coronavirus-beijing-market-outbreak-investigation-zeroes-seafood (2020).
- 80.Li H, et al. Human-animal interactions and bat coronavirus spillover potential among rural residents in Southern China. Biosafety Health. 2019;1:84–90. doi: 10.1016/j.bsheal.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li H-Y, et al. A qualitative study of zoonotic risk factors among rural communities in southern China. Int. Health. 2020;12:77–85. doi: 10.1093/inthealth/ihaa001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang N, et al. Serological evidence of bat SARS-related coronavirus infection in humans China. Virol. Sin. 2018;33(1):104–107. doi: 10.1007/s12250-018-0012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kwok YLA, Gralton J, McLaws M-L. Face touching: a frequent habit that has implications for hand hygiene. Am. J. Infect. Control. 2015;43(2):112–114. doi: 10.1016/j.ajic.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ding, Z. et al. Toilets dominate environmental detection of SARS-CoV-2 virus in a hospital. Ding Z, Qian H, Xu B, et al. Toilets dominate environmental detection of severe acute respiratory syndrome coronavirus 2 in a hospital. Sci. Total Environ. 753:141710 (2020). 10.1016/j.scitotenv.2020.141710 [DOI] [PMC free article] [PubMed]
- 85.Mushi V, Shao M. Tailoring of the ongoing water, sanitization and hygiene interventions for prevention and control of COVID-19. Trop. Med. Health. 2020;48:47. doi: 10.1186/s41182-020-00236-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.De Albuquerque N, et al. Hepatitis virus strain 1 produces a clinically relevant model of severe acute respiratory syndrome in A/J mice. J. Virol. 2006;80(21):120382–210394. doi: 10.1128/JVI.00747-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.DeDiego ML, et al. Pathogenicity of severe acute respiratory coronavirus deletion mutants in hACE-2 transgenic mice. Virology. 2008;376:379–389. doi: 10.1016/j.virol.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stadnytskyi V, Bax CE, Bax A, Anfinrud P. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc. Natl. Acad. Sci. USA. 2020;117(22):11857–11877. doi: 10.1073/pnas.2006874117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Anonymous. SARS dose response experiments. QMRA Wiki. http://qmrawiki.org/experiments/sars (2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).