Abstract
Physical activity is important for physical function and pain relief in people with lower extremity osteoarthritis (OA). Unfortunately, people with OA are not as active as their peers without OA. The objective of this study was to determine whether aerobic capacity and fatigability are associated with physical activity in women with hip OA. We conducted a cross-sectional analysis of 36 women with hip OA. We assessed aerobic fitness as predicted VO2max from a six-minute walk test. We assessed fatigability using a treadmill test. Finally, we assessed self-reported physical activity using the UCLA activity scale, and quantified steps per day and activity intensity using accelerometers. We used Pearson correlations to determine associations. We used regression analysis to determine whether fatigability mediated the association between aerobic fitness and physical activity. On average, subjects were moderately active via the UCLA score (5.2 ± 1.3 out of 10). Aerobic fitness (R = 0.582, p < 0.001) and fatigability (R = 0.516, p = 0.003) were significantly correlated with UCLA scores. However, aerobic fitness was the best predictor of UCLA scores, as well as sedentary time, and time spent in light activity. Fatigability was not a mediator between aerobic fitness and UCLA scores. Aerobic fitness and fatigability may be modifiable barriers to physical activity in people with OA. Future interventional studies should examine whether improving aerobic fitness improves physical activity or fatigability.
Keywords: hip osteoarthritis, physical activity, aerobic fitness, fatigue
Introduction
People with osteoarthritis (OA) are at risk for low physical activity and for failing to meet physical activity recommendations.1-3 This is a serious problem because physical activity is critical for healthy aging. Physical activity promotes cardiovascular health, cognitive health, and living independence.4-6 Moreover, increasing physical activity is beneficial for those suffering from OA because physical activity has positive effects on physical function and pain.7-9 OA is associated with increased cardiovascular morbidity and mortality,10, 11 in part through its adverse effect on mobility.12, 13 Together, these findings suggest that improving physical activity is an important goal in the care of the older adult with OA. However, the reasons for reduced physical activity in people with OA are not well understood.
Fatigue is one factor that can contribute to lower physical activity in older adults generally.14-16 Fatigue is a common symptom associated with OA,17, 18 and has been linked to lower physical activity in people with OA.14, 19 Fatigability is a separate but related construct.20, 21 While fatigue can arise from a number of mental and physical sources, fatigability refers to the process of becoming more fatigued with activity. Fatigability has been associated with lower physical activity in general samples of older adults22 but has not yet been related to physical activity in people with OA.
Separately, energy availability, or specifically the reduction in energy availability associated with aging, has been proposed as a factor limiting physical activity in older adults.23 It has been postulated that people who require more energy relative to their total energy capacity for activities of daily living, such as walking, have less energy reserve and are therefore less likely to participate in physical activity.24 This hypothesis is dubbed the energetic model of mobility limitation.23 Wert and colleagues linked energy cost of walking to physical function in older adults with slow gait,24 however to our knowledge, the impact of aerobic capacity (energy availability) on physical activity has not been studied in OA.
There is a dearth of successful physical activity interventions for people with OA. Many have taken a behavioral approach,25 and have not taken into account the specific physical impairments that may characterize OA. Perhaps, as a result, these approaches have had mixed results.25 New targeted interventions are needed to improve physical activity in this unique population and to create such new interventions, more insight is needed into the factors that limit physical activity in people with OA. Hence, the rationale for this study is that fatigability may be a modifiable factor associated with physical activity in people with OA and could thereby provide a new target for intervention. As stated above, fatigability has been shown to be a key limiting factor in physical activity in older adults,22 and has been shown to be modifiable in other populations.26 Further, there is literature demonstrating reduced aerobic capacity in people with OA and that aerobic capacity is modifiable in people with OA.27 Thus establishing a relationship between fatigability and physical activity, and between fatigability and aerobic capacity in people with OA could uncover a mechanism contributing to reduced physical activity in this group and a framework for interventions. As a first step, in this study we sought to establish potential associations among fatigability, aerobic capacity, and physical activity in a subset of people with OA.
The primary purpose of this study was to investigate the association between fatigability and physical activity in women with hip OA. We focus on women in this study because some studies show that older adult women are at higher risk for low levels of physical activity than older adult men.28-30 The secondary purpose was to investigate whether or not aerobic capacity, a potentially modifiable factor, is associated with fatigability and physical activity. This would suggest that fatigability may be amenable to treatments targeting aerobic capacity. We hypothesized that insufficient energy capacity (poorer aerobic capacity) could adversely affect physical activity through its effect on fatigability. Thus, the specific hypotheses tested were that in women with hip OA (i) increased fatigability is associated with decreased physical activity levels, (ii) lower levels of aerobic capacity are associated with decreased physical activity levels, and (iii) fatigability mediates the association between aerobic capacity and physical activity.
Methods
Participants
This Level I prospective study was approved by the Institutional Review Board of the University of Illinois at Chicago. We recruited women from local surgical practices, radio and public transport advertising, and an IRB-approved contact list obtained from medical records. Inclusion criteria were doctor-diagnosed hip OA. Exclusion criteria included other actively symptomatic joints, history of total joint replacement within 2 years, inability to walk without assistive devices, and any medical condition that interfered with gait or the ability to safely complete the protocol. Data from 36 women who satisfied enrollment criteria and provided written informed consent to participate were used in this study (Table 1).
Table 1.
Characteristics of (N = 36) women with hip OA
| Mean ± SD | |
|---|---|
| Age (years) | 60.3 ± 9.3 |
| Body Mass Index (kg/m2) | 29.9 ± 5.8 |
| Aerobic Capacity (mg/ml*kg) | 21.2 ±10.1 |
| UCLA scores | 5.0 ± 1.3 |
Clinical Status
The Hip disability and Osteoarthritis Outcome Score (HOOS) was used to characterize clinical status of participants. HOOS is a patient-reported assessment about the participant’s hip and hip-associated problems.31 The HOOS questionnaire consists of 5 subscales: Pain, Symptoms, Function in activities of daily living (ADL), Function in Sport and Recreation (Sport/Rec), and hip-related quality of life (QOL) in which higher scores indicate fewer hip-related problems or symptoms.
Fatigability
We modified a fatigability test that was previously developed and validated in older adults.32 The major change between the original fatigability test and the test as administered in this study was that we used a treadmill and asked people to walk for a set time rather than a set distance. Participants were aware that fatigability was being assessed. Women walked on a treadmill for 10 minutes, after a 2-3 minute acclimation period. They began walking at their preferred speed. Every 2.5 minutes they were given the opportunity to reduce or increase their speed to their comfort but were encouraged to do their best with regard to walking speed throughout the evaluation. participants were asked to indicate verbally or using hand gestures whether they wanted the speed increased or decreased. Speed was changed accordingly by the investigator in 0.2 m/s increments until the participant indicated that they should stop. Environmental factors were the same for all participants. The performance fatigability score was calculated as the average speed during the 10-minute period divided by the speed during the first 2.5 minutes. Lower scores reflected increased levels of fatigability.
Aerobic capacity
Aerobic capacity was assessed by predicting the VO2max from a treadmill-based six-minute walk test using a published regression equation.33 For the six-minute walk test, participants were asked to walk for as far as possible. They could adjust speed as needed but were encouraged to maximize distance. Heart rate was measured during the test. The regression equation estimates VO2max based on heart rate, body mass, and distance walked. Calculated VO2max was then normalized to body mass and reported as ml/min*kg.
Physical activity
Physical activity was our primary outcome measure. Physical activity was characterized in three ways. First, we used the UCLA activity score.34 This score is widely used in this population and has been validated against pedometers.34, 35 The UCLA activity score assesses self-reported activity level ranging from a score of 1 – “Wholly inactive; dependent on others; cannot leave residence” to 10 “Regularly participate in impact sports such as jogging, tennis, skiing, acrobatics, ballet, heavy labor, or backpacking.” Thus both perceived frequency and perceived intensity are included in this scale. Next, participants were provided with an accelerometer-based activity monitor (ActiGraph wGT3X-BT, ActiGraph, Pensacola, FL) to be worn for 7 days. We used manufacturer-provided software to extract average number of steps per day to represent quantity of activity. We also extracted intensity of activity characterized as percent sedentary time, percent time spent in light, moderate, or vigorous activity.
Statistical Analysis
We used SPSS version 26 (IBM Corp, Armonk, NY, USA) for all analyses. First, descriptive statistics were computed for all variables. Next, before testing the hypotheses, we assessed the effect of age, BMI, and HOOS pain scores on all variables of interest to determine whether they should be included as covariates in our analyses. Where the associations were statistically significant at the level of 0.05, we used regression analyses including these terms to verify that they did not confound the primary associations sought.
To test hypothesis (i), that increased fatigability is associated with decreased physical activity levels we used Pearson correlations to assess associations between performance fatigability scores and UCLA scores. (By convention based on Cohen’s recommendations, a medium correlation coefficient was defined as between 0.3 and 0.5 and a large correlation coefficient was defined as higher than 0.5.36) To test hypothesis (ii), that lower levels of aerobic capacity are associated with physical activity, we again used Pearson correlations to assess associations between predicted VO2max and UCLA scores. In both cases we used regression analysis including any potentially confounding terms (i.e. age, BMI, or pain) to verify these associations as stated above. Finally, to test hypothesis (iii) that fatigability mediates the association between aerobic capacity and activity levels we used nonparametric bootstrapping analysis with bias-corrected confidence estimates.37 The 95% confidence interval of the indirect effect was obtained with 1000 bootstrap resamples and the mediation was considered significant if the confidence interval of the indirect effect did not cross zero.
Results
UCLA scores ranged from 2, representing “mostly inactive or restricted to minimum activities of daily living” to 8, representing “sometimes participates in impact sports…” (mean 5 ± 1, representing “sometimes participates in moderate activities…” Based on the ActiGraph measures, participants walked 4440 to 16,967 steps per day (mean 9697 ± 3436 steps per day) and spent 43.1 ±15.2% of time sedentary, 43.7 ± 9.7 % of time in light activity, and 13.2 ± 7.8 % of time in moderate activity. No participants engaged in vigorous activity. UCLA scores were significantly correlated with sedentary time (−0.376, p = 0.041), but not with light activity (R = 0.318, p = 0.087), moderate activity time (R = 0.350, p = 0.058) or number of steps per day (R = 0.243, p = 0.195). Age and BMI were not associated with UCLA scores, quantity or intensity of physical activity (R = −0.021 to 0.307, p = 0.087 to 0.910).
HOOS profiles indicated a moderate level of impairment in all domains (Figure 1). There were significant associations between some physical activity variables and HOOS scores (Table 2). UCLA scores were associated with HOOS ADL function, Sport/Rec Function, and Hip Related Quality of Life. Steps per day were associated with all domains of the HOOS. More sedentary time and lower UCLA scores were associated with worst sport/rec function and quality of life. Notably, no domains of the HOOS were associated with light activity time. Further, pain was associated with percent of time in moderate activity, along with ADL and sport/rec function, and hip related quality of life.
Figure 1.
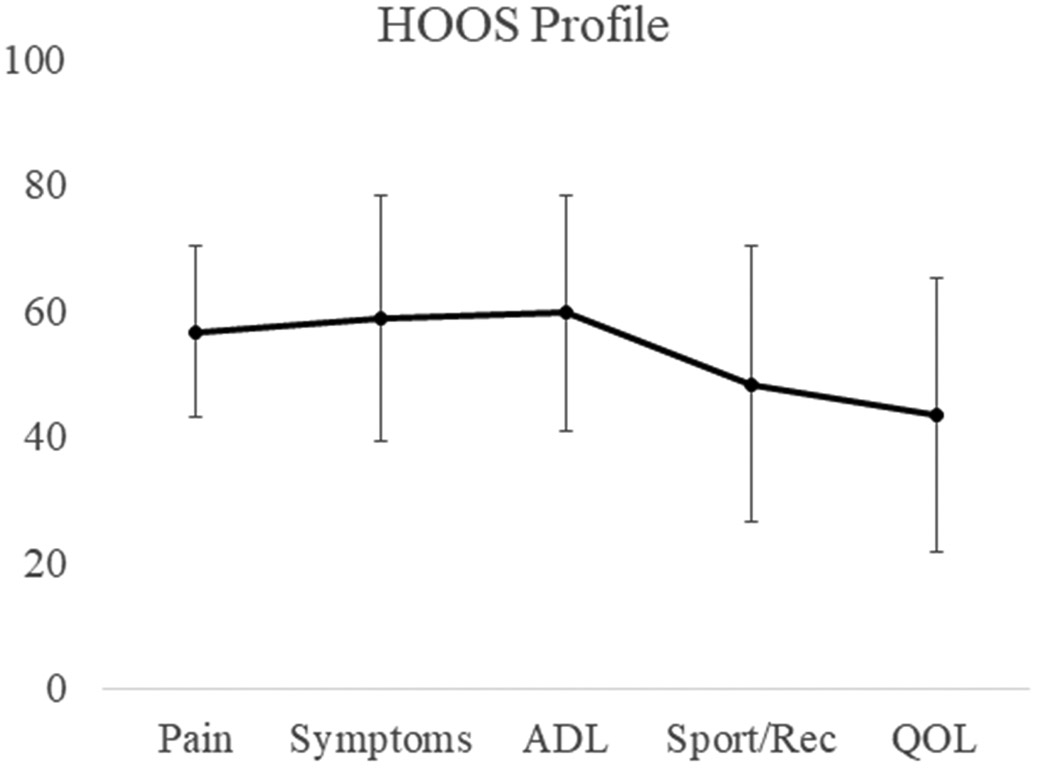
HOOS profiles (mean ± standard deviation). Lower scores indicate a greater level of impairment.
Table 2.
Associations between physical activity variables and HOOS domain scores
| Pain | Symptoms | Function: ADL |
Function: Sport/Rec |
Quality of Life |
|
|---|---|---|---|---|---|
| UCLA Score | R = 0.275 | R = 0.086 | R = 0.357 | R = 0.475 | R = 0.467 |
| p = 0.121 | p = 0.633 | p = 0.041 | p = 0.005 | p = 0.006 | |
| Steps per day | R = 0.443 | R = 0.402 | R = 0.506 | R = 0.460 | R = 0.419 |
| p = 0.013 | p = 0.025 | p = 0.004 | p = 0.009 | p = 0.019 | |
| %Sedentary time | R = −0.245 | R = −0.253 | R = −0.325 | R = −0.384 | R = −0.370 |
| p = 0.185 | p = 0.170 | p = 0.074 | p = 0.033 | p = 0.041 | |
| %Light activity time | R = 0.095 | R = 0.127 | R = 0.157 | R = 0.223 | R = 0.262 |
| p = 0.613 | p = 0.497 | p = 0.400 | p = 0.229 | p = 0.155 | |
| %Moderate activity time | R = 0.356 | R = 0.332 | R = 0.435 | R = 0.468 | R = 0.391 |
| p = 0.049 | p = 0.068 | p = 0.014 | p = 0.008 | p = 0.030 |
There was a significant correlation between fatigability scores and UCLA scores. More fatigable women reported less physical activity (Figure 2). Fatigability was not associated with quantity or intensity of accelerometer-based activity measures at a statistically significant level (p = 0.122 to 0.432).
Figure 2.
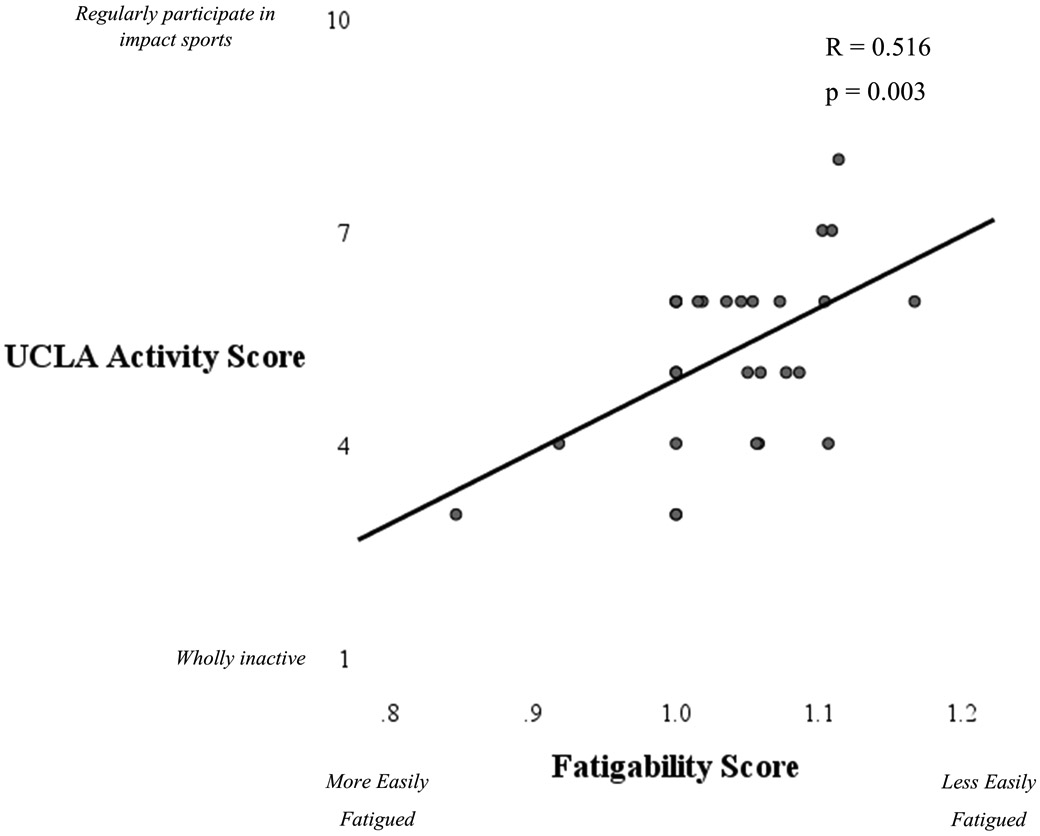
Scatterplot illustrating the association between fatigability and activity levels. Women with OA who were more fatigable (lower scores) had lower activity levels.
Aerobic capacity, as measured by predicted VO2max, ranged from 8.9-42.9 (mean 21.6 ± 9.5) ml/kg*min. Aerobic capacity was significantly associated with UCLA scores with women with lower aerobic capacity reporting less physical activity levels (Figure 3). Aerobic capacity was also associated with percent sedentary time (R = −0.477, p = 0.006), and percent light activity time (R = 0.501, p = 0.004). There was no association between aerobic capacity and steps per day (p = 0.264) and there was a weak but not statistically significant association between aerobic capacity and percent moderate activity time (R = 0.302, p = 0.094). Notably, lower aerobic capacity was related to increased fatigability scores (R = 0.619, p < 0.001, Figure 4).
Figure 3.
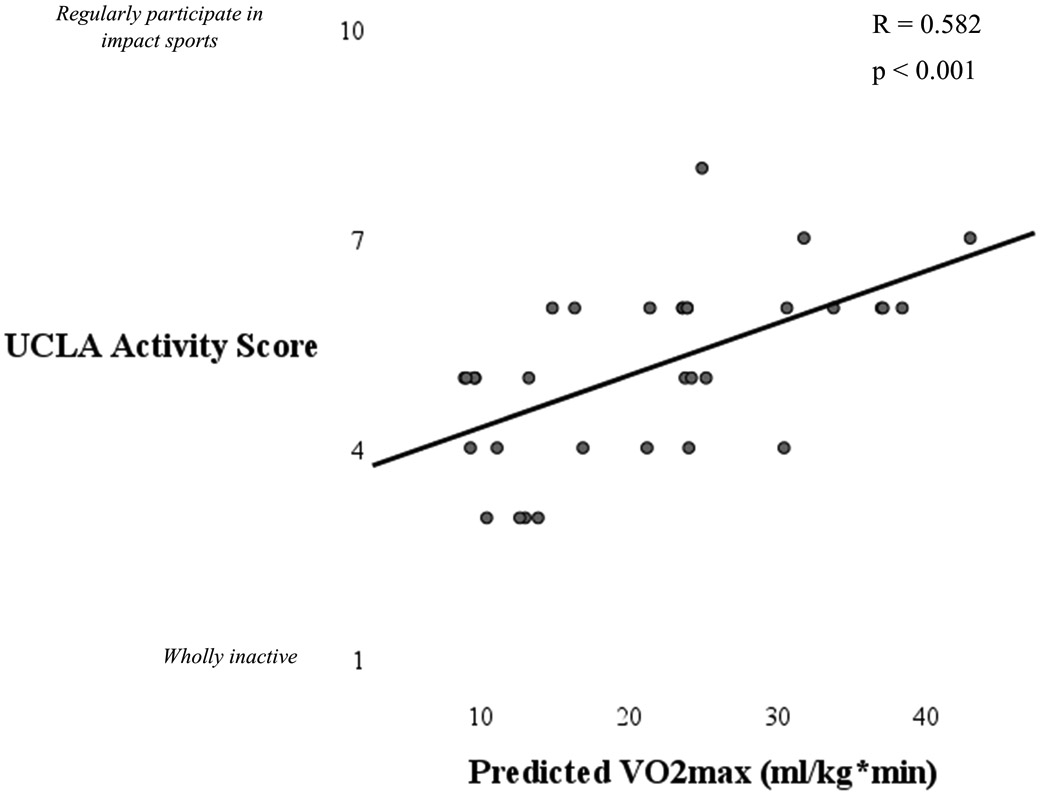
Scatterplot illustrating the association between aerobic capacity and activity levels. Women with OA who had lower aerobic capacity had lower activity levels.
Figure 4.
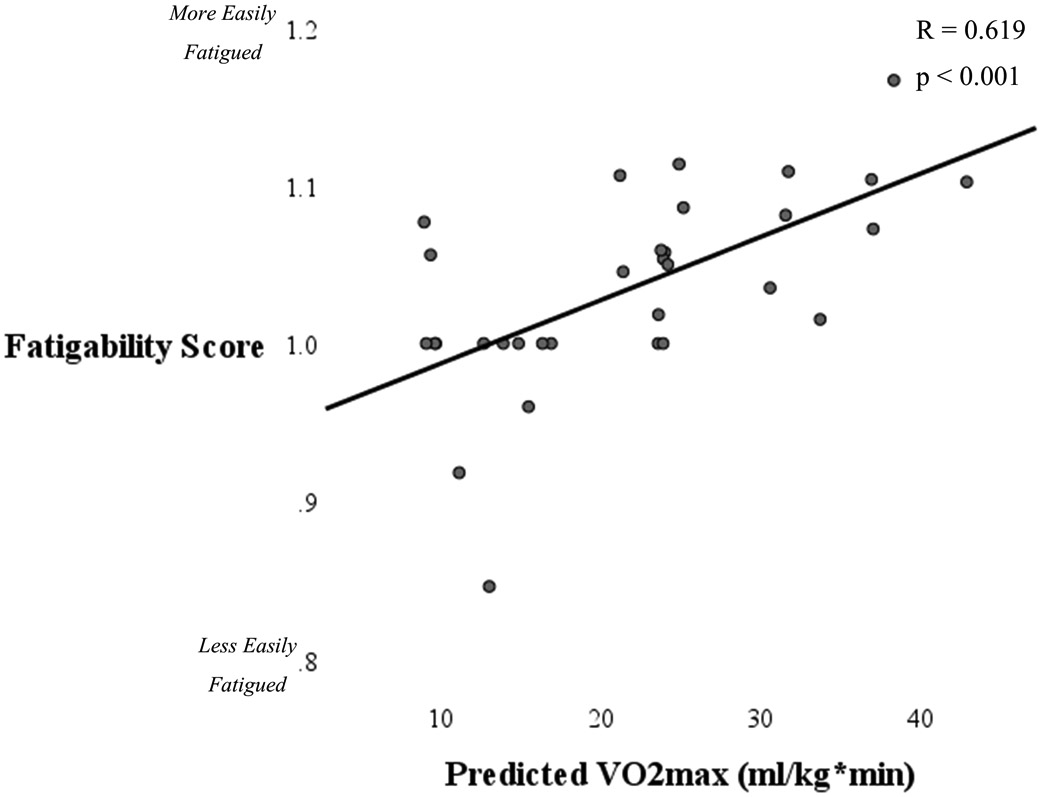
Scatterplot illustrating the association between aerobic capacity and fatigability. Women with OA who had lower aerobic capacity were more fatigable.
Finally, we sought to determine the combined influence of aerobic capacity and fatigability on physical activity and whether fatigability functioned as a mediator between aerobic capacity and physical activity. Together aerobic capacity and fatigability predicted UCLA scores with an R2 of 0.404 (adj R2 = 0.360, SEE = 0.969, p = 0.001). We note, however, that aerobic capacity was the stronger predictor of UCLA scores with a standardized coefficient of 0.464 compared to a standardized coefficient of 0.236 for fatigability. Moreover, the coefficient for fatigability was not statistically significant (p = 0.215). Further, there was not a significant indirect effect of aerobic capacity on UCLA through fatigability (b = 0.018, SE = 0.018, 95% CI [−0.020, 0.055]).
Discussion
This study characterized physical activity in a group of women with hip OA and investigated potentially modifiable physical predictors of physical activity. The main purpose of this study was to investigate the association between fatigability and physical activity, and to test whether aerobic capacity could be a modifiable factor associated with fatigability and physical activity. Our hypothesis was partially supported. Fatigability was associated with self-reported physical activity as assessed through the UCLA score. Aerobic capacity was independently associated with physical activity and there was no evidence that fatigability mediated this association. Moreover, when aerobic capacity and fatigability were both used as predictors in the same regression model, the coefficient representing fatigability was no longer statistically significant. This suggests that aerobic capacity is a key contributor to physical activity in women with hip OA.
Several studies have shown that people with OA have reduced aerobic capacity compared to healthy controls. Values in the literature range from ~12-16 ml/min*kg for people with hip OA vs. ~17-21 ml/min*kg for controls.38, 39 Most of these studies, however, have been performed in people with advanced disease. One study showed that those with mild to moderate symptoms do not necessarily show a decrease in aerobic capacity.40 With a mean aerobic capacity of 21.6 ± 9.5 ml/min*kg, our cohort of participants do not necessarily exhibit reduced aerobic capacity. This is in line with their moderate level of impairment as assessed via HOOS scores. However, it is important to note that there are various ways to assess aerobic capacity (e.g. submaximal or maximum effort cycling tests, six-minute walk test, etc.); different methods were used in each study, so direct comparison is difficult.
Aerobic capacity was independently associated with physical activity. A potential mechanism by which reduced aerobic capacity could reduce physical activity is via the energetic model of activity limitation.23 This model posits that people whose energy cost of walking is high relative to their total energy capacity have less energy reserve for physical activity. Thus, this model suggests that reduced total energy capacity is implicated in lower physical activity levels in older adults. Since OA is associated with reduced aerobic capacity – total energy capacity – this mechanism could be an explanation for reduced physical activity in women with hip OA.
This study adds to the growing body of work linking fatigability to physical activity in various ways in people with OA. Momentary self-reported fatigue, induced fatigue, and self-reported fatigue have been linked to reduced physical activity in people with hip OA.14, 19, 41, 42 Fatigue or fatigability are also found to predict subsequent physical activity.19, 43 This is the case even when confounders such as pain and physical function are included in regression models.41 The contribution of the present work is the use of a performance-based fatigability measure to assess the effects of movement-associated fatigue. This is important because other factors associated with OA, such as sleep-related problems, and depression could be linked to self-reported fatigue.44 Fatigability, movement-associated fatigue may be a more direct determinant of physical activity limitation compared to the construct of fatigue. Moreover, if linked to modifiable factors, fatigability may in turn be modifiable. Indeed, in this study aerobic capacity, which is modifiable in OA,25, 27 was associated with fatigability. The present findings suggest, however, that targeting aerobic capacity may be the most direct way to improve physical activity.
Although this was a cross-sectional study, findings suggest that fatigability could be modifiable by targeting aerobic capacity. It is further expected that this would have positive effects on physical activity. This is important because it is well established that exercise improves physical function in OA.7, 8, 45, 46 Moreover, exercise has a positive impact on OA biomarkers in animal studies,47 and in some human studies.47, 48 Unfortunately, current interventions to improve physical activity in people with OA have had limited effectiveness.25 The present findings emphasize the importance of aerobic fitness and suggest that improving fitness may have positive effects on physical activity as well.
In contrast with physical activity as represented by UCLA scores, accelerometer-based physical activity was not associated with aerobic capacity and fatigability. HOOS scores, however, were significantly associated with several aspects of accelerometer-based physical activity. Notably, pain was associated with average number of steps per day and moderate activity times. This is in contrast with studies of knee OA and of mixed hip and knee cohorts. In a study using data from the Multicenter Osteoarthritis (MOST) Study, pain was not highly associated with walking quantity.49 The impact of momentary pain on physical activity in women with hip or knee OA was studied in a series of work by Murphy and colleagues.14, 15 In one study, pain was found to be associated with physical activity once momentary fatigue was accounted for in regression models.15 A later study from this group found that older women with hip or knee OA higher levels of pain actually had higher levels of activity early in the day when they had low levels of pain interference.14 In these studies, results were not reported separately by affected joint so it is not possible to determine whether or not the results were different in women with hip OA compared to women with knee OA. In any case, the relationship between pain and physical activity in OA is complex and is governed by a variety of physical and psychological factors. More work is needed to understand factors related to physical activity specifically in hip OA.
The discordance in results between the association between fatigability and UCLA scores and ActiGraph data suggests that these tools may be measuring different constructs related to physical activity. We expect that the UCLA score and the ActiGraph are measuring different aspects of the construct of physical activity. There are several aspects of physical activity including quantity, intensity, duration, type. The ActiGraph primarily reflects walking activity so could miss other types of activities that are important to participants that may be picked up by the UCLA score. The fact that there were only moderate correlations between UCLA scores were more closely associated with objectively measured physical activity intensity and no correlation with steps per day suggests that the UCLA score may be a better indicator of perceived intensity than perceived quantity of activity. We also note that the UCLA score assesses overall perceived/desired physical activity levels rather than a snapshot of one week’s worth of activity that may not be fully representative of a longer timespan. Murphy et al., reported that fatigability was associated with increased self-pacing behaviors, wherein participants slowed down, took breaks, or otherwise paced their activity levels.50 While self-pacing was not assessed in the present study, it is possible that variation in self-pacing behaviors could confound the ActiGraph measures, but would not be as likely to affect the UCLA measures, as the latter assesses an overall perceived level of physical activity. Studies using the UCLA score to characterize physical activity should keep this in mind when interpreting findings. Combinations of both subjective and objective frequency and intensity measures may be needed to fully characterize physical activity in an OA population.
With the cross-sectional design of this study, we cannot rule out an alternative framework, in which reduced physical activity leads to deconditioning and reduced aerobic capacity which could in turn increases fatigability. However, based on the existing literature in older adults, we believe that our proposed hypothesis is the most plausible. It has already been established that reduced energy capacity or increased effort in walking is associated with reduced physical activity in older adults, via the energetic model.23, 51 Because of literature showing that people with OA have reduced aerobic capacity,38, 39 it seems likely that the energetic model could be applied here as well. We concede that it is not possible to determine the initiating event regarding aerobic capacity and physical activity. However, because improving one necessarily improves the other, it may be reasonable to attempt to improve the capacity for physical activity by addressing aerobic capacity.
There were several important limitations to consider when interpreting these results and their generalizability. First selection bias may have been an issue. For inclusion in the study participants needed to be able to tolerate the fatigability test by being able to walk on a treadmill without assistive devices. This introduced a risk of biasing the study toward higher functioning individuals. However, we do not believe this was the case. The wide range of HOOS scores and UCLA scores indicate that a broad spectrum of functional levels were represented. Second, we note that we did not assess radiographic severity of OA. Thus, we do not know how physical activity may vary with radiographic severity. While other studies have shown, that physical function does not vary strongly with radiographic severity of OA,52 whether or not radiographic severity of OA impacts physical activity has yet to be determined. Third, there is no consensus on how to assess fatigability, other than that performance measures are necessary.50, 53 In addition , others who have used the same type of fatigability assessment have modified it to suit their experimental design.26, 50, 54 As a result, we cannot make direct numerical comparisons between our results and others that have been described in the literature. Finally, only women were included in this study. The rationale was that being female is a risk factor for having lower physical activity in older adults,29, 55 as well as for having osteoarthritis. Future studies should evaluate men as well and should specifically examine potential sex differences in physical activity predictors.
In conclusion, we found that performance-based fatigability was associated with physical activity engagement in women with hip OA. Moreover, aerobic capacity was associated with fatigability and physical activity. Although 40% of the variance in physical activity level was explained by aerobic capacity and fatigability together, aerobic capacity was the best predictor of activity levels. It is also important to note that a substantial amount of variance can be accounted for by factors not considered in this study. Among these may be behavioral factors addressed in many other approaches to improving physical activity in this population.25 Addressing physical factors such as aerobic capacity and fatigability may improve the effectiveness of behavioral interventions. This study is important because physical activity is associated with better outcomes in OA.7-9 Further OA is associated with increased cardiovascular mortality,10, 11 which in turn has low physical activity as a risk factor. Although this was a cross-sectional study, these results suggest that addressing aerobic capacity, could be an avenue to improve physical activity in women with hip OA, with potential additional benefits to fatigability. Longitudinal and interventional studies are needed to determine whether targeting aerobic capacity results in an increase in physical activity levels in women with hip OA.
Acknowledgements
This research was supported by NIH R21AG052111 (KCF) and UL1TR002003.
References
- 1.Centers for Disease Control and Prevention, (CDC). 2011. Arthritis as a potential barrier to physical activity among adults with obesity--united states, 2007 and 2009. MMWR Morb Mortal Wkly Rep 60: 614–618. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention, (CDC). 2006. Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation--united states, 2003-2005. MMWR Morb Mortal Wkly Rep 55: 1089–1092. [PubMed] [Google Scholar]
- 3.Wallis JA, Webster KE, Levinger P, Taylor NF. 2013. What proportion of people with hip and knee osteoarthritis meet physical activity guidelines? A systematic review and meta-analysis. Osteoarthritis and Cartilage 21: 1648–1659. [DOI] [PubMed] [Google Scholar]
- 4.Miller ME, Rejeski WJ, Reboussin BA, Ten Have TR, Ettinger WH. 2000. Physical activity, functional limitations, and disability in older adults. J Am Geriatr Soc 48: 1264–1272. [DOI] [PubMed] [Google Scholar]
- 5.Buchman AS, Boyle PA, Yu L, et al. 2012. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology 78: 1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee I-, Shiroma EJ, Lobelo F, et al. 2012. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. The Lancet 380: 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fransen M, McConnell S, Harmer AR, et al. 2015. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev 1: CD004376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fransen M, McConnell S, Hernandez-Molina G, Reichenbach S. 2014. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev (4):CD007912. doi: CD007912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hurley M, Dickson K, Hallett R, et al. 2018. Exercise interventions and patient beliefs for people with hip, knee or hip and knee osteoarthritis: A mixed methods review. Cochrane Database Syst Rev 4: CD010842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall AJ, Stubbs B, Mamas MA, Myint PK, Smith TO. 2016. Association between osteoarthritis and cardiovascular disease: Systematic review and meta-analysis. Eur J Prev Cardiol 23: 938–946. [DOI] [PubMed] [Google Scholar]
- 11.Rahman MM, Kopec JA, Anis AH, Cibere J, Goldsmith CH. 2013. Risk of cardiovascular disease in patients with osteoarthritis: A prospective longitudinal study. Arthritis Care Res (Hoboken) 65: 1951–1958. [DOI] [PubMed] [Google Scholar]
- 12.Fernandes GS, Valdes AM. 2015. Cardiovascular disease and osteoarthritis: Common pathways and patient outcomes. Eur J Clin Invest 45: 405–414. [DOI] [PubMed] [Google Scholar]
- 13.Corsi M, Alvarez C, Callahan LF, et al. 2018. Contributions of symptomatic osteoarthritis and physical function to incident cardiovascular disease. BMC Musculoskelet Disord 19: 393–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy SL, Schepens Niemiec S, Lyden AK, Kratz AL. 2016. Pain, fatigue, and physical activity in osteoarthritis: The moderating effects of pain- and fatigue-related activity interference. Arch Phys Med Rehabil 97: 201. [DOI] [PubMed] [Google Scholar]
- 15.Murphy SL, Smith DM, Clauw DJ, Alexander NB. 2008. The impact of momentary pain and fatigue on physical activity in women with osteoarthritis. Arthritis Rheum 59: 849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egerton T, Helbostad JL, Stensvold D, Chastin SF. 2016. Fatigue alters the pattern of physical activity behavior in older adults: Observational analysis of data from the generation 100 study. J Aging Phys Act 24: 633–641. [DOI] [PubMed] [Google Scholar]
- 17.Snijders GF, van den Ende CH, Fransen J, et al. 2011. Fatigue in knee and hip osteoarthritis: The role of pain and physical function. Rheumatology (Oxford) 50: 1894–1900. [DOI] [PubMed] [Google Scholar]
- 18.Power JD, Badley EM, French MR, Wall AJ, Hawker GA. 2008. Fatigue in osteoarthritis: A qualitative study. BMC Musculoskelet Disord 9: 63–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy SL, Alexander NB, Levoska M, Smith DM. 2013. Relationship between fatigue and subsequent physical activity among older adults with symptomatic osteoarthritis. Arthritis Care Res (Hoboken) 65: 1617–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eldadah BA. 2010. Fatigue and fatigability in older adults. PM R 2: 406–413. [DOI] [PubMed] [Google Scholar]
- 21.Kim I, Hacker E, Ferrans CE, et al. 2018. Evaluation of fatigability measurement: Integrative review. Geriatr Nurs 39: 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buchowski MS, Simmons SF, Whitaker LE, et al. 2013. Fatigability as a function of physical activity energy expenditure in older adults. Age (Dordr) 35: 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schrack JA, Simonsick EM, Ferrucci L. 2010. The energetic pathway to mobility loss: An emerging new framework for longitudinal studies on aging. J Am Geriatr Soc 58 Suppl 2: 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wert DM, Brach JS, Perera S, VanSwearingen J. 2013. The association between energy cost of walking and physical function in older adults. Arch Gerontol Geriatr 57: 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williamson W, Kluzek S, Roberts N, et al. 2015. Behavioural physical activity interventions in participants with lower-limb osteoarthritis: A systematic review with meta-analysis. BMJ open 5: e007642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keyser RE, Christensen EJ, Chin LMK, et al. 2015. Changes in fatigability following intense aerobic exercise training in patients with interstitial lung disease. Respiratory Medicine 109: 517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Escalante Y, Garcia-Hermoso A, Saavedra JM. 2011. Effects of exercise on functional aerobic capacity in lower limb osteoarthritis: A systematic review. J Sci Med Sport 14: 190–198. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan MS, Newsom JT, McFarland BH, Lu L. 2001. Demographic and psychosocial correlates of physical activity in late life. Am J Prev Med 21: 306–312. [DOI] [PubMed] [Google Scholar]
- 29.Lee YS. 2005. Gender differences in physical activity and walking among older adults. J Women Aging 17: 55–70. [DOI] [PubMed] [Google Scholar]
- 30.Rosenfeld CS. 2017. Sex-dependent differences in voluntary physical activity. J Neurosci Res 95: 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nilsdotter A, Lohmander LS, Klssbo M, Roos E. 2003. Hip disability and osteoarthritis outcome score (HOOS)--validity and responsiveness in total hip replacement. BMC musculoskeletal disorders 4: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schnelle JF, Buchowski MS, Ikizler TA, et al. 2012. Evaluation of two fatigability severity measures in elderly adults. J Am Geriatr Soc 60: 1527–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laskin JJ, Bundy S, Marron H, et al. 2007. Using a treadmill for the 6-minute walk test: Reliability and validity. J Cardiopulm Rehabil Prev 27: 407–410. [DOI] [PubMed] [Google Scholar]
- 34.Zahiri CA, Schmalzried TP, Szuszczewicz ES, Amstutz HC. 1998. Assessing activity in joint replacement patients. J Arthroplasty 13: 890–895. [DOI] [PubMed] [Google Scholar]
- 35.Naal FD, Impellizzeri FM, Leunig M. 2009. Which is the best activity rating scale for patients undergoing total joint arthroplasty? Clin Orthop Relat Res 467: 958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu Q. 2010. Measures of effect size. In: Salkind NJ editor. Encyclopedia of research design, Thousand Oaks, CA: SAGE Publications, Inc.; . [Google Scholar]
- 37.Preacher KJ, Hayes AF. 2004. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior research methods, instruments, & computers 36: 717–731. [DOI] [PubMed] [Google Scholar]
- 38.Philbin EF, Groff GD, Ries MD, Miller TE. 1995. Cardiovascular fitness and health in patients with end-stage osteoarthritis. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology 38: 799–805. [DOI] [PubMed] [Google Scholar]
- 39.Horstmann T, Vornholt-Koch S, Brauner T, Grau S, Mundermann A. 2012. Impact of total hip arthroplasty on pain, walking ability, and cardiovascular fitness. J Orthop Res 30: 2025–2030. [DOI] [PubMed] [Google Scholar]
- 40.Rydevik K, Fernandes L, Nordsletten L, Risberg MA. 2010. Functioning and disability in patients with hip osteoarthritis with mild to moderate pain. J Orthop Sports Phys Ther 40: 616–624. [DOI] [PubMed] [Google Scholar]
- 41.Smith DM, DeCaro JA, Murphy SL, Parmelee PA. 2019. Momentary reports of fatigue predict physical activity level: Wrist, waist, and combined accelerometry. J Aging Health 898264319863609. [DOI] [PubMed] [Google Scholar]
- 42.Murphy SL, Kratz AL, Williams DA, Geisser ME. 2012. The association between symptoms, pain coping strategies, and physical activity among people with symptomatic knee and hip osteoarthritis. Front Psychol 3: 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schepens SL, Kratz AL, Murphy SL. 2012. Fatigability in osteoarthritis: Effects of an activity bout on subsequent symptoms and activity. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences 67: 1114–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fertelli TK, Tuncay FO. 2019. Fatigue in individuals with knee osteoarthritis: Its relationship with sleep quality, pain and depression. Pak J Med Sci 35: 1040–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skou ST, Roos EM. 2019. Physical therapy for patients with knee and hip osteoarthritis: Supervised, active treatment is current best practice. Clin Exp Rheumatol 37 Suppl 120: 112–117. [PubMed] [Google Scholar]
- 46.Golightly YM, Allen KD, Caine DJ. 2012. A comprehensive review of the effectiveness of different exercise programs for patients with osteoarthritis. Phys Sportsmed 40: 52–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bricca A, Struglics A, Larsson S, et al. 2019. Impact of exercise therapy on molecular biomarkers related to cartilage and inflammation in individuals at risk of, or with established, knee osteoarthritis: A systematic review and meta-analysis of randomized controlled trials. Arthritis Care Res (Hoboken) 71: 1504–1515. [DOI] [PubMed] [Google Scholar]
- 48.Mazor M, Best TM, Cesaro A, Lespessailles E, Toumi H. 2019. Osteoarthritis biomarker responses and cartilage adaptation to exercise: A review of animal and human models. Scand J Med Sci Sports 29: 1072–1082. [DOI] [PubMed] [Google Scholar]
- 49.White DK, Neogi T, Zhang Y, et al. 2012. The association of obesity with walking independent of knee pain: The multicenter osteoarthritis study. J Obes 2012: 261974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murphy SL, Kratz AL, Schepens Niemiec SL. 2017. Assessing fatigability in the lab and in daily life in older adults with osteoarthritis using perceived, performance, and ecological measures. J Gerontol A Biol Sci Med Sci 72: 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Julius LM, Brach JS, Wert DM, VanSwearingen JM. 2012. Perceived effort of walking: Relationship with gait, physical function and activity, fear of falling, and confidence in walking in older adults with mobility limitations. Phys Ther 92: 1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Odding E, Valkenburg HA, Algra D, et al. 1998. Associations of radiological osteoarthritis of the hip and knee with locomotor disability in the rotterdam study. Ann Rheum Dis 57: 203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Geel F, Moumdjian L, Lamers I, Bielen H, Feys P. 2019. Measuring walking-related performance fatigability in clinical practice: A systematic review. Eur J Phys Rehabil Med . [DOI] [PubMed] [Google Scholar]
- 54.Barbosa JFd, Bruno SS, Cruz NSO, et al. 2016. Perceived fatigability and metabolic and energetic responses to 6-minute walk test in older women. Physiotherapy 102: 294–299. [DOI] [PubMed] [Google Scholar]
- 55.Kaplan MS, Newsom JT, McFarland BH, Lu L. 2001. Demographic and psychosocial correlates of physical activity in late life. Am J Prev Med 21: 306–312. [DOI] [PubMed] [Google Scholar]


