Abstract
Background:
Persons with serious mental illness (SMI) die 10–20 years earlier than the general population; cancer is the second leading cause of death. Differences in cancer screening between SMI and the general population are not well understood.
Objectives:
To describe receipt of cancer screening among individuals with versus without SMI and to explore clinicians’ perceptions around cancer screening for people with SMI.
Methods:
Mixed-methods study using 2010–2017 MarketScan commercial insurance administrative claims data and semi-structured clinician interviews. In the quantitative analyses, we used multivariate logistic regression analyses to calculate the likelihood of receiving cervical, breast, colorectal, or prostate cancer screening among people with versus without SMI, defined as schizophrenia or bipolar disorder. We conducted semi-structured interviews with 17 primary care physicians and 15 psychiatrists. Interview transcripts were coded using a hybrid deductive/inductive approach.
Results:
Relative to those without SMI, individuals with SMI were less likely to receive screening for cervical cancer (adjusted Odds Ratio [aOR] 0.80; 95% CI:0.80–0.81), breast cancer (aOR 0.79; 95% CI:0.78–0.80), colorectal cancer (aOR 0.90; 95% CI:0.89–0.91), and prostate cancer (aOR 0.85; 95% CI:0.84–0.87). Clinicians identified five themes that may help explain the lower rates of cancer screening in persons with SMI: access to care, available support, prioritization of other issues, communication, and patient concerns.
Conclusions:
People with SMI were less likely to receive four common types of cancer screening. Improving cancer screening rates in the SMI population will likely require a multi-disciplinary approach to overcome barriers to screening.
1. INTRODUCTION
People with serious mental illnesses (SMIs) such as schizophrenia and bipolar disorder experience premature mortality at rates two to three times higher than the general population.1–3 For the 11.2 million U.S. adults with SMI,4 cancer is the second leading cause of death.1,3 Risk factors for cancer, including tobacco use,5 obesity, poor diet, and comorbidity are highly prevalent in the SMI population.6–9
While cancer screening is a key strategy for early detection,10,11 people with SMI appear to have suboptimal screening rates.12–20 Yet, prior studies are limited to small clinical network,12 single county,13 single-state Medicaid,14–18 single-state commercial insurance,19 or Veterans Health Administration20 populations. Moreover, the clinical and psychosocial context underlying these findings are not well understood. Qualitative work has focused on general access to care,21–23 perceptions of preventive care24 or engagement with cancer care after a diagnosis among those with SMI but not cancer screening specifically.25
Work is needed to understand drivers of disparate cancer screening rates in the SMI population. Our study (1) examines cancer screening rates in a national sample of commercially insured adults, and (2) identifies perceived barriers and facilitators to cancer screening for the SMI and general population through interviews with primary care providers (PCPs) and psychiatrists.
2. METHODS
2.1. Quantitative Methods
2.1.1. Data and Study Population
We conducted a retrospective study using MarketScan commercial claims data from 2010–2017. Data was constructed at the person-year level; individuals were included if enrolled for the entire year. We did not require continuous enrollment across multi-year intervals because vulnerable populations, such as those with SMI, are more likely to have gaps in insurance coverage.26 We identified eligible sub-populations based on US Preventive Services Task Force (USPSTF) guidelines: cervical cancer screening among women ages 21–64 years, breast cancer screening among women 50–64 years, colorectal cancer screening among men and women ages 50–64 years, and prostate cancer screening among men ages 55–64 years.10,11 We included prostate cancer screening because the USPSTF recommends a shared-decision making approach for men at elevated risk,11 and people with SMI may be at elevated mortality risk due to increased tobacco use27 and reduced access to care.28 We excluded individuals with a history of relevant cancers or symptoms three months prior to testing that suggested that testing was for non-screening purposes (Appendix, Supplemental Digital Content 1, http://links.lww.com/MLR/C167). All data collection analyses were approved by the [blinded for review] Institutional Review Board.
2.1.2. Serious Mental Illness Classification
Based on International Classification of Diseases, Tenth edition (ICD-9 and 10) codes, we defined SMI by a diagnosis of schizophrenia or bipolar disorder (Appendix, Supplemental Digital Content 1, http://links.lww.com/MLR/C167). We included major depressive disorder (Appendix, Supplemental Digital Content 1, http://links.lww.com/MLR/C167) in this definition in sensitivity analyses.
2.1.3. Cancer Screening Outcomes
Our outcomes were receipt of cancer screening: pap smear for cervical cancer screening; mammography for breast cancer screening; either colonoscopy or sigmoidoscopy or fecal occult blood test (FOBT) for colorectal cancer screening; and prostate specific antigen (PSA) test for prostate cancer screening (Appendix, Supplemental Digital Content 1, http://links.lww.com/MLR/C167)10,11 In sensitivity analyses, we stratified colorectal cancer screening into individual modalities. We excluded procedure claims from emergency or inpatient settings that were unlikely to represent routine screening.
2.1.4. Covariates
The MarketScan administrative claims database provided age, sex, region of residence, and primary insurance holder. Substance use disorder was identified by the HEDIS Chemical Dependency Value Set, toxic ingestion, poisoning, or overdose diagnosis code (Appendix, Supplemental Digital Content 1, http://links.lww.com/MLR/C167). We measured Charlson Comorbidity Index and number of primary care and obstetrics-gynecology (OB-GYN) visits per year. Primary care and OB-GYN visits (cervical and breast cancer screening) were dichotomized (yes/no). In a sensitivity analysis, we adjusted for the number of yearly primary care visits (0, 1–5, and >5 visits) with upper limit determined by the top 10% of primary care users in the general population, who may have greater medical complexity or require more outpatient resources.29,30
2.1.5. Statistical Analysis
We compared baseline characteristics of the study population stratified by SMI status using t-test and chi-square analyses for continuous and categorical variables, respectively. We used multivariable regression analyses to calculate the association between SMI and the odds of cancer screening, adjusting for age, sex, region of residence, substance use disorder, Charlson comorbidity index, primary care visit, ob-gyn visit, year of study entry, and time in cohort. We used general estimating equation (GEE) models to account for clustering of individuals across years. To improve interpretability of results, we calculated the adjusted predicted screening rate; this is the expected screening rate if all participants had SMI versus did not have SMI. Analyses were performed using Stata version 15.
2.2. Qualitative Methods
2.2.1. Study Population
To understand the healthcare needs of individuals with SMI, we conducted interviews with 32 attending physicians (17 PCPs and 15 psychiatrists) across three primary care clinics and four psychiatry clinics affiliated with an academic medical center between February and April 2019.
2.2.2. Data Collection
We emailed physicians, following up with another email if no response. Participants received a $50 gift card. We used structured interview protocols (Appendix, Supplemental Digital Content 1, http://links.lww.com/MLR/C167) aimed at understanding barriers and facilitators to cancer screening and potential strategies to overcoming barriers. One researcher conducted the 20–30 minute interviews. With oral consent, interviews were audio-recorded and transcribed.
2.2.3. Analysis
We coded interviews using a hybrid deductive/inductive approach.31 We developed an initial codebook based on the literature and a priori knowledge. Two researchers piloted this codebook on five transcripts and added new codes that emerged. The coders compared and reconciled coding differences. Researchers reviewed the final codebook, applied it to transcripts, and organized codes into themes and sub-themes. Data was analyzed with NVivo version 12.
3. RESULTS
3.1. Sample population characteristics
Persons with versus without SMI differed on most demographic, geographic, and health status characteristics (Table 1). People with SMI were more likely to be younger, female, have more comorbidities, and less likely to be the primary insurance holder. More people with SMI saw a PCP and had more frequent primary care visits.
Table 1:
Baseline characteristics of Marketscan populations stratified by cancer screening population
| Cervical Cancer (N=32,224,180) | Breast Cancer (N=12,050,595) | Colorectal Cancer (N=22,882,818) | Prostate Cancer (N=7,189,649) | |||||
|---|---|---|---|---|---|---|---|---|
| SMI (N=274,643) | Non-SMI (N=31,949,537) | SMI (N=94,921) | Non-SMI (N=11,955,674) | SMI (N=151,377) | Non-SMI (N=22,731,441) | SMI (N=34,870) | Non-SMI (N=7,154,779) | |
| Age (SD) | 41.2 (12.5) | 41.5 (12.6)** | 54.9 (4.2) | 55.2 (4.3)** | 55.0 (4.2) | 55.2 (4.3)** | 58.8 (2.6) | 58.9 (2.6)** |
| Female | 100 | 100 | 100 | 100 | 64.1 | 52.8** | - | - |
| SMI diagnosis, % | ||||||||
| Schizophrenia | 15.8 | - | 20.9 | - | 22.8 | - | 26.6 | - |
| Bipolar disorder | 84.2 | - | 79.1 | - | 77.2 | - | 73.4 | - |
| Major depression, % | 17.7 | 2.0** | 16.1 | 2.0** | 14.9 | 1.6** | 12.9 | 1.0** |
| Substance use disorder, % | 14.7 | 0.9** | 10.7 | 0.9** | 12.6 | 1.2** | 13.6 | 1.4** |
| Region, % | ||||||||
| Northeast | 20.4 | 18.5** | 22.0 | 19.5** | 23.0 | 19.7** | 26.4 | 20.1** |
| North Central | 22.7 | 21.9** | 23.2 | 22.4** | 23.0 | 22.6* | 24.0 | 22.9** |
| South | 37.7 | 39.3** | 36.3 | 38.5** | 33.7 | 37.9** | 31.3 | 37.0** |
| West | 17.3 | 18.9** | 16.8 | 18.3** | 16.5 | 18.4** | 16.7 | 18.6** |
| Unknown | 1.9 | 1.4** | 1.8 | 1.4** | 1.7 | 1.3** | 1.5 | 1.3** |
| Primary ins. holder, % | 48.3 | 57.5** | 53.1 | 62.8** | 57.1 | 68.8** | 67.6 | 74.9** |
| Charlson comorbidity index, mean (SD) | 0.6 (1.2) | 0.4 (1.0)** | 0.9 (1.6) | 0.6 (1.2)** | 1.0 (1.6) | 0.6 (1.3)** | 1.2 (1.8) | 0.8 (1.4)** |
| Primary care visit (≥1) in one year, % | 72.1 | 56.3** | 76.2 | 64.1** | 73.2 | 62.0** | 73.5 | 62.3** |
| Primary care visits per year, mean (SD) | 3.3 (4.0) | 1.8 (2.5)** | 3.8 (4.3) | 2.2 (2.7)** | 3.5 (4.1) | 2.0 (2.6)** | 3.3 (3.8) | 2.0 (2.5)** |
| Ob-gyn visit (≥1) in one year, % | 31.9 | 32.0 | 24.7 | 25.4** | - | - | - | - |
| Year in sample, mean (SD) | 2.5 (1.9) | 2.7 (1.9)** | 2.7 (1.9) | 2.8** (1.9) | 2.6 (1.8) | 2.7** (1.9) | 2.5 (1.7) | 2.5** (1.7) |
All covariates based on initial calendar year patient is eligible. T-test or chi-square test was used to determine statistical significance with
p<0.05 and
p<0.001
3.2. Receipt of cancer screening
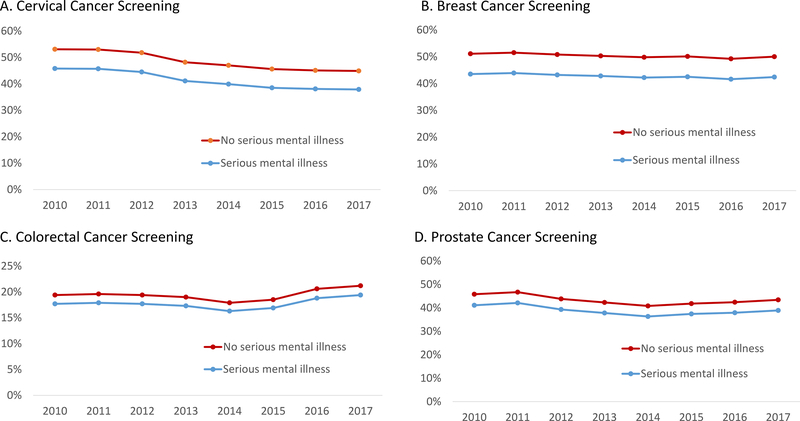
After adjustment for demographics, comorbidities, and healthcare utilization, people with SMI were less likely to receive all types of cancer screening (Table 2). Specifically, people with SMI (versus those without SMI) were less likely to receive cervical cancer screening (aOR 0.80; 95% CI: 0.80–0.81) with predicted rates of 42% versus 49%; breast cancer screening (aOR 0.79; 95% CI:0.78–0.80) with predicted rates of 43% vs 51%; colorectal cancer screening (aOR 0.90; 95% CI: 0.89–0.91) with predicted rates of 18% vs 19%; and prostate cancer screening (aOR 0.85; 95% CI: 0.84–0.87) with predicted rates of 39% vs 44% (Table 2). Predicted screening rates were similar across time. (Figure 1). Similar trends were observed for colonoscopies and FOBT but not for the small proportion (<1%) of patients who received sigmoidoscopies (Appendix, Supplemental Digital Content 1, http://links.lww.com/MLR/C167). Inclusion of major depression in the definition of SMI and primary care utilization levels were similar to main results. (Appendix, Supplemental Digital Content 1, http://links.lww.com/MLR/C167).
Table 2:
Use of preventive screening from 2010–2017 among people with and without serious mental illness in unadjusted and adjusted models
| Serious Mental Illness N individuals (%) | No Serious Mental Illness N individuals (%) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|---|---|
| Cervical Cancer | 141,665 (51.6%) | 19,623,131 (61.4%) | 0.92 (0.92–0.93) | 0.80 (0.80–0.81) |
| Breast Cancer | 48,514 (51.1%) | 7,347,148 (61.5%) | 0.88 (0.87–0.89) | 0.79 (0.78–0.80) |
| Colorectal Cancer | 48,624 (32.1%) | 8,487,887 (37.3%) | 1.00 (0.99–1.01) | 0.90 (0.89–0.91) |
| Prostate Cancer | 18,382 (52.7%) | 4,125,137 (57.7%) | 1.02 (1.00–1.04) | 0.85 (0.84–0.87) |
GEE multivariate model adjusted for age, sex, employment, region, substance use, comorbidity, primary care utilization, and calendar year. Models for cervical and breast cancer screening were also adjusted for ob-gyn utilization.
Figure 1:
Predicted probability of receiving cancer screening from 2010 to 2017 after adjustment for patient characteristics and healthcare utilization.
3.3. Clinician-reported factors related to cancer screening
Clinicians described five major themes: access to care, available support, prioritization of other medical issues, communication, and patient concerns. PCPs and psychiatrists identified similar themes, though we highlight differences between clinical specialties. Themes were applicable to individuals with and without SMI but differentially impacted individuals with SMI.
3.3.1. Access to care
Clinicians noted that continuous insurance coverage and accessibility to health services, particularly to in-network providers, were critical for cancer screening. They linked screening and timely follow-up with access to services and reliable transportation. Psychiatrists perceived that patients with SMI did not see PCPs regularly. Clinicians also expressed concern that patients with SMI face stigma with new healthcare providers.
3.3.2. Available support from family and community
All patients were felt to benefit from social support, but this was especially important for patients with SMI who were perceived to have limited support networks. A support person could be a family member, friend, or case manager to assist with appointments, logistics, and education.
3.3.3. Prioritization of other medical issues
Clinicians discussed challenges where acute issues (e.g. knee pain) or a poorly controlled chronic disease (e.g. diabetes) took precedent over cancer screening. PCPs noted that mental illness symptoms were a high priority topic during visits.
3.3.4. Communication
Clinicians identified clear communication and shared-decision making as necessary components for cancer screening and noted that education should be tailored to individuals with SMI. Psychiatrists noted that some providers may feel uncomfortable engaging in shared-decision making or have misconceptions about capacity assessment in patients with SMI. Some PCPs expressed concern for how to assess capacity or tailor conversations, particularly when discussing risks and benefits.
3.3.5. Patient concern
Clinicians also identified individual concerns, such as testing logistics (e.g. bowel preparation for colonoscopy), personal experience (e.g. trauma, stigma), lack of motivation, and fear of a positive test result. PCPs noted that mental health symptoms, such as apathy or anxiety, influenced motivation. Personal experience and the presence of delusions and paranoia were thought to influence a patient’s decision-making process around cancer screening.
4. DISCUSSION
In this mixed-methods study, we found that commercially insured persons with SMI are less likely to receive screening for four common cancers and were perceived to face additional barriers to screening around access to care, family and community support, competing clinical priorities, communication issues, and patient concerns. Overcoming cancer screening disparities for the SMI population likely will require a multi-faceted approach.
Our national study in a commercially insured population extended smaller studies that demonstrated lower rates of cancer screening in the SMI population.12,15–20 In our study, while psychiatrists perceived that SMI patients were not seeing PCPs and prior qualitative work identified access to care as a potential driver of disparities,21–23 our quantitative findings suggest that disparities extend beyond insurance and merely having a PCP visit.
Even within visits, prioritizing cancer screening may be particularly challenging with the SMI population due to high comorbidity, limited time, communication and executive function concerns, and other patient-driven concerns. Solmi and colleagues hypothesized that interventions to improve cancer screening in the general population, such as paper or electronic reminders or educational materials, may not extend to those with SMI without modification.32 Strategies should account for psychosocial factors (e.g. trauma history, social support), barriers to care (e.g. transportation, insurance), and neurocognitive impairments that may be present in the SMI population.33 Specific interventions could include educational material with high readability and simplified messaging34 or care managers with experience with the SMI population. Health systems can facilitate coordination and integration with mental health providers, particularly when mental health symptoms inhibit preventive or chronic care.
Our study has several limitations. Data reflect a commercially insured population; the findings may not generalize to publicly insured individuals or those in the individual insurance market. We analyzed data at one-year rather than the multi-year intervals recommended by clinical guidelines; importantly our results are consistent with studies examining single and multi-year screening intervals.14,15,17,32,35 We were unable to identify individuals who had a colonoscopy prior to the study and would be less likely to be eligible or if a colonoscopy was conducted after a positive screening test (e.g. FOBT).32 In addition, we could not assess if the test was ordered by the provider but not completed or if other medical issues were prioritized during a visit. Finally, interview subjects were affiliated with an academic institution which may limit generalizability. Qualitative interviews may be limited by social desirability bias, though themes were consistent with prior work observed in access to primary care21–24
In conclusion, individuals with SMI were less likely to receive cancer screening than the general population. People with SMI were noted to be a particularly vulnerable group with barriers to cancer screening in and out of the primary care setting. Future work to address integrated physical and mental healthcare and supportive coordination services, communication, and shared-decision making are needed to address the persistent disparities.
Supplementary Material
Table 3:
Primary care physicians’ (PCPs) (N= 17) and psychiatrists’ (N=15) perceptions of key factors that influence cancer screening for individuals with serious mental illness.
| Key Theme | Summary | Representative Statements |
|---|---|---|
|
Access to Care (Mentioned by 65% of PCPs and 60% of Psychiatrists) |
Clinicians identified insurance coverage, affordable care, and reliable transportation as necessary components to access to cancer screening. Stigma around mental illness was also perceived to influence access to care. | “But there are certain tests that cost more or less money or are not covered...other than that, I think it’s more like an insurance versus not insurance.” “I mean I do worry about my patients with schizophrenia how they're going to be perceived and treated by health professionals” |
|
Support Available
(Mentioned by 88% of PCPs and 40% of Psychiatrists) |
Clinicians noted the role of a support person (e.g. family, friend, case manager) as important for preventive cancer screening. Serious mental illness often was linked with highneed for social support. | “So, [patients with serious mental illness] are sometimes more likely to make it to appointments, or there’s a case manager that I can connect with to follow up with… and to try to build an argument for why the cancer screening is important.” “And then I think for particularly the schizophrenic that might not have a strong support network just by virtue of their isolation from their mental illness, getting them to the tests, having them prep for the tests, that is more difficult.” |
|
Prioritization of other medical issues (Mentioned by 71% of PCPs and 33% of Psychiatrists) |
Acute and chronic medical issues were often prioritized over preventive screening. Clinicians frequently identified control of mental illness symptoms as a high priority. | “…there are more immediate things like making sure that their blood pressure isnť 180 over 100 and their blood sugar isnť 400… I'm just spending my time trying to figure out how to get those under better control.” “I think about it as a hierarchy of needs and then in terms of life expectancy…. I'll say that if their mental illness is really unstable, then that takes the priority until thaťs stabilized.” |
|
Communication (Mentioned by 76% of PCPs and 73% of Psychiatrists) |
Clinicians viewed shared decision making as critical to discussions around cancer screening. Clients with serious mental illness were perceived as more likely to have difficulties with executive functioning and require more time to discuss risks and benefits of screening. | “For example, if we do a stool test for a colon cancer screening, if it’s positive, then that’s going to mean we’re going to do a colonoscopy, and so making sure that people understand testing can lead to more testing...” “I do wonder if our perceptions of their decisional capacity as it relates to their ability or inability to manage their mental health… that we may assume that they have inability to make good decisions about screenings.” |
|
Patient Concerns (Mentioned by 47% of PCPs and 33% of Psychiatrists) |
Clinicians identified logistics of obtaining testing, procedure-related concerns, and personal experience as common factors voiced by clients. Apathy, anxiety, delusions, and paranoia were noted to affect motivation. | “So most of my patients I have seen, they donť-- they do not want to do colonoscopy. They absolutely do not want to have anything put in them. There are-- many female patients do not want a pap smear done because they have PTSD.” “I think that there are some unique issues with trust and/or paranoia that include strong beliefs, perhaps even delusional beliefs, that people attach to procedure…. Mammography or colon cancer screening might be the best examples.” |
Acknowledgments
This study was supported by the National Institutes of Health: P50MH115842 (Daumit), K01MH106631 (McGinty), 2T32HL007180-41A (Murphy), T32MH109436 (Stone, Presskreischer), and the Maryland Cigarette Restitution Fund Program.
Footnotes
Disclosures
To the best of our knowledge, no conflict of interest, financial or other, exists. We have included acknowledgements, conflicts of interest, and funding sources after the discussion.
References
- 1.Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Preventing chronic disease. 2006;3(2):A42. [PMC free article] [PubMed] [Google Scholar]
- 2.Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Archives of general psychiatry. 2007;64(10):1123–1131. [DOI] [PubMed] [Google Scholar]
- 3.Olfson M, Gerhard T, Huang C, Crystal S, Stroup TS. Premature Mortality Among Adults With Schizophrenia in the United States. JAMA psychiatry. 2015;72(12):1172–1181. [DOI] [PubMed] [Google Scholar]
- 4.National Institute of Mental Health. Mental Health Information: Statistics. National Institute of Mental Health Information Resource Center. https://www.nimh.nih.gov/health/statistics/mental-illness.shtml. Published 2019. Accessed September 17, 2019. [Google Scholar]
- 5.Dickerson F, Schroeder J, Katsafanas E, et al. Cigarette Smoking by Patients With Serious Mental Illness, 1999–2016: An Increasing Disparity. Psychiatr Serv. 2018;69(2):147–153. [DOI] [PubMed] [Google Scholar]
- 6.Daumit GL, Anthony CB, Ford DE, et al. Pattern of mortality in a sample of Maryland residents with severe mental illness. Psychiatry research. 2010;176(2–3):242–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mangurian C, Newcomer JW, Modlin C, Schillinger D. Diabetes and Cardiovascular Care Among People with Severe Mental Illness: A Literature Review. Journal of General Internal Medicine. 2016;31(9):1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. Jama. 2007;298(15):1794–1796. [DOI] [PubMed] [Google Scholar]
- 9.Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA psychiatry. 2015;72(4):334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. Preventive Services Task Force. USPSTF A and B Recommendations. USPSTF. https://www.uspreventiveservicestaskforce.org/Page/Name/uspstf-a-and-b-recommendations/. Published 2019. Updated December 2019. Accessed February 17, 2020. [Google Scholar]
- 11.U.S. Preventive Services Task Force. Final Recommendation Statement- Prostate Cancer: Screening. USPSTF. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1. Published 2018. Updated October 2018. Accessed February 17, 2020. [Google Scholar]
- 12.Tilbrook D, Polsky J, Lofters A. Are women with psychosis receiving adequate cervical cancer screening? Can Fam Physician. 2010;56(4):358–363. [PMC free article] [PubMed] [Google Scholar]
- 13.Xiong GL, Bermudes RA, Torres SN, Hales RE. Use of cancer-screening services among persons with serious mental illness in Sacramento County. Psychiatr Serv. 2008;59(8):929–932. [DOI] [PubMed] [Google Scholar]
- 14.Thomas M, James M, Vittinghoff E, Creasman JM, Schillinger D, Mangurian C. Mammography Among Women With Severe Mental Illness: Exploring Disparities Through a Large Retrospective Cohort Study. Psychiatr Serv. 2018;69(1):48–54. [DOI] [PubMed] [Google Scholar]
- 15.James M, Thomas M, Frolov L, et al. Rates of Cervical Cancer Screening Among Women With Severe Mental Illness in the Public Health System. Psychiatr Serv. 2017;68(8):839–842. [DOI] [PubMed] [Google Scholar]
- 16.Salsberry PJ, Chipps E, Kennedy C. Use of general medical services among Medicaid patients with severe and persistent mental illness. Psychiatr Serv. 2005;56(4):458–462. [DOI] [PubMed] [Google Scholar]
- 17.Abrams MT, Myers CS, Feldman SM, et al. Cervical cancer screening and acute care visits among Medicaid enrollees with mental and substance use disorders. Psychiatr Serv. 2012;63(8):815–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Domino ME, Beadles CA, Lichstein JC, et al. Heterogeneity in the quality of care for patients with multiple chronic conditions by psychiatric comorbidity. Med Care. 2014;52 Suppl 3:S101–109. [DOI] [PubMed] [Google Scholar]
- 19.Carney CP, Jones LE. The influence of type and severity of mental illness on receipt of screening mammography. Journal of General Internal Medicine. 2006;21(10):1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Druss BG, Rosenheck RA, Desai MM, Perlin JB. Quality of preventive medical care for patients with mental disorders. Med Care. 2002;40(2):129–136. [DOI] [PubMed] [Google Scholar]
- 21.Kaufman EA, McDonell MG, Cristofalo MA, Ries RK. Exploring barriers to primary care for patients with severe mental illness: frontline patient and provider accounts. Issues Ment Health Nurs. 2012;33(3):172–180. [DOI] [PubMed] [Google Scholar]
- 22.Mojtabai R, Cullen B, Everett A, et al. Reasons for not seeking general medical care among individuals with serious mental illness. Psychiatr Serv. 2014;65(6):818–821. [DOI] [PubMed] [Google Scholar]
- 23.Lester H, Tritter JQ, Sorohan H. Patients’ and health professionals’ views on primary care for people with serious mental illness: focus group study. BMJ (Clinical research ed). 2005;330(7500):1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stumbo SP, Yarborough BJH, Yarborough MT, Green CA. Perspectives on Providing And Receiving Preventive Health Care From Primary Care Providers and Their Patients With Mental Illnesses. Am J Health Promot. 2018;32(8):1730–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irwin KE, Park ER, Fields LE, et al. Bridge: Person-Centered Collaborative Care for Patients with Serious Mental Illness and Cancer. The oncologist. 2019;24(7):901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schoen C, DesRoches C. Uninsured and unstably insured: the importance of continuous insurance coverage. Health Serv Res. 2000;35(1 Pt 2):187–206. [PMC free article] [PubMed] [Google Scholar]
- 27.Foerster B, Pozo C, Abufaraj M, et al. Association of Smoking Status With Recurrence, Metastasis, and Mortality Among Patients With Localized Prostate Cancer Undergoing Prostatectomy or Radiotherapy: A Systematic Review and Meta-analysis. JAMA Oncol. 2018;4(7):953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dess RT, Hartman HE, Mahal BA, et al. Association of Black Race With Prostate Cancer-Specific and Other-Cause Mortality. JAMA Oncol. 2019;5(7):975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearson SD, Katzelnick DJ, Simon GE, Manning WG, Helstad CP, Henk HJ. Depression among high utilizers of medical care. Journal of General Internal Medicine. 1999;14(8):461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smeets RGM, Elissen AMJ, Kroese MEAL, Hameleers N, Ruwaard D. Identifying subgroups of high-need, high-cost, chronically ill patients in primary care: A latent class analysis. PloS one. 2020;15(1):e0228103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coffey A, Atkinson P. Making Sense of Qualitative Data: Complementary Research Strategie. Thousand Oaks, CA: Sage Publications; 1996. [Google Scholar]
- 32.Solmi M, Firth J, Miola A, et al. Disparities in cancer screening in people with mental illness across the world versus the general population: prevalence and comparative meta-analysis including 4 717 839 people. The lancet Psychiatry. 2020;7(1):52–63. [DOI] [PubMed] [Google Scholar]
- 33.Mueser KT, McGurk SR. Schizophrenia. Lancet. 2004;363(9426):2063–2072. [DOI] [PubMed] [Google Scholar]
- 34.Velligan DI, Bow-Thomas CC, Huntzinger C, et al. Randomized controlled trial of the use of compensatory strategies to enhance adaptive functioning in outpatients with schizophrenia. Am J Psychiatry. 2000;157(8):1317–1323. [DOI] [PubMed] [Google Scholar]
- 35.Murphy KA, Daumit GL, Bandara SN, et al. Association Between the Maryland Medicaid Behavioral Health Home Program and Cancer Screening in People With Serious Mental Illness. Psychiatr Serv. 2020:appips201900299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.