Abstract
Background
Atrial fibrillation (AF) is correlated with a poor biventricular pacing and inadequate response to cardiac resynchronization therapy (CRT). Biventricular pacing improvement can be achieved by conducting the atrioventricular junction ablation (AVJA). We aimed to investigate the benefit of AVJA for permanent AF and heart failure with reduced ejection fraction (HFrEF) patients receiving CRT.
Methods
In August 2020, a systematic review and meta-analysis study comparing CRT plus AVJA versus CRT for permanent AF and HFrEF patients was conducted. Relevant articles were identified through the electronic scientific database such as ClinicalTrials.gov, ProQuest, ScienceDirect, PubMed, and Cochrane. The pooled risk ratio (RR) and pooled mean difference (MD) were estimated.
Results
A total of 3199 patients from 14 cohort studies were involved in this study. Additional AVJA reduced cardiovascular mortality (RR = 0.75, 95% confidence interval [CI] = 0.61 to 0.93, P < 0.01) in permanent AF and HFrEF patients receiving CRT. Biventricular pacing rate was higher in CRT plus AVJA group (MD = 8.65%, 95% CI = 5.62 to 11.67, P < 0.01) than in CRT alone group. The reverse remodeling characterized by the reduction of left ventricular end-diastolic diameter (LVEDD) was greater in the CRT plus AVJA group (MD = −2.11 mm, 95% CI = −3.79 to −0.42, P = 0.01).
Conclusion
In permanent AF and HFrEF patients receiving CRT, AVJA effectively increased the biventricular pacing rate. Adequate biventricular pacing rate provided a better response to the CRT marked by the greater ventricular reverse remodeling and survival from cardiovascular mortality.
Keywords: Atrioventricular junction ablation, Permanent atrial fibrillation, Heart failure, Cardiac resynchronization therapy
1. Introduction
Both heart failure (HF) and atrial fibrillation (AF) are well known as the “perfect partner in crime.” About 15% to 50% of HF patients also suffer from AF [1]. The presence of AF is correlated with increased HF severity, cardiovascular death, and rehospitalization due to the worsening of HF [2,3]. Heart failure with reduced ejection fraction (HFrEF) patients have a poor prognosis, despite being treated using an optimal medical treatment (OMT). Several guidelines strongly recommend cardiac resynchronization therapy (CRT) for HFrEF patients with New York Heart Association (NYHA) functional class II to IV despite OMT, left ventricular ejection fraction (LVEF) ≤35%, sinus rhythm, QRS duration ≥130 msec, and left bundle branch block (LBBB) morphology [4,5]. However, for patients with HFrEF and AF, the current guidelines give the lower class of recommendation because several randomized controlled trials (RCTs) for CRT excluded patients with AF [[4], [5], [6], [7], [8]].
In HFrEF patients, AF is correlated with a high risk of death, poor biventricular pacing, and inadequate response to CRT [[9], [10], [11]]. The previous studies revealed that consistent and effective delivery of biventricular pacing significantly contributed to the successful CRT [[10], [11], [12], [13]]. For patients with AF and HFrEF receiving CRT, atrioventricular junction ablation (AVJA) could be the therapeutic choice for heart rate control and biventricular pacing improvement by creating a complete atrioventricular (AV) block [[14], [15], [16]]. However, the AVJA procedure has been limited by its permanent character and the need for long-life ventricular pacing [17]. It has not been answered whether AVJA is a procedure that must be done in conjunction with CRT implantation or is a procedure that can be conducted if pharmacologic therapy has been unable to control heart rate. Therefore, we performed a systematic review and meta-analysis to investigate the benefit of AVJA for permanent AF and HFrEF patients receiving CRT.
2. Methods
2.1. Design
This systematic review and meta-analysis study had been conducted based on the guidance from Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) [18]. We looked for and identified relevant articles published in the electronic scientific database such as ClinicalTrials.gov, ProQuest, ScienceDirect, PubMed, and Cochrane. Articles that met the eligibility criteria were included in the study quality assessment and data extraction process. The pooled effect was determined using risk ratio (RR) or mean difference (MD) for categorical data or continuous data, respectively.
2.2. Search strategy
Up to August 2020, relevant articles about the comparison between CRT plus AVJA and CRT for permanent AF and HFrEF patients were collected from the electronic scientific database such as ClinicalTrials.gov, ProQuest, ScienceDirect, PubMed, and Cochrane. These keywords: "atrioventricular junction ablation" OR "AVJA," AND "catheter ablation" OR “ablation,” AND "permanent atrial fibrillation" OR "permanent AF," AND “systolic heart failure” OR "heart failure" OR "HF," AND “heart failure with reduced ejection fraction” OR “HFrEF,” AND "cardiac resynchronization therapy" OR "CRT" were used to identify the relevant articles. We also identified potentially relevant information from the reference lists of all collected full-text articles.
2.3. Eligibility criteria
We involved articles that fulfill the following criteria: (1) articles compared CRT plus AVJA versus CRT in patients with HFrEF and permanent AF; (2) the shortest follow-up duration was six months; and (3) availability of data about mortality, rehospitalization, biventricular pacing rate, functional status changes, or echocardiographic parameter changes. The exclusion criteria included: (1) duplications; (2) non-English language; (3) unavailable full-text; (4) review articles; (5) editorials; (6) case reports; (7) sub-study of the included studies; (8) treatment group and control group were incomparable; or (9) outcomes of interest were not reported.
2.4. Exposure and outcomes
AVJA was the exposure in this systematic review and meta-analysis. Therefore, patients were divided into “CRT plus AVJA” and “CRT” groups. The all-cause mortality, cardiovascular mortality, and rehospitalization because of the worsening of HF were the primary outcomes of this study. The secondary outcomes included: (1) biventricular pacing rate; (2) improvement of LVEF; (3) reduction of left ventricular end-diastolic diameter (LVEDD); (4) improvement of NYHA functional class; (5) improvement of walking distance in six-minute walk test (SMWT); and (6) improvement of Minnesota living with heart failure questionnaire (MLHFQ).
2.5. Study quality assessment and data extraction
The Newcastle-Ottawa Scale (NOS) was used to evaluate the study quality in this systematic review and meta-analysis [19,20]. The quality of studies was considered as good (NOS ≥7), moderate (NOS 5 to 6), and poor (NOS ≤4) [21]. The important information about: (1) the first author name; (2) year of publication; (3) study design; (4) center involved; (5) CRT implantation criteria (6) indications for AVJA; (7) duration of the follow-up period; (8) sample size; (9) age; (10) gender; (11) LVEF; (12) etiology of HF; (13) chronic AF and HF medications such as diuretic, angiotensin-converting enzyme inhibitor (ACEI), mineralocorticoid receptor antagonist (MRA), angiotensin receptor blocker (ARB), β-blocker, digoxin, and negative chronotropic drugs; (14) cardiac resynchronization therapy defibrillator (CRT-D); (15) all-cause mortality; (16) cardiovascular mortality; (17) rehospitalization because of worsening HF; (18) biventricular pacing rate; (19) improvement of LVEF; (20) reduction of LVEDD; (21) improvement of NYHA functional class; (22) improvement of walking distance in SMWT; and (23) improvement of MLHFQ were extracted from each article. Numeric data were shown using mean and standard deviation (SD), while categorical data were shown using number and percentage. The mean and SD could also be calculated from the median and interquartile range (IQR) using the Tiejun Tong group formula [[22], [23], [24]].
2.6. Statistical analysis
The statistical analysis process was conducted based on the standard guideline [25]. Before final conclusion determination, data were evaluated for heterogeneity and publication bias. The Q test was used to identify heterogeneity. We used P for heterogeneity (PHet) of 0.1 as the cut-off point. The random-effect analysis model was used in the presence of heterogeneity (PHet < 0.1). On the contrary, the fixed-effect analysis model was used in the absence of heterogeneity (PHet ≥ 0.1) [26]. The assessment of publication bias was conducted using a combination of the Egger test and the funnel plot analysis. The P Egger (pE) < 0.05 and/or the asymmetric funnel plot indicates the presence of publication bias [27]. For continuous data, the inverse variance statistical method was used to determine the pooled MD and its 95% confidence interval (CI). For categorical data, the pooled RR and its 95% CI were estimated using the Mantel-Haenszel statistical method. Statistically significant was considered if a p-value < 0.05. The data analysis process was conducted using Review Manager Version 5.3 (Cochrane, Copenhagen, Denmark) and Comprehensive Meta-Analysis version 3.0 (CMA, New Jersey, US).
3. Results
3.1. Eligible articles
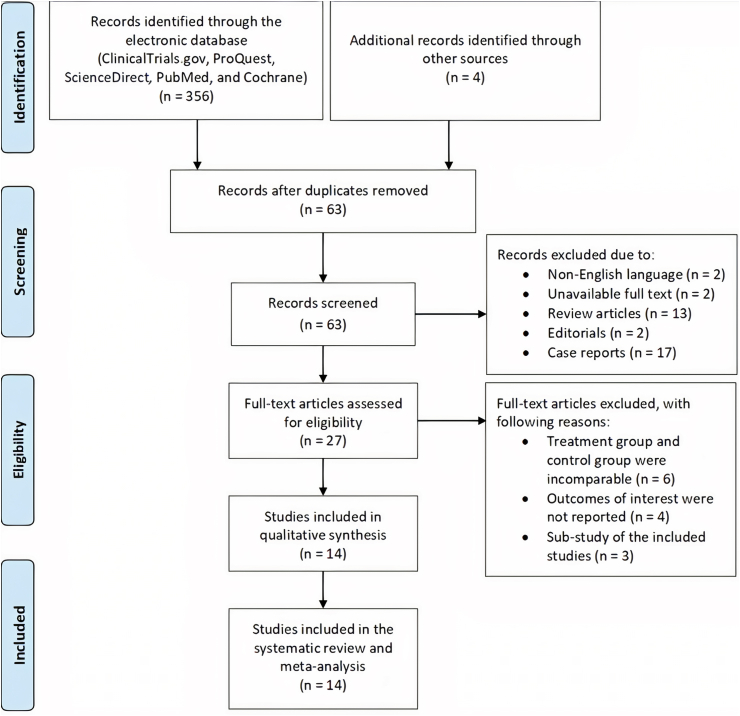
Through ClinicalTrials.gov, ProQuest, ScienceDirect, PubMed, and Cochrane, we successfully obtained 356 records. From the reference lists of the accessed full-text article, we identified four articles. A total of 297 records were excluded due to duplications. In the initial screening, we excluded a total 36 articles because of: (1) written in non-English language (n = 2); (2) unavailable full text (n = 2); (3) review articles (n = 13); (4) editorials (n = 2); and (5) case reports (n = 17). In further screening and assessment, 13 articles were excluded due to: (1) treatment group and control group were incomparable (n = 6); (2) outcomes of interest were not reported (n = 4); and (3) sub-study of the included studies (n = 3). Finally, 14 studies were involved in this systematic review and meta-analysis [[28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41]]. Fig. 1 shows the flow diagram of the study selection process.
Fig. 1.
Flowchart of the study selection process.
3.2. Baseline characteristics
Only good-quality studies were involved in this systematic review and meta-analysis. Ten prospective cohort studies [28], [31], [32], [33], [34], [35], [36], [37], [40], [41] and four retrospective cohort studies [29,30,38,39] were included in this study. Eight studies were single-center studies [28], [29], [30], [35], [36], [37], [38], [41], while six studies were multicenter studies [[31], [32], [33], [34],39,40]. Generally, the main indications of CRT implantation included the presence of HF symptoms despite OMT, severe left ventricular systolic dysfunction, and long QRS duration [28,30,31,[33], [34], [35], [36], [37], [38], [39], [40], [41]]. In most studies, the main indication to perform AVJA was inadequate biventricular pacing [31], [32], [33], [34], [35], [36], [38], [40] and/or poor heart rate control [28,39,41]. The shortest follow-up period was six months [[28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41]]. Baseline characteristics of the involved studies are summarized in Table 1.
Table 1.
Baseline characteristics of the involved studies.
| Study | Design | Center | CRT implantation criteria | Indications for AVJA | Follow up | NOS |
|---|---|---|---|---|---|---|
| Dong, 2010 [28] | Prospective cohort | Single-center |
|
Poor rate control |
|
8 |
| Eisen, 2013 [29] | Retrospective cohort | Single-center | NA | NA | 2 years | 9 |
| Ferreira, 2008 [30] | Retrospective cohort | Single-center |
|
NA | 29 ± 18 months | 8 |
| Gasparini, 2006 [31] | Prospective cohort | Multicenter |
|
Biventricular pacing ≤85% | 48 months | 8 |
| Gasparini, 2008 [32] | Prospective cohort | Multicenter | NA | Biventricular pacing ≤85% | 34 (10 - 40) months | 7 |
| Gasparini, 2013 [33] | Prospective cohort | Multicenter |
|
Clinical improvement and/or adequate biventricular pacing percentage did not occur with rate slowing drugs within 3 months | 37 (14 - 58) months | 7 |
| Gasparini, 2018 [34] | Prospective cohort | Multicenter |
|
Adequate biventricular pacing percentage (>95%) did not occur with rate-slowing drugs within 3 months | 18 (12–18) months | 8 |
| Himmel, 2012 [35] | Prospective cohort | Single-center |
|
Biventricular pacing ≤80% | 12 ± 3 months | 7 |
| Jędrzejczyk-Patej, 2014 [36] | Prospective cohort | Single-center |
|
Biventricular pacing <95% | 6 months | 7 |
| Molhoek, 2004 [37] | Prospective cohort | Single-center |
|
NA | 6 months | 7 |
| Schütte, 2009 [38] | Retrospective cohort | Single-center |
|
Biventricular pacing <90% | 11 ± 0.34 months | 7 |
| Tolosana, 2008 [39] | Retrospective cohort | Multicenter |
|
Poor rate control despite negative chronotropic therapy | 12 months | 8 |
| Tolosana, 2012 [40] | Prospective cohort | Multicenter |
|
Biventricular pacing ≤85% | 12 months | 9 |
| Tolosana, 2013 [41] | Prospective cohort | Single-center |
|
Poor rate control | 30 (13 - 51) months | 8 |
AVJA = atrioventricular junction ablation; CRT = cardiac resynchronization therapy; HF = heart failure; LBBB = left bundle branch block; LV = left ventricle; LVEF = left ventricular ejection fraction; NA = not available; NOS = Newcastle-Ottawa scale; NYHA = New York Heart Association.
A total of 3199 patients, including 1207 patients in CRT plus AVJA group and 1992 patients in CRT group, were involved in this systematic review and meta-analysis study. The mean age ranged from 57.1 to 71.5 years old [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [39], [40], [41]. About 54% to 96% of the included participants were male [28], [29], [30], [31], [32], [33], [34], [36], [37], [40], [41]. The mean LVEF varied from 20% to 28% [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [39], [40], [41]. Ischemic heart disease was the etiology for HF in 10% to 72.1% of the included patients [28], [29], [30], [31], [32], [33], [34], [35], [36], [39], [40], [41]. β-blocker, digoxin, and/or other negative chronotropic were the pharmacologic treatment of choice for rate control [28], [29], [30], [31], [32], [33], [34], [35], [36], [38], [39], [40], [41]. In 10 studies, CRT-D was implanted in 40.7% to 100% patients, but the indication of the CRT-D implantation was not explicitly described [[28], [29], [30], [31], [32], [33], [34], [39], [40], [41]]. The summary of baseline characteristics of the included patients is shown in Table 2.
Table 2.
Baseline characteristics of the included patients.
| Study | Arm | Size, n | Age (years old) | Male, % | LVEF (%) | Ischemic etiology, % | β - blocker, % | ACEI or ARB, % | MRA, % | Diuretic, % | Digoxin, % | Negative chronotropic, % | CRT-D |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dong, 2010 [28] | CRT + AVJA | 45 | 68.1 ± 10.5 | 84 | 25.5 ± 8.1 | 56 | 60 | 82 | NA | NA | 64 | 9 | 100 |
| CRT | 109 | 71.5 ± 9.4 | 87 | 22.6 ± 6.4 | 63 | 80 | 88 | NA | NA | 63 | 20 | 100 | |
| Eisen, 2013 [29] | CRT + AVJA | 10 | 69.5 ± 12.7 | 78.8 | 23.5 ± 11 | 72.1 | 80.3 | 83.3 | 47 | NA | 35.4 | NA | 48.5 |
| CRT | 56 | ||||||||||||
| Ferreira, 2008 [30] | CRT + AVJA | 26 | 67 ± 9 | 92 | 24 ± 9 | 58 | 50 | 96 | 46 | 100 | 54 | 54 | 77 |
| CRT | 27 | 70 ± 8 | 96 | 26 ± 9 | 48 | 67 | 100 | 30 | 100 | 63 | 33 | 85 | |
| Gasparini, 2006 [31] | CRT + AVJA | 114 | 66.8 ± 9.0 | 86.8 | 26.8 ± 7.1 | 36 | 83.3 | 93.8 | 52.5 | 91.4 | NA | 98.7 | 48.8 |
| CRT | 48 | 64.1 ± 6.3 | 83.3 | 25.1 ± 5.7 | 39.6 | ||||||||
| Gasparini, 2008 [32] | CRT + AVJA | 118 | 66.5 ± 9.2 | 86.4 | 27 ± 12 | 41.5 | 78 | 94.1 | 61.9 | 89.8 | 66.9 | 96.6 | 40.7 |
| CRT | 125 | 65.9 ± 8.6 | 77.6 | 24.8 ± 7.6 | 38.4 | 81.6 | 93.5 | 49.6 | 96.6 | 73.6 | 98.4 | 55.2 | |
| Gasparini, 2013 [33] | CRT + AVJA | 443 | 68.4 ± 9.1 | 84.2 | 27.0 ± 6.6 | 41 | 76.3 | 87.3 | 47.6 | 89.6 | 17.8 | 26 | 68.2 |
| CRT | 895 | 69.7 ± 9.3 | 85.4 | 25.9 ± 6.9 | 36.4 | 74.8 | 84 | 47.8 | 93.2 | 25.8 | 31.7 | 70.9 | |
| Gasparini, 2018 [34] | CRT + AVJA | 262 | 69 ± 10 | 83.8 | 28 ± 5 | 43 | 71.2 | 73.1 | NA | 87.4 | 33.9 | 19.4 | 100 |
| CRT | 402 | 69 ± 9 | 87.3 | 27 ± 6 | 44.1 | 69.8 | 72.2 | NA | 86 | 30.9 | 23.3 | 100 | |
| Himmel, 2012 [35] | CRT + AVJA | 15 | 70 ± 7 | NA | 23.7 ± 6.9 | 60 | 87 | 87 | 40 | 100 | 47 | 20 | NA |
| CRT | 31 | 69 ± 9 | NA | 23.6 ± 6.2 | 65 | 97 | 94 | 42 | 77 | 58 | 45 | NA | |
| Jędrzejczyk-Patej, 2014 [36] | CRT + AVJA | 40 | 62.2 ± 2.8 | 77.5 | 23.6 ± 1.7 | 47.5 | 97.5 | 92.5 | 90 | 97.5 | 52.5 | 25 | NA |
| CRT | 40 | 57.1 ± 3.9 | 77.5 | 24.1 ± 2.1 | 45 | 95 | 90 | 87.5 | 95 | 22.5 | 37.5 | NA | |
| Molhoek, 2004 [37] | CRT + AVJA | 17 | 63 ± 10 | 90 | 20 ± 11 | NA | NA | NA | NA | NA | NA | NA | NA |
| CRT | 13 | ||||||||||||
| Schütte, 2009 [38] | CRT + AVJA | 9 | NA | NA | NA | NA | 97 | 94 | 33 | 75 | 53 | 36 | NA |
| CRT | 27 | ||||||||||||
| Tolosana, 2008 [39] | CRT + AVJA | 19 | 69.7 ± 7 | NA | 26 ± 8 | 10 | 32 | 73 | 49 | 100 | 68 | 0 | 52 |
| CRT | 107 | 68.2 ± 10 | NA | 27 ± 12 | 35 | 54 | 73 | 42 | 91 | 63 | 30 | ||
| Tolosana, 2012 [40] | CRT + AVJA | 13 | 68 ± 10 | 84 | 24 ± 5 | 31 | 54 | 69 | 31 | NA | 69 | NA | 61 |
| CRT | 33 | 67 ± 9 | 67 | 25 ± 7 | 33 | 70 | 88 | 57 | NA | 57 | NA | 76 | |
| Tolosana, 2013 [41] | CRT + AVJA | 76 | 69.7 ± 7.5 | 82 | 23.7 ± 6.3 | 32 | 64 | 84 | 47 | NA | 45 | 31 | 49 |
| CRT | 79 | 68 ± 8.3 | 81 | 25.3 ± 7.5 | 39 | 76 | 91 | 57 | NA | 45 | 25 | 62 |
ACEI = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; AVJA = atrioventricular junction ablation; CRT = cardiac resynchronization therapy; CRT-D = cardiac resynchronization therapy - defibrillator; LVEF = left ventricular ejection fraction; MRA = mineralocorticoid receptor antagonist; NA = not available.
3.3. Heterogeneity and publication bias
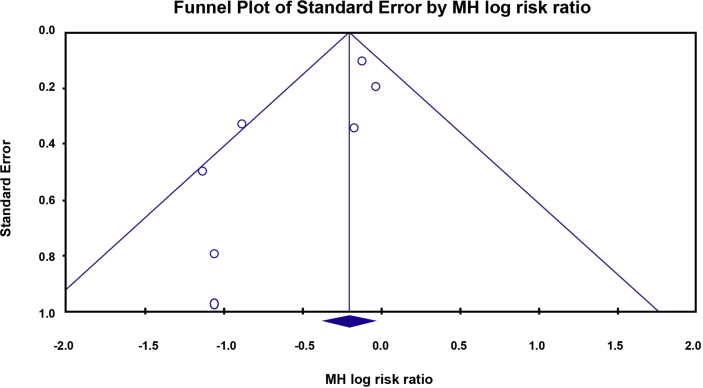
Heterogeneity was found during performing meta-analysis of all-cause mortality, biventricular pacing rate, improvement of LVEF, reduction of LVEDD, and improvement of NYHA functional class. Therefore, effect estimation was determined using a random-effect model. The other outcomes did not reveal any heterogeneity. Therefore, the effect estimation was determined using the fixed-effect model (Fig. 2, Fig. 3, Fig. 4). We found the publication bias only in the all-cause mortality analysis. It was identified by the PE = 0.04 (Table 3) and the asymmetric funnel plot (Fig. 5). Table 3, Table 4 demonstrate the summary of heterogeneity and publication bias assessment.
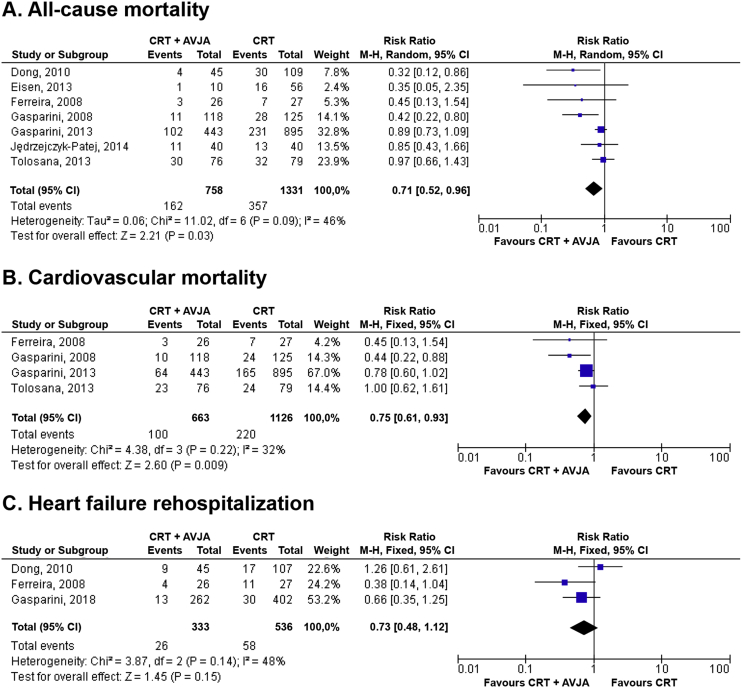
Fig. 2.
Forest plot of primary outcomes: (A) all-cause mortality; (B) cardiovascular mortality; and (C) heart failure rehospitalization. AVJA = atrioventricular junction ablation; CI = confidence interval; CRT = cardiac resynchronization therapy; M-H = Mantel-Haenszel.
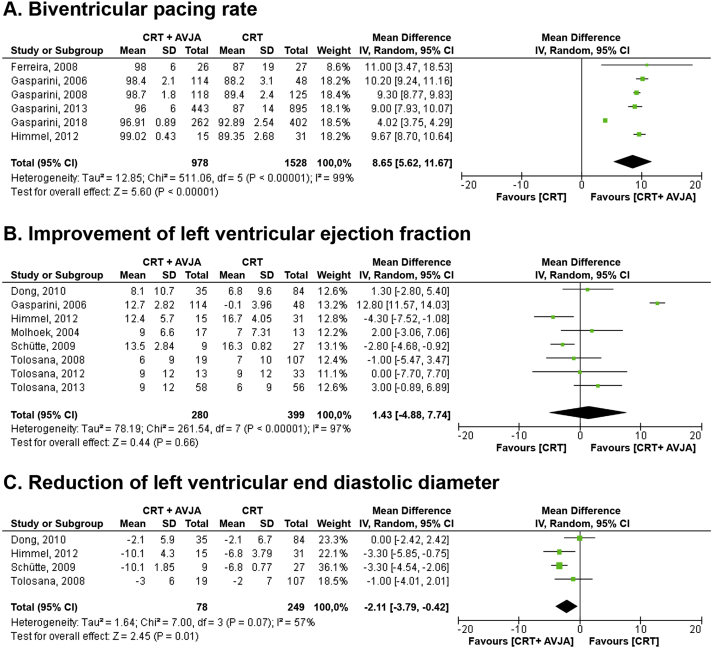
Fig. 3.
Forest plot of secondary outcomes: (A) biventricular pacing rate; (B) improvement of left ventricular ejection fraction; and (C) reduction of left ventricular end diastolic diameter. AVJA = atrioventricular junction ablation; CI = confidence interval; CRT = cardiac resynchronization therapy; IV = inverse variance; SD = standard deviation.
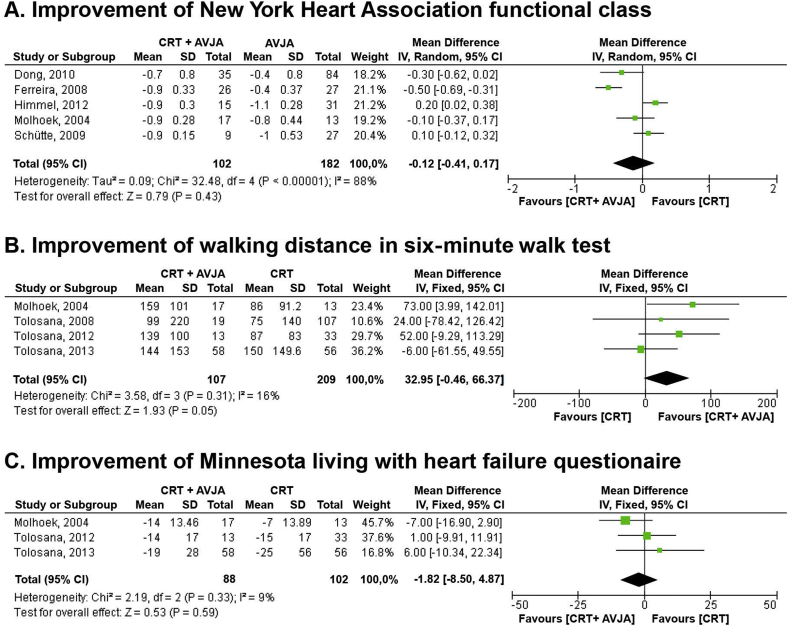
Fig. 4.
Forest plot of secondary outcomes: (A) improvement of New York Heart Association functional class; (B) improvement of walking distance in six-minute walk test; and (C) improvement of Minnesota living with heart failure questionnaire. AVJA = atrioventricular junction ablation; CI = confidence interval; CRT = cardiac resynchronization therapy; IV = inverse variance; SD = standard deviation.
Table 3.
Summary of the primary outcomes.
| Primary outcomes | Number of studies | CRT + AVJA |
CRT |
Model | RR | 95% CI |
PHet | PE | P | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Event, n (%) | Total, n | Event, n (%) | Total, n | Lower limit | Upper limit | |||||||
| All-cause mortality | 7 | 162 (21.4) | 758 | 357 (26.8) | 1331 | Random | 0.71 | 0.52 | 0.96 | 0.09 | 0.04 | 0.03 |
| Cardiovascular mortality | 4 | 100 (15.1) | 663 | 220 (19.5) | 1126 | Fixed | 0.75 | 0.61 | 0.93 | 0.22 | 0.42 | < 0.01 |
| HF rehospitalization | 3 | 26 (7.8) | 333 | 58 (10.8) | 536 | Fixed | 0.73 | 0.48 | 1.12 | 0.14 | 0.67 | 0.15 |
AVJA = atrioventricular junction ablation; CRT = cardiac resynchronization therapy; CI = confidence interval; HF = heart failure; PE = P Egger; PHet = P for heterogeneity, RR = risk ratio.
Fig. 5.
Funnel plot analysis showing asymmetrical funnel plot for all-cause mortality.
Table 4.
Summary of the secondary outcomes.
| Secondary outcomes | Number of studies | CRT + AVJA, n | CRT, n | Model | Mean difference | 95% CI |
PHet | PE | P | |
|---|---|---|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||||||
| Biventricular pacing rate | 6 | 978 | 1528 | Random | 8.65 | 5.62 | 11.67 | <0.01 | 0.19 | <0.01 |
| Improvement of LVEF | 8 | 280 | 399 | Random | 1.43 | -4.88 | 7.74 | < 0.01 | 0.49 | 0.66 |
| Reduction of LVEDD | 4 | 78 | 249 | Random | -2.11 | -3.79 | -0.42 | 0.07 | 0.25 | 0.01 |
| Improvement of NYHA functional class | 5 | 102 | 182 | Random | -0.12 | -0.41 | 0.17 | < 0.01 | 0.97 | 0.43 |
| Improvement of walking distance in SMWT | 4 | 107 | 209 | Fixed | 32.95 | -0.46 | 66.37 | 0.31 | 0.63 | 0.05 |
| Improvement of MLHFQ | 3 | 88 | 102 | Fixed | -1.82 | -8.5 | 4.87 | 0.33 | 0.37 | 0.59 |
AVJA = atrioventricular junction ablation; CRT = cardiac resynchronization therapy; CI = confidence interval; LVEDD = left ventricular end-diastolic diameter; LVEF = left ventricular ejection fraction; MLHFQ = Minnesota living with heart failure questionnaire; NYHA = New York Heart Association; SMWT = six-minute wak test; PE = P Egger; PHet = P for heterogeneity.
3.4. Outcome
The meta-analysis of seven studies revealed that the AVJA reduced all-cause mortality in permanent AF and HFrEF patients receiving CRT (RR = 0.71, 95% CI = 0.52 to 0.96, P = 0.03). Through the meta-analysis of four studies, we found that the AVJA also reduced cardiovascular mortality (RR = 0.75, 95% CI = 0.61 to 0.93, P < 0.01). However, from the meta-analysis of three studies, AVJA did not significantly reduce the HF rehospitalization (RR = 0.73, 95% CI = 0.48 to 1.12, P = 0.15). The forest plot of the primary outcomes is shown in Fig. 2, and the summary of the primary outcomes is summarized in Table 3.
We assessed the overall effect of additional AVJA for permanent AF and HFrEF patients receiving CRT on biventricular pacing rate and echocardiographic parameters, including LVEF and LVEDD (Fig. 3). Data from meta-analysis of six studies revealed that biventricular pacing rate was higher in CRT plus AVJA group (MD = 8.65%, 95% CI = 5.62 to 11.67, P < 0.01). Through the meta-analysis of eight studies, we found the comparable improvement of LVEF between both groups (MD = 1.43%, 95% CI = −4.88 to 7.74, P = 0.66). However, a meta-analysis of four studies revealed that the reduction of LVEDD was greater in the CRT plus AVJA group (MD = −2.11 mm, 95% CI = −3.79 to −0.42, P = 0.01). The additional AVJA for permanent AF and HFrEF patients receiving CRT did not give a significant improvement on the several clinical or functional parameters including: (1) NYHA functional class (MD = −0.12, 95% CI = −0.41 to 0.17, P = 0.43); (2) walking distance in SMWT (MD = 32.95 m, 95% CI = −0.46 to 66.37, P = 0.05); and (3) MLHFQ (MD = −1.82 mm, 95% CI = −8.5 to 4.87, P = 0.59) (Fig. 4). The summary of the secondary outcomes is summarized in Table 4.
4. Discussion
4.1. Atrioventricular junction ablation for rate control strategy in atrial fibrillation
The prevention of thromboembolism, rate control, and rhythm control are the current AF treatment strategies [16,42] The decision to adopt a rate control or rhythm control strategy is very important for patients with AF and HFrEF. The Atrial Fibrillation and Congestive Heart Failure trial revealed that compared to the rate control strategy, the routine rhythm control strategy did not decrease cardiovascular mortality in patients who suffered from AF and HF with LVEF ≤35% [43]. The Resynchronization for Ambulatory Heart Failure Trial (RAFT), which included 114 patients with CRT-D, provided important lessons for us that even though rate control strategy was achieved, the biventricular pacing rate ≥95% could be achieved in 34.3% of patients. In contrast, a biventricular pacing rate of ≥90% could be achieved in 47.1% of patients [44]. For AF patients with LVEF <40%, digoxin and/or β-blockers are recommended for rate control strategy [16].
Since 1982, intentional devastation of the atrioventricular (AV) junction to establish the total AV block has been used as a rate control strategy for AF [45]. In the correctly selected patient, it is a simple approach that can relieve symptoms and may also improve left ventricular systolic function [14]. Moreover, for patients with permanent AF and HFrEF receiving CRT, AVJA ensures the regular ventricular rhythm. In the studies involved in this meta-analysis, the decision to conduct AVJA was based on the patient’s poor heart rate control, with a cut-off point of biventricular pacing rate ranged from 80% to 95% . The retrospective analysis of 2 RCTs for CRT showed that the >92% biventricular pacing rate was associated with a significant clinical benefit [13]. The LATITUDE study provided us a valuable lesson in which the most significant impact of the reduction in mortality was observed with a biventricular pacing rate reaching more than 98% of all ventricular beats [10]. However, according to the current guideline from the European Society of Cardiology (ESC), AVJA should be added to incomplete biventricular pacing (Class of recommendation IIa; Level of evidence B). AVJA can be conducted during the CRT implantation or several weeks later [15].
4.2. Primary outcomes and comparison with the previous studies
Through this meta-analysis of 14 studies with the follow-up period ranged from 6 to 48 months, we directly compared CRT plus AVJA versus CRT alone in AF and HFrEF patients. We found that AVJA effectively reduced all-cause mortality in permanent AF and HFrEF patients receiving CRT. For the all-cause mortality outcome, our result supported the previous meta-analysis study from Ganesan et al. [46] and Yin et al. [47]. A study from Xue et al. revealed that AVJA was correlated with a a higher survival rate in permanent AF and HFrEF patients receiving CRT [48]. However, we found the publication bias in the analysis of all-cause mortality. The possible explanation for this bias might be due to: (1) old aged patients (mean age 63 to 72 years old); (2) risk of bleeding because of the effect of the long-term anticoagulant treatment as the consequences of rate control strategy; and (3) inadequate data about the comorbid condition.
Our result demonstrated that conducting AVJA in permanent AF and HFrEF patients receiving CRT effectively reduced cardiovascular mortality. Our result in cardiovascular mortality was different from the prior meta-analysis study from Yin et al. which revealed that AVJA did not reduce cardiovascular mortality in these populations [47]. It was because we were able to add a study with a larger sample [33]. In our meta-analysis, AVJA did not significantly reduce the HF rehospitalization in permanent AF and HFrEF patients receiving CRT. Several factors could precipitate worsening of HF, such as acute coronary syndrome, respiratory tract infection, arrhythmia, diet non-compliance, medication non-compliance, renal failure, and uncontrolled hypertension [49], were not specifically reported [28,30,34]. To the best of our knowledge, our study is the only meta-analysis study that provide data about HF rehospitalization in this population.
4.3. Secondary outcomes and comparison with the previous studies
We did not assess the CRT response because the definition of responders was not available in 8 studies [28,29,[32], [33], [34], [35], [36],38]. Moreover, the definition of responders in each study was not the same [30,31,37,[39], [40], [41]]. We proved that AVJA could effectively increase the biventricular pacing rate in permanent AF and HFrEF patients receiving CRT through this meta-analysis. In our meta-analysis, the mean biventricular pacing rate was ranged from 96% to 99% in the CRT plus AVJA group (Fig. 3). We thought that the patients with permanent AF and HFrEF receiving CRT could get more benefit from AVJA because the previous study revealed that the >92% biventricular pacing rate was associated with a significant clinical benefit [13].
In our meta-analysis, the reduction in all-cause and cardiovascular mortality with AVJA were not accompanied by a significant improvement of LVEF. Both groups shared a similar improvement in LVEF. Our result supported the previous meta-analysis study from Ganesan et al. [46] The explanation for the mortality reduction without concomitant significant LVEF improvement is still not clear. However, the possible explanation was the diversity in: (1) LVEF assessment modalities; (2) LVEF measurement method; and (3) interobserver variability. Our result showed that the additional AVJA in permanent AF and HFrEF patients receiving CRT provided the more significant reverse remodeling on the left ventricle. Our work supported the previous study from Ruwald et al. which revealed an improvement of biventricular pacing rate (≥97%) was correlated with an increased ventricular reverse remodeling [50].
Our study results proved that AVJA in permanent AF and HFrEF patients receiving CRT did not offer any benefit in improving the functional parameters of patients consisting of: (1) improvement of NYHA functional class; (2) improvement of walking distance in SMWT, and (3) improvement of MLHFQ. Our results did not support the previous meta-analysis study from Ganesan et al. which stated that AVJA in permanent AF and HFrEF patients receiving CRT was associated with the improvement of NYHA functional class. Compared to the meta-analysis by Ganesan et al. [46], we were able to add two new studies to our meta-analysis [35,38]. As far as we are concerned, our study was the only meta-analysis that provided data about the impact of AVJA on the improvement of SMWT and MLHFQ in permanent AF and HFrEF patients receiving CRT. There were several possible explanations for the failure of AVJA to improve functional parameters in permanent AF and HFrEF patients receiving CRT. First, several studies did not exclude patients who had comorbidities that could influence those parameters. Second, patients with permanent AF are at high risk for stroke, which may worsen functional parameters [28,30,35,[37], [38], [39], [40], [41]].
4.4. Strengths and limitations
Our study had several strengths. First, to the best of our knowledge, our study represents the biggest pooled analysis of cohort studies of the benefit of AVJA for patients with permanent AF and HFrEF receiving CRT. Second, we provided the data about HF rehospitalization, improvement of walking distance in SMWT, and improvement of MLHFQ data that were not available in the prior meta-analysis studies [46], [47], [48]. Apart from the strengths mentioned above, our study also had some drawbacks. First, all studies involved in this systematic review and meta-analysis were cohort studies, causing unwanted referral bias or selection bias. Second, publication bias was also unavoidable. However, we had anticipated that situation by conducting a strict publication bias assessment using the combination of Egger test and funnel plot analysis. We found the publication bias only in the meta-analysis of all-cause mortality. Third, the complete data could not be extracted for several parameters, such as CRT implantation criteria, CRT programming, indication for performing AVJA, the method for assessing LVEF, and interobserver variability. Fourth, the inability to access the individual patient-level data limited our ability to evaluate patient-level real effects on our outcomes.
5. Conclusion
Our study revealed that in permanent AF and HFrEF patients receiving CRT, AVJA reduced cardiovascular mortality, decreased LVEDD, and increased the biventricular pacing rate. The possible association among those parameters improvement was AVJA increased biventricular pacing rate. Adequate biventricular pacing rate improved the response to the CRT marked by the greater ventricular reverse remodeling and survival from cardiovascular mortality. Prospective evaluation of AVJA in permanent AF and HFrEF patients receiving CRT by a large size and well-designed RCT is required.
Declaration of competing interest
All authors declare that there is no conflict of interest regarding the publication of this manuscript.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Trulock K.M., Narayan S.M., Piccini J.P. Rhythm control in heart failure patients with atrial fibrillation. J Am Coll Cardiol. 2014;64(7):710–721. doi: 10.1016/j.jacc.2014.06.1169. [DOI] [PubMed] [Google Scholar]
- 2.Olsson L.G., Swedberg K., Ducharme A. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction. J Am Coll Cardiol. 2006;47(10):1997–2004. doi: 10.1016/j.jacc.2006.01.060. [DOI] [PubMed] [Google Scholar]
- 3.Sartipy U., Dahlström U., Fu M., Lund L.H. Atrial fibrillation in heart failure with preserved, mid-range, and reduced ejection fraction. JACC Heart Fail. 2017;5(8):565–574. doi: 10.1016/j.jchf.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Yancy C.W., Jessup M., Bozkurt B. ACCF/AHA guideline for the management of heart failure. J Am Coll Cardiol. 2013;62(16):e147–e239. doi: 10.1016/j.jacc.2013.05.019. 2013. [DOI] [PubMed] [Google Scholar]
- 5.Ponikowski P., Voors A.A., Anker S.D. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. 2016. [DOI] [PubMed] [Google Scholar]
- 6.Cleland J.G.F., Erdmann E., Kappenberger L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352(15):1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 7.Moss A.J., Hall W.J., Cannom D.S. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361(14):1329–1338. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 8.Tang A.S.L., Wells G.A., Talajic M. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363(25):2385–2395. doi: 10.1056/NEJMoa1009540. [DOI] [PubMed] [Google Scholar]
- 9.Wilton S.B., Leung A.A., Ghali W.A., Faris P., Exner D.V. Outcomes of cardiac resynchronization therapy in patients with versus those without atrial fibrillation: a systematic review and meta-analysis. Heart Rhythm. 2011;8(7):1088–1094. doi: 10.1016/j.hrthm.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Hayes D.L., Boehmer J.P., Day J.D. Cardiac resynchronization therapy and the relationship of percent biventricular pacing to symptoms and survival. Heart Rhythm. 2011;8(9):1469–1475. doi: 10.1016/j.hrthm.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Mullens W., Grimm R.A., Verga T. Insights from a cardiac resynchronization optimization clinic as part of a heart failure disease management program. J Am Coll Cardiol. 2009;53(9):765–773. doi: 10.1016/j.jacc.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 12.Kamath G.S., Cotiga D., Koneru J.N. The utility of 12-lead holter monitoring in patients with permanent atrial fibrillation for the identification of nonresponders after cardiac resynchronization therapy. J Am Coll Cardiol. 2009;53(12):1050–1055. doi: 10.1016/j.jacc.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 13.Koplan B.A., Kaplan A.J., Weiner S., Jones P.W., Seth M., Christman S.A. Heart failure decompensation and all-cause mortality in relation to percent biventricular pacing in patients with heart failure. J Am Coll Cardiol. 2009;53(4):355–360. doi: 10.1016/j.jacc.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 14.Betts T.R. Atrioventricular junction ablation and pacemaker implant for atrial fibrillation: still a valid treatment in appropriately selected patients. Europace. 2008;10(4):425–432. doi: 10.1093/europace/eun063. [DOI] [PubMed] [Google Scholar]
- 15.Brignole M., Auricchio A., Baron-Esquivias G., Bordachar P., Boriani G. ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA) Eur Heart J. 2013;34(29):2281–2329. doi: 10.1093/eurheartj/eht150. 2013. [DOI] [PubMed] [Google Scholar]
- 16.Kirchhof P., Benussi S., Kotecha D. ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–2962. doi: 10.1093/eurheartj/ehw210. 2016. [DOI] [PubMed] [Google Scholar]
- 17.Garcia B., Clementy N., Benhenda N. Mortality after atrioventricular nodal radiofrequency catheter ablation with permanent ventricular pacing in atrial fibrillation: outcomes from a controlled nonrandomized study. Circ Arrhythm Electrophysiol. 2016;9(7) doi: 10.1161/CIRCEP.116.003993. [DOI] [PubMed] [Google Scholar]
- 18.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting Items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):6. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wells G., Shea B., O’Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 20.Bae J.-M. A suggestion for quality assessment in systematic reviews of observational studies in nutritional epidemiology. Epidemiol Health. 2016;38 doi: 10.4178/epih.e2016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azagury D., Morton J.M. Bariatric surgery outcomes in US accredited vs non-accredited centers: a systematic review. J Am Coll Surg. 2016;223(3):469–477. doi: 10.1016/j.jamcollsurg.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Tiejun Tong group Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. http://www.math.hkbu.edu.hk/∼tongt/papers/median2mean.html [DOI] [PMC free article] [PubMed]
- 23.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 27(6):1785–1805. doi:10.1177/0962280216669183. [DOI] [PubMed]
- 24.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cleophas T.J., Zwinderman A.H. Springer International Publishing; 2017. Modern meta-analysis: review and update of methodologies. [DOI] [Google Scholar]
- 26.Fletcher J. What is heterogeneity and is it important? BMJ. 2007;334:94–96. doi: 10.1136/bmj.39057.406644.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin J., Sun Y., Zhao S., Xu J., Zhao C. Safety and efficacy of thrombolysis in cervical artery dissection-related ischemic stroke: a meta-analysis of observational studies. Cerebrovasc Dis. 2016;42(3–4):272–279. doi: 10.1159/000446004. [DOI] [PubMed] [Google Scholar]
- 28.Dong K., Shen W.-K., Powell B.D. Atrioventricular nodal ablation predicts survival benefit in patients with atrial fibrillation receiving cardiac resynchronization therapy. Heart Rhythm. 2010;7(9):1240–1245. doi: 10.1016/j.hrthm.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Eisen A., Nevzorov R., Goldenberg G. Cardiac resynchronization therapy in patients with atrial fibrillation: a 2-year follow-up study: CRT IN patients with AF. Pacing Clin Electrophysiol. 2013;36(7):872–877. doi: 10.1111/pace.12136. [DOI] [PubMed] [Google Scholar]
- 30.Ferreira A.M., Adragao P., Cavaco D.M. Benefit of cardiac resynchronization therapy in atrial fibrillation patients vs. patients in sinus rhythm: the role of atrioventricular junction ablation. Europace. 2008;10(7):809–815. doi: 10.1093/europace/eun135. [DOI] [PubMed] [Google Scholar]
- 31.Gasparini M., Auricchio A., Regoli F. Four-year efficacy of cardiac resynchronization therapy on exercise tolerance and disease progression. J Am Coll Cardiol. 2006;48(4):734–743. doi: 10.1016/j.jacc.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 32.Gasparini M., Auricchio A., Metra M. Long-term survival in patients undergoing cardiac resynchronization therapy: the importance of performing atrio-ventricular junction ablation in patients with permanent atrial fibrillation. Eur Heart J. 2008;29(13):1644–1652. doi: 10.1093/eurheartj/ehn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gasparini M., Leclercq C., Lunati M. Cardiac resynchronization therapy in patients with atrial fibrillation. JACC Heart Fail. 2013;1(6):500–507. doi: 10.1016/j.jchf.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Gasparini M., Kloppe A., Lunati M. Atrioventricular junction ablation in patients with atrial fibrillation treated with cardiac resynchronization therapy: positive impact on ventricular arrhythmias, implantable cardioverter-defibrillator therapies and hospitalizations: atrioventricular junction ablation in CRT patients with AF. Eur J Heart Fail. 2018;20(10):1472–1481. doi: 10.1002/ejhf.1117. [DOI] [PubMed] [Google Scholar]
- 35.Himmel F., Reppel M., Mortensen K., Schunkert H., Bode F. A strategy to achieve CRT response in permanent atrial fibrillation without obligatory atrioventricular node ablation: CRT IN permanent atrial fibrillation. Pacing Clin Electrophysiol. 2012;35(8):943–947. doi: 10.1111/j.1540-8159.2012.03433.x. [DOI] [PubMed] [Google Scholar]
- 36.Jędrzejczyk-Patej E., Lenarczyk R., Pruszkowska P. Long-term outcomes of cardiac resynchronization therapy are worse in patients who require atrioventricular junction ablation for atrial fibrillation than in those with sinus rhythm. Cardiol J. 2014;21(3):309–315. doi: 10.5603/CJ.a2013.0110. [DOI] [PubMed] [Google Scholar]
- 37.Molhoek S.G., Bax J.J., Bleeker G.B. Comparison of response to cardiac resynchronization therapy in patients with sinus rhythm versus chronic atrial fibrillation. Am J Cardiol. 2004;94(12):1506–1509. doi: 10.1016/j.amjcard.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 38.Schütte F., Lüdorff G., Grove R., Kranig W., Thale J. Atrioventricular node ablation is not a prerequisite for cardiac resynchronization therapy in patients with chronic atrial fibrillation. Cardiol J. 2009;16(3):246–249. https://journals.viamedica.pl/cardiology_journal/article/view/21502/17106 [PubMed] [Google Scholar]
- 39.Tolosana J.M., Hernandez Madrid A., Brugada J. Comparison of benefits and mortality in cardiac resynchronization therapy in patients with atrial fibrillation versus patients in sinus rhythm (results of the Spanish atrial fibrillation and resynchronization [SPARE] study) Am J Cardiol. 2008;102(4):444–449. doi: 10.1016/j.amjcard.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Tolosana J.M., Arnau A.M., Madrid A.H. Cardiac resynchronization therapy in patients with permanent atrial fibrillation. Is it mandatory to ablate the atrioventricular junction to obtain a good response? Eur J Heart Fail. 2012;14(6):635–641. doi: 10.1093/eurjhf/hfs024. [DOI] [PubMed] [Google Scholar]
- 41.Tolosana J.M., Trucco E., Khatib M. Complete atrioventricular block does not reduce long-term mortality in patients with permanent atrial fibrillation treated with cardiac resynchronization therapy. Eur J Heart Fail. 2013;15(12):1412–1418. doi: 10.1093/eurjhf/hft114. [DOI] [PubMed] [Google Scholar]
- 42.January C.T., Wann L.S., Calkins H. AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. J Am Coll Cardiol. 2019;74(1):104–132. doi: 10.1016/j.jacc.2019.01.011. 2019. [DOI] [PubMed] [Google Scholar]
- 43.Roy D., Lee K.L., Camm A.J., Guerra P.G., O’Hara G., Stevenson L.W. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358(25):2667–2677. doi: 10.1056/nejmoa0708789. [DOI] [PubMed] [Google Scholar]
- 44.Healey J.S., Hohnloser S.H., Exner D.V. Cardiac resynchronization therapy in patients with permanent atrial fibrillation: results from the resynchronization for ambulatory heart failure trial (RAFT) Circ Heart Fail. 2012;5(5):566–570. doi: 10.1161/CIRCHEARTFAILURE.112.968867. [DOI] [PubMed] [Google Scholar]
- 45.Gallagher J.J., Svenson R.H., Kasell J.H. Catheter technique for closed-chest ablation of the atrioventricular conduction system — a therapeutic alternative for the treatment of refractory supraventricular tachycardia. N Engl J Med. 1982;306:194–200. doi: 10.1056/NEJM198201283060402. [DOI] [PubMed] [Google Scholar]
- 46.Ganesan A.N., Brooks A.G., Roberts-Thomson K.C., Lau D.H., Kalman J.M., Sanders P. Role of AV nodal ablation in cardiac resynchronization in patients with coexistent atrial fibrillation and heart failure. J Am Coll Cardiol. 2012;59(8):719–726. doi: 10.1016/j.jacc.2011.10.891. [DOI] [PubMed] [Google Scholar]
- 47.Yin J., Hu H., Wang Y. Effects of atrioventricular nodal ablation on permanent atrial fibrillation patients with cardiac resynchronization therapy: a systematic review and meta-analysis: the role of AVN ablation for CRT in PAF. Clin Cardiol. 2014;37(11):707–715. doi: 10.1002/clc.22312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xue Y., Wang J., Wang J. Comparison of survival for cardiac resynchronization therapy in atrial fibrillation patients with or without atrio-ventricular junction ablation and patients in sinus rhythm: a systematic review and network meta-analysis. Heart Fail Rev. 2019;24(3):335–342. doi: 10.1007/s10741-018-9761-5. [DOI] [PubMed] [Google Scholar]
- 49.Kapoor J.R., Kapoor R., Ju C. Precipitating clinical factors, heart failure characterization, and outcomes in patients hospitalized with heart failure with reduced, borderline, and preserved ejection fraction. JACC Heart Fail. 2016;4(6):464–472. doi: 10.1016/j.jchf.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 50.Ruwald A.-C., Kutyifa V., Ruwald M.H. The association between biventricular pacing and cardiac resynchronization therapy-defibrillator efficacy when compared with implantable cardioverter defibrillator on outcomes and reverse remodeling. Eur Heart J. 2015;36(7):440–448. doi: 10.1093/eurheartj/ehu294. [DOI] [PubMed] [Google Scholar]