Abstract
Background
Parkinson's disease (PD) is a rare, neurodegenerative disease with various occupational and environmental risk factors. Exposure to specific pesticides contributes significantly to the incidence of PD. However, it is difficult to measure the level of pesticide exposure in workers. This study presents the first case recognized the work-relatedness between PD and pesticide exposure.
Case presentation
A 68-year-old male was diagnosed with PD after working with pesticides at a tomato greenhouse for 12 years and 5 months. From the results of a field study, it was reasonable to assume that the patient had been exposed to a significant level of various insecticides. In the present report, we described the first accepted case of work-relatedness between PD and exposure to pesticides. The evaluation was conducted using the following steps: we ruled out other possible risk factors including additional occupational history and personal risk factors, we assessed the work environment, surveyed possible exposures, found proper epidemiological evidence, and calculated the probability of causation. The work-relatedness was determined through the review of epidemiological evidence and estimation of exposure situation and level, and biological plausibility. We also decided work-relatedness based on the exposure of PD related pesticides with identified biologically plausible and the presumption that the exposure level would be high due to the working process.
Conclusions
In this case, the field study and epidemiological results supported the work-relatedness of PD and exposure to pesticides. Moreover, the results of previous studies have confirmed a causal relationship between exposure to pesticides and PD.
Keywords: Parkinson disease; Pesticides; Occupational exposure, Farmers
BACKGROUND
Parkinson's disease (PD) is an idiopathic, neurodegenerative disease that occurs in the central nervous system. After the introduction of levodopa (L-dopa) in the late 1960's, the mortality rate of PD declined, but incidences have steadily been increasing [1]. In South Korea, the incidence rate was 20.2 per 100,000 in 2004, which increased to 53.1 per 100,000 in 2013, with 426 new cases in 2013 [2].
The pathogenesis of PD is still unclear, but it is considered to be a multifactorial disease to which both genetic and environmental factors contribute [3]. The following factors have been reported to affect the occurrence of PD: environmental risk factors (including exposure to pesticides), use of beta-blockers, occupational farming, and drinking water from wells [4]. Since the association between PD and exposure to pesticides is supported by significant epidemiological evidence, France and the Netherlands have broadly compensated pesticide workers for PD [5,6,7]. However, there was no previously accepted case of work-relatedness between PD and pesticides in South Korea until July 2019.
In this study, we described the first accepted case of work-relatedness between PD and exposure to insecticides. We evaluated the work-relatedness by comprehensively considering the results of previous observational epidemiological studies, estimation of occupational exposure methods and levels, causal probability, and the use of pesticides with known toxicological risks.
CASE PRESENTATION
Patient information
The patient was a 68-year-old male.
Chief complaints
In 2014, the patient first complained of arthralgia, followed by dyskinesia.
Present illness
Since March 2014, the patient felt weakness in both legs and reported painful gait. In addition, it was difficult for him to smell and to speak quickly before he visited the neurology department. His symptoms got worse, so he was admitted to the hospital for further examination. Based on radiological findings and neurological symptoms, the patient was diagnosed with PD in August 2014.
After initial admission to the hospital, his illness was managed with medications after hospital discharge. The prescribed medications were Pramipexole for PD, and Itopride hydrochloride and Pantoprazole for gastroenteric symptoms. A review of his medical records through 2019 revealed no apparent deterioration of the illness.
Neurological examinations
There were no specific findings in both the motor and sensory nerves from the nerve conduction study. The Seoul Neuropsychological Screening Battery was completed to evaluate for dementia and other psychiatric disorders. The results indicated that his language and concentration ability were intact, but his semantic generative naming ability was degraded to below the normal range, which suggested a brain function disability of his bilateral temporal and frontal lobes [8].
Radiological examinations
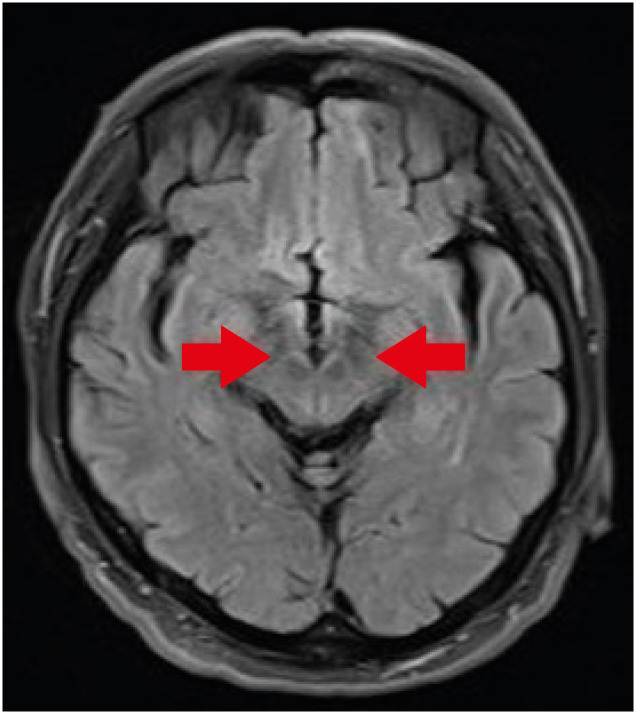
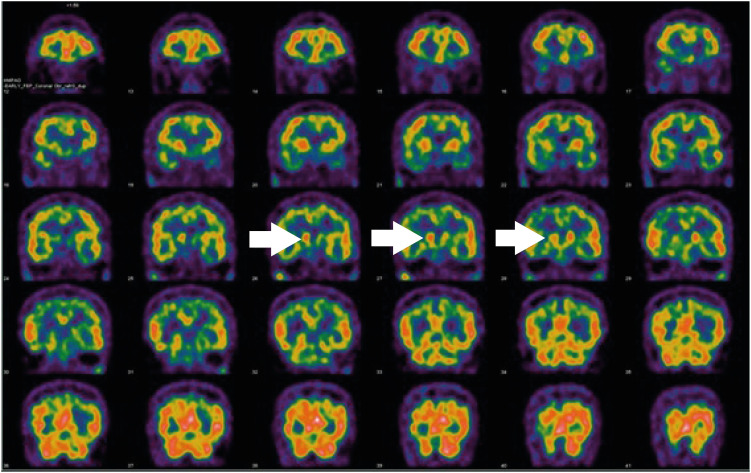
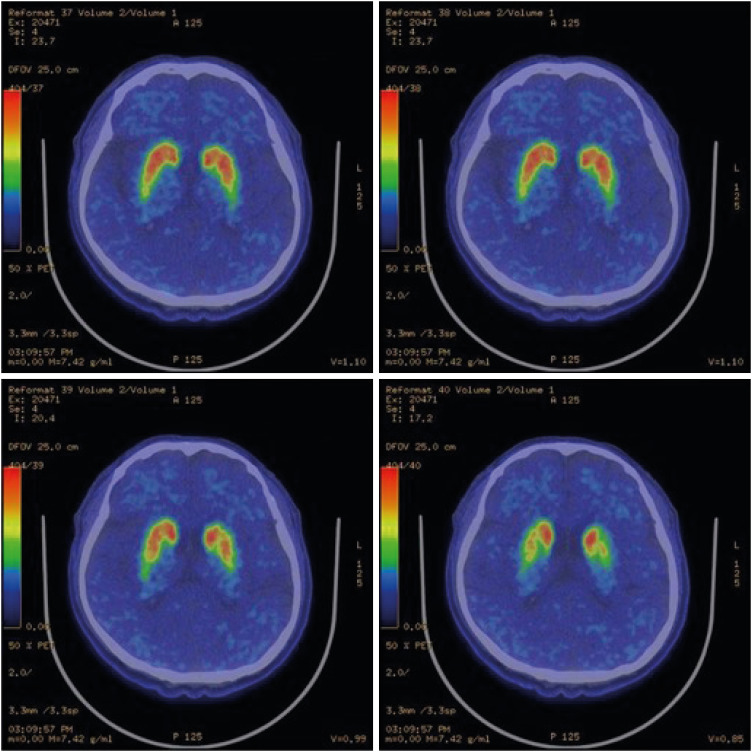
Special radiological examinations can be used to diagnose PD. In the present case, the patient underwent magnetic resonance imaging (MRI), positron emission tomography (PET)-computed tomography, and single-photon emission computed tomography (SPECT) upon his first clinical manifestation in 2014. Transverse T2 MRI images of the patient showed no swallow-tail signs indicating degeneration of the reticular parts of the substantia nigra and red nuclei (Fig. 1) [9]. In addition, SPECT and PET images showed asymmetrical oval-shaped, rather than comma-shaped, dopamine uptake (Figs. 2 and 3). These radiological characteristics are common signs of PD, so it was assumed that the disease course of the patient was typical [10,11].
Fig. 1. Patient transverse T2 magnetic resonance imaging results. The swallow-tail sign (red arrow) was absent.
Fig. 2. Patient single-photon emission computed tomography images. There was asymmetrical uptake in the caudate nucleus (white arrows).
Fig. 3. Patient positron emission tomography-computed tomography images. The comma-shape deteriorated to an oval-shape.
Past medical history
Based on his records from health screenings up until 2014, the patient had no other risk factors that might be associated with PD. His history noted hypertension, and chronic obstructive pulmonary disease was diagnosed in 2016, after the onset of PD. He had no history of head injuries.
Social history & family history
The patient was a 25 pack per year ex-smoker and a social drinker who consumed one bottle of Soju per month. He denied drinking coffee and he performed some regular exercise. There was no family history of neurodegenerative diseases including PD.
Occupational history
Basically, the occupational history was written by self-reporting. It obtained additional history through his health insurance and income tax data (Table 1). He had been a coal miner for 16 years before becoming a pesticide worker at a tomato greenhouse for 12 years and 5 months. His work duties included mixing various pesticides and spraying pesticides on tomato plants.
Table 1. Patient occupational history.
| Period | Occupational history | Evidence |
|---|---|---|
| 1972–1980 | Coal miner | Self-reported |
| 1981–1983 | Unclear | - |
| 1985–1998 | Coal miner | Health insurance |
| 2001–2013 | Pesticide worker | Income tax data |
Data were collected from the patient with consent.
Work-relatedness evaluation
How to evaluate work-relatedness
Many case reports in the discipline of occupational medicine have focused on assuming the level of exposure without previous epidemiological evidence, and hypothetical exposure models have been more critical than causal relationships between exposure and the disease itself for determining work-relatedness [12,13,14,15]. If exposure could not be estimated or assumed, however, epidemiological evidence was more significant. For example, a retrospective cohort study played an essential role in evaluating work-relatedness between cancers and working in the semiconductor fabrication industry [16]. There have been previous suggestions by occupational physicians in South Korea to use the causal inference theory [17,18,19]. In this study, a process for evaluating work-relatedness was suggested with a concrete example in which the workplace was within traditional agriculture, exposure assessment was limited, and previous epidemiological evidence was relatively plentiful.
Occupational history
Before evaluating the relationship between PD and exposure to pesticides, it should first be confirmed whether other potential risk factors affected this patient's disease. First, the possibility of another occupational history that could be related to PD other than exposure to pesticides should be identified. The patient had worked as a coal miner, so this occupational history should be reviewed.
As a result of our investigation of epidemiological literature, there was no evidence of a causal relationship between coal mining and PD. For example, a multi-center case-control study in South Korea showed that the odds ratio (OR) of laborers in mining and construction was 1.36 (95% CI: 0.67–2.75), and the OR of farmers was 1.64 (95% CI: 0.96–2.81) [20].
The reason that coal mining was suspected as a cause of PD is that miners are exposed to manganese, and manganese toxicity occurs with PD. There have been several case reports of PD and parkinsonism in welders who have been exposed to manganese in South Korea, and their mean age of onset was their late 40's, earlier than that of most PD cases, which occur in a person's late 60's [21,22]. In addition, a systematic review of manganese and PD confirmed a T1-weighted MRI at the level of globus pallidus, which was not found in the radiological examinations of this case [23]. However, occupational exposure to manganese, such as welding, does not have any significant epidemiological evidence as a risk factor for PD and even manganese-induced parkinsonism, nor share any biological plausibility and mechanism of PD [4,24,25].
The clinical features of the patient did not match with the few case reports related to exposure to manganese, and there was no epidemiological evidence between coal mining and PD. In conclusion, it was reasonable to rule out the possibility that his coal mining experience contributed to the onset of PD in his case.
Personal risk factors
Furthermore, other personal risk factors, such as age, sex, medical history, and genetic factors might have contributed to the patient's risk. For example, aging is the most significant cause of the onset and aggravation of PD, and many genetic factors affect PD [3,11]. Nevertheless, such personal risk factors should not devalue the work-relatedness both for medical and legal reasons (Detailed descriptions and suggestions are noted below in the Discussion and Conclusion). Exposure to pesticides was determined to be the only risk factor that should be taken into consideration rather than any other potential risk factors including additional occupational history and personal risk factors.
Work environment assessment
Investigation of a work environment should include physical and chemical assessment, the specific details of the work, and the labor conditions of the workers, such as the form of employment and the working time. Unfortunately, it was hard to directly assess the physical and chemical environment of the workplace because it had since closed. Problems with pesticides are very sensitive topics for farmers who sell fruit as a commodity, so it was also impossible to find a similar greenhouse to estimate pesticide levels. Previous studies were referred to in order to assume the workplace environment.
In general, the inside temperature of the tomato greenhouse was about 21ºC–24ºC, which is the sub-optimal condition for tomato cultivation [26]. A review of statements by the patient and his previous employer revealed that the only protections provided were raincoats and cotton masks. The patient stated that the temperature in the greenhouse was too high to wear this protective equipment. Therefore, it was reasonable to assume that the patient rarely used personal protective equipment during his work. Moreover, the tomatoes were cultivated in a stair-shaped structure, so the workers sprayed pesticides above other workers' heads, worsening their exposure.
Tomato cultivation was carried out in the following steps: sowing, planting, harvesting, and loading. Sowing is the process of arranging seeds at regular intervals and planting young seedlings to help germination. Planting is the process of re-planting germinated seedlings that are about 70 days old. In each of these steps, pesticide workers regularly apply insecticides and fungicides [27]. The patient was primarily responsible for mixing and spraying pesticides in his workplace. Depending on the cultivation period, the patient continuously sprayed pesticides for about 7–10 days. There were 4 glass greenhouses in which the patient alternated, spraying pesticides in short intervals of 1–2 days.
The patient worked at the tomato greenhouse from June 11, 2001, to November 1, 2013, for 12 years and 5 months. Typically, he started work at 8:00 am and left at 5:00 pm, although he stayed until 7:00 pm during harvest periods. Thus, the patient was not typically exposed to long working hours and he did not work over 60 hours per a week, which is the specific time value associated with compensation for cardiovascular diseases in South Korea [28,29,30].
Possible exposure investigation: pesticide survey
The primary concern for work-relatedness in this case was exposure to pesticides. Direct assessment of the pesticides was impossible, so interviews with the employer, employee, pesticide experts, and pesticide dealers, as well as previous studies of pesticide-use in South Korea were collected. Records of the workplace and workers had been discarded because the workplace had since closed.
The employer claimed that the only pesticides used in their facilities were 2 fungicides (polyoxin B and iminoctadine tris [albesilate]) and 2 insecticides (spiromesifen and dinotefuran), although the patient was not sure which pesticides he had used. Spiromesifen and dinotefuran, the 2 neonicotinoid insecticides claimed by the employer, have limited epidemiological evidence for PD. Although the mechanism is unclear, some animal studies on dinotefuran in rats have demonstrated the destruction of nerve cells [31,32].
In addition, rotenone is one of the insecticides that is the most related to PD [33,34,35]. Interviews with pesticide experts and pesticide dealers were completed to identify the lists of pesticides used for tomato cultivation; it was determined that rotenone is currently used in tomato cultivation, although it was not shown in the previous pesticide survey.
The Rural Development Administration of South Korea examined pesticide surveys, and published their results in 2012 and 2016 [36,37]. They reviewed the types and amounts of pesticides used in agricultural industries. As a result, the 5 most used classes of pesticides in tomato cultivation changed from 2012 to 2016 [38]. Table 2 describes the neurotoxicity of the pesticides present in the Pesticides Properties Database provided by the Agriculture & Environment Research Unit of the University of Hertfordshire in England [39].
Table 2. Pesticides used in tomato greenhouses.
| Source | Name | Amounta | Class | Chemistry | Neurotoxicityc |
|---|---|---|---|---|---|
| Pesticide survey in 2008 | Triflumizole | 0.3 | Fungicide | Imidazole | X |
| Dimethomorphb | 0.2 | Fungicide | Morpholine | - | |
| Dithianonb | Fungicide | Quinone | X | ||
| Ethoprophos | 0.2 | Insecticide, Nematicide | Organo-phosphate | ? | |
| Sulfurb | 0.1 | Fungicide, acaricide | Element | X | |
| Thiophanate methylb | Fungicide | Benzimidazole | - | ||
| Cymoxanilb | 0.1 | Fungicide | Cyanoacetamide oxime | X | |
| Famoxadoneb | Fungicide | Oxazole | ? | ||
| Pesticide survey in 2012 | Chlorothalonil | 0.2 | Fungicide | Chloronitrile | X |
| Fludioxonil | 0.1 | Fungicide | Phenylpyrrole | X | |
| Cymoxanilb | 0.1 | Fungicide | Cyanoacetamide oxime | X | |
| Famoxadoneb | Fungicide | Oxazole | ? | ||
| Dinotefuran | 0.1 | Insecticide | Neo-nicotinoid | X | |
| Propamocarb hydrochloride | 0.1 | Fungicide | Carbamate | ? | |
| Pesticides employer claimed to use | Polyoxin B | - | Fungicide | Micro-organism derived | X |
| Iminoctadine tris (albesilate) | - | Fungicide | Guanidine | - | |
| Spiromesifen | - | Insecticide | Tetronic acid | X | |
| Dinotefuran | - | Insecticide | Neo-nicotinoid | X |
From the Rural Development Administration of South Korea's pesticide surveys [40,41].
-: insufficient data; X: no effect; ?: effect unclear.
aThe units are kg a.i./ha; bThe doses of the pesticides used together are presented in one column; cFrom the Pesticides Properties Database provided by the Agriculture & Environment Research Unit of the University of Hertfordshire in England [43].
As indicated by the collected information, the most used pesticides in tomato greenhouses were insecticides and fungicides, and the most frequently used insecticides and fungicides changed year to year. It was assumed that, in the real workplace, there were various kinds of pesticides used in addition to those claimed by the employer. When investigations on the workplace environment and pesticide surveys were examined together, it was plausible that the patient was significantly exposed to various fungicides and insecticides, including potentially neurotoxic substances, such as rotenone, over the course of 12 years.
Citing adequate epidemiological evidence
Although exact exposure assessment was impossible, it was reasonable to assume that the patient was exposed to various fungicides and insecticides over the course of 12 years, according to the information discussed above. After that, adequate epidemiological evidence needed to be cited in which the patient and work environment could be matched to what the epidemiological study had investigated.
Three meta-analyses (MAs) were reviewed to evaluate the work-relatedness of this case. The first MA of 19 studies conducted between 1989 and 1999, revealed an OR of 1.94 (95% CI: 1.49–2.53) indicating that all kinds of pesticide exposure were risk factors for PD [40].
The second MA of 39 patient-control studies, 4 cohort studies, and 3 cross-sectional studies reported a 1.62 (95% CI: 1.40–1.88) relative risk (RR) of PD for all types of pesticides [41]. The results of this study are shown in Table 3. In addition, the study provided results for the RR according to the type of pesticides: herbicide was 1.40 (95% CI: 1.08–1.81), insecticide was 1.50 (95% CI: 1.07–2.11), and fungicide was 0.99 (95% CI: 0.71–1.40). The analysis did not reveal statistical significance for fungicides, but herbicides and insecticides were significantly associated with PD. In addition, the RR was different according to the method of evaluating pesticide exposure. When pesticide exposure was self-reported, the RR was lower at 1.5 (95% CI: 1.30–1.80). However, this previous MA did not address the dose-response relationship.
Table 3. Results of a meta-analysis for exposure to pesticides and Parkinson's disease.
| Variables | Odds ratio (95% confidence interval) | |
|---|---|---|
| All pesticides | 1.62 (1.40–1.88) | |
| Classification | ||
| Herbicides | 1.40 (1.08–1.81) | |
| Insecticides | 1.50 (1.07–2.11) | |
| Fungicides | 0.99 (0.71–1.40) | |
| Exposure assessment | ||
| Self-report (experience) | 1.50 (1.26–1.78) | |
| Self-report (regular exposure) | 1.71 (1.30–2.25) | |
| Occupation | 2.50 (1.54–4.05) | |
| Region | ||
| North America | 1.44 (1.19–1.75) | |
| Europe | 1.76 (1.41–2.20) | |
| Others | 1.75 (1.12–2.75) | |
| Study type | ||
| Case-control studies | 1.67 (1.43–1.96) | |
| Cohort studies | 1.39 (0.92–2.10) | |
| Cross-sectional studies | 1.64 (0.37–7.29) | |
From Environ Health Perspect 2012;120(3):340-7 [41].
The third MA of observational studies published in September 2017 evaluated low-level and long-term pesticide exposure, and associated risk of PD [42]. This study examined the dose-response relationship for exposure. The OR for pesticide exposure by time-period was as follows: 1 year, 1.01 (95% CI: 1.00–1.02); 5 years, 1.05 (95% CI: 1.02–1.09); and 10 years, 1.11 (95% CI: 1.05–1.18). The risk increased by 5% and 11% at 5 and 10 years, respectively. In another analysis, 3 research papers were synthesized to investigate the relationship between the exposure period and occurrence by subdividing cumulative exposures by 15 days [43,44,45]. As a result, when the cumulative exposure period increased by 15 days, the OR increased to 1.04 (95% CI: 1.01–1.07), which was about 4% per 15-day exposure. Overall, statistical significance was achieved at 1.05 (95% CI: 1.03–1.07).
Probability of causation (PC)
The index of the contribution of a specific factor to a disease in an individual case is called the PC. In contrast, the formulation of the contribution of factors to a disease in exposed groups is called the attributable fraction (AF) and is calculated as the RR. A specific relationship is established between the PC of the individual with a particular disease and the AF, and this can be expressed as follows [46,47,48]:
In cases of a rare disease like PD, however, the OR is an alternative epidemiological value for the RR [49].
| RR ≈ OR where the prevalence is under 10% (the disease is rare) |
Therefore, it was suggested to reformulate the inequation of PC to evaluate this case as follows:
When the result of the first MA was applied, the PC was 48.5% (95% CI: 32.9%–60.5%). The patient was exposed to fungicides and insecticides, so the specific values of these pesticides were cited using the second MA, and the PC of exposure to only fungicides was −1.01% (95% CI: −40.8%–28.6%) and that of only insecticides was 33.3% (95% CI: 6.54%–52.6%). The third MA showed the dose-response relationship, and the PC using the third MA was 9.9% (95% CI: 4.76%–15.3%).
Summary
The worker was diagnosed with PD in 2014 when he was 68 years old. For approximately 12 years from 2001 to 2013, the worker primarily sprayed pesticides in a tomato greenhouse. There have been many epidemiological studies that have shown that pesticides are the most frequently studied occupational risk factors for PD, and have included MAs of all pesticide exposure.
There is a possibility that the pesticide's respiratory and skin exposure levels were high, considering the use of pesticides and that it was difficult to wear protective gear in the workplace conditions. The worker may have been exposed to rotenone, which are thought to cause PD. Therefore, we applied pesticide exposure situation, the results of epidemiologic studies, biological plausibility to evaluate work-relatedness, and there was a considerable scientific basis for the link between the worker's condition and his work environment.
Ethics statement
This study was approved by the Institutional Review Board of Hanyang University (HYU-2019-07-014), and written informed consent was obtained from the patient for publication of this case report and any accompanying data.
DISCUSSION AND CONCLUSION
Pesticides cause various kinds of health effects including chronic diseases, such as cancer and nervous system diseases [50]. As a result, agricultural workers have a higher mortality rate than other occupational groups, and their suicide rates are also higher by self-poisoning with pesticides [51]. The first report in this study disclosed that the relationship between exposure to pesticides and PD was that the active metabolite of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine affected the human nervous system [52]. This result provided an initial clue to the relationship between pesticides and PD. The levels of oxidative stress, mitochondrial dysfunction, α-synuclein fibrosis, and neuronal cell destruction observed in pesticide-damaged nervous systems were similar to those of PD patients. After several decades, from the biochemical level to the MA, evidence of the relationship between exposure to pesticides and PD has been well-established and abundant.
However, direct exposure assessments of pesticides often have limitations, so many studies have relied on self-report questionnaires. Some studies in South Korea have used mathematical models to estimate exposure levels, such as the job-exposure matrix. However, they were also based on questionnaires [53,54]. Based on the results of one of these studies, insecticides used in fruit cultivation in South Korea have been underestimated, so the official statistics of insecticide use could be 10 times less than the real amount of insecticides used [53]. In conclusion, it was reasonable to assume that the patient in this case was significantly exposed to pesticides.
One of the misunderstandings when using the causal inference theory is that it only suggests a final value, and there is no room for analysis of this value. For example, the causation must be accepted when the RR is over 2, because then the PC is over 50%. The PC is not a cut-off or absolute value. However, the PC should be interpreted in both medical and legal ways [18,46,55]. How to use and apply these PCs should be discussed.
Consequently, finding a legal interpretation is the last goal. This relates to the question of how to look at pesticide workers. The conclusions differ depending on whether the patient is considered to be a general pesticide worker or a specific type of insecticide worker, since epidemiological evidence varies depending on the type of pesticide. Judging by the data collected so far, the patient who worked in a tomato greenhouse could be regarded as an insecticide worker.
In the MA of 34 studies conducted in 2011, the RRs of shift work for myocardial infarction and ischemic stroke were 1.23 (95% CI: 1.15–1.31) and 1.05 (95% CI: 1.01–1.09) [56]. Epidemiological evidence did not have a high enough PC, however, considering other social contexts in South Korea, the presumption of disease was applied when exposure to long working hours was confirmed [18].
The Committee for the Evaluation of Epidemiological Surveys and the Committee for the Determination of Diseases have decided to accept the work-relatedness of this case for the following reasons: the relatively poor working conditions, the enclosed spaces of glass greenhouses, the provision of improper protective equipment and industrial hygiene education, the unfairness of the strict distinction between general pesticides and insecticides, the possible exposure to highly neurotoxic insecticides, such as rotenone, and the long-term exposure for over 10 years.
Previous studies have confirmed the causal relationship between exposure to pesticides and PD. The ORs related to this relationship were between 1.50 (95% CI: 1.07–2.11) (insecticides) and 1.94 (95% CI: 1.49–2.53) (all pesticides) based on the results of previous MAs. When direct measurement is impossible, an epidemiological model should be adopted to evaluate work-relatedness. In the present case, the lower limits of the PC were estimated to be 48.5% (95% CI: 32.9%–60.5%) for all pesticides and 33.3% (95% CI: 6.54%–52.6%) for insecticides. If we treated the patient as broadly exposed to pesticides, it would be more plausible his PD had the work-relatedness than only to insecticides. Considering the difficulty of the survey for the agriculture industry, it needs to set up a standard for treating pesticide workers and applying epidemiological evidence to them. The PC might be one of the evidence for establishing the standard for pesticide workers.
As a result, the pesticide survey from a field study and the explanations based on biological plausibility played an essential role in the acceptance of this work-relatedness case. This case was the first case recognized the work-relatedness between PD and pesticide exposure.
ACKNOWLEDGMENTS
This study was performed under the commission of the Occupational Safety & Health Research Institute.
Footnotes
Competing interests: The authors declare that they have no competing interests.
- Conceptualization: Kim Y, Kim I, Song J.
- Data curation: Kim Y.
- Investigation: Kim Y, Sung JM.
- Writing - original draft: Kim Y.
References
- 1.Dorsey ER, Sherer T, Okun MS, Bloem BR. The emerging evidence of the Parkinson pandemic. J Parkinsons Dis. 2018;8(s1):S3–S8. doi: 10.3233/JPD-181474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park JH, Kim DH, Kwon DY, Choi M, Kim S, Jung JH, et al. Trends in the incidence and prevalence of Parkinson's disease in Korea: a nationwide, population-based study. BMC Geriatr. 2019;19(1):320. doi: 10.1186/s12877-019-1332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ascherio A, Schwarzschild MA. The epidemiology of Parkinson's disease: risk factors and prevention. Lancet Neurol. 2016;15(12):1257–1272. doi: 10.1016/S1474-4422(16)30230-7. [DOI] [PubMed] [Google Scholar]
- 4.Nandipati S, Litvan I. Environmental exposures and Parkinson's disease. Int J Environ Res Public Health. 2016;13(9):881. doi: 10.3390/ijerph13090881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kab S, Spinosi J, Chaperon L, Dugravot A, Singh-Manoux A, Moisan F, et al. Agricultural activities and the incidence of Parkinson's disease in the general French population. Eur J Epidemiol. 2017;32(3):203–216. doi: 10.1007/s10654-017-0229-z. [DOI] [PubMed] [Google Scholar]
- 6.Brouwer M, Kromhout H, Vermeulen R, Duyzer J, Kramer H, Hazeu G, et al. Assessment of residential environmental exposure to pesticides from agricultural fields in the Netherlands. J Expo Sci Environ Epidemiol. 2018;28(2):173–181. doi: 10.1038/jes.2017.3. [DOI] [PubMed] [Google Scholar]
- 7.Vlaar T, Kab S, Schwaab Y, Fréry N, Elbaz A, Moisan F. Association of Parkinson's disease with industry sectors: a French nationwide incidence study. Eur J Epidemiol. 2018;33(11):1101–1111. doi: 10.1007/s10654-018-0399-3. [DOI] [PubMed] [Google Scholar]
- 8.Lee AY, Lee J, Oh E, Yoon SJ, Yoon B, Yu SD. Clinical utility of Seoul Neuropsychological Screening Battery-Core for dementia management project in the community. J Korean Neurol Assoc. 2019;37(3):277–283. [Google Scholar]
- 9.Schwarz ST, Afzal M, Morgan PS, Bajaj N, Gowland PA, Auer DP. The ‘swallow tail’ appearance of the healthy nigrosome - a new accurate test of Parkinson's disease: a case-control and retrospective cross-sectional MRI study at 3T. PLoS One. 2014;9(4):e93814. doi: 10.1371/journal.pone.0093814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarz ST, Rittman T, Gontu V, Morgan PS, Bajaj N, Auer DP. T1-weighted MRI shows stage-dependent substantia nigra signal loss in Parkinson's disease. Mov Disord. 2011;26(9):1633–1638. doi: 10.1002/mds.23722. [DOI] [PubMed] [Google Scholar]
- 11.Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- 12.Park S, Park J, Lee E, Eom H, Shin MY, Kim J, et al. Ovarian cancer in a former asbestos textile factory worker: a case report. Ann Occup Environ Med. 2018;30:65. doi: 10.1186/s40557-018-0277-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee N, Baek K, Park S, Hwang I, Chung I, Choi W, et al. Pneumoconiosis in a polytetrafluoroethylene (PTFE) spray worker: a case report with an occupational hygiene study. Ann Occup Environ Med. 2018;30:37. doi: 10.1186/s40557-018-0248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee S, Kim I, Park D, Song J, Lee SG. A case of acute organic solvent poisoning during epoxy coating. Ann Occup Environ Med. 2019;31:e9. doi: 10.35371/aoem.2019.31.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park S, Kim J. A case of extensor pollicis longus (EPL) tendon rupture in an automotive assembly line worker: an ergonomic evaluation through job strain index (JSI) and musculoskeletal risk factor survey. Ann Occup Environ Med. 2019;31:e2. doi: 10.35371/aoem.2019.31.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee HE, Kim EA, Park J, Kang SK. Cancer mortality and incidence in Korean semiconductor workers. Saf Health Work. 2011;2(2):135–147. doi: 10.5491/SHAW.2011.2.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bae S, Kim HC, Ye B, Choi WJ, Hong YS, Ha M. Causal inference in environmental epidemiology. Environ Health Toxicol. 2017;32:e2017015. doi: 10.5620/eht.e2017015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myong JP, Kim H, Lee K, Chang SH. Trends in the of epidemiological perspectives on the causality of occupational diseases. J Korean Med Assoc. 2018;61(8):466. [Google Scholar]
- 19.Choi BJ, Lee S, Lee IJ, Park SW, Lee S. Gastric and rectal cancers in workers exposed to asbestos: a case series. Ann Occup Environ Med. 2020;32:e4. doi: 10.35371/aoem.2020.32.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park J, Yoo CI, Sim CS, Kim HK, Kim JW, Jeon BS, et al. Occupations and Parkinson's disease: a multi-center case-control study in South Korea. Neurotoxicology. 2005;26(1):99–105. doi: 10.1016/j.neuro.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Yim HW, Kim JH, Phee YG, Koo JW, Lee KS, Park CY, et al. An association between brain MRI and neurologic findings in welders exposed to manganese fume. Korean J Occup Environ Med. 1998;10(2):161–171. [Google Scholar]
- 22.Racette BA, McGee-Minnich L, Moerlein SM, Mink JW, Videen TO, Perlmutter JS. Welding-related parkinsonism: clinical features, treatment, and pathophysiology. Neurology. 2001;56(1):8–13. doi: 10.1212/wnl.56.1.8. [DOI] [PubMed] [Google Scholar]
- 23.Guilarte TR. Manganese and Parkinson's disease: a critical review and new findings. Environ Health Perspect. 2010;118(8):1071–1080. doi: 10.1289/ehp.0901748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mortimer JA, Borenstein AR, Nelson LM. Associations of welding and manganese exposure with Parkinson disease: review and meta-analysis. Neurology. 2012;79(11):1174–1180. doi: 10.1212/WNL.0b013e3182698ced. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jankovic J. Searching for a relationship between manganese and welding and Parkinson's disease. Neurology. 2005;64(12):2021–2028. doi: 10.1212/01.WNL.0000166916.40902.63. [DOI] [PubMed] [Google Scholar]
- 26.Van Ploeg D, Heuvelink E. Influence of sub-optimal temperature on tomato growth and yield: a review. J Hortic Sci Biotechnol. 2005;80(6):652–659. [Google Scholar]
- 27.Jones JB., Jr . Tomato Plant Culture: In the Field, Greenhouse, and Home Garden. Boca Raton, FL: CRC press; 2007. [Google Scholar]
- 28.Park J, Kim Y, Han B. Long working hours in Korea: based on the 2014 Korean Working Conditions Survey. Saf Health Work. 2017;8(4):343–346. doi: 10.1016/j.shaw.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang MY, Cho SH, Yoo MS, Kim T, Hong YC. Long working hours may increase risk of coronary heart disease. Am J Ind Med. 2014;57(11):1227–1234. doi: 10.1002/ajim.22367. [DOI] [PubMed] [Google Scholar]
- 30.Jeong I, Rhie J, Kim I, Ryu I, Jung PK, Park YS, et al. Working hours and cardiovascular disease in Korean workers: a case-control study. J Occup Health. 2014;55(5):385–391. doi: 10.1539/joh.12-0245-oa. [DOI] [PubMed] [Google Scholar]
- 31.Tomizawa M, Casida JE. Neonicotinoid insecticide toxicology: mechanisms of selective action. Annu Rev Pharmacol Toxicol. 2005;45(1):247–268. doi: 10.1146/annurev.pharmtox.45.120403.095930. [DOI] [PubMed] [Google Scholar]
- 32.Rodrigues KJ, Santana MB, Do Nascimento JL, Picanço-Diniz DL, Maués LA, Santos SN, et al. Behavioral and biochemical effects of neonicotinoid thiamethoxam on the cholinergic system in rats. Ecotoxicol Environ Saf. 2010;73(1):101–107. doi: 10.1016/j.ecoenv.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 33.Nisticò R, Mehdawy B, Piccirilli S, Mercuri N. Paraquat- and rotenone-induced models of Parkinson's disease. Int J Immunopathol Pharmacol. 2011;24(2):313–322. doi: 10.1177/039463201102400205. [DOI] [PubMed] [Google Scholar]
- 34.Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, et al. Rotenone, paraquat, and Parkinson's disease. Environ Health Perspect. 2011;119(6):866–872. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson ME, Bobrovskaya L. An update on the rotenone models of Parkinson's disease: their ability to reproduce the features of clinical disease and model gene-environment interactions. Neurotoxicology. 2015;46:101–116. doi: 10.1016/j.neuro.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 36.Ha HY, Park SE, You AS, Gil GH, Park JE, Lee IY, et al. Survey of pesticide use in leaf and fruit vegetables, fruits, and rice cultivation areas in Korea. Weed Turfgrass Sci. 2016;5(4):203–212. [Google Scholar]
- 37.Ha HY, Ra DS, Shin WC, Im GJ, Park JE. Survey of pesticide use in fruit vegetables, fruits, and rice cultivation areas in Korea. Korean J Pestic Sci. 2012;16(4):395–400. [Google Scholar]
- 38.Jankowska M, Kaczyński P, Łozowicka B. Metabolic profile and behavior of clethodim and spirotetramat in herbs during plant growth and processing under controlled conditions. Sci Rep. 2020;10(1):1323. doi: 10.1038/s41598-020-58130-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agriculture & Environment Research Unit (AERU), University of Hertfordshire. Pesticides Properties Database. [Updated 2020]. [Accessed March 10, 2020]. http://sitem.herts.ac.uk/aeru/ppdb/en/index.htm.
- 40.Priyadarshi A, Khuder SA, Schaub EA, Shrivastava S. A meta-analysis of Parkinson's disease and exposure to pesticides. Neurotoxicology. 2000;21(4):435–440. [PubMed] [Google Scholar]
- 41.van der Mark M, Brouwer M, Kromhout H, Nijssen P, Huss A, Vermeulen R. Is pesticide use related to Parkinson disease? Some clues to heterogeneity in study results. Environ Health Perspect. 2012;120(3):340–347. doi: 10.1289/ehp.1103881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan D, Zhang Y, Liu L, Shi N, Yan H. Pesticide exposure and risk of Parkinson's disease: Dose-response meta-analysis of observational studies. Regul Toxicol Pharmacol. 2018;96:57–63. doi: 10.1016/j.yrtph.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 43.Kamel F, Tanner C, Umbach D, Hoppin J, Alavanja M, Blair A, et al. Pesticide exposure and self-reported Parkinson's disease in the agricultural health study. Am J Epidemiol. 2007;165(4):364–374. doi: 10.1093/aje/kwk024. [DOI] [PubMed] [Google Scholar]
- 44.Hancock DB, Martin ER, Mayhew GM, Stajich JM, Jewett R, Stacy MA, et al. Pesticide exposure and risk of Parkinson's disease: a family-based case-control study. BMC Neurol. 2008;8:6. doi: 10.1186/1471-2377-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Mark M, Vermeulen R, Nijssen PC, Mulleners WM, Sas AM, van Laar T, et al. Occupational exposure to pesticides and endotoxin and Parkinson disease in the Netherlands. Occup Environ Med. 2014;71(11):757–764. doi: 10.1136/oemed-2014-102170. [DOI] [PubMed] [Google Scholar]
- 46.Broadbent A. Epidemiological evidence in proof of specific causation. Leg Theory. 2011;17(4):237–278. [Google Scholar]
- 47.Greenland S. Underestimating effects: Why causation probabilities need to be replaced in regulation, policy, and the law. Bull At Sci. 2015;68(3):76–83. [Google Scholar]
- 48.Greenland S, Robins JM. Epidemiology, justice, and the probability of causation. Jurimetrics. 1999;40:321–340. [Google Scholar]
- 49.Norman GR, Streiner DL. Biostatistics: The Bare Essentials. New Haven, CT: PMPH USA; 2008. [Google Scholar]
- 50.Mostafalou S, Abdollahi M. Pesticides: an update of human exposure and toxicity. Arch Toxicol. 2017;91(2):549–599. doi: 10.1007/s00204-016-1849-x. [DOI] [PubMed] [Google Scholar]
- 51.Kim Y, Min J, Lee SJ. Suicide overall and suicide by pesticide rates among South Korean workers: a 15-year population-based study. Int J Environ Res Public Health. 2019;16(23):4866. doi: 10.3390/ijerph16234866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219(4587):979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 53.Choi JH, Kim KY. Fundamental research for establishing job-exposure matrix (JEM) of farmer related to insecticide of pesticide (III): fruit. J Korean Soc Occup Environ Hyg. 2016;26(3):317–323. [Google Scholar]
- 54.Kim SK, Park S, Chang SJ, Kim SK, Song JS, Kim HR, et al. Pesticides as a risk factor for metabolic syndrome: population-based longitudinal study in Korea. Mol Cell Toxicol. 2019;15(4):431–441. [Google Scholar]
- 55.Broadbent A. Epidemiological evidence in law: a comment on Supreme Court Decision 2011Da22092, South Korea. Epidemiol Health. 2015;37:e2015025. doi: 10.4178/epih/e2015025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vyas MV, Garg AX, Iansavichus AV, Costella J, Donner A, Laugsand LE, et al. Shift work and vascular events: systematic review and meta-analysis. BMJ. 2012;345:e4800. doi: 10.1136/bmj.e4800. [DOI] [PMC free article] [PubMed] [Google Scholar]