Abstract
Background
Currently, there is no consensus on the role of postoperative adjuvant radiotherapy (PORT) for resected stage IIIA/N2 non‐small cell lung cancer (NSCLC). Our study sought to determine which patients may be able to benefit from PORT, based on a patient prognostic score.
Methods
A retrospective cohort study was conducted to identify patients diagnosed with IIIA/N2 NSCLC between 1988 and 2016 in the SEER database. Eligible patients were divided into the following two groups: PORT group and non‐PORT group. We classified patient prognostic scores as an ordinal factor and stratified patients based on prognostic scores. A Cox proportional hazards model with propensity score weighting was performed to evaluate cancer‐specific mortality (CSM) between the two groups.
Results
We identified 7060 eligible patients with IIIA/N2 NSCLC, 2833 (40.1%) in the PORT group and 4227 (59.9%) in the non‐PORT group. Overall, the 10‐year CSM rate in the weighted cohorts was 70.4% in the PORT group, 72.0% in the non‐PORT group, and patients who received PORT had a lower CSM rate (p = 0.001). Compared with the non‐PORT group, significant survival improvements in the PORT group were observed in patients with higher age, grade, T stage and lymph node ratio (LNR), and without chemotherapy. The improved survival of patients receiving PORT was significantly correlated with patient prognostic scores (p < 0.001).
Conclusions
In our population‐based study, the prognostic score was associated with the survival improvement offered by PORT in IIIA/N2 NSCLC, suggesting that prognostic scores and clinicopathological characteristics may be helpful in proper candidate selection for PORT.
Keywords: cancer‐specific mortality, non‐small cell lung cancer, patient prognostic scores, postoperative radiation, stage IIIA/N2
The role of postoperative radiotherapy for IIIA/N2 non‐small cell lung cancer is unclear. Treatments for IIIA/N2 non‐small cell lung cancer should be individualized. Patients who received postoperative radiotherapy had a lower CSM rate. The improved survival of patients receiving PORT was correlated with patient prognostic scores. Prognostic scores and clinical characteristics may be helpful in candidate selection for PORT.

INTRODUCTION
Lung cancer is currently the most common cancer and remains the leading cause of cancer‐related mortality worldwide. 1 About 85% of patients with lung cancer are diagnosed with non‐small cell lung cancer (NSCLC), and approximately one third of these cases have been diagnosed with locally advanced disease (stage IIIA/IIIB). 2 Locally advanced NSCLC, specifically N2 disease, has a higher risk of local relapse and distant metastasis after treatment and a poorer prognosis. 3 , 4 Surgery‐based multimodal therapies are considered as the optimal therapeutic strategy for patients with locally advanced disease. 4 , 5 However, there is currently no consensus on the role of postoperative adjuvant radiotherapy (PORT) for locally advanced NSCLC, as the benefit on survival remains uncertain. 6 , 7 , 8 , 9 , 10 A survival benefit from PORT in patients with stage N2 has been previously reported by some population‐based studies and a subgroup analysis from the Adjuvant Navelbine International Trialist Association (ANITA) trial where patients were treated with PORT in a nonrandomized fashion. 6 , 7 , 8 However, a Phase III study which included 37 patients showed that there was no survival difference between the observation and RT arms. 9 Additionally, a meta‐analysis based on nine clinical trials demonstrated an adverse effect of PORT on the two‐year survival rate with a 7% lower survival rate of the whole patient group (48% vs. 55%), but the subgroup of patients with stage N2 showed a trend towards a better outcome in terms of survival. 10
In addition to the differences revealed by these clinical studies, some clinical guidelines also hold different views on the role of PORT in multidisciplinary therapy. 11 , 12 , 13 PORT was recommended as a treatment option for N2 patients in the National Comprehensive Cancer Network (NCCN) guidelines. 11 On the contrary, the American Society for Radiation Oncology and American Society of Clinical Oncology (ASCO) guidelines did not routinely recommend PORT for patients with stage IIIa/N2, as it was not considered to improve survival. 12 , 13 However, patients with stage IIIA/N2 NSCLC are a heterogeneous population with different clinicopathological characteristics. Some clinicopathological factors have been previously reported to be associated with significant variability of survival, such as age, sex, grade, T stage, chemotherapy and positive/resected lymph node ratio (LNR). 14 , 15 , 16 , 17 , 18 This indicates that treatments for IIIA/N2 NSCLC should be individualized. Currently, some studies have attempted to predict the outcomes of patients receiving PORT based on different clinicopathological features and prognostic scoring models have been developed to help predict the survival of patients receiving PORT, including the patient prognostic score reported by Deng et al. 14 , 15 , 16 Now, it is necessary to select patients who can benefit most from PORT. Therefore, our study sought to assess the survival benefit from PORT in a population‐based database and determine which, if any, subpopulations of patients with resected IIIA/N2 NSCLC might benefit from PORT based on a reported prognostic score model.
METHODS
Patient selection
After receiving the exemption from informed consent and institutional review, a retrospective cohort study was performed by using data obtained from the Surveillance, Epidemiology, and End Results (SEER) database of the National Cancer Institute (National Cancer Institute, version 8.3.5; www.seer.cancer.gov), which was routinely collected from multiple population‐based cancer registries. Patients with IIIA/N2 NSCLC were included in this study, according to the inclusion criteria: (i) Patients with stage IIIA/N2 lung cancer who received surgery between 1988 and 2016; (ii) NSCLC that was pathologically proven; (iii) NSCLC that was the only cancer or the first primary cancer; and (iv) complete informational records (such as race, grade, T staging, number of lymph nodes removed or positive lymph nodes, and radiation status/methods). Exclusion criteria were: (i) Not diagnosed by histology; (ii) not first primary cancer; (iii) small cell cancer or non‐small cell lung cancer was unable to be determined; and (iv) unknown informational records regarding race, grade, T staging and number of positive lymph nodes. Based on these criteria, a total of 7060 patients were enrolled in the final cohort (Figure S1). We divided all the patients into two groups: the PORT group and the non‐PORT group. The PORT group was defined as patients receiving surgery and postoperative radiotherapy and the non‐PORT group was defined as patients who received surgical resection without postoperative radiotherapy.
Variables and outcome
Data on age, sex, race, grade, pathological type, T stage, number of lymph nodes removed or positive lymph nodes, LNR, the cause of patient death, survival month, surgery, radiotherapy and chemotherapy were collected in this study. All variables were categorized as shown in Table 1. We restaged all the patients as being stage IIIA/N2 according to the seventh edition of the American Joint Committee on Cancer (AJCC) staging. 19 , 20 LNR was defined as the ratio of the number of positive lymph nodes to the number of lymph nodes removed. The number of positive lymph nodes was calculated according to the code manual of regional nodes positive or CS lymph nodes, and the number of lymph nodes removed was calculated according to the record of regional nodes examined and scope of lymph node surgery. To assess the benefit of PORT on the basis of different clinicopathological features, we used a patient prognostic score, as reported by Deng et al14, and classified the prognostic score as an ordinal factor, as shown in Figure 1.
TABLE 1.
Baseline characteristics of patients with resected IIIA/N2 non‐small cell lung cancer
| Variables | PORT group (n = 2833) | Non‐PORT group (n = 4227) | p‐value |
|---|---|---|---|
| Age, years | <0.001 | ||
| <65 | 1440 (50.8%) | 1744 (41.3%) | |
| ≥65 | 1393 (49.2%) | 2483 (58.7%) | |
| Sex | 0.610 | ||
| Male | 1395 (49.2%) | 2055 (48.6%) | |
| Female | 1438 (50.8%) | 2172 (51.4%) | |
| Race | 0.095 | ||
| White | 2310 (81.5%) | 3470 (82.1%) | |
| Black | 260 (9.2%) | 421 (10.0%) | |
| Other | 263 (9.3%) | 336 (7.9%) | |
| Grade | 0.789 | ||
| I–II | 1352 (47.7%) | 2031 (48.0%) | |
| III–IV | 1481 (52.3%) | 2196 (52.0%) | |
| Histological type | 0.008 | ||
| SCC | 660 (23.3%) | 1109 (26.2%) | |
| Adenocarcinoma | 1835 (64.8%) | 2590 (61.3%) | |
| Other | 338 (11.9%) | 528 (12.5%) | |
| T stage | 0.602 | ||
| T1–2 | 2422 (85.5%) | 3633 (85.9%) | |
| T3 | 411 (14.5%) | 594 (14.1%) | |
| Chemotherapy | <0.001 | ||
| Yes | 2296 (81.0%) | 2412 (57.1%) | |
| No | 537 (19.0%) | 1815 (42.9%) | |
| No. of LNs resected | 0.216 | ||
| <18 | 2277 (80.4%) | 3346 (79.2%) | |
| ≥18 | 556 (19.6%) | 881 (20.8%) | |
| No. of positive LNs | <0.001 | ||
| <3 | 1400 (49.4%) | 2275 (53.8%) | |
| ≥3 | 1433 (50.6%) | 1952 (46.2%) | |
| LNR | <0.001 | ||
| <0.31 | 1271 (44.9%) | 2292 (54.2%) | |
| ≥0.31 | 1562 (55.1%) | 1935 (45.8%) |
Abbreviations: LNR, lymph node ratio; LNs, lymph nodes; PORT, postoperative radiation; SCC, squamous cell carcinoma.
FIGURE 1.
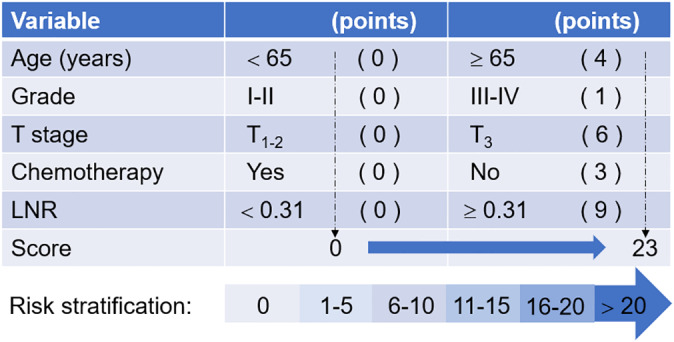
Patient prognostic scores: risk stratification. Modified from Deng et al. 14
In this study, the primary outcome of interest was the cancer‐specific mortality (CSM) of lung cancer. The SEER database defined mortality data according to the International Classification of Diseases Revisions,8–10 and the SEER cause of death was categorized as cancer‐specific death, other‐cancer death, death due to heart disease, or noncancer cause of death. The time of overall mortality (OM) and CSM was calculated as the time from the date of diagnosis to the date of interesting death, the time of last follow‐up, or November 31, 2018. The date of interesting death, for OM, is the date of death, and for CSM, is the date of death because of lung cancer.
Statistical analysis
In this study, we used the same statistical analytical methods as reported in previous studies. 21 , 22 For descriptive variables, the absolute number and percentage were used to describe categorical variables. Chi‐square or Fisher's exact tests were conducted to compare the baseline characteristics of patients. The inverse probability propensity score weighting was then performed to balance patient characteristics between the PORT and non‐PORT groups. 21 , 23 To calculate propensity scores, a logistic regression model was applied based on baseline characteristics of patient, such as age, sex, race, grade, histological type, T stage, chemotherapy and LNR.
The Kaplan–Meier plots and log‐rank test were conducted for survival analyses. After weighted by propensity score, OM and CSM were compared between the two groups by using propensity score‐weighted log‐rank tests and Cox proportional hazards models, and adjusted hazard ratios (HR) were generated from multivariable Cox proportional hazards models. Subsequently, we performed the interaction tests to explore whether any survival benefit conferred by PORT varied across subgroups. Treatment effect modification of PORT was assessed using categorical factors and interaction tests in bivariable weighted Cox models. All statistical analyses were performed with R statistical software for Windows (version 3.6.0, https://cran.R-project.org). Statistical significance was indicated by p < 0.05.
RESULTS
Patient characteristics
We identified 7060 eligible patients with IIIA/N2 NSCLC based on inclusion and exclusion criteria (Figure S1). Of the initial cohort, 2833 patients (40.1%) were enrolled into the PORT group and 4227 patients (59.9%) were enrolled into the non‐PORT group. Patient clinicopathological characteristics are shown in Table 1. Clinicopathological factors were comparable between two groups, except that there were more younger patients, adenocarcinoma, patients receiving chemotherapy, patients with three or more positive lymph nodes, and patients with LNR ≥0.31 in the PORT group (p < 0.05). The inverse probability propensity‐score weighting was then performed and the balance in patient characteristics between two groups was achieved for estimating the treatment effect, as shown in Table 2.
TABLE 2.
Clinical characteristics of patients weighted by propensity score
| Variables | PORT group (n = 2833) | Non‐PORT group (n = 4227) | p‐value |
|---|---|---|---|
| Age, years | 0.609 | ||
| <65 | 1303 (46.0%) | 1919 (45.4%) | |
| ≥65 | 1530 (54.0%) | 2308 (54.6%) | |
| Sex | 0.903 | ||
| Male | 1391 (49.1%) | 2068 (48.9%) | |
| Female | 1442 (50.9%) | 2159 (51.1%) | |
| Race | 0.274 | ||
| White | 2311 (81.6%) | 3472 (82.1%) | |
| Black | 262 (9.3%) | 412 (9.7%) | |
| Other | 260 (9.1%) | 343 (8.1%) | |
| Grade | 0.734 | ||
| I–II | 1339 (47.2%) | 2016 (47.7%) | |
| III–IV | 1494 (52.8%) | 2211 (52.3%) | |
| Histological type | 0.461 | ||
| SCC | 701 (24.8%) | 1080 (25.5%) | |
| Adenocarcinoma | 1801 (63.6%) | 2626 (62.1%) | |
| Other | 331 (11.7%) | 521 (12.3%) | |
| T stage | 0.945 | ||
| T1–2 | 2429 (85.8%) | 3622 (85.7%) | |
| T3 | 404 (14.2%) | 605 (14.3%) | |
| Chemotherapy | 0.959 | ||
| Yes | 1893 (66.8%) | 2820 (66.7%) | |
| No | 940 (33.2%) | 1407 (33.3%) | |
| No. of LNs resected | 0.239 | ||
| <18 | 2279 (80.5%) | 3353 (79.3%) | |
| ≥18 | 554 (19.5%) | 874 (20.7%) | |
| No. of positive LNs | 0.422 | ||
| <3 | 1499 (52.9%) | 2195 (51.9%) | |
| ≥3 | 1334 (47.1%) | 2032 (48.1%) | |
| LNR | 0.961 | ||
| <0.31 | 1431 (50.5%) | 2133 (50.4%) | |
| ≥0.31 | 1402 (49.5%) | 2094 (49.6%) |
Abbreviations: LNR, lymph node ratio; LNs, lymph nodes; PORT, postoperative radiation; SCC, squamous cell carcinoma.
Survival benefit of PORT
We further studied the impact of PORT on the prognosis of patients with IIIA/N2 NSCLC. The 10‐year CSM rate was 70.4% in the PORT group, 72.1% in the non‐PORT group, and patients who received PORT had a lower CSM than those who did not (p = 0.001, Figure S2A). Patients who received PORT had a lower 10‐year OM rate than those who did not (79.5% vs. 81.2%, p < 0.001, Figure S2B). After adjustments based on propensity score, the balance in patient characteristics between two groups was achieved. The 10‐year CSM rate weighted by inverse propensity‐score was 70.4% in the PORT group and 72.0% in the non‐PORT group, and patients who received PORT had a lower CSM (p = 0.001, Figure 2(a)). Patients in the PORT group had a lower 10‐year OM rate than those in the non‐PORT group (80.2% vs. 80.7%, p < 0.001, Figure 2(b)).
FIGURE 2.
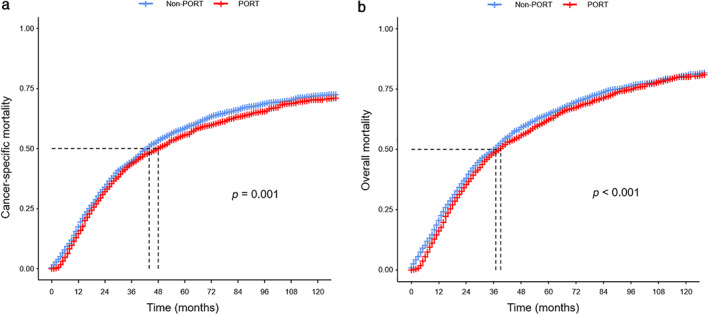
Comparison of (a) cancer‐specific mortality and (b) overall mortality weighted by propensity score in patients with resected IIIA/N2 NSCLC
Survival benefit of PORT according to prognostic score
When examining the benefit of PORT stratified by prognostic factors used in the patient prognostic score, we found that the survival observed in the PORT group was significantly better than that in the non‐RT group for patients with higher age, grade, T stage and LNR, and without chemotherapy (Tables S1 and S2). Moreover, the magnitude of improved survival of patients receiving PORT was significantly associated with the patient prognostic scores (interaction tests: p < 0.001, Figures 3 and 4). Patients with lower prognostic scores presented no significant difference in CSM and OM between two groups (score 0: CSM, 56.3% vs. 62.6%; p = 0.524; OM, 66.0% vs. 70.3%; p = 0.638; score 1–5: CSM, 60.3% vs. 59.5%; p = 0.103; OM, 70.4% vs. 70.3%; p = 0.145; score 6–10: CSM, 73.3% vs. 73.3%; p = 0.574; OM, 81.6% vs. 81.7%; p = 0.136, respectively), but among patients with higher prognostic scores, the PORT brought a significant reduction in CSM and OM (score 11–15: CSM, 72.5% vs. 77.6%; p = 0.001; OM, 83.5% vs. 85.8%; p = 0.004; score 16–20: CSM, 85.6% vs. 87.1%; p = 0.047; OM, 93.5% vs. 92.7%; p = 0.048, respectively, Figures 3 and 4). Before inverse probability propensity‐score weighting was performed, the Cox proportional hazards analyses and interaction tests yielded consistent results (Figures S3 and S4).
FIGURE 3.
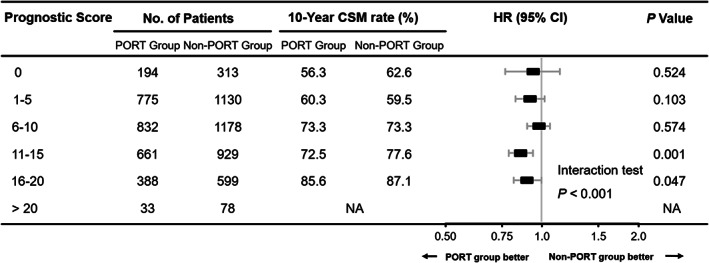
Comparison of cancer‐specific mortality weighted by inverse propensity score between the PORT group and the non‐PORT group based on prognostic scores
FIGURE 4.
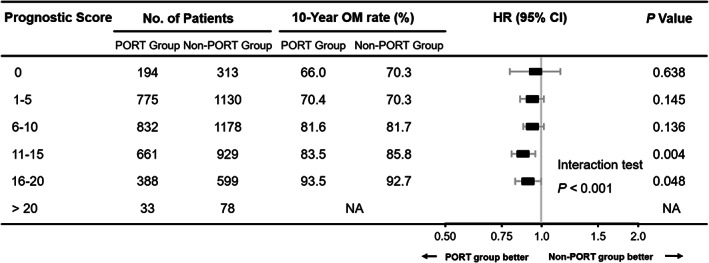
Comparison of overall mortality weighted by inverse propensity score between the PORT group and the non‐PORT group based on prognostic scores
DISCUSSION
In this large population‐based cohort study, the results of survival analyses showed that the long‐term survival of the entire cohort of IIIA/N2 NSCLC patients who received PORT was significantly better than that of the entire cohort of patients who did not, which was consistent with previous reports. 6 , 7 , 8 Furthermore, we observed a heterogeneous treatment effect of PORT that might be most important, when some potential risk factors (such as higher age, nuclear grade, T stage and LNR, and without chemotherapy) were present. These clinicopathological factors had been used together to establish a patient prognostic‐scoring system for IIIA/N2 NSCLC patients who received PORT. 14 Our findings suggested that among patients with lower prognostic scores, there was no statistical difference in cancer‐specific survival between the PORT group and the non‐RT group, whereas patients with higher prognostic scores treated with PORT demonstrated a significantly statistical difference in the survival, compared with those in whom PORT was not used. Therefore, taken together with previous findings, 15 , 16 , 17 , 18 these results indicated that treatments for IIIA/N2 NSCLC should be individualized. Further studies on the basis of patient prognostic‐score to determine which patients with resected IIIA/N2 NSCLC may be able to benefit most from PORT are required.
Patients with stage IIIA NSCLC, specifically N2 disease, had a higher risk of locoregional recurrence, even after complete resection, the locoregional failure rate has been reported to reach as high as 20%–40%, and locoregional recurrence was correlated independently with worse survival of patients. 24 , 25 , 26 , 27 Currently, surgery combined with adjuvant therapies is the standard treatment for patients with stage IIIA/N2 NSCLC. However, the role of postoperative radiotherapy in adjuvant therapies remained controversial. Some studies have been conducted to evaluate the effect of PORT on the survival of patients. A Phase III study, including a small sample cohort of 37 patients, showed that there was no survival difference between the observation and RT arms. 9 In addition, some previous meta‐analyses of randomized trials demonstrated no benefit with PORT, and the use of PORT could even result in a decrease in survival, due to the cardiac and pulmonary toxicity from PORT. 10 , 28 Although one of two meta‐analyses observed that patients with stage III and pN2 had a better survival rate with PORT, the difference was not statistically significant. 10 However, in these studies, either a small sample of patients were included, or patients were treated with PORT in a nonrandomized fashion. At present, a recent randomized trial evaluating the efficacy of PORT for N2 NSCLC patients (NCT00410683, Lung ART in Paris) is still recruiting patients, and is expected to provide stronger evidence. Before this, some population‐based retrospective studies reported that the use of PORT could improve survival for patients with stage N2. 6 , 7 These results are similar to our findings. In this study, we found that patients who received PORT had a lower CSM and OM rate than those who did not. Our results were consistent after the balance in patient characteristics between two groups was achieved. These results made us more confident that patients who received PORT had a better survival rate. The reasons for this may be that PORT can eliminate regions of microscopic disease, and thus reduce locoregional recurrence and improve survival. 16 , 29 , 30 In addition, compared with older techniques that increased the risk of cardiac and pulmonary toxicity, more modern radiation techniques have become safer and bring a greater survival benefit for patients treated with PORT. 8 , 31
Moreover, patients with stage IIIA/N2 NSCLC are a heterogeneous population with different clinicopathological characteristics. Some clinicopathological factors have been reported to be independent risk factors for patients with poor prognosis, such as age, sex, grade, T stage, chemotherapy and LNR. 14 , 15 , 16 , 17 , 18 Mao et al. 32 analyzing data from 1809 patients, developed a nomogram to predict the survival of stage IIIA–N2 NSCLC after surgery, based on certain clinical factors, such as age, sex, grade, histology, positive lymph nodes and lymph nodes examined. Furthermore, a prognostic scoring model, based on age, grade, T stage, chemotherapy and LNR, to predict cancer‐specific survival in resected N2 NSCLC patients who received PORT, has been developed by Deng et al. 14 These prognostic scoring systems showed a higher efficiency in the verification cohort they reported. 14 , 32
In the Asian Thoracic Oncology Research Group expert consensus statement on optimal management of stage III NSCLC by Tan et al., the consensus statement emphasized that PORT should be considered for stage N2 NSCLC patients in whom the benefit outweighs risk of excess toxicity. 5 As IIIA/N2 NSCLC were diseases heterogeneous in levels of age, grade, T stage and LNR, identifying patients who might derive the greatest (or least) benefit from PORT, based on the disease characteristics, was necessary. Some studies had tried to obtain answers. Wisnivesky et al. 33 found that PORT was not associated with improved survival of elderly (age ≥ 65 years) patients with stage III/N2 NSCLC between 1992 and 2005. Although using the identical database, there were also some contrary results because their study only included earlier patients in the SEER database. Our results showed that PORT improved the survival in elderly patients age ≥65 years. The LNR was considered as a practicable prognostic indicator in NSCLC patients and some studies have reported that a higher LNR predicted the benefit of PORT to patients with stage III/N2 NSCLC. 16 , 34 , 35 , 36 A recent study reported that PORT was more suitable for patients with a higher LNR ≥1/3 and a poorer tumor histological differentiation grade. 30 These results were in close agreement with our findings. Furthermore, another recently published retrospective study conducted by Hui et al. 15 suggested that PORT significantly improved patients' survival with three or more of five factors of smoking index ≤400, cN2, stage T3, SCC, and ≥4 positive lymph nodes. However, the sample size of patients for their analysis was small (n = 221), which possibly reduced the explanatory power of prediction model. In this study, based on a population‐based database, we used a patient prognostic score which was developed by Deng et al., 14 and transformed the prognostic score as ordinal factors, as shown in Figure 1. The results showed that the improved survival of patients receiving PORT was significantly associated with the prognostic scores (interaction tests: p < 0.001). Patients with lower prognostic scores presented no significant difference in CSM and OM between the PORT and non‐PORT groups, but among patients with higher prognostic scores, PORT brought a significant reduction in CSM and OM. Before adjusting for potential confounding factors, the Cox proportional hazards analyses and interaction tests yielded consistent results which provided us with more credible evidence.
There were some limitations in this study. First, the major limitation of this study was its retrospective nature. The patients were included in this study between 1988 and 2016. During this period (almost 30 years), the treatment strategy of NSCLC has changed in many aspects. Patients were not grouped in a random way, which might cause confounding factors that affected the results. We applied the inverse scoring weighted matching method to minimize the influence of confounding factors. Second, the surgical margin status was not available in the SEER database which could make a difference in the use of PORT. If surgical margins were positive, the physician would be more likely to recommend PORT and the benefit would therefore be underestimated in our study. Third because patients from the same database were analyzed, there was an overlap in the study population. There are also similarities and any difference in the eligibility criteria or study design. Finally, because of missing detailed information of the surgical approach (lobectomy/pneumonectomy), targeted therapy, immunotherapy, chemotherapy, and PORT (dose and radiation field), our results may be affected.
The strength of our study is that this is the first time the survival benefit of PORT in resected IIIA/N2 NSCLC patients according to a patient prognostic score has been explored. By using a large population‐based database, detection of the absolute difference of survival between the PORT and non‐PORT groups was possible. Furthermore, our results provide information to guide individual treatment options according to prognostic scores that predict which patients could benefit from PORT.
In conclusion, the results of this study validated that the long‐term survival of the entire cohort of IIIA/N2 NSCLC patients who received PORT was significantly better than that of the entire cohort of patients who did not. Furthermore, the patient prognostic score could be used not only to predict the survival of patients receiving PORT, but also determine which patients with resected IIIA/N2 NSCLC may benefit from PORT. Therefore, patient prognostic scores and clinicopathological characteristics may be helpful in proper patient selection for PORT. Given the limitations of this study, larger prospective studies are needed in the future to confirm our findings.
CONFLICT OF INTEREST
All authors declare no conflict of interest.
Supporting information
Figure S1. Flowchart of selection of patients with resected IIIA/N2 NSCLC.
Figure S2. Comparison of (a), cancer‐specific mortality and (b), overall mortality in patients with resected IIIA/N2 NSCLC.
Figure S3. Comparison of cancer‐specific mortality between the PORT group and the non‐PORT group based on prognostic scores.
Figure S4. Comparison of overall mortality between the PORT group and the non‐PORT group based on prognostic scores.
Table S1. Comparison of CSM between two groups according to clinicopathologic factors.
Table S2. Comparison of OM between two groups according to clinicopathologic factors.
ACKNOWLEDGMENTS
This study was funded by the Medical and Health Technology Innovation Project of Chinese Academy of Medical Sciences (2018‐I2M‐3‐003) (Spatial–Temporal Mapping Analysis on Chinese Cancer Burden). We are very grateful to Hui‐min Qu for her assistance with the language. Additionally, for her support in terms of analysis, we would like to express our deep gratitude to Na An (Shanghai Lung Tumor Clinical Medical Center, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China).
Xu L, Xie H‐n, Chen X‐k, Bi N, Qin J‐j, Li Y. Patient prognostic scores and association with survival improvement offered by postoperative radiotherapy for resected IIIA/N2 non‐small cell lung cancer: A population‐based study. Thoracic Cancer. 2021;12:12:760–767. 10.1111/1759-7714.13835
Lei Xu, Hou‐nai Xie, Xian‐kai Chen contributed equally to this work.
Funding information Chinese Academy of Medical Sciences, Grant/Award Number: 2018‐I2M‐3‐003
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2. Auperin A, Le Pechoux C, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta‐analysis of concomitant versus sequential radiochemotherapy in locally advanced non‐small‐cell lung cancer. J Clin Oncol. 2010;28(13):2181–90. [DOI] [PubMed] [Google Scholar]
- 3. Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami‐Porta R, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2(8):706–14. [DOI] [PubMed] [Google Scholar]
- 4. Xu Z, Xing P, Ma D, Zhu Y, Ying J, Li J. Review on treatment modalities for resectable IIIa/N2 non‐small cell lung cancer. Zhongguo Fei Ai Za Zhi. 2019;22(2):111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tan WL, Chua KLM, Lin CC, Lee VHF, Tho LM, Chan AW, et al. Asian Thoracic Oncology Research Group Expert Consensus Statement on Optimal management of stage III NSCLC. J Thorac Oncol. 2020;15(3):324–43. [DOI] [PubMed] [Google Scholar]
- 6. Lally BE, Zelterman D, Colasanto JM, Haffty BG, Detterbeck FC, Wilson LD. Postoperative radiotherapy for stage II or III non‐small‐cell lung cancer using the surveillance, epidemiology, and end results database. J Clin Oncol. 2006;23:2998–3006. [DOI] [PubMed] [Google Scholar]
- 7. Herskovic A, Mauer E, Christos P, Nagar H. Role of postoperative radiotherapy in pathologic stage IIIA (N2) non‐small cell lung cancer in a prospective nationwide oncology outcomes database. J Thorac Oncol. 2017;12(2):302–13. [DOI] [PubMed] [Google Scholar]
- 8. Douillard JY, Rosell R, De Lena M, Riggi M, Hurteloup P, Mahe MA. Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, stage II, or stage IIIA non‐small‐cell lung cancer treated with adjuvant chemotherapy: the adjuvant Navelbine International Trialist Association (ANITA) Randomized Trial. Int J Radiat Oncol Biol Phys. 2008;72:695–701. [DOI] [PubMed] [Google Scholar]
- 9. Perry MC, Kohman LJ, Bonner JA, Gu L, Wang X, Vokes EE, et al. A phase III study of surgical resection and paclitaxel/carboplatin chemotherapy with or without adjuvant radiation therapy for resected stage III non‐small‐cell lung cancer: cancer and Leukemia Group B 9734. Clin Lung Cancer. 2007;8(4):268–72. [DOI] [PubMed] [Google Scholar]
- 10. PORT Meta‐analysis Trialists Group . Postoperative radiotherapy in non‐small‐cell lung cancer: systematic review and meta‐analysis of individual patient data from nine randomised controlled trials. Lancet. 1998;352(9124):257–63. [PubMed] [Google Scholar]
- 11. National Comprehensive Cancer Network . NCCN guidelines version 4. 2016. Non‐small cell lung cancer. http://www.nccn.org. Accessed 22 Feb 2016. [DOI] [PMC free article] [PubMed]
- 12. Kris MG, Gaspar LE, Chaft JE, Kennedy EB, Azzoli CG, Ellis PM, et al. Adjuvant systemic therapy and adjuvant radiation therapy for stage I to IIIA completely resected non‐small‐cell lung cancers: American Society of Clinical Oncology/Cancer Care Ontario clinical practice guideline update. J Clin Oncol. 2017;35(25):2960–74. [DOI] [PubMed] [Google Scholar]
- 13. Rodrigues G, Choy H, Bradley J, Rosenzweig KE, Bogart J, Curran WJ Jr, et al. Adjuvant radiation therapy in locally advanced non‐small cell lung cancer: executive summary of an American Society for Radiation Oncology (ASTRO) evidence‐based clinical practice guideline. Pract Radiat Oncol. 2015;5(3):149–55. [DOI] [PubMed] [Google Scholar]
- 14. Deng W, Xu T, Xu Y, Wang Y, Liu X, Zhao Y, et al. Survival patterns for patients with resected N2 non‐small cell lung cancer and postoperative radiotherapy: a prognostic scoring model and heat map approach. J Thorac Oncol. 2018;13(12):1968–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hui Z, Dai H, Liang J, Lv J, Zhou Z, Feng Q, et al. Selection of proper candidates with resected pathological stage IIIA‐N2 non‐small cell lung cancer for postoperative radiotherapy. Thorac Cancer. 2015;6(3):346–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zeng WQ, Feng W, Xie L, Zhang CC, Yu W, Cai XW, et al. Postoperative radiotherapy for resected stage IIIA‐N2 non‐small‐cell lung cancer: a population‐based time‐trend study. Lung. 2019;197(6):741–51. [DOI] [PubMed] [Google Scholar]
- 17. Liu T, Mu Y, Dang J, Li G. The role of postoperative radiotherapy for completely resected pIIIA‐N2 non‐small cell lung cancer patients with different clinicopathological features: a systemic review and meta‐analysis. J Cancer. 2019;10(17):3941–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kou P, Wang H, Lin J, Zhang Y, Yu J. Male patients with resected IIIA‐N2 non‐small‐cell lung cancer may benefit from postoperative radiotherapy: a population‐based survival analysis. Future Oncol. 2018;14(23):2371–81. [DOI] [PubMed] [Google Scholar]
- 19. AJCC, UICC . Cancer survivor analysis. In: Edge S, Byrd D, Compton C, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. New York: Springer; 2009. p. 17. [Google Scholar]
- 20. Boffa DJ, Detterbeck FC, Smith EJ, Rami‐Porta R, Crowley J, Zelterman D, et al. Should the 7th edition of the lung cancer stage classification system change treatment algorithms in non‐small cell lung cancer? J Thorac Oncol. 2010;5(11):1779–83. [DOI] [PubMed] [Google Scholar]
- 21. Sagara Y, Freedman RA, Vaz‐Luis I, Mallory MA, Wong SM, Aydogan F, et al. Patient prognostic score and associations with survival improvement offered by radiotherapy after breast‐conserving surgery for ductal carcinoma in situ: a population‐based longitudinal cohort study. J Clin Oncol. 2016;34(11):1190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sagara Y, Mallory MA, Wong S, Aydogan F, DeSantis S, Barry WT, et al. Survival benefit of breast surgery for low‐grade ductal carcinoma in situ: a population‐based cohort study. JAMA Surg. 2015;150(8):739–45. [DOI] [PubMed] [Google Scholar]
- 23. Stürmer T, Rothman KJ, Avorn J, Glynn RJ. Treatment effects in the presence of unmeasured confounding: dealing with observations in the tails of the propensity score distribution—a simulation study. Am J Epidemiol. 2010;172(7):843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arriagada R, Bergman B, Dunant A, le Chevalier T, Pignon JP, Vansteenkiste J, et al. Cisplatin‐based adjuvant chemotherapy in patients with completely resected non‐small‐cell lung cancer. N Engl J Med. 2004;350:351–60. [DOI] [PubMed] [Google Scholar]
- 25. Winton T, Livingston R, Johnson D, Rigas J, Johnston M, Butts C, et al. Vinorelbine plus cisplatin vs. observation in resected non‐small‐cell lung cancer. N Engl J Med. 2005;352:2589–97. [DOI] [PubMed] [Google Scholar]
- 26. Douillard JY, Rosell R, De Lena M, Carpagnano F, Ramlau R, Gonzáles‐Larriba JL. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB‐IIIA non‐small‐cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7:719–27. [DOI] [PubMed] [Google Scholar]
- 27. Le Péchoux C. Role of postoperative radiotherapy in resected non‐small cell lung cancer: a reassessment based on new data. Oncologist. 2011;16(5):672–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Group PM‐aT . Postoperative radiotherapy for non‐small cell lung cancer. Cochrane Database Syst Rev. 2005;2:CD002142. [DOI] [PubMed] [Google Scholar]
- 29. Liu H, Xu Y, Wang M, Hu K, Ma M, Zhong W, et al. Postoperative survival of patients with stage IIIa non‐small cell lung cancer. Zhongguo Fei Ai Za Zhi. 2013;16(11):596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wei W, Zhou J, Zhang Q, Liao DH, Liu QD, Zhong BL, et al. Postoperative intensity‐modulated radiation therapy reduces local recurrence and improves overall survival in III‐N2 non‐small‐cell lung cancer: a single‐center, retrospective study. Cancer Med. 2020;9(8):2820–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lally BE, Detterbeck FC, Geiger AM, Thomas CR, Machtay M, Miller AA, et al. The risk of death from heart disease in patients with nonsmall cell lung cancer who receive postoperative radiotherapy: analysis of the surveillance, epidemiology, and end results database. Cancer. 2007;110:911–7. [DOI] [PubMed] [Google Scholar]
- 32. Mao Q, Xia W, Dong G, Chen S, Wang A, Jin G, et al. A nomogram to predict the survival of stage IIIA‐N2 non‐small cell lung cancer after surgery. J Thorac Cardiovasc Surg. 2018;155(4):1784–92.e3. [DOI] [PubMed] [Google Scholar]
- 33. Wisnivesky JP, Halm EA, Bonomi M, Smith C, Mhango G, Bagiella E. Postoperative radiotherapy for elderly patients with stage III lung cancer. Cancer. 2012;118(18):4478–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Urban D, Bar J, Solomon B, Ball D. Lymph node ratio may predict the benefit of postoperative radiotherapy in non‐small‐cell lung cancer. J Thorac Oncol. 2013;8(7):940–6. [DOI] [PubMed] [Google Scholar]
- 35. Jonnalagadda S, Arcinega J, Smith C, Wisnivesky JP. Validation of the lymph node ratio as a prognostic factor in patients with N1 nonsmall cell lung cancer. Cancer. 2011;117(20):4724–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shinde A, Horne ZD, Li R, Glaser S, Massarelli E, Koczywas M, et al. Optimal adjuvant therapy in clinically N2 non‐small cell lung cancer patients undergoing neoadjuvant chemotherapy and surgery: the importance of pathological response and lymph node ratio. Lung Cancer. 2019;133:136–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flowchart of selection of patients with resected IIIA/N2 NSCLC.
Figure S2. Comparison of (a), cancer‐specific mortality and (b), overall mortality in patients with resected IIIA/N2 NSCLC.
Figure S3. Comparison of cancer‐specific mortality between the PORT group and the non‐PORT group based on prognostic scores.
Figure S4. Comparison of overall mortality between the PORT group and the non‐PORT group based on prognostic scores.
Table S1. Comparison of CSM between two groups according to clinicopathologic factors.
Table S2. Comparison of OM between two groups according to clinicopathologic factors.


